Abstract
Background: Fibroblast growth factor 21 (FGF21) is a regulator of carbohydrate and lipid metabolism; however, the regulation of Fgf21 gene expression by diet remains incompletely understood.
Objective: We investigated the effect of a high-carbohydrate (HC) liquid diet, with and without supplementation with a lipid emulsion (LE), and of a high-fat diet (HFD) compared with a low-fat diet (LFD) on the regulation of Fgf21 gene expression in the liver of intact mice.
Methods: C57BL/6 male mice were fed standard feed pellets (SFPs), a purified HC liquid diet (adequate in calories and protein), or an HC liquid diet containing an LE at either 4% or 13.5% of energy for 5 wk (Expt. 1) or 1 wk (Expt. 2). In Expt. 3, mice were fed a purified LFD (∼10% fat) or HFD (∼60% fat) or were fed an HFD and given access to a running wheel for voluntary exercise for 16 wk.
Results: Fgf21 mRNA in liver and FGF21 protein in plasma were increased by 3.5- to 7-fold in HC mice compared with SFP mice (P < 0.001), whereas the LE dose-dependently attenuated the induction of Fgf21 expression (P < 0.05). After 16 wk, hepatic Fgf21 mRNA did not differ between LFD and HFD mice but was dramatically reduced in the HFD+exercise group to <20% of the level in the HFD group (P < 0.0001).
Conclusions: In mice, hepatic Fgf21 expression was upregulated by 1 and 5 wk of feeding a lipogenic HC diet but not by 16 wk of feeding an obesogenic HFD, whereas the addition of fat as an LE to the HC formula significantly reduced Fgf21 gene expression and the plasma FGF21 protein concentration. Our results support a strong and reversible response of hepatic Fgf21 expression to shifts in dietary glucose intake.
Keywords: fibroblast growth factor, high-carbohydrate diet, high-fat diet, steatosis, exercise
Introduction
Fibroblast growth factor 21 (FGF21)11 is a peptide hormone that belongs to an evolutionarily conserved gene family consisting of 22 members that regulate a variety of physiologic processes (1). Several lines of evidence suggest that FGF21 is a metabolic regulator of glucose and lipid homeostasis (1). FGF21 has attracted attention due to reports that pharmacologic administration of FGF21 can promote weight loss (2), improve insulin resistance (3), and ameliorate dyslipidemia and reduce hepatic steatosis (4) in rodents or primates. In particular, functional studies of FGF21 have reported that FGF21 facilitates glucose uptake in differentiated mouse 3T3-L1 cells and primary human adipocytes through upregulation of glucose transporter 1 (GLUT1) gene expression (3). Collectively, these studies suggest that FGF21 could be a promising candidate for the treatment of metabolic disorders.
Although the pharmacologic function of FGF21 has been extensively studied, the physiologic role of FGF21 in energy homeostasis is controversial and incompletely understood (5, 6). Relatively little is known about how FGF21 expression itself is regulated. Most studies on the nutritional regulation of FGF21 have focused on how fasting and refeeding conditions regulate FGF21 in mouse and human tissues (7–9). During fasting, hepatic FGF21 is dramatically induced, suggesting that FGF21 regulates gluconeogenesis, ketogenesis, and FA oxidation (8, 10, 11). However, to our knowledge, no studies have yet investigated if hepatic Fgf21 gene expression is regulated by a lipogenic or an obesogenic diet. In the present study, we took advantage of tissues that had been collected in studies in mice fed a high-carbohydrate (HC) liquid-formula diet, which induces hepatic lipid accumulation, and in mice fed an obesogenic high-fat diet (HFD) compared with a low-fat diet (LFD) to investigate the Fgf21 gene expression response in mice with mild and more severe hepatic steatosis, characteristic of nonalcoholic fatty liver disease. In mice fed an HFD for 16 wk, we also examined whether physical activity (exercise) can modulate Fgf21 transcript expression levels.
Methods
Animal protocol.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University. Male C57BL/6 mice (from Taconic in Expts. 1 and 2 and from Jackson Laboratories in Expt. 3) were housed in plastic cages in humidity-controlled rooms with a 12-h light/dark cycle. Mice were killed with carbon dioxide in Expts. 1 and 2 or anesthetized and killed by exsanguination in Expt. 3.
Diets and experimental designs.
