Abstract
Background and objective
Contrast-induced nephropathy (CIN) is an acute major complication following intravascular administration of iodinated contrast agents; however, the best approach for preventing CIN is not clear. Remote ischemic pre-conditioning (RIPC) is a new, non-pharmacological method that has been considered for the prevention of CIN following coronary angiography. This study assessed the effects of RIPC with four brief episodes of upper limb ischemia and reperfusion in the prevention of contrast-induced nephropathy (CIN) after coronary angiography and/or angioplasty.
Methods
In this double-blind randomized clinical trial, we enrolled 51 patients with chronic stable angina and non-ST elevation acute coronary syndrome (NSTE.ACS), and they underwent coronary angiography and/or angioplasty. Standard fluid therapy with normal saline was prescribed for all patients before and after the procedure. The patients were divided into two groups, i.e., a study group of patients who had undergone RIPC intervention and a control group of patients who had not undergone RIPC. One hour before the procedure, a sphygmomanometer cuff was placed around one arm and inflated up to 50 mmHg above the systolic pressure for five minutes; then, the cuff was deflated for another five minutes, and this cycle was repeated four times. The patients’ serum creatinine levels were measured at baseline and 48 hours after the procedure, and the incidence of CIN was calculated.
Results
Twenty-one males and 30 females were studied in two groups, i.e., an RIPC intervention group (n = 25) and a control group (n = 26) that were homogenous considering baseline characteristics. No significant difference was observed in the mean level of serum creatinine between the two groups at a post-intervention time of 48 hours (RICP: 1.74 ± 0.70 mg/dL vs. Control: 1.75 ± 0.87 mg/dL; P = 0.64). However, a lower incidence rate of CIN was observed 48 hours after the administration of the contrast medium in the RIPC group, but it was not statistically significant (RIPC: 23.1% vs. Control: 12.0%; P = 0.30).
Conclusion
It seems that adequate fluid therapy is still the most effective strategy for preventing CIN and that RIPC might have additional protective effects in very high risk patients, such as those with severe renal insufficiency and heart failure.
Keywords: percutaneous coronary intervention, coronary angiography, kidney diseases, contrast-induced nephropathy, remote ischemic pre-conditioning
1. Introduction
Contrast-induced nephropathy (CIN) is an acute major complication that follows the intravascular administration of iodinated contrast agents (1). CIN is defined as an absolute increase of 0.3 mg/dL in serum creatinine within 48 hours of exposure to the contrast agents (2, 3). It is the third leading cause of acute renal failure in hospitalized patients, and its occurrence is accompanied by a significant increase in mortality, longer hospitalization, and accelerated progression to end stage renal disease (ESRD) (4, 5). The incidence of CIN is estimated to be in the range of 10 to 15%; however, it also has been reported to be as high as 50% in patients with cardiovascular risk factors (2, 6). To date, several methods have been proposed to prevent CIN, including hydration with normal saline or sodium bicarbonate; the use of drugs, such as N-acetylcysteine (NAC), vitamin C, theophylline, and acetazolamide; and the administration of iso-osmolar contrast agents (2, 7, 8). However, there is still uncertainty regarding the best approach for preventing CIN, and research is ongoing to identify better modalities. In recent years, a comparatively new, non-pharmacological method has been proposed for the prevention of CIN following coronary angiography (9–14). The method is called remote ischemic pre-conditioning (RIPC), and it produces a protective effect on tissue and organs by creating short, consecutive cycles of ischemia and reperfusion. Its beneficial effect may result from the release of humoral factors, such as adenosine or bradykinin, into the bloodstream or to its anti-inflammatory or anti-oxidant effects (9, 10). The initial results have shown that this method can be effective in reducing the incidence of CIN by 15–30%; however, due to the small number of studies in this field, it still cannot be recommended for general, routine use. (9–14). Therefore, the aim of this study was to assess the effect of RIPC intervention on the incidence of CIN in the first 48 hours following coronary angiography and/or angioplasty.
