Abstract
Background
The combination of skeletal muscle wasting and compromised function plays a role in the health decline commonly observed in chronic kidney disease (CKD) patients, but the pathophysiology of muscle mass/strength changes remains unclear. The purpose of this study was to characterize muscle properties in the Cy/+ rat model of spontaneously progressive CKD.
Methods
Leg muscle function and serum biochemistry of male Cy/+ (CKD) rats and their nonaffected littermates (NLs) were assessed in vivo at 25, 30 and 35 weeks of age. Architecture and histology of extensor digitorum longus (EDL) and soleus (SOL) muscles were assessed ex vivo at the conclusion of the experiment. We tested the hypothesis that animals with CKD have progressive loss of muscle function, and that this functional deficit is associated with loss of muscle mass and quality.
Results
Thirty-five-week-old CKD rats produced significantly lower maximum torque in ankle dorsiflexion and shorter time to maximum torque, and longer half relaxation time in dorsiflexion and plantarflexion compared with NL rats. Peak dorsiflexion torque (but not plantarflexion torque) in CKD remained steady from 25 to 35 weeks, while in NL rats, peak torque increased. Mass, physiologic cross-sectional area (CSA) and fiber-type (myosin heavy chain isoform) proportions of EDL and SOL were not different between CKD and NL. However, the EDL of CKD rats showed reduced CSAs in all fiber types, while only MyHC-1 fibers were decreased in area in the SOL.
Conclusions
The results of this study demonstrate that muscle function progressively declines in the Cy/+ rat model of CKD. Because whole muscle mass and architecture do not vary between CKD and NL, but CKD muscles show reduction in individual fiber CSA, our data suggest that the functional decline is related to increased muscle fiber atrophy.
Keywords: atrophy, chronic kidney disease, muscle function, muscle quality, rat models
INTRODUCTION
Over 20 million American adults (or 1 in 10 adults) suffer from chronic kidney disease (CKD). As CKD progresses, patients manifest phenotypes of accelerated aging with increased burden of falls, immobility and loss of functional independence that leads to hospitalizations and mortality. Studies of human patients have documented that the loss of muscle mass is common and progressive in CKD [1–4]. This is accompanied by compromised muscle function in patient populations [5–7] leading to the common conclusion that loss of mass is the key factor driving functional loss. Yet disease-associated muscle functional capacity changes depend on the relative changes of both muscle mass and intrinsic properties of the muscle [7–10] (also referred to as muscle quality [11, 12]). While the metabolic properties of CKD skeletal muscle biopsies are altered [7, 13, 14], few studies have systematically assessed muscle mass, function and intrinsic properties to understand the tissue-level changes that occur to muscle in CKD.
Recent investigations have capitalized on in vivo imaging modalities such as dual X-ray absorptiometry and peripheral quantitative computed tomography to quantify lean mass or anatomical cross-sectional area (CSA), respectively [15–17]. These imaging-based estimates of muscle mass or size have yielded mixed results, with some studies finding lean muscle deficits in CKD and others finding no deficits when compared with age-matched normal controls [15–17]. A recent study by Lee et al. [18] showed that muscle torque relative to muscle (anatomical) CSA is lower in CKD patients and progressively decreases with the advance of CKD. This study emphasizes that muscle functional deficits in CKD may be due to more than simply changes in muscle mass, but in vitro studies are required to confirm.
The purpose of the present study was to characterize muscle function and structure in a rat model of spontaneously progressive CKD. We hypothesized that animals with CKD would experience progressive loss of muscle function and that this would be associated with loss of muscle mass and quality.
MATERIALS AND METHODS
Animals
This study used Cy/+ rats (CKD)—Han Sprague-Dawley rats with autosomal dominant polycystic disease due to genetic defects in the samcystin gene [19, 20]—and their normal nonaffected littermates (NLs) (n = 7 per group). Male heterozygotes (Cy/+) develop azotemia at ∼10 weeks of age with a progressive rise in blood urea nitrogen (BUN) and creatinine to terminal uremia by ∼40 weeks of age. As we have shown previously, by 30 weeks of age, untreated CKD rats develop biochemical abnormalities that parallel advanced CKD (Stage 4) in humans, including secondary hyperparathyroidism and hyperphosphatemia [21]; these abnormalities further progress by 35 weeks of age [22]. In addition to biochemical abnormalities, these animals have been shown to spontaneously develop both of the other manifestations of CKD-Mineral Bone Disorder: extraskeletal calcification and abnormal bone [21, 23–26]. Therefore, this animal model faithfully represents the course of progressive kidney disease in humans.
For the present study, animals were fed a casein diet (Purina AIN-76A, Purina Animal Nutrition, Shreveport, LA, USA; 0.53% Ca and 0.56% P) beginning at 24 weeks, which has been shown to produce a more consistent kidney disease in this rat model [21]. At 25, 30 and 35 weeks of age, animals underwent in vivo assessment of leg muscle function (ankle torque) in dorsiflexion and plantarflexion. At the end of the experiment, animals were euthanized by CO2 and muscles were collected for analysis. All procedures were approved by the Indiana University School of Medicine Animal Care and Use Committee prior to the start of this study.