In Expts. 1 and 2, the liquid HC diet was the only source of nutrition and hydration for the HC and HC + lipid emulsion (LE) groups, whereas the standard feed pellet (SFP) group, included as a reference group, was fed LabDiet (Purina), as previously reported (12). Access to food and water was unrestricted. The composition of diets used in these studies has been published (12–14) and is shown in brief in Table 1. In Expts. 1 and 2, the liquid HC diet was Clinimix E (Baxter, Abbott) composed of 76.8% of energy as carbohydrate in the form of dextrose, 22.7% protein, and electrolytes, supplemented with multiple vitamins (Pediatric Influvite; Baxter, Abbott) and minerals and trace elements (Supplemental Table 1). The basal HC diet contained 0.5% LE, which was included as a source of essential FAs. Five-week-old male C57BL/6 mice were randomly assigned into 4 groups: 1) SFP diet; 2) HC diet, as above; 3) HC diet supplemented with 4% LE [designated as HC+4%LE, where the LE was Intralipid 20% (Baxter)]; or 4) HC diet supplemented with 13.5% LE (designated as HC+13.5%LE). The liquid diet was freshly prepared and replaced daily, and any amount not consumed was recorded. Mice were fed under these conditions for 5 wk (Expt. 1) or 1 wk (Expt. 2). In Expt. 3, liver tissue was from a previously reported study of HFD-induced metabolic syndrome (13). Briefly, male C57BL/6 mice were randomly assigned to 3 groups, which were fed a purified LFD or an HFD (Supplemental Table 2) or the same HFD plus exercise, by access to a running wheel that mice used voluntarily, for the study period of 16 wk. After mice were killed, tissues were rapidly dissected. For histologic analysis, in Expts. 1 and 2 a portion of the left lobe of each liver was embedded in Optimal Cutting Temperature compound (Sakura) (12), whereas in Expt. 3, a portion of liver was fixed in phosphate-buffered formalin. In each experiment, samples of liver were rapidly frozen in liquid nitrogen and stored at −80°C before analysis.
TABLE 1.
Summary of macronutrient composition of diets used in Expts. 1, 2, and 31
| Expts. 1 and 2 |
Expt. 3 |
|||||
| SFP | HC | HC+4%LE | HC+13.5%LE | LFD | HFD | |
| Macronutrients, % of energy | ||||||
| Carbohydrate | 58 | 77 | 74 | 67 | 70 | 20 |
| Protein | 28.5 | 22.5 | 22 | 20 | 20 | 20 |
| Fat | 13.5 | 0.5 | 4.0 | 13.5 | 10 | 60 |
| Source of carbohydrate | Mixed | Dextrose | Dextrose | Dextrose | Corn starch, maltodextrin, sucrose | Maltodextrin, sucrose |
| Source of fat | Mixed | Intralipid | Intralipid | Intralipid | Soybean oil/lard | Soybean oil/lard |
HC, high-carbohydrate diet; HFD, high-fat diet; LE, lipid emulsion; LFD, low-fat diet; SFP, standard feed pellet.
RNA extraction and real-time PCR.
RNA extraction and real-time qPCR have been described elsewhere (14). Briefly, total RNA was extracted from mouse liver and adipose tissue by using TRIzol reagent (Life Technologies). The reverse-transcription reaction and real-time PCR assays were performed as in reference 14 by using an input of 1 μg total RNA and the Reverse Transcription System (Promega). The resultant cDNA was amplified by using the iQ SYBR Green Supermix (Bio-Rad). The PCR reaction conditions for each cycle were as follows: 94°C for 5 min, followed by 40 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. Primer sequences for mouse Fgf21 were as follows: 5′-ACACAATTCCAGCTGCCTTG-3′, 5′-TAGAGGCTTTGACACCCAGG-3′. Each value for the mRNA of analyzed genes was normalized relative to the mRNA for Gapdh (14), which was analyzed on the same plate. To determine tissue distribution of mouse Fgf21 mRNA, relative levels of Fgf21 transcripts were determined by qPCR in different tissues and organs of 10-wk-old male C57BL/6 mice fed the SFP diet.
Protein analysis.
Liver tissue lysates (40 μg protein) were separated by electrophoresis on 10% SDS-PAGE gels and transferred to a Millipore Immobilon-FL membrane. The blots were blocked with Odyssey blocking buffer (LI-COR Biosciences) at room temperature for 1 h and then incubated with monoclonal anti-FGF21 antibody (1:1000 dilution; ab171941; Abcam) and monoclonal anti–β-actin antibody (1:1500 dilution; A2228; Sigma) overnight at 4°C. After washing 4 times for 7 min each in Phosphate buffered saline-Tween 20 (PBS-T) (0.1%, vol:vol), the membranes were incubated with goat anti-rabbit IgG or rabbit anti-mouse IgG at 1:7000 dilutions at room temperature for 1 h. After washing, the protein bands were visualized (Odyssey Classic Imaging System; LI-COR Biosciences), and all blots were normalized with immunoblotted β-actin to adjust for protein loading. The protein band was observed to comigrate with an authentic FGF21 protein standard (ab63277; Abcam). For mouse plasma FGF21, an ELISA was run with the use of a commercial kit from R&D Systems/Bio-Techne (catalog MF2100; Mouse/Rat FGF-21 immunoassay), following the protocol provided.