2. Material and Methods
2.1. Study Design
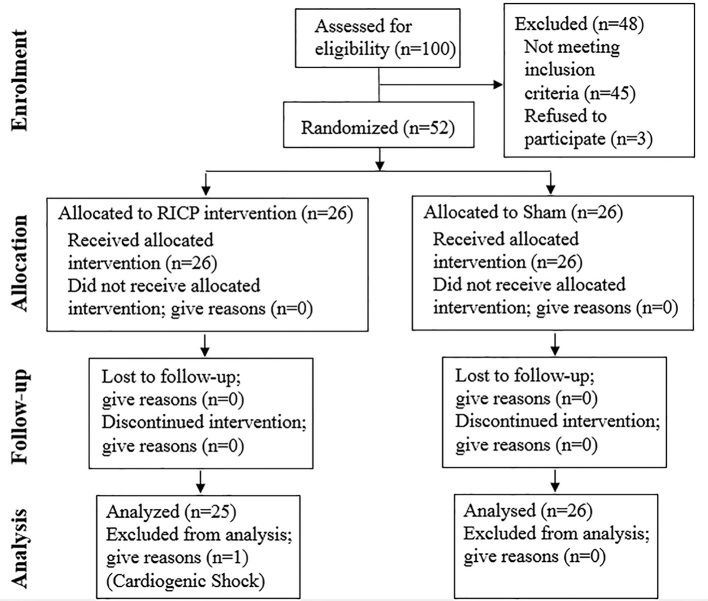
This double-blind randomized clinical trial was conducted in 2014 on patients who were referred to the Cardiology Department at Imam Reza University Hospital in Mashhad, Iran. Specifically, the patients selected for the study were those who had been diagnosed with chronic stable angina or non-ST elevation acute coronary syndrome (NSTE.ACS) and were candidates for coronary angiography and/or angioplasty. CONSORT statement flow diagram is illustrated in Figure 1.
Figure 1.
Follow-up of candidates for angiography (according to consort statement)
2.2. Sampling
Among patients described in the Study Design, we included those who had a risk score ≥ 6 based on Mehran et al. study (4). We excluded patients who had cardiogenic shock, had ST elevation myocardial Infarction (STEMI), had a kidney transplant, or required dialysis. We also excluded patients who had undergone an imaging study with contrast agents during the week before the study or who had experienced bleeding complications and lost 1 g per dL or more of hemoglobin. Those who could not tolerate an inflated cuff for five minutes were withdrawn from the study. The calculation of the required sample size was based on Fikert et al.’s study (15). According to the “comparison of two proportions” formula for determining the sample size and by assuming an alpha error of 5%, a study power of 80%, and a loss of 20%, we determined that 84 patients were required in each group. In order to ensure blinding and random allocation, all angiography candidates were included in a separate list by the head nurse of the unit who was blind to the study protocol. The patients were divided in one of the two groups by using numbered envelopes that contained information identifying one of the two methods of intervention. All of the patients who entered the catheterization laboratory received either RIPC intervention or a placebo (according to the group specified in the envelope), and then they underwent angiography and/or angioplasty by an interventional cardiologist. After the procedure, a cardiology resident who was also blind to the interventions collected the required data. After the data were entered into the software and the statistical analyses were performed, the group members were revealed by the interventional cardiologist in charge.
2.3. Interventions
All of the patients underwent hydration with normal saline at a rate of 1.5 cc per kg per hr for 3 to 12 hr before coronary angiography. The hydration with the saline was continued at the rate of 1.5 ml per kg per hr for 6 to 24 hr after the intervention. According to the aforementioned protocol, the patients were divided into two groups. In the intervention group with RIPC, a blood pressure cuff was fastened around the patient’s arm an hour before coronary angiography, and it was inflated to 50 mmHg above systolic pressure for 5 min, and then the cuff was deflated for 5 min; this cycle was repeated four times. In the placebo group, a pressure cuff also was fastened around the patient’s arm, and, despite repeated cycles by pressure gages, the pressure transferring wire was cut off from the cuff in order to prevent ischemia/reperfusion in this group of patients. The contrast agent Visipaque (TM320 mg/ml, GE Health Care, Ireland) was used for all of the patients. Before coronary angiography and/or angioplasty, none of the patients received nephrotoxic drugs, such as non-steroidal anti-inflammatory drugs or aminoglycosides.