Serum biochemistry
Blood was collected at 25, 30 and 35 weeks of age via tail vein. Blood plasma was analyzed for BUN using colorimetric assays (BioAssay Systems, Hayward, CA, USA). Intact parathyroid hormone (PTH) was determined by enzyme-linked immunosorbent assay (Alpco, Salem, NH, USA).
In vivo muscle function
Under inhalation isoflurane anesthesia, the skin of the right leg of each rat was shaved and prepared aseptically, and the animal was transferred to a muscle function testing system (1305A Whole Rat Test System, Aurora Scientific Inc., Aurora, ON, Canada). With the animal in supine position, its right foot was secured to a foot pedal attached to a servomotor. With the ankle in 90° of dorsiflexion and the leg positioned perpendicular to the foot pedal, the knee was clamped in 90° of flexion and two sterile shielded monopolar stimulated electrodes were inserted near the common peroneal nerve under the skin in order to stimulate a dorsiflexion twitch response of the anterior crural musculature. To illicit plantarflexion, electrodes were placed subcutaneously near the tibial nerve in the posterior crural compartment. Electrode placement and stimulation current were adjusted to achieve maximum twitch response and then increased by an additional ∼20% to ensure supramaximal stimulation of muscle fibers (∼15 mA for dorsiflexion; ∼60 mA for plantarflexion). Supramaximal twitch responses were monitored carefully to ensure that antagonist muscles were not recruited during function testing. Maximum isometric torque (N m) was recorded for various stimulation frequencies ranging between 10 and 200 Hz, with a pulse width of 0.2 ms and train duration of 200 ms. Following testing, time to maximum torque and half relaxation time were extracted from the peak isometric torque data. In vivo muscle testing protocols were modified from previously published protocols [27, 28].
Muscle harvesting
At sacrifice, extensor digitorum longus (EDL) and soleus (SOL) muscles were excised for immunohistochemical and histological examination. Muscles were blotted dry, proximal and distal tendons were removed, and muscle belly length, belly mass and surface pinnation angles of fascicles were measured for the calculation of physiologic CSA (PCSA) [29]. Because assessment of muscle architecture is destructive by nature, fascicle length was estimated from published muscle-specific fascicle-to-tendon ratios [29] following previous studies [30] in order to preserve muscle bellies for histologic measurement of fiber CSAs. The middle of the muscle bellies (∼1 cm3) was removed, mounted to cork blocks using tissue freezing medium (TBS, Durham, NC, USA), flash frozen using isopentane cooled in liquid nitrogen and then stored at −80°C.
Immunohistochemistry and histology
Using a cryostat, muscle blocks were cut into transverse serial cross sections (10 µm) for immunohistochemical reactions. Serial muscle sections for each animal were reacted with primary monoclonal antibodies (mAbs) (DSHB, University of Iowa) specific to slow myosin heavy chain 1 [MyHC-1 (S58)], fast MyHC-2A (SC-71) and MyHC-1, -2A, -2B (BF35) to determine fiber-type percentages. Concentrates of mAbs were diluted 1:200 in phosphate-buffered saline (pH 7.4). Using the Histostain Plus kit (Invitrogen), serial cross sections were blocked using 5% goat serum, incubated with primary mAbs overnight at 4°C and then reacted with a biotinylated (anti-mouse) secondary antibody labeled with a streptavidin horseradish peroxidase enzyme conjugate. Positive reactions were visualized using DAB chromagen and hematoxylin counter stain to visualize fiber morphology and nuclei (Supplementary data, Figure S1).
Muscle fiber-type percentage was determined in the entire muscle cross section by counting the total number of positive reacted fibers for each specific mAb and dividing that number by the total number of fibers. Muscle fiber-type-specific CSAs were traced and measured manually (∼300 fibers per muscle) in ImageJ (v.1.43).
Statistics
All statistics were performed in SPSS software. Possible interactions between genotype and age for body mass, serum biochemistries and muscle function parameters were examined using a two-factor mixed-model repeated-measures analysis of variance (ANOVA). Data were further evaluated using single-factor repeated-measures ANOVAs and post hoc multiple pairwise comparisons (Bonferroni correction) within genotype to assess changes in parameter means across time points, and independent samples t-tests were used to compare parameter means between genotypes at a given age. Histomorphometric data were also evaluated with independent sample t-tests between genotypes. Pearson product–moment correlations were used to compare serum biochemistry outcomes with muscle function parameters. A priori α levels for statistical tests were set at 0.05.