Liver TG quantification.
Liver TGs were quantified as previously described (12) by extracting total lipid from 100–200 mg liver (15) and applying the lipid extract to a column of 5% water-deactivated aluminum oxide to separate TGs and phospholipid; TGs were eluted with 25% diethyl ether in hexane. Finally, the glycerol of the TG–containing eluate was determined spectrophotometrically by using triolein as a standard (16).
Liver histology.
For Oil Red O staining, frozen tissues embedded in Optimal Cutting Temperature compound were cut into 8-μm-thick sections, stained with Oil Red O, and counterstained with hematoxylin dye. For hematoxylin and eosin staining, formalin-fixed and paraffin-embedded liver tissues were cut into 6-μm sections and stained with hematoxylin and eosin dyes.
Statistical analysis.
Data are presented as means ± SEMs for the number of replicates indicated. Statistical analysis was performed by using 1-factor ANOVA or Student’s t test, as appropriate (Prism 5 software; GraphPad). Student’s t test was used to compare the difference between the SFP reference group and HC diet only, because these diets are fundamentally different in composition; the HC diet serving as the control group for a 1-factor ANOVA used to analyze the results for all treatments in Expts. 1 and 2 and for Expt. 3. A significant difference was defined as P < 0.05.
Results
Body weight and hepatic lipid accumulation in mice fed the HC diet, without and with LE, for 5 and 1 wk.
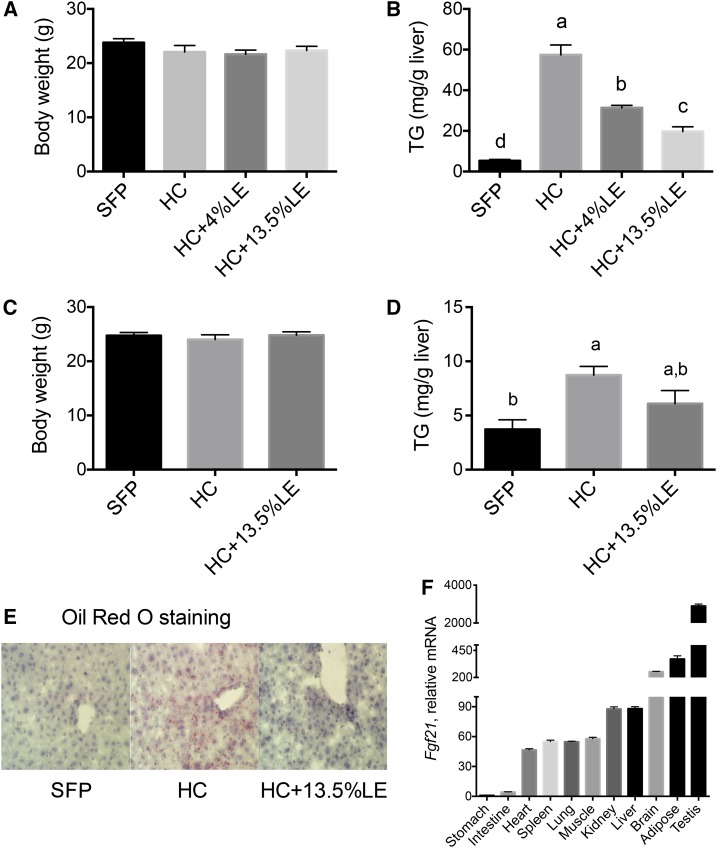
In Expt. 1, body weight did not differ between any of the 4 groups (Figure 1A). Hepatic TGs were significantly higher in HC mice than in SFP mice, whereas the administration of LE ameliorated hepatic TG content dose-dependently (Figure 1B). In the 1-wk study (Expt. 2), body weight also did not differ with treatment (Figure 1C). Liver TGs were significantly elevated after 1 wk of the HC diet, although not to the extent after 5 wk (note y axis scale, Figure 1D). Oil Red O staining in liver sections, as shown for representative sections from Expt. 2 in Figure 1E, showed less red staining in the liver of HC+13.5%LE mice relative to the other 2 groups. Fgf21 transcripts were present in several organs that are active in energy metabolism, including skeletal muscle, liver, and adipose tissue, as well as in several other tissues (Figure 1F). Thus, Fgf21 has a wide distribution of expression, including substantial expression in liver, the tissue on which we focused for most of the rest of our studies.
FIGURE 1.