2.4. Baseline variables
Before the intervention, the demographic characteristics of all of the patients were recorded, including age; gender; history of diabetes mellitus, hypertension, dyslipidemia, heart disease, and related health interventions; smoking; drug history, including aspirin, clopidogrel, statins, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and loop diuretics of recent use. The day before the angiography, baseline tests were performed for each patient, including hematocrit, hemoglobin, fasting blood sugar, hemoglobin A1c (HbA1c), total cholesterol, triglycerides (TGs), low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c), erythrocyte sedimentation rate (ESR), serum creatinine, and calculation of estimated glomerular filtration rate (eGFR) by the Modification of Diet in Renal Disease (MDRD) equation. If new diagnoses were made, such as anemia (hemoglobin < 13 g/dL or hematocrit < 39% for men and hemoglobin < 12 g/dL or hematocrit < 36% for women), diabetes mellitus(FBS > 126 mg/dL or HbA1c > 6.5%), or dyslipidemia (TGs > 150 mg/dL, LDL-c > 130 mg/dL, or HDL-c < 40 mg/dL for men and HDL-c < 50 mg/dL for women), they were recorded in the patients’ files. Transthoracic echocardiography also was performed for all of the patients before catheterization. The findings were recorded based on the left ventricular ejection fraction (LVEF) and diastolic function. Then, the intervention was conducted, and the amount of contrast agent used for each patient was recorded.
2.5. Outcomes
Serum creatinine, eGFR, CIN incidence, Mehran score, and the volume of saline infused within the 48 hr following the angiography were recorded for both groups. In this study, CIN was defined as an absolute increase in serum creatinine of 0.3 mg/dL within 48 hr of exposure to the contrast agent. The length of the hospital stay also was recorded for both groups.
2.6. Research ethics
The study protocol was approved by the Ethics Committee of Mashhad University of Medical Sciences (Ref code: 920581), and a signed informed consent form was obtained from all participants prior to their participation in the study. The patients’ data were entered into the statistical software using a code, and they were published as a general conclusion.
2.7. Statistical analysis
The required data were collected using a designed checklist. SPSS ver. 16 was used for data analysis. The t-test was used for quantitative data, and the chi-squared test or its non-parametric equivalent was used for the qualitative variables. The significance level was set at P < 0.05.
3. Results
3.1. Baseline characteristics
During the 18-month study period, 100 patients were studied who had stable angina or NSTE.ACS fulfilling the Mehran criteria for CIN and were candidates for coronary angiography or angioplasty. Out of these 100 patients, 52 patients who had a Mehran Risk Score greater than 6 were included in the study. Only one patient was excluded based on the exclusion criteria (Figure 1). In this study, 51 patients (30 females, 21 males) with a mean age of 68.7± 11.8 were divided into two groups, i.e., an intervention group (n = 25) and a control group (n = 26). The two groups were matched for age, gender, laboratory findings, Mehran rating, past medical history, echocardiography findings, and the volume of saline administered before and after coronary angiography (Tables 1 and 2).
Table 1.
Demographic and laboratory characteristics of participants in both the RIPC and Control Groups
| Variable | All studied patients, (n = 51) | Intervention group with RIPC, (n = 25) | Control group, (n = 26) | p-value |
|---|---|---|---|---|
| Age, years (SD) | 68.73 ( 11.83 ) | 67.08 (12.49) | 70.31 (11.18) | 0.33 |
| Sex, male, n (%) | 30 (41.2) | 18 (49.0) | 12 (51.0) | 0.99 |
| FBS, mg/dL (SD) | 155.1 (60.0) | 150.2 (46.0) | 159.7 (71,7) | 0.59 |
| HbA1c, % (SD) | 14.8 (2.0) | 7.8 (1.6) | 8.4 (2.5) | 0.43 |
| TG, mg/dL (SD) | 138.7 (101.62) | 134.0 (48.4) | 143.8 (139.6) | 0.74 |
| HDL, mg/dL (SD) | 36.0 (9.8) | 37.1 (12.5) | 35.0 (6.6) | 0.47 |
| LDL, mg/dL (SD) | 100.1 (26.8) | 96.3 (31.9) | 104.1 (28.20) | 0.32 |
| Total Cholesterol, mg/dL (SD) | 169.4 (38.2) | 166.8 (43.0) | 172.1 (33.2) | 0.63 |
| ESR, mm/h (SD) | 34.1 (22.5) | 36.4 (22.9) | 31.1 (22.3) | 0.51 |
Table 2.
