Abstract
Background
The aim of this study was to analyze device conformability in TEVAR of acute and chronic (a/c) type B aortic dissections (TBAD) using the Gore Conformable Thoracic Aortic Stent-graft (CTAG).
Material/Methods
From January 1997 to February 2014, a total of 90 out of 405 patients in our center received TEVAR for TBAD. Since November 2009, 23 patients (16 men; median age: 62 years) were treated with the CTAG. Indications were complicated aTBAD in 15 (65%) and expanding cTBAD in 8 (35%) patients. Primary endpoints were the assessment of device conformability by measuring the distance (D) from the radiopaque gold band marker (GM) at the proximal CTAG end to the inner curvature (IC) of the arch on parasagittal multiplanar reformations of CT angiography, as well as the evaluation of aortic diameter changes following TEVAR. Median follow-up was 13.3 months (range: 2 days to 35 months).
Results
Primary and secondary success rates were 91.3% (21/23) and 95.6% (22/23), respectively. There was 1 type Ia endoleak, retrograde dissection or primary conversion was not observed. Median GM-IC-D was 0 mm (range: 0 mm to 10 mm). GM-IC-D was associated with zone 2 placement compared to zone 3 (P=0.036). There was no association between GM-IC-D formation and arch type. In aTBAD cases the true lumen significantly increased after TEVAR (P=0.017) and the false lumen underwent shrinkage (P=0.025). In cTBAD patients the false lumen decreased after TEVAR (P=0.036).
Conclusions
The CTAG shows favorable conformability and wall apposition in challenging arch pathologies such as TBAD.
MeSH Keywords: Aneurysm, Dissecting; Cardiovascular Diseases; Endovascular Procedures
Background
Thoracic endovascular aortic repair (TEVAR) has evolved as the treatment modality of choice for various pathologies of the thoracic aorta, including Stanford type B aortic dissections (TBAD) [1]. Increasing evidence, including studies like ADSORB and INSTEAD XL, of TEVAR in acute and chronic TBAD (aTBAD)/cTBAD) patients defines more and more subgroups of patients benefiting from TEVAR [2–5]. Nevertheless, TEVAR for TBAD remains a technically demanding endeavor. The dynamic character of the disease, the fragility of the aortic wall, and the anatomical features of the aortic arch represent challenges for the devices used. Experimental and clinical data have shown that poor endograft apposition within the aortic arch increases the risk of technical failure after TEVAR [6–8]. The requirement for enhanced conformability has influenced the design of next-generation devices [9]. In November 2009, the Conformable TAG Thoracic Endoprosthesis (CTAG; W. L. Gore & Associates, Inc., Flagstaff, USA) received CE mark approval for TEVAR in Europe. Compared to the former Gore TAG endoprosthesis, the device had undergone several modifications for improved conformability and adaptable radial force with respect to the underlying aortic pathology [10]. So far, there are limited data available on the performance of the CTAG in clinical practice [11–13]. The aim of this analysis was to report our preliminary experience in stent-grafting of TBAD with the CTAG endoprosthesis focusing on the conformability of the device to the aortic arch in this specific pathology.
Material and Methods
Study design
The study design was a retrospective single-center analysis enrolling consecutive patients who underwent TEVAR for TBAD with the CTAG device. Data were extracted from a prospectively maintained departmental database.
Patient population
Between March 1997 and February 2014, 90 patients underwent TEVAR for TBAD at our institution. Of these, a cohort of 23 consecutive patients was treated with the CTAG device (Table 1). Fifteen patients underwent TEVAR for complicated aTBAD; in 8 cases the indication for treatment was cTBAD (Table 2). Within the cTBAD group, 5 patients were treated for aortic diameter expansion ≥60 mm, 2 patients had large entry tears (>10 mm), and 1 patient presented with true lumen collapse and renal malperfusion (Table 2). Demographic, preoperative clinical, operative, and follow-up (FU) data were analyzed and supplemented from patient records and computed tomography (CT) data. FU included medical history, physical examination, and CT angiography (CTA) before discharge and at 6 months, 1 year, and annually thereafter. Median postoperative FU was 13 months (range: 2 days to 35 months). The median CT imaging interval (preoperative baseline CT scan to last postoperative CT scan) was 11 months, ranging from 0 days to 39 months.
Table 1.
Patients’ demographics.
| N | % | |
|---|---|---|
| Patient population | 23 | |
| Median age, years | 62 | |
| Age range, years | 32–79 | |
| Male sex | 16 | 69.6 |
| Patients ≥ASA III | 22 | 95.7 |
| Previous aortic surgery | 10 | 43.5 |
| Arterial hypertension | 22 | 95.7 |
| Smoking | 13 | 56.5 |
| Coronary artery disease | 8 | 34.8 |
| Renal insufficiency | 5 | 21.7 |
| COPD | 3 | 13.0 |
| Diabetes | 2 | 8.7 |
ASA – American Society of Anesthesiologists risk index; COPD – chronic obstructive pulmonary disease.
Table 2.
Case-by-case presentation of underlying treatment indications (N=23).
| Patient N. | Sex/age | ASA | Indication | Max. AD (mm) | Complicating factor | Urgency |
|---|---|---|---|---|---|---|
| 1 | ♀/45 | 3 | aTBAD | 30 | Paraparesis | Urgent |
| 2 | ♂/59 | 3 | aTBAD | 64 | TLC/visceral ischemia | Urgent |
| 3 | ♂/68 | 4 | aTBAD | 58 | Recurrent pain | Urgent |
| 4 | ♂/62 | 3 | aTBAD | 41 | FL rupture | Urgent |
| 5 | ♂/32 | 3 | aTBAD | 53 | TLC/visceral ischemia | Urgent |
| 6 | ♂/75 | 3 | aTBAD | 40 | Pleural effusion | Urgent |
| 7 | ♂/63 | 3 | aTBAD | 39 | Recurrent pain | Urgent |
| 8 | ♂/68 | 4 | aTBAD | 39 | Recurrent pain | Elective |
| 9 | ♀/70 | 2 | aTBAD | 53 | Recurrent pain | Elective |
| 10 | ♂/53 | 3 | aTBAD | 42 | TLC/Visceral ischemia | Urgent |
| 11 | ♀/63 | 2 | aTBAD | 46 | Recurrent pain | Elective |
| 12 | ♂/59 | 3 | aTBAD | 41 | Visceral ischemia | Urgent |
| 13 | ♀/79 | 3 | aTBAD | 39 | Recurrent pain | Urgent