Final body weights (A, C), hepatic TG concentrations (B, D), Oil Red O staining in liver sections (E), and Fgf21 mRNA expression (F) in mice fed SFPs or a liquid HC diet alone or with an LE. Final body weights (A), liver TGs (C), and Fgf21 mRNA (F) were measured in Expt. 1 (5 wk); and final body weights (B), liver TGs (D), and liver Oil Red O staining (E) were determined in Expt. 2 (1 wk). Panel E: red, lipid droplets; purple, nuclei. Original magnification ×400. Representative images from each group are shown. Values are means ± SEMs, n = 5/group. In panels A–D, 1-factor ANOVA was used to compare the treatments. Different letters indicate differences between the treatment groups, P < 0.05. Fgf21, fibroblast growth factor 21; HC, high-carbohydrate diet; LE, lipid emulsion; SFP, standard feed pellet.
Fgf21 gene expression is increased in liver in an HC diet–induced mouse model of nonalcoholic fatty liver disease.
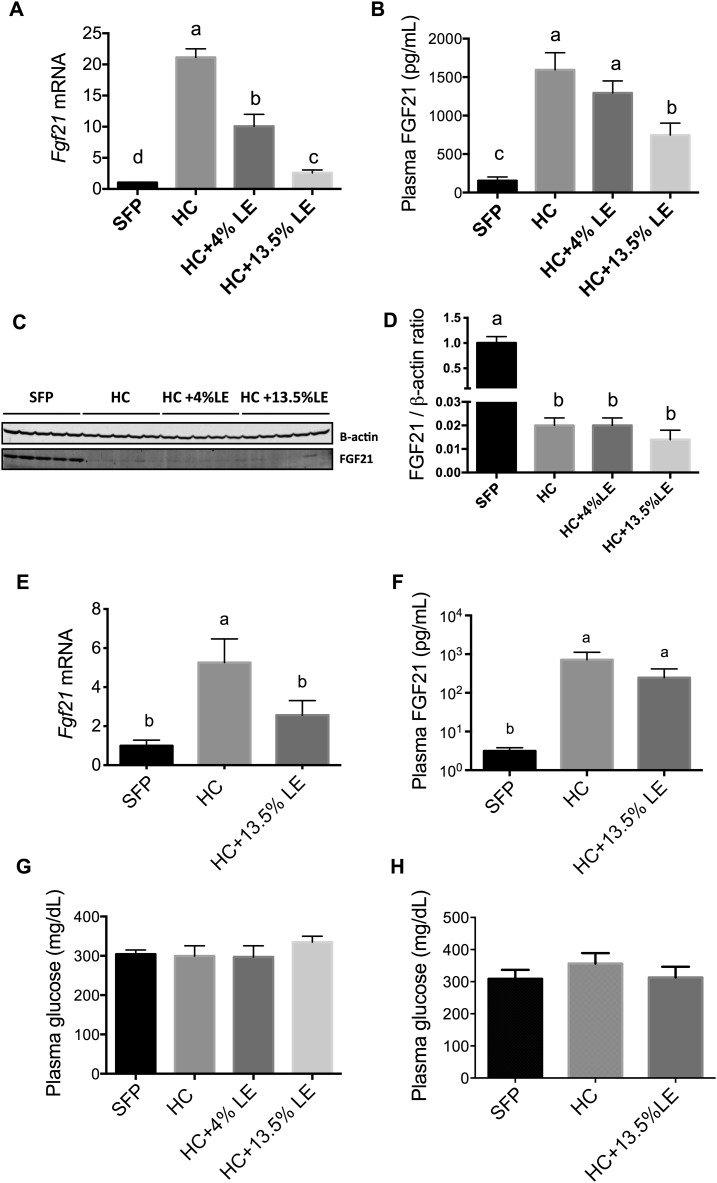
In Expt. 1, hepatic Fgf21 mRNA increased by >20-fold in mice fed an HC diet for 5 wk (P < 0.001 compared with SFP; Figure 2A). However, supplementation of the HC diet with either 4% or 13.5% LE attenuated Fgf21 levels dose-dependently (Figure 2A). Consistent with our observations on hepatic Fgf21 expression, plasma FGF21 protein was also significantly higher in mice fed an HC diet and was lower with the addition of LE to the HC diet (Figure 2B). In contrast, immunoblotting results showed that FGF21 protein in liver was significantly reduced in HC compared with SFP mice (P < 0.001; Figure 2C). To determine if this metabolic response was very rapid, mice in Expt. 2 were fed an HC diet for only 1 wk, with and without 13.5% LE. Hepatic Fgf21 mRNA was 5-fold higher after 1 wk in the HC group than in the SFP group. However, Fgf21 expression was lower in mice fed HC+13.5%LE compared with HC alone (Figure 2E). As observed in the 5-wk study, plasma FGF21 also increased after 1 wk of feeding the HC diet (Figure 2F). Plasma glucose did not differ between groups in either of the experiments (Figure 2G, H).