Clinical characteristics of participants in the RIPC and Control Groups
| Variable | All patients, (n = 51) | Intervention group with RIPC, (n = 25) | Control group, (n = 26) | p | |
|---|---|---|---|---|---|
| Mehran Score, mean (SD) | 9.9 (3.0) | 9.9 (2.2) | 9.8 (3.5) | 0.20 | |
| History of heart disease and related interventions | Angioplasty | 8 (16.7) | 3 (12.5) | 5 (20.8) | 0.70 |
| Coronary artery bypass surgery | 7 (14.6) | 2 (8.3) | 5 (20.8) | 0.22 | |
| Heart failure | 10 (21.3) | 5 (20.8) | 5 (21.7) | 0.94 | |
| Coronary artery disease | 21 (43.8) | 10 (41.7) | 11 (45.8) | 0.77 | |
| Hypertension | 37 (75.5) | 18 (75.0) | 19 (76.0) | 0.94 | |
| Dyslipidemia | 26 (52.0) | 13 (52.0) | 13 (52.0) | 0.99 | |
| Diabetes | 37 (72.5) | 19 (76.0) | 18 (69.2) | 0.59 | |
| Anemia | 35 (68.6) | 17 (68.0) | 18 (69.2) | 0.92 | |
| Cigarettes | 4 (8.0) | 3 (12.0) | 1 (4.0) | 0.30 | |
| Left ventricular ejection fraction (LVEF) (Echocardiographic findings) [n (%)] | Less than 30% | 7 (14.9) | 4 (16.7) | 3 (13.0) | 0.40 |
| 31 to 44% | 14 (29.8) | 9 (37.5) | 5 (21.7) | ||
| 45 to 55% | 14 (29.8) | 5 (20.8) | 9 (39.1) | ||
| More than 55% | 12 (25.5) | 6 (25.0) | 6 (26.1) | ||
| Diastolic function (Echocardiographic findings) | Normal diastolic function, n (%) | 4 (8.9) | 2 (8.3) | 2 (9.5) | 0.69 |
| Mild diastolic dysfunction | 36 (80.0) | 20 (83.3) | 16 (76.2) | ||
| Moderate diastolic dysfunction | 4 (8.9) | 2 (8.3) | 2 (9.5) | ||
| Severe diastolic dysfunction | 1 (2.2) | 0 (0) | 1 (8.4) | ||
| Medication Records [n (%)] | Aspirin | 44 (88.0) | 22 (88.0) | 22 (88.2) | 0.99 |
| Clopidogrel | 22 (45.8) | 13 (54.2) | 9 (37.5) | 0.25 | |
| Statins | 42 (82,4) | 22 (88.0) | 20 (76.9) | 0.30 | |
| ACE* and ARB** inhibitors | 36 (70.6) | 19 (76.0) | 17 (65.4) | 0.40 | |
| Loop Diuretic | 14 (27.5) | 7 (28.0) | 7 (26.9) | 0.93 | |
Angiotensin-converting-enzyme,
Angiotensin II receptor blockers
3.2. The outcomes that were studied
There was no significant difference in the mean serum creatinine level between the intervention and control groups within 48 hours after the intervention (1.74 ± 0.70 and 1.75 ± 0.87, respectively, P = 0.64). Also, the eGFR levels were not meaningfully different during the same period of time (41.25 ± 15.33 and 39.78 ± 15.56, P = 0.73). The serum creatinine level was increased in both groups, but, again, the differences were not statistically significant (0.30 ± 0.03 and 0.44 ± 0.10, respectively, P = 0.20). The overall incidence of CIN in our study was 17.6% (nine patients); however, no statistically meaningful difference in CIN incidence was obtained between the two groups (RICP and Control: 12% and 23%, P = 0.30). The hospital stay was longer in the control group than in the intervention group, but the difference was not statistically significant (4.35 ± 6.79 and 2.60 ± 1.95 days, respectively, P = 0.64).
3.3. Comparison of variables between the two groups with and without CIN
Several variables were compared between the two groups of patients with and without CIN following sham and RICP intervention. These variables included age, past medical history, volume of contrast agent/saline, creatinine level, eGFR, Mehran score, and LVEF. Post-intervention creatinine level (P = 0.003) and a positive past medical history for heart failure (P = 0.02) were the only variables that were significantly different in the two groups (Table 3).
Table 3.