RESULTS
Body mass and serum biochemistry
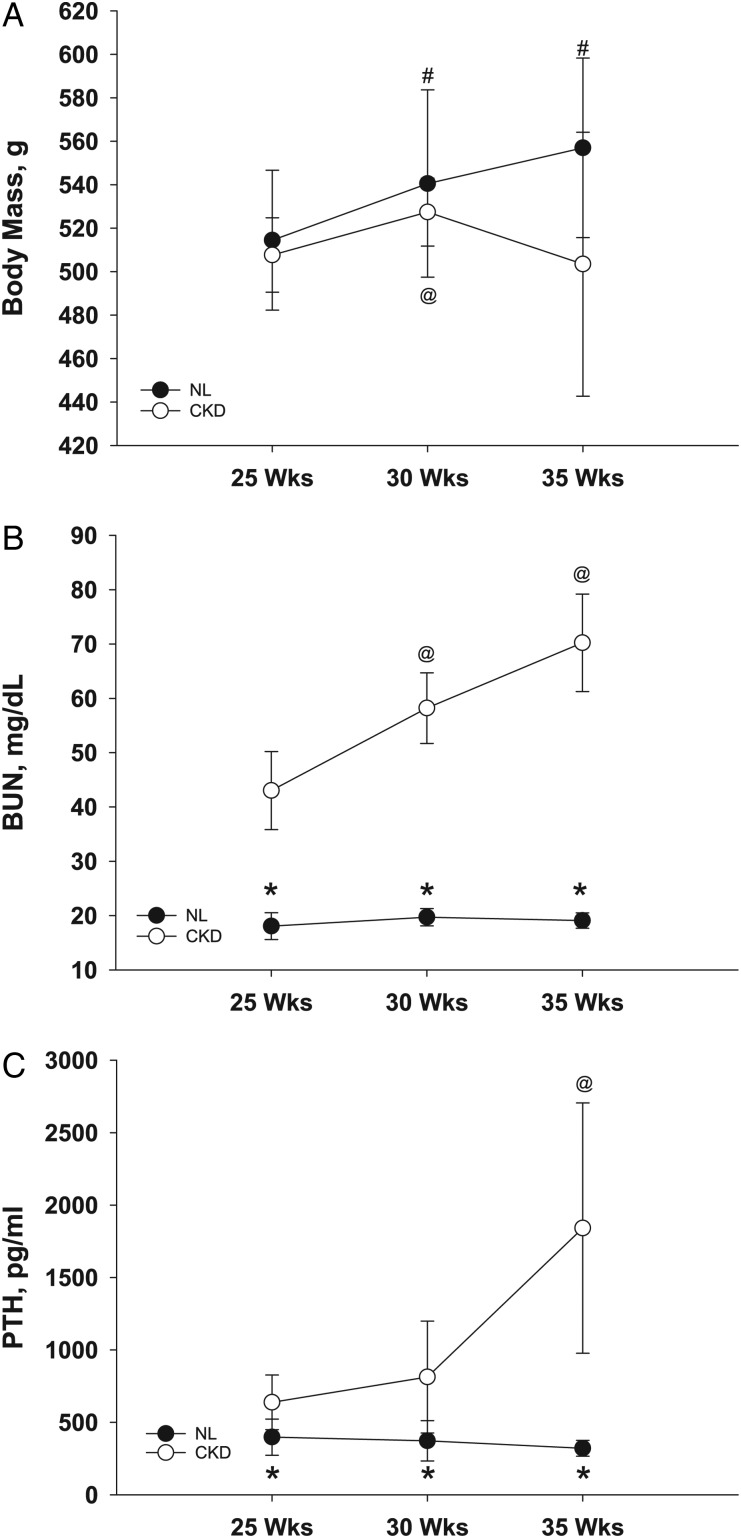
There was no interaction between genotype and age for body mass (two-factor repeated-measures ANOVA). NL animals gained body mass throughout the experiment (F[2,5] = 50.42, P < 0.001), and CKD animals gained body mass from 25 to 30 weeks, but at 35 weeks, they had begun to lose mass (F[2,5] = 15.27, P = 0.007) (Figure 1A). Yet even with the drop in body mass at 35 weeks, there was no difference in body mass between CKD and NL animals at any age (Figure 1A).
FIGURE 1:
Body mass (A) and serum levels of BUN (B) and PTH (C) of CKD and NL rats. Data are shown for 25, 30 and 35 weeks of age. Values are mean ± SD. *Significantly different from CKD at same age (P < 0.05); #significantly different from NL at 25 weeks (P < 0.05); @significantly different from CKD at 25 weeks (P < 0.05).
There was a significant interaction between genotype and age for plasma BUN levels (F[2,11] = 22.32, P < 0.001) as well as PTH levels (F[2,11] = 7.52, P = 0.009). Neither of the plasma biochemistries changed with age in NL animals, while in CKD animals, BUN (F[2,5] = 25.31, P = 0.002) (Figure 1B) and PTH (F[2,5] = 6.25, P = 0.04) (Figure 1C) both increased with age. Additionally, BUN and PTH levels were both significantly higher in CKD animals at all ages: 25 weeks (BUN: t[12] = −8.67, P < 0.001; PTH: t[12] = −2.82, P = 0.016), 30 weeks (BUN: t[12] = −15.27, P < 0.001; PTH: t[12] = −2.84, P = 0.015) and 35 weeks (BUN: t[12] = −14.89, P < 0.001; PTH: t[12] = −4.64, P = 0.001) (Figure 1B and C).