FIGURE 2.
Fgf21 mRNA expression (A, E) and FGF21 protein (C, D) in liver, FGF21 protein in plasma (B, F), and plasma glucose (G, H) in mice fed SFPs or a liquid HC diet alone or with an LE in Expt. 1 (5 wk) (A–D, G) and Expt. 2 (1 wk) (E, F, H). For qPCR data, values were first normalized to Gapdh mRNA for each sample; the mean for the SFP group was set to 1.0 for each experiment. Values are means ± SEMs, n = 5. One-factor ANOVA was used to compare the treatments. Different letters indicate differences between the treatment groups, P < 0.05. FGF21, fibroblast growth factor 21; HC, high-carbohydrate diet; LE, lipid emulsion; SFP, standard feed pellet.
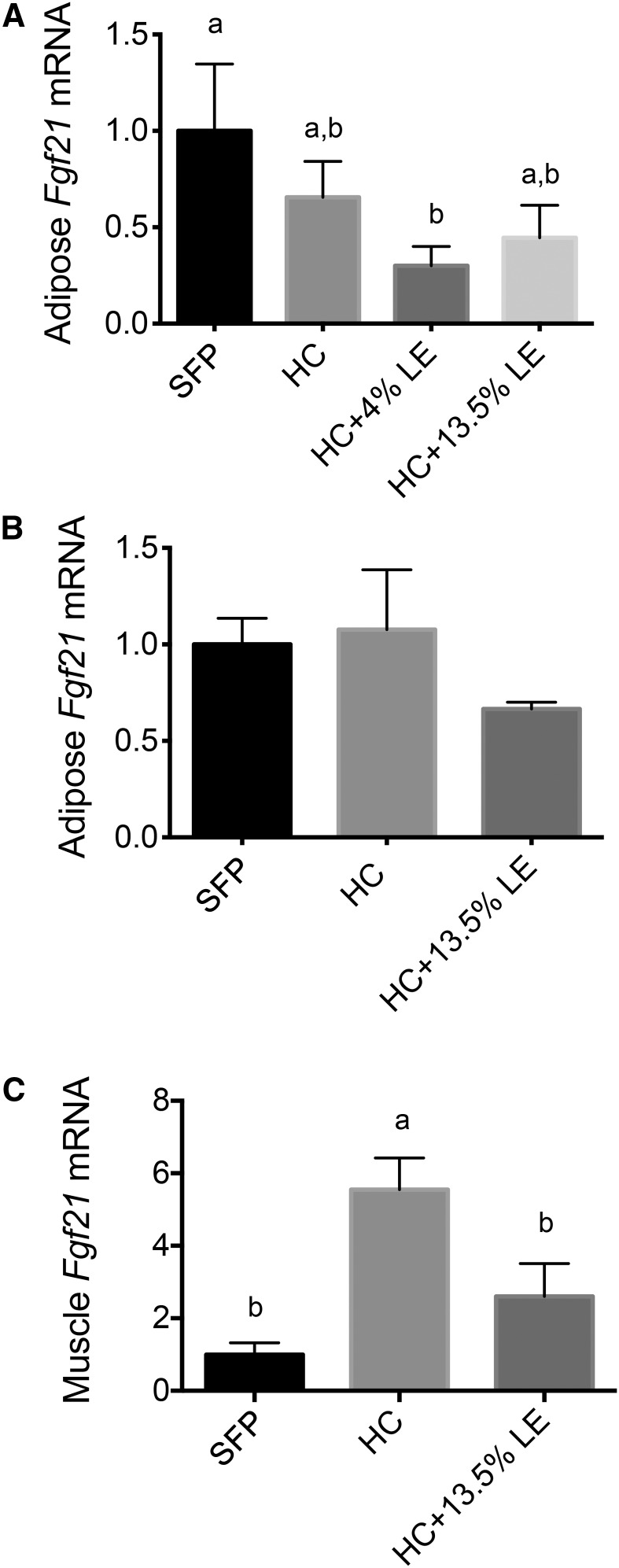
Fgf21 transcripts were also determined in adipose tissue in both our 5- and 1-wk experiments and in muscle in the 1-wk experiment. Fgf21 expression in adipose tissue did not differ in either experiment (Figure 3A, B), whereas Fgf21 expression in muscle tissue was higher in mice fed the HC diet and lower in mice fed the HC+13.5%LE diet (Figure 3C), which is similar to that shown for Fgf21 mRNA in liver (Figure 2E).