Characteristics of patients with and without contrast-induced nephropathy (CIN)
| Variable | Patients with CIN (n = 9) | Patients without CIN (n = 42) | p | |
|---|---|---|---|---|
| Age, years (SD) | 68.2 (14.01) | 68.8 (11.5) | 0.89 | |
| Baseline creatinine level, mg/dL (SD) | 1.94 (0.8) | 1.65 (0.6) | 0.20 | |
| Baseline eGFR, mL/min/1·73m2 (SD) | 39.14 (23.7) | 41.28 (13.8) | 0.72 | |
| History of heart disease and related interventions | Heart failure, n (%) | 4 (57.1) | 6 (15.0) | 0.02 |
| Diabetes, n (%) | 6 (66.7) | 31 (73.8) | 0.69 | |
| Anemia, n (%) | 3 (33.3) | 13 (31.0) | 0.99 | |
| Left ventricular ejection fraction (LVEF) | Less than 30%, n (%) | 1 (11.1) | 6 (15.8) | 0.74 |
| 31 to 44%, n (%) | 3 (33.3) | 11 (28.9) | ||
| 45 to 55%, n (%) | 2 (22.2) | 12 (31.6) | ||
| More than 55%, n (%) | 3 (33.3) | 9 (23.7) | ||
| Mehran Score | Average, n (%) | 6 (66.7) | 2 (59.5) | 0.32 |
| High, n (%) | 2 (22.2) | 16 (38.1) | ||
| Too high, n (%) | 1 (11.1) | 2 (2.4) | ||
| Volume of contrast agent, ml (SD) | 61.7 (32.8) | 81.1 (76.8) | 0.78 | |
| Volume of saline, ml (SD) | 1100.0 (692.8) | 986.9 (824.9) | 0.35 | |
3.2. The outcomes that were studied
There was no significant difference in the mean serum creatinine level between the intervention and control groups within 48 hours after the intervention (1.74 ± 0.70 and 1.75 ± 0.87, respectively, P = 0.64). Also, the eGFR levels were not meaningfully different during the same period of time (41.25 ± 15.33 and 39.78 ± 15.56, P = 0.73). The serum creatinine level was increased in both groups, but, again, the differences were not statistically significant (0.30 ± 0.03 and 0.44 ± 0.10, respectively, P = 0.20). The overall incidence of CIN in our study was 17.6% (nine patients); however, no statistically meaningful difference in CIN incidence was obtained between the two groups (RICP and Control: 12% and 23%, P = 0.30). The hospital stay was longer in the control group than in the intervention group, but the difference was not statistically significant (4.35 ± 6.79 and 2.60 ± 1.95 days, respectively, P = 0.64).
3.3. Comparison of variables between the two groups with and without CIN
Several variables were compared between the two groups of patients with and without CIN following sham and RICP intervention. These variables included age, past medical history, volume of contrast agent/saline, creatinine level, eGFR, Mehran score, and LVEF. Post-intervention creatinine level (P = 0.003) and a positive past medical history for heart failure (P = 0.02) were the only variables that were significantly different in the two groups (Table 3).
3.4. Logistic regression model of the incidence of CIN
Baseline creatinine levels and a history of heart failure (HF) were the only two factors that significantly affected the incidence of CIN. The risk of CIN increased up to 0.004 times for each 1.0 mg/dL increase in creatinine levels. The risk of CIN in those with a history of HF was 0.004 times higher (Table 4).
Table 4.