Muscle function
There was no interaction between genotype and age for any of the dorsiflexion measures: maximum torque, time to maximum torque or half relaxation time (two-factor repeated-measures ANOVA). Maximum dorsiflexion torque increased with age among NL animals (F[2,5] = 20.21, P = 0.004) (single-factor repeated-measures ANOVA), although there was no such effect of age among CKD animals (Figure 2A). At 25 and 30 weeks of age, there was no significant main effect of genotype on maximum dorsiflexion torque, although by 35 weeks, CKD animals produced significantly lower (−21%) torque (t[11] = 2.456, P = 0.03) (Figure 2A). Time to maximum dorsiflexion torque decreased with age in NL animals (F[2,5] = 103.63, P < 0.001) and CKD animals (F[2,4] = 521.17, P < 0.001), and CKD animals exhibited shorter times to maximum torque at 30 weeks (−54%, t[12] = 8.19, P < 0.001) and 35 weeks (−44%, t[11] = 2.67, P = 0.02) of age compared with NL animals (Figure 2B). Dorsiflexion half relaxation time increased with age in CKD animals (F[2,4] = 19.68, P < 0.001) (single-factor repeated-measures ANOVA), but there was no effect of age on half relaxation time in NL animals. CKD animals had significantly longer dorsiflexion half relaxation times than NL animals at 30 weeks (+79%, t[12] = −7.99, P < 0.001) and 35 weeks (+34%, t[11] = −2.66, P = 0.02) of age (Figure 2C).
FIGURE 2:
In vivo dorsiflexion maximum isometric torque (A), time to maximum torque (B) and half relaxation time (C). Data are shown for CKD and NL rats at 25, 30 and 35 weeks of age. Values are mean ± SD. *Significantly different from CKD at same age (P < 0.05); #significantly different from NL at 25 weeks (P < 0.05); @significantly different from CKD at 25 weeks (P < 0.05).
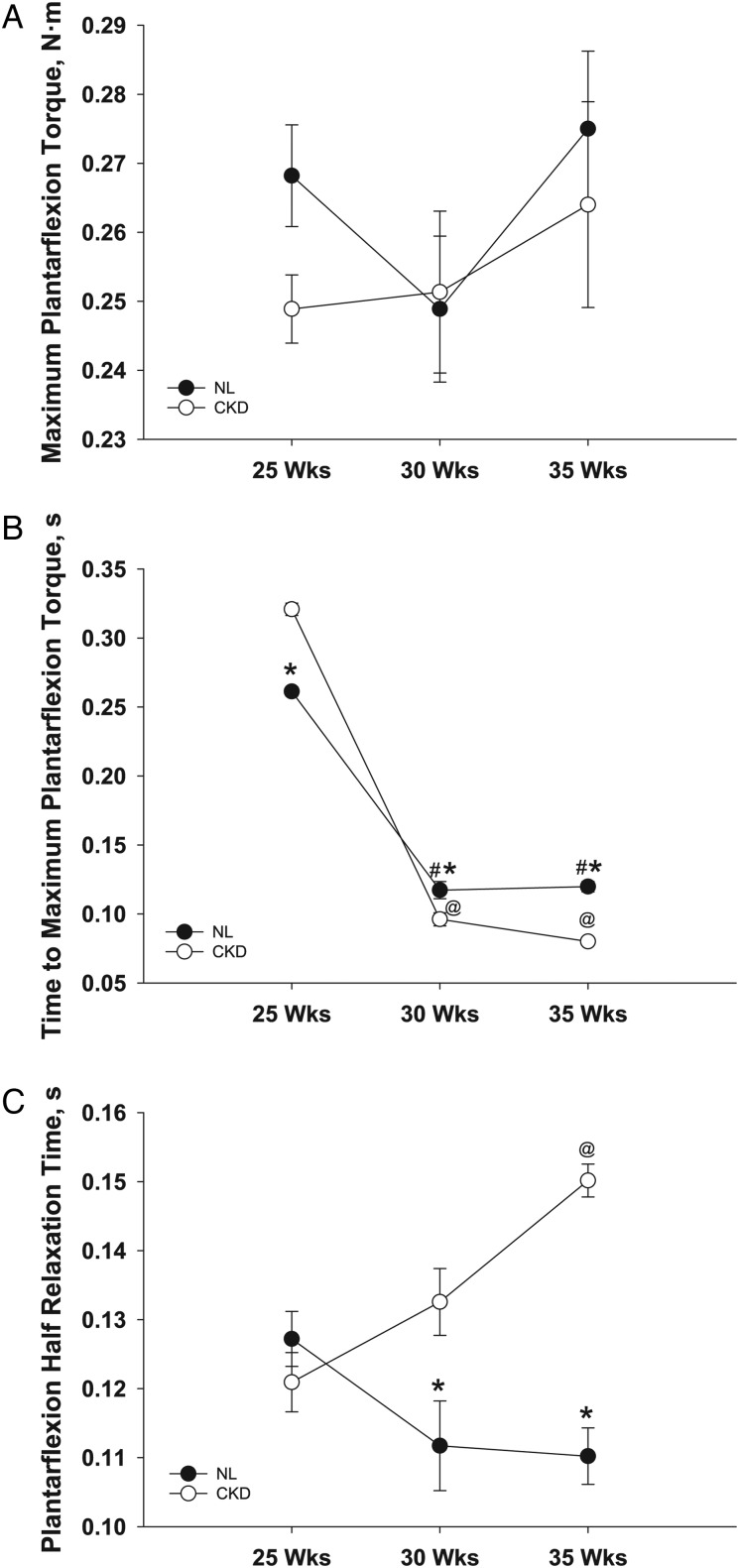
There was no interaction between genotype and age for maximum plantarflexion torque (two-factor repeated-measures ANOVA), no significant main effect of age within either genotype (single-factor repeated-measures ANOVA) and no differences between genotypes at any age (t-test) (Figure 3A). There were significant interactions, however, between the two factors for time to maximum plantarflexion torque (F[2,10] = 