FIGURE 3.
Fgf21 transcript levels in adipose tissue (A, B) and muscle (C) of mice fed SFPs or a liquid HC diet alone or with an LE. Fgf21 expression was measured in adipose tissue in Expt. 1 (5 wk) (A) and Expt. 2 (1 wk) (B) and in muscle in Expt. 2 (C). Values are means ± SEMs, n = 5/group. One-factor ANOVA was used to compare the treatments. Different letters indicate differences between the treatment groups, P < 0.05. Fgf21, fibroblast growth factor 21; HC, high-carbohydrate diet; LE, lipid emulsion; SFP, standard feed pellet.
Fgf21 gene expression is not upregulated in the liver of mice fed an HFD but is suppressed by physical activity.
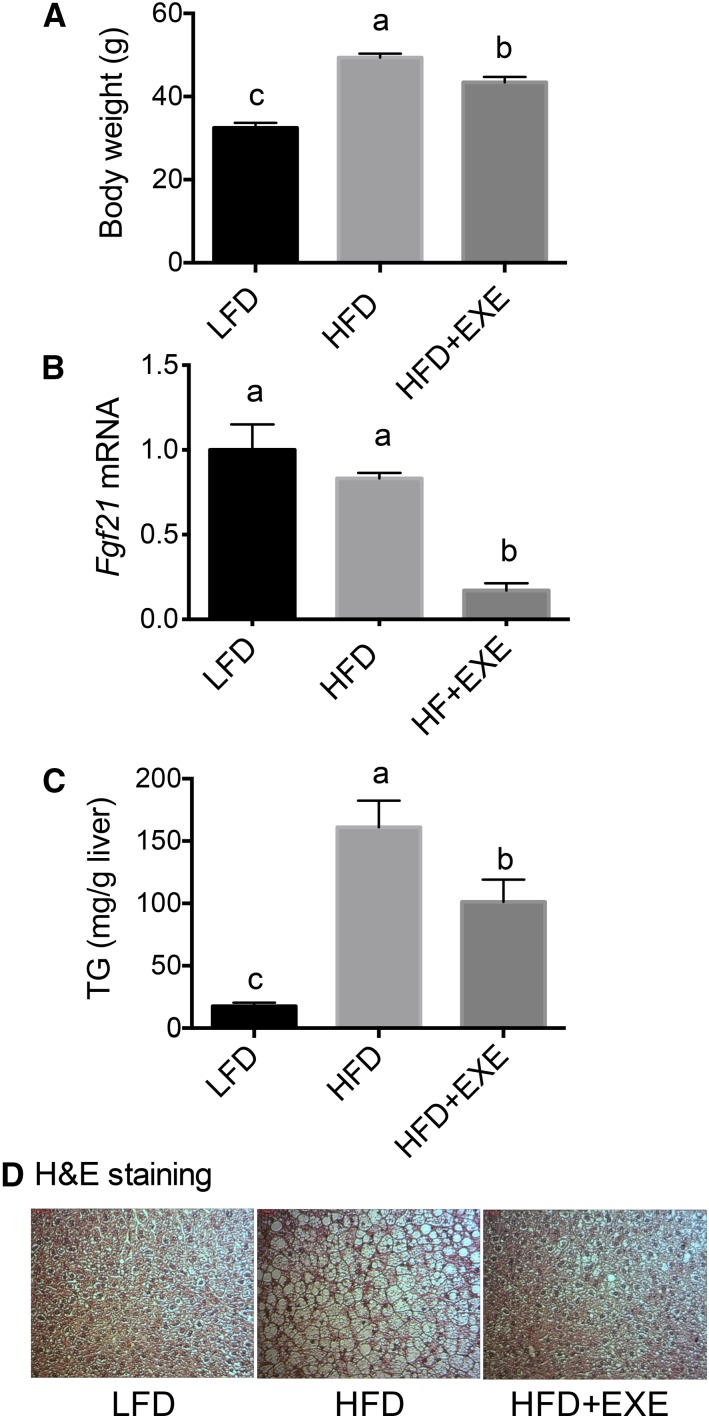
Next, we examined whether Fgf21 transcript levels are regulated by an obesogenic HFD and by physical activity in a mouse model of nonalcoholic fatty liver disease with metabolic syndrome (13). Mice fed a purified HFD for 16 wk weighed more than mice fed an otherwise equivalent LFD, whereas the addition of exercise to the HFD, in the form of voluntary running, partially but significantly ameliorated the increase in body weight (Figure 4A). As shown by both hepatic TGs (Figure 4C) and liver histology (Figure 4D), mice fed an HFD for 16 wk developed steatosis and exercise attenuated lipid accumulation in these mice. Fgf21 mRNA in liver did not differ between HFD and LFD mice; however, exercise reduced Fgf21 mRNA significantly (HFD compared with HFD+exercise, P < 0.0001) (Figure 4B).