Logistic Regression Model of the Incidence of Contrast-Induced Nephropathy
| Variable | B | Wald | P-value | Odds Ratio | 95% C.I. for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.116 | 2.232 | 0.135 | 0.891 | 0.765 | 1.037 |
| Baseline creatinine level | 5.410 | 5.145 | 0.023 | 0.004 | 0.000 | 0.479 |
| Baseline eGFR | 0.141 | 3.471 | 0.062 | 0.869 | 0.749 | 1.007 |
| Smoking | 2.798 | 1.843 | 0.175 | 0.061 | 0.001 | 3.462 |
| Dyslipidemia | −0.004 | 0.106 | 0.745 | 1.004 | 0.980 | 1.029 |
| Heart failure | 5.651 | 5.011 | 0.025 | 0.004 | 0.000 | 0.495 |
| Saline/contrast agent volume | 0.001 | 0.797 | 0.372 | 0.999 | 0.998 | 1.001 |
| −0.007 | 0.307 | 0.580 | 1.007 | 0.982 | 1.032 | |
| Baseline Mehran score | −0.582 | 2.580 | 0.108 | 1.789 | 0.880 | 3.639 |
4. Discussion
This study was performed to investigate whether RIPC intervention can reduce the incidence of acute CIN in the 48 hours after coronary angiography and/or angioplasty. In this clinical trial, the intervention and control groups were matched for underlying factors and Mehran’s score. Also, there was no difference between the two groups in the use of drugs, mainly statins, ACE inhibitors, or ARBs, and injectable loop diuretics. Also, there was no difference in the cardiac function, which can have either a positive or negative impact on the incidence of CIN. Our results indicated a 17.6% incidence rate for CIN in the study population; this was higher than the rates reported by other studies, which indicated that the incidence of CIN in patients after undergoing coronary angiography is expected to be in the range of 10–15% (1, 2, 4, 6, 16). The reason for the higher incidence of CIN in our study may be related to our inclusion criteria, a point that also was mentioned in Mehran et al.’s study (4). In Mehran’s study, the CIN incidence was reported to be as high as 57.3%, which was attributed to the presence of underlying risk factors (4). Therefore, the higher incidence of CIN seems reasonable according to the inclusion criterion of the study, i.e., a Mehran score higher than the average (≥6). The role of RIPC intervention in the incidence of CIN in high-risk patients, based on Mehran’s rating system, was the primary outcome measure studied. Recent studies of patients who underwent coronary angiography with no STEMI have reported a 60% decrease in CIN incidence following RIPC (6, 8, 11, 12). However, there was a strikingly high incidence rate of CIN in such studies. This indicates that these studies evaluated the protective role of RIPC in patients with a higher risk of CIN, giving rise to the hypothesis that RIPC can be used to prevent CIN among higher risk groups. However, our results showed no significant difference in the incidence of CIN between the intervention and control groups, i.e., among the nine CIN cases diagnosed, six were controls and three were from the intervention group. However, we obtained a 10% lower incidence rate of CIN in the intervention group than in the control group. This difference might have been more significant in a larger population or in patients at higher risk for CIN, as was the case in previous studies. Nevertheless, it should be noted that the findings on the protective role of RIPC against the development of CIN are still insufficient, and further studies will be required to confirm this hypothesis. Furthermore, in this study, no meaningful difference was found in the serum creatinine levels of the two groups after catheterization, a finding that is consistent with that of other studies. In a study by Igarashi et al., no significant differences in serum creatinine, eGFR, and other biomarkers associated with nephropathy, such as Cystatin C, and the L-FABP, were reported between the study and control groups before and after the intervention (11). However, it seems that the current definition of CIN does not adequately include other biomarkers. Thus, for a closer look at RIPC’s protective role in kidney damage, future studies should evaluate additional biomarkers, such as Cystatin C and L-FABP. However, the difference in time of calculation of CIN incidence can be another reason for the different results reported by various studies. In recent publications, such as Fikret et al. (15) and Igarashi et al. (11), the incidence of CIN was calculated in the short term and within the first 48 hours after angiography, while, in other studies, the incidence of CIN was measured within 30 days after angiography. Our results indicated that there was no significant difference in the amount of contrast agent that was received by the intervention and control groups with CIN; the mean volume of the contrast agent used was 62 ml in the affected group and 81 ml in the unaffected group. This indicates a high risk of the development of CIN in susceptible individuals, even with contrast volumes less than 100 ml; therefore, if either angiography or angioplasty is essential, the volume of contrast agent administered should be less than 50 ml. This can be achieved by using diluted contrast agents, bone markers, or other markers for the placement of balloons and stents, as well as using intra-vascular ultrasound studies. In general, the mean volume of saline administered before and after coronary angiography or angioplasty is 1 and 1.5 l, respectively, which are considerable amounts. Given that hydration currently is the most important and most effective strategy for preventing CIN, adequate hydration in our study population may have minimized the protective role of RIPC against the development of CIN. As was the case in other studies (6, 9, 11), our patients did not experience any adverse complications or side effects related to RIPC.