45.87, P < 0.01) and plantarflexion half relaxation time (F[2,10] = 13.88, P < 0.01) (two-factor repeated-measures ANOVA) (Figure 3B and C). Time to maximum plantarflexion torque decreased with age in both genotypes (CKD: F[2,4] = 782.04, P < 0.01; NL: F[2,5] = 194.87, P < 0.01) (single-factor repeated-measures ANOVA), and there were significant differences between the genotypes at all three ages. However, because of the interaction between factors, it took a longer time for CKD animals to reach maximum plantarflexion torque at 25 weeks of age (+23%, t[13] = −5.44, P < 0.01), but a shorter time at 30 weeks (−18%, t[12] = 2.71, P = 0.02) and 35 weeks (−33%, t[11] = 8.62, P < 0.01) of age (Figure 3B). Plantarflexion half relaxation time increased with age in CKD animals (F[2,4] = 14.79, P = 0.01) (single-factor repeated-measures ANOVA), but there was no effect of age on half relaxation time in NL animals. CKD animals had significantly longer plantarflexion half relaxation times than NL animals at 30 weeks (+19%, t[12] = −2.58, P = 0.02) and 35 weeks (+36%, t[11] = −8.06, P < 0.01) of age (Figure 3C).
FIGURE 3:
In vivo plantarflexion maximum isometric torque (A), time to maximum torque (B) and half relaxation time (C). Data are shown for CKD and NL rats at 25, 30 and 35 weeks of age. Values are mean ± SD. *Significantly different from CKD at same age (P < 0.05); #significantly different from NL at 25 weeks (P < 0.05); @significantly different from CKD at 25 weeks (P < 0.05).
There was no correlation between these muscle parameters and serum levels of BUN or PTH.
Muscle mass, fiber type and architecture
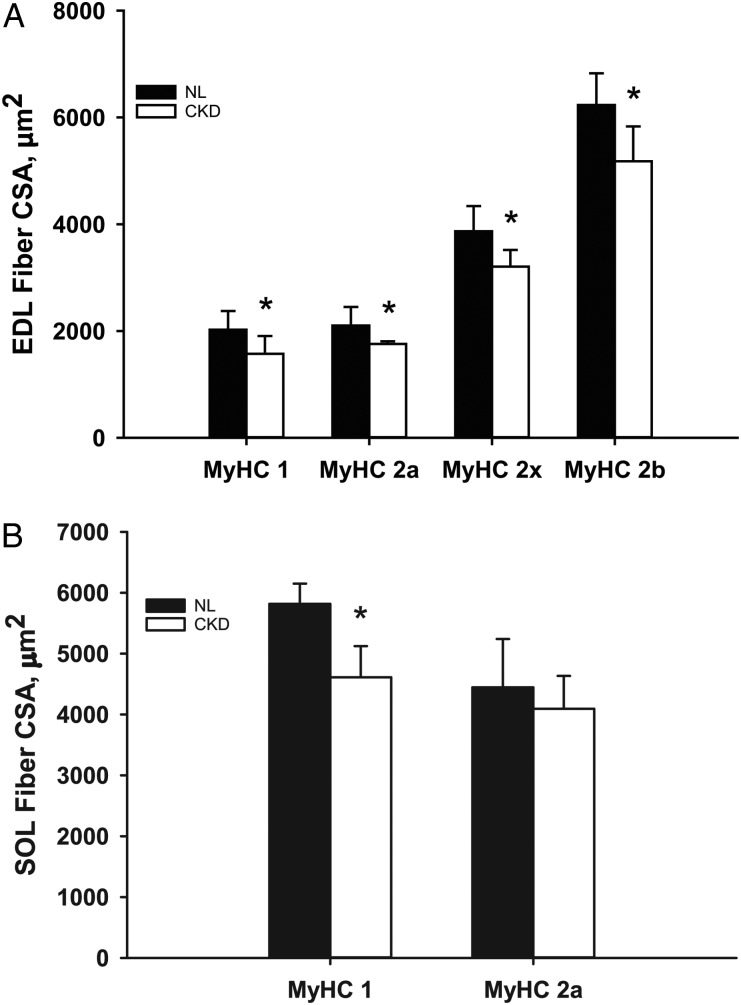
There was no difference between CKD and NL animals in whole muscle mass (Figure 4) or PCSA (Figure 5) of EDL or SOL muscles. Although PCSA was not different, there were significant differences in fiber-type-specific CSA. All fiber types of the EDL (MyHC-1, MyHC-2A, MyHC-2X and MyHC-2B) had smaller CSAs in CKD animals compared with NL animals (Figure 6A), while only MyHC-1 fibers of the SOL muscle were smaller in CKD animals (Figure 6B). There was no difference in fiber-type proportions between CKD and NL animals (Figure 7).