FIGURE 4.
Final body weights (A), Fgf21 mRNA expression (B), liver TGs (C), and H&E staining (D) in the liver of mice fed an LFD or an HFD (with or without exercise) in Expt. 3 (16 wk). In panel B, values were normalized to Gapdh mRNA, and the mean for the LFD group was set to 1.0. Values are means ± SEMs, n = 9/group. One-factor ANOVA was used to compare the treatments. Different letters indicate differences between the treatment groups, P < 0.05. EXE, exercise; Fgf21, fibroblast growth factor 21; H&E, hematoxylin and eosin; HFD, high-fat diet; LFD, low-fat diet.
Discussion
Since FGF21 was discovered more than a decade ago (17), a number of studies have focused on its pharmacologic effects (18, 19), whereas fewer studies have addressed the regulation of FGF21 expression under dietary or physiologic conditions. Fgf21 mRNA is broadly expressed (20) (Figure 1F), suggesting involvement with physiologic processes that are shared by numerous tissues. Previous studies have shown that Fgf21 expression is elevated in the adaptive response to starvation, under the control of the transcription factor PPAR-α (7, 9, 11). In the present study, we tested whether a lipogenic HC diet, rich in simple carbohydrate (dextrose), and an obesogenic HFD containing an excess of calories as fat would similarly affect Fgf21 expression in vivo. In liver, Fgf21 mRNA was dramatically upregulated in mice fed our lipogenic HC diet, which is in agreement with results from studies conducted in isolated rat hepatocytes cultured with high glucose (21). In contrast, Fgf21 mRNA levels did not differ between mice fed the obesogenic HFD and those fed the LFD. Together, these results suggest a relation between fat produced de novo from excess carbohydrate, likely in the liver, and Fgf21 expression in liver, but not necessarily with hepatic steatosis per se. Moreover, we have shown that the addition of exogenous lipid in the form of LE to the HC diet reduced Fgf21 gene expression. However, causal relations still must be tested. It should be noted that the mice in our HC feeding study were not obese, although hepatic lipogenesis was elevated as shown by the expression of lipogenic enzymes and other factors and hepatic lipid accumulation (12, 14). Thus, a positive energy balance per se was not the cause of these results. Interestingly, the level of Fgf21 mRNA in liver was not predictive for FGF21 protein in this tissue, although the pattern of Fgf21 mRNA in liver was similar to that for plasma FGF21 protein. This might not be a paradoxical finding because FGF21 is a peptide hormone that is secreted into blood where it may regulate glucose uptake by extrahepatic tissues, such as adipose and muscle, thus contributing to homeostasis. Indeed, we observed that plasma glucose concentrations remained unchanged in mice fed the HC compared with the SFP diet, which may suggest that increased plasma FGF21 facilitated glucose uptake by adipose and muscle tissues. Recently, it has been suggested that FGF21 expression is related to protein adequacy (22). Of note, protein was adequate in all of the diets in our studies, and yet shifts in carbohydrate and lipid availability, or exercise in the presence of HFD, were sufficient to alter Fgf21 gene expression.
In our studies LE supplementation suppressed Fgf21 expression in the liver of mice fed an HC diet. The LE we used, Intralipid, is a soybean oil–based emulsion that is rich in C-18:1 and C-18:2(n–6) unsaturated FAs. The addition of this LE to the HC diet was previously shown to reduce the expression of several lipogenic genes, such as sterol-response element binding protein 1C (SREBPF1), fatty acid synthase (FAS), acetyl-CoA carboxylase 1 (ACC1), and patatin-like phospholipase domain containing 3 (PNPLA3/adiponutrin) (14). Alternatively, the suppression of FGF21 expression could be the consequence of reduced de novo lipogenesis, due to the presence of sufficient amounts of exogenous FAs. Nevertheless, we did not observe an effect of the obesogenic HFD that was fed in Expt. 3 on hepatic Fgf21 gene expression. Compared with other studies in which mice were fed a ketogenic diet that contained only 0.78% carbohydrate (7), the obesogenic diet fed to mice in Expt. 3 is still higher in carbohydrate (20% carbohydrate, 20% protein, and 60% fat). This may suggest that hepatic FGF21 expression remains stable when there is no demand for de novo glucose production. In Expt. 3, voluntary exercise dramatically reduced the level of Fgf21 transcripts in the liver of mice fed an HFD. Consistent with this, a recent study showed an ∼65% lower hepatic Fgf21 mRNA due to exercise in Otsuka Long-Evans Tokushima Fatty rats (23), a model of type 2 diabetes with obesity (24). Conversely, the FGF21 concentration in serum was significantly increased after 2 wk of physical activity in young healthy women (25). However, elevated serum/plasma FGF21 could be derived from other organs, such as adipose, muscle, and pancreas. A limitation of our study is that plasma FGF21 was not measured in Expt. 3. Our data from Expt. 3 do not support the idea that the expression of FGF21 is associated with insulin resistance or is due simply to general overnutrition. Instead, our data support that the change in FGF21 expression in the liver is related to the diet composition, in particular the amount of carbohydrate, which, in the case of our HC diet, has been shown to be lipogenic. In summary, the present experiments add to our understanding of FGF21 regulation by showing that the expression of Fgf21 mRNA is regulated in mouse liver by a lipogenic HC diet, but not by an obesogenic HFD, although both of these diets resulted in hepatic lipid accumulation.