CIN is a serious problem, and it is essential to anticipate and, if possible, prevent its progression. Several studies have shown that old age, renal dysfunction, and diabetes are the three main risk factors for CIN. Accordingly, in this study, we evaluated the incidence of CIN in relation to age, renal dysfunction, and diabetes. In a study performed on 16,592 patients who underwent percutaneous coronary intervention (PCI), the incidence of CIN that required dialysis in was significantly higher in patients with basic renal dysfunction than in those without renal dysfunction (17). In addition, an inverse relationship was found between kidney function and the risk of CIN. Diabetes mellitus is another known risk factor for CIN as confirmed by many studies. Dangas et al. showed the rate of occurrence of CIN to be 15% in patients with diabetes and normal renal function (serum creatinine < 1.5 mg/dL or eGFR > 60 ml/min per 1.73 m2 of the body’s surface area) (1). Another study stated that diabetes is an independent risk factor in patients with normal renal function (Odds Ratio (OR): 1.55 and 95% CI: 1.26 – 1.91)(4). However, our results did not confirm the hypothesis that diabetes and chronic renal failure are associated with a greater incidence of CIN, because there was no significant difference in the incidence of CIN between our diabetic and non-diabetic patients. In addition, there was no difference in the incidence of CIN between the chronic kidney disease (CKD) group and the group of patients with normal renal function. This also applied to mild-to-severe cases of CKD. Even so, it is worth noting that, among all of the patients with severe CKD in the two groups, only two patients in the control group developed CIN; however, it is likely that, in the case of a larger sample size, the difference between the two groups would be significant, suggesting a protective role for RIPC in patients with severe CKD.
In our study, only one case (2% of the patients) required dialysis due to CIN, and this was similar to previous studies (2). Given that there was no relationship between diabetes and the incidence of CIN in this study, as was the case in several previous studies, the duration of DM and the associated complications were associated strongly with the incidence of CIN (2, 16). In a prospective observational study that was performed recently, acute disrupted blood sugar level was reported as a risk factor for CIN (2, 16). However, in our study, we only addressed the history of DM, and the duration of DM and the associated complications were not evaluated. It is considered likely that the evaluation of these factors would have led to a stronger association between diabetes and CIN.
Age is another risk factor that affects the incidence of CIN, and it has been discussed in several studies. This association may be related to the progression of renal failure with aging (2). In fact, aging is associated to a greater extent with stenosis and vascular endothelial dysfunction than renal failure. However, this finding has not been confirmed in all related studies, and no relationship was found between age and the incidence of CIN in this study. This seems to be due to the simultaneous impact of age on the incidence of coronary artery-related disorders. The mean age of the patients in the study was 68.7 ± 11.8 years, thus the age range was around 60 to 80 years, and no other patients were available in other age groups, so it was not actually possible to examine the effect of age on the incidence of CIN. Considering that there was no significant difference in the amount of saline infused into individuals with CIN and those without CIN, it is important to note that the average volume of saline infused before and after coronary angiography and angioplasty was about 1 and 1.5 l, respectively, which is a considerable amount; given that hydration is currently the most important and most effective strategy for the prevention of CIN, it is likely that the adequate hydration of the patients in our study minimized the effect of other factors, such as diabetes and CKD, on the incidence of CIN. The higher incidence of CIN in patients with a history of HF was addressed in this study; in this group of patients. Multivariate analysis introduced HF as the only independent predictor of CIN. Among HF patients who developed CIN, one had undergone RIPC, while three others were in the control group. The difference between the two groups may have been significant if the study population had been larger. Furthermore, there was no significant difference in the Mehran score between the CIN and CIN-free groups; out of the nine patients with CIN, six had moderate Mehran scores, two had high Mehran scores, and one had a very high Mehran score. The Mehran score is not a good parameter for predicting the risk of CIN in patients with moderate to high risk. Moreover, proper hydration in our study may have minimized the effects of factors that could have interfered with the Mehran score; this should be confirmed in studies with a larger sample size. Another finding of this study was the relationship between the incidence of CIN and longer hospital stays; patients with CIN had a mean 8-day longer hospital stay than CIN-free patients, and this also was in agreement with the findings of other studies (2).
Due to budget limitations of this research project, a smaller sample size and a shorter follow-up period were necessary. Also, other biomarkers associated with CIN were not investigated. Therefore, we highly recommend that additional studies be conducted to assess the protective role of RIPC in the development of CIN using a larger sample size and with a longer follow-up period and to evaluate other biomarkers.