FIGURE 4:
Whole muscle mass of the EDL and SOL muscles of 35-week-old CKD and NL rats. Values are mean ± SD.
FIGURE 5:
Whole muscle PCSA of the EDL and SOL muscles of 35-week-old CKD and NL rats. Values are mean ± SD.
FIGURE 6:
Muscle fiber-type-specific CSA measured in the EDL (A) and SOL (B) muscles of 35-week-old CKD and NL rats. Values are mean ± SD. Asterisks indicate values that are significantly different (P < 0.05) between CKD and NL animals.
FIGURE 7:
Fiber-type proportions of the EDL (A) and SOL (B) muscles of 35-week-old CKD and NL rats. Values are mean ± SD.
DISCUSSION
The purpose of this study was to characterize muscle structure and function in an animal model of progressive kidney disease. Compared with their normal NLs, Cy/+ (CKD) rats have compromised muscle function, as illustrated by reduced maximum muscle torque (muscle strength) and increased half relaxation time. These functional deficits can be explained in part by changes in muscle quality, those characteristics of muscle that influence function independent of muscle mass.
Morphometric parameters, such as whole muscle mass, whole muscle PCSA and muscle fiber-type (myosin heavy chain isoform) proportions of the SOL and EDL muscles did not differ between CKD and NL rats. It is possible that the CKD animals had increased total body water and, in turn, muscle water and that may have accounted for the equivalent muscle mass. However, in a study of kidney transplant recipients with Stage 2–3 CKD, the total body water measured by bioimpedence analysis was actually significantly lower in patients with sarcopenia [31]. These data suggest that the progressive decline in muscle function is not related to a loss of muscle mass. Instead, it is possible that reduced muscle function in the CKD rats is related to other factors, such as protein–energy wasting (protein degradation) or changes in the metabolic properties of the muscles themselves [1, 10]. Metabolic diseases such as CKD are often associated with shifts in fiber-type proportions. Fiber-type proportion data presented here contrast with previous studies in humans that have demonstrated shifts in the proportions of fast and slow fiber types, although those studies have had mixed results. One study showed a significant shift toward a higher proportion of fast contracting fibers (MyHC-2A/X/B) in the quadriceps femoris muscles of dialysis patients [13], another showed a nonsignificant shift toward faster contracting fibers [7] and still another study showed the opposite shift, with a significant transition toward a higher proportion of slow contracting fibers (MyHC-1) [14]. The disagreement among human studies and between humans and rats (presented here) may be due in part to species differences, and also could be the result of examining different muscles. Our study measured fiber-type proportions in SOL and EDL, which have different functions than the quadriceps (traditionally assessed in the human studies), and therefore could be expected to have different metabolic profiles. SOL is composed of predominantly MyHC-1 fibers, and EDL is composed of predominantly MyHC-2 fibers. It is possible that shifts in fiber-type proportions are difficult to see with such small percentages of the other types of fibers in these muscles. Future examination of muscles with more mixed fiber-type proportions, such as gastrocnemius and tibialis anterior, may allow us to more easily detect transitions in fiber-type proportions, which could help us better interpret the conflicting reports in human patient studies.
Additionally, the current study measured fiber-type proportions from a cross section of the midbelly of these muscles, whereas the human studies were conducted on muscle biopsies. There is some evidence that fiber-type proportions vary along the length and depth of skeletal muscles [32, 33]. Therefore, if the location of muscle biopsy is not standard within and among studies, results characterizing fiber-type proportions could be affected. Our data are not subject to this potential problem as they were all collected from complete midbelly sections of the select musculature. Yet, even without differences in fiber-type proportions, fiber-type-specific CSAs were significantly smaller (all fiber types in EDL; Type I fibers only in SOL) in CKD rats, indicating uniform abnormalities across fiber types.
Muscle contraction is initiated by calcium release from the sarcoplasmic reticulum in response to an action potential. Muscle relaxation occurs when the sarcoplasmic reticulum sequestrates calcium following contraction. Time to maximum torque and half relaxation time, therefore, can be affected by factors that change or disrupt the processes of calcium release and sequestration. Muscle function data presented here are in general agreement with studies in patient populations. As predicted, muscle strength is diminished in our CKD rats. Time to maximum torque is shorter in the CKD rats, as well. But perhaps the more interesting result is the increased half relaxation time of CKD muscles. If time to maximum torque and half relaxation time were both increased or both decreased (i.e. altered in the same direction), we might expect to see transitions between fiber types when examining muscles histologically (i.e. from slower to faster MyHC isoforms, or vice versa). However, our data demonstrate a decrease in time to maximum torque and an increase in half relaxation time in CKD animals. We interpret shorter time to peak torque as a result of producing lower maximum torque in dorsiflexion (nonsignificant in plantarflexion)—it takes less time to reach the maximum. Longer half relaxation times in CKD animals, however, suggest that there may be a problem with calcium uptake by the sarcoplasmic reticulum at the termination of contraction. We have previously identified abnormalities in calcium transport mRNA and protein expression in cardiac muscle [34] in these animals leading to increased calcium transients, strongly suggesting abnormal intracellular calcium signaling. Specifically, the mRNA levels of the sodium calcium-exchanger type 1 were upregulated in CKD rats, and the L-type calcium channel, sarco/endoplasmic reticulum calcium ATPase type 2a, was downregulated in CKD rats [34]. Furthermore, we cannot rule out the possibility that pathological post-translational modifications of actin and myosin, such as occurs in normal aging [35], could affect muscle function. Future studies should be undertaken to explore these potential mechanisms further.