Acknowledgments
LH and K-HH designed and conducted the study, analyzed data, and wrote the manuscript; KI, SS-t, and JDL designed and conducted the study and analyzed data; and ACR designed the study, analyzed data, edited the manuscript, and takes responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ACC1, acetyl-CoA carboxylase 1; FAS, fatty acid synthase; FGF21, fibroblast growth factor 21; GLUT1, glucose transporter 1; HC, high-carbohydrate; HFD, high-fat diet; LE, lipid emulsion; LFD, low-fat diet; SFP, standard feed pellet; SREBPF1, sterol-response element binding protein 1C .
References
- 1.Iglesias P, Selgas R, Romero S, Diez JJ. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur J Endocrinol 2012;167:301–9. [DOI] [PubMed] [Google Scholar]
- 2.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–27. [DOI] [PubMed] [Google Scholar]
- 3.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009;58:250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 2012;26:312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Zhang J, Jia W. Fibroblast growth factor 21: a novel metabolic regulator from pharmacology to physiology Front Med 2013;7:25–30. [DOI] [PubMed] [Google Scholar]
- 7.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 2009;150:4931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–37. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007;5:415–25. [DOI] [PubMed] [Google Scholar]
- 10.Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008;8:169–74. [DOI] [PubMed] [Google Scholar]
- 11.Lundåsen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 2007;360:437–40. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Hao L, Wray AE, Ross AC. Lipid emulsion administered intravenously or orally attenuates triglyceride accumulation and expression of inflammatory markers in the liver of nonobese mice fed parenteral nutrition formula. J Nutr 2013;143:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sae-Tan S, Rogers CJ, Lambert JD. Voluntary exercise and green tea enhance the expression of genes related to energy utilization and attenuate metabolic syndrome in high fat fed mice. Mol Nutr Food Res 2014;58:1156–9. [DOI] [PubMed] [Google Scholar]
- 14.Hao L, Ito K, Huang KH, Sae-tan S, Lambert JD, Ross AC. Shifts in dietary carbohydrate-lipid exposure regulate expression of the non-alcoholic fatty liver disease-associated gene PNPLA3/adiponutrin in mouse liver and HepG2 human liver cells. Metabolism 2014;63:1352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 16.Sardesi VM, Manning JA. The determination of triglycerides in plasma and tissues. Clin Chem 1968;14:156–61. [Google Scholar]
- 17.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 2000;1492:203–6. [DOI] [PubMed] [Google Scholar]
- 18.Gimeno RE, Moller DE. FGF21-based pharmacotherapy—potential utility for metabolic disorders. Trends Endocrinol Metab 2014;25:303–11. [DOI] [PubMed] [Google Scholar]
- 19.Kharitonenkov A, Shanafelt AB. Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. BioDrugs 2008;22:37–44. [DOI] [PubMed] [Google Scholar]
- 20.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 2010;24:2050–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma L, Robinson LN, Towle HC. ChREBP•Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem 2006;281:28721–30. [DOI] [PubMed] [Google Scholar]
- 22.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Münzberg H, Hutson SM, Gettys TW, Schwartz MW, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014;124:3913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher JA, Meers GM, Laughlin MH, Ibdah JA, Thyfault JP, Rector RS. Modulating fibroblast growth factor 21 in hyperphagic OLETF rats with daily exercise and caloric restriction. Appl Physiol Nutr Metab 2012;37:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 1992;41:1422–8. [DOI] [PubMed] [Google Scholar]
- 25.Cuevas-Ramos D, Almeda-Valdes P, Meza-Arana CE, Brito-Cordova G, Gomez-Perez FJ, Mehta R, Oseguera-Moguel J, Aguilar-Salinas CA. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 2012;7:e38022. [DOI] [PMC free article] [PubMed] [Google Scholar]