5. Conclusions
It seems that RIPC may have an additive protective role in high-risk patients, such as those with severe CKD or heart failure. However, regarding the favorable short-term and long-term effects reported by some studies and the simple, non-invasive technique without side effects or undue costs reported by other studies, the use of RIPC seems reasonable in patients who clinically are considered to be high risk patients for the administration of the contrast medium.
Acknowledgments
This study was the result of a thesis by Dr. Sayed Masoud Sadjadi for the fulfillment of the requirements for a specialty degree in Cardiology (thesis No. T-3069). The authors thank the Research Council at Mashhad University of Medical Sciences for its financial support of this study.
Footnotes
iThenticate screening: August 28, 2015, English editing: October 10, 2015, Quality control: November 10, 2015
Funding: This research was supported financially by the Research Council of Mashhad University of Medical Sciences. The authors received no financial support for writing or publishing this article.
Trial registration: The trial was registered at the Iranian Clinical Trial Registry (http://www.irct.ir) with the IRCT identification number IRCT2015061422713N1.
Conflict of Interest: There is no conflict of interest to be declared.
Authors’ contributions: All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95(1):13–9. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 2.Aurelio A, Durante A. Contrast-induced nephropathy in percutaneous coronary interventions: pathogenesis, risk factors, outcome, prevention and treatment. Cardiology. 2014;128(1):62–72. doi: 10.1159/000358042. [DOI] [PubMed] [Google Scholar]
- 3.Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263–72. doi: 10.1093/ndt/gfs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–9. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SR. Past and present attempts to prevent radiocontrast nephropathy. Rev Cardiovasc Med. 2001;2( Suppl 1):S14–8. [PubMed] [Google Scholar]
- 6.Bell RM, Rear R, Cunningham J, Dawnay A, Yellon DM. Effect of remote ischaemic conditioning on contrast-induced nephropathy in patients undergoing elective coronary angiography (ERICCIN): rationale and study design of a randomised single-centre, double-blind placebo-controlled trial. Clin Res Cardiol. 2014;103(3):203–9. doi: 10.1007/s00392-013-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomsen H, Morcos S. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2014;87(1042):20140307. doi: 10.1259/bjr.20140307. [DOI] [PubMed] [Google Scholar]
- 8.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. 2011;21(12):2527–41. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 9.Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote Ischemic Conditioning. J Am Coll Cardiol. 2015;65(2):177–95. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seino Y. Remote Ischemic Conditioning. Circ J. 2013;77(12):2883–5. doi: 10.1253/circj.CJ-13-1297. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi G, Iino K, Watanabe H, Ito H. Remote ischemic pre-conditioning alleviates contrastinduced acute kidney injury in patients with moderate chronic kidney disease. Circ J. 2013;77(12):3037–44. doi: 10.1253/circj.CJ-13-0171. [DOI] [PubMed] [Google Scholar]
- 12.Deftereos S, Giannopoulos G, Tzalamouras V, Raisakis K, Kossyvakis C, Kaoukis A, et al. Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2013;61(19):1949–55. doi: 10.1016/j.jacc.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Saxena P, Newman MA, Shehatha JS, Redington AN, Konstantinov IE. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg. 2010;25(1):127–34. doi: 10.1111/j.1540-8191.2009.00820.x. [DOI] [PubMed] [Google Scholar]
- 14.Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther. 2009;16(6):680–9. doi: 10.1583/09-2817.1. [DOI] [PubMed] [Google Scholar]
- 15.Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, et al. Ischemic Preconditioning for Prevention of Contrast Medium–Induced Nephropathy: Randomized Pilot RenPro Trial (Renal Protection Trial) Circulation. 2012;126(3):296–303. doi: 10.1161/CIRCULATIONAHA.112.096370. [DOI] [PubMed] [Google Scholar]
- 16.Turcot DB, Kiernan FJ, McKay RG, Grey NJ, Boden W, Perdrizet GA. Acute Hyperglycemia Implications for contrast-induced nephropathy during cardiac catheterization. Diabetes care. 2004;27(2):620–1. doi: 10.2337/diacare.27.2.620. [DOI] [PubMed] [Google Scholar]
- 17.Freeman RV, O’Donnell M, Share D, Meengs WL, Kline-Rogers E, Clark VL, et al. Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol. 2002;90(10):1068–73. doi: 10.1016/S0002-9149(02)02771-6. [DOI] [PubMed] [Google Scholar]