Slow relaxation of muscles has been reported for a variety of diseases, including osteomalacia [36], hypothyroidism [37] and muscular dystrophies [38, 39]. In the context of CKD, the quadriceps femoris muscles of dialysis patients demonstrated a significant increase in muscle relaxation time compared with healthy subjects [7]. The increased muscle relaxation time in dialysis patients was hypothesized to be due to one of two potential mechanisms: atrophy of MyHC-2A fibers (those with fast contractile properties and high oxidative capacity) or lower ATP concentrations in muscle [7]. In the present study, there were no differences between CKD and NL rats with regard to fiber-type proportions, but all fiber types (not just MyHC-2A fibers) were atrophied in CKD rats. Therefore, the muscle atrophy observed here might reflect activation of protein degradation (protein–energy wasting) via the ubiquitin–proteasome system in muscle [1, 4]. Alternatively or additionally, these results could occur with decreased protein synthesis, disturbed autophagic pathways and/or disrupted myofiber regeneration. The pathogenesis of the differences in muscle quality and torque—whether related to calcium uptake by the sarcoplasmic reticulum, muscle fiber atrophy, protein synthesis, muscle regeneration or with regard to ATP concentration—will require further study.
Our results should be interpreted in the context of some limitations. Although our sample size was sufficient to show significant differences in key muscle function parameters, it is possible that larger studies may be needed to show differences in other parameters, especially in the widely variable biochemical changes. Further, we did not examine the architecture of the two major muscles producing dorsiflexion and plantarflexion: tibialis anterior and gastrocnemius, respectively. Therefore, our conclusion of reduced muscle quality in CKD is based on two relatively smaller muscles in the dorsiflexor and plantarflexor groups. Future studies will need to incorporate histological and architectural assessments of tibialis anterior and gastrocnemius. And along those same lines, assessing muscle contractions of SOL and extensor digitorum in vitro would allow us to make more definitive statements about muscle function differences that result from altered muscle quality, as the torque measurements that we made in vivo characterized the function of more than just these two muscles.
In conclusion, our data demonstrate that muscle function—particularly maximum isometric dorsiflexion torque—progressively declines in a rat model of CKD, mirroring the functional phenotype in human patients. We also demonstrate that muscle functional deficits are associated with a decline in muscle quality. In particular, individual muscle fibers experience atrophy, but whole muscles do not change in size (mass or PCSA). Future work with this model will focus on the pathogenesis of abnormal muscle quality and function to facilitate the development of appropriate therapies to treat the muscle wasting phenotype of CKD.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
AUTHORS’ CONTRIBUTIONS
J.M.O., S.M.M. and M.R.A. conceived and designed the experiments; A.S., P.P., J.E.J., K.C.B., J.E.R. and N.X.C. performed the experiments; J.M.O., N.X.C., K.G.A., S.M.M. and M.R.A. analyzed the data and J.M.O., J.E.R, K.G.A., S.M.M. and M.R.A. contributed to the writing of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report. The results of this research have not been published previously in whole or in part, except in abstract format.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Joey Wallace, Drew Brown, Chris Newman, Keith Condon, Shannon Roy and Kali O'Neill for technical assistance and Vince Gattone II, who developed the Cy/+ rat model and inspired us all. This study was funded by a pilot grant from the National Skeletal Muscle Research Center at the University of California-San Diego, as a subcontract of NIH 5R24HD050837-10. The animal colony is funded on NIH R01AR058005.
REFERENCES
- 1.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010; 91: 1128S–1132S [DOI] [PubMed] [Google Scholar]
- 2.Lin J, Curhan GC. Kidney function decline and physical function in women. Nephrol Dial Transplant 2008; 23: 2827–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley RN, Wang C, Ishani A et al. Kidney function and sarcopenia in the United States General Population: NHANES III. Am J Nephrol 2007; 27: 279–286 [DOI] [PubMed] [Google Scholar]
- 4.Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 2014; 10: 504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkelhammer C, Leiter L, Jeejeebhoy K et al. Skeletal muscle function in chronic renal failure: an index of nutritional status. Am J Clin Nutr 1985; 42: 845–854 [DOI] [PubMed] [Google Scholar]
- 6.Kettner-Melsheimer A, Weiss M, Huber W. Physical work capacity in chronic renal disease. Int J Artif Organs 1987; 10: 23–30 [PubMed] [Google Scholar]
- 7.Fahal I, Bell G, Bone J et al. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrol Dial Transplant 1997; 12: 119–127 [DOI] [PubMed] [Google Scholar]
- 8.Gosker HR, Engelen MP, van Mameren H et al. Muscle fiber type IIX atrophy is involved in the loss of fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr 2002; 76: 113–119 [DOI] [PubMed] [Google Scholar]
- 9.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 2013; 24: 337–351 [DOI] [PubMed] [Google Scholar]
- 10.Isoyama N, Qureshi AR, Avesani CM et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014; 9: 1720–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbat-Artigas S, Rolland Y, Zamboni M et al. How to assess functional status: a new muscle quality index. J Nutr Health Aging 2012; 16: 67–77 [DOI] [PubMed] [Google Scholar]
- 12.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 2014; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molsted S, Eidemak I, Sorensen HT et al. Myosin heavy-chain isoform distribution, fibre-type composition and fibre size in skeletal muscle of patients on haemodialysis. Scand J Urol Nephrol 2007; 41: 539–545 [DOI] [PubMed] [Google Scholar]
- 14.Lewis MI, Fournier M, Wang H et al. Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol 2012; 112: 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster BJ, Kalkwarf HJ, Shults J et al. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol 2011; 22: 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ et al. Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int 2013; 83: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetzsteon RJ, Kalkwarf HJ, Shults J et al. Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res 2011; 26: 2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DY, Wetzsteon RJ, Zemel BS et al. Muscle torque relative to cross-sectional area and the functional muscle-bone unit in children and adolescents with chronic disease. J Bone Miner Res 2015; 30: 575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley B, Gudapaty S, Kraybill AL et al. Autosomal-dominant polycystic kidney disease in the rat. Kidney Int 1993; 43: 522–534 [DOI] [PubMed] [Google Scholar]
- 20.Cowley BD Jr, Grantham JJ, Muessel MJ et al. Modification of disease progression in rats with inherited polycystic kidney disease. Am J Kidney Dis 1996; 27: 865–879 [DOI] [PubMed] [Google Scholar]
- 21.Moe SM, Chen NX, Seifert MF et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int 2008; 75: 176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moe SM, Chen NX, Newman CL et al. Anti-sclerostin antibody treatment in a rat model of progressive renal osteodystrophy. J Bone Miner Res 2015; 30: 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen MR, Chen NX, Gattone VH II et al. Skeletal effects of zoledronic acid in an animal model of chronic kidney disease. Osteoporos Int 2013; 24: 1471–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen MR, Newman CL, Chen N et al. Changes in skeletal collagen cross-links and matrix hydration in high-and low-turnover chronic kidney disease. Osteoporos Int 2015; 26: 977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moe SM, Radcliffe JS, White KE et al. The pathophysiology of early-stage chronic kidney disease–mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res 2011; 26: 2672–2681 [DOI] [PubMed] [Google Scholar]
- 26.Newman CL, Moe SM, Chen NX et al. Cortical bone mechanical properties are altered in an animal model of progressive chronic kidney disease. PLoS One 2014; 9: e99262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willems MET, Stauber WT. Isometric and concentric performance of electrically stimulated ankle plantar flexor muscles in intact rat. Exp Physiol 1999; 84: 379–389 [PubMed] [Google Scholar]
- 28.Warren G, Stallone J, Allen M et al. Functional recovery of the plantarflexor muscle group after hindlimb unloading in the rat. Eur J Appl Physiol 2004; 93: 130–138 [DOI] [PubMed] [Google Scholar]
- 29.Eng CM, Smallwood LH, Rainiero MP et al. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol 2008; 211: 2336–2345 [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Roy RR, Zhong H et al. Electromechanical stimulation ameliorates inactivity-induced adaptations in the medial gastrocnemius of adult rats. J Appl Physiol 2007; 103: 195–205 [DOI] [PubMed] [Google Scholar]
- 31.Ozkayar N, Altun B, Halil M et al. Evaluation of sarcopenia in renal transplant recipients. Nephrourol Mon 2014; 6: e20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raub RR, Bechtel PJ, Lawrence LM. Variation in the distribution of muscle fiber types in equine skeletal muscles. J Equine Vet Sci 1985; 5: 34–37 [Google Scholar]
- 33.Levine JM, Taylor RA, Elman LB et al. Involvement of skeletal muscle in dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy). Muscle Nerve 2004; 30: 569–577 [DOI] [PubMed] [Google Scholar]
- 34.Hsueh C-H, Chen NX, Lin S-F et al. Pathogenesis of arrhythmias in a model of CKD. J Am Soc Nephrol 2014; 25: 2812–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson LV. Age-related muscle dysfunction. Exp Gerontol 2009; 44: 106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodman JS, Baker T. Changes in the kinetics of muscle contraction in vitamin D-depleted rats. Kidney Int 1978; 13: 189–193 [DOI] [PubMed] [Google Scholar]
- 37.Johansson C, Lunde PK, Göthe S et al. Isometric force and endurance in skeletal muscle of mice devoid of all known thyroid hormone receptors. J Physiol 2003; 547: 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor R, Abresch R, Lieberman J et al. In vivo quantification of muscle contractility in humans: healthy subjects and patients with myotonic muscular dystrophy. Arch Phys Med Rehabil 1992; 73: 233–236 [PubMed] [Google Scholar]
- 39.Grady RM, Teng H, Nichol MC et al. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 1997; 90: 729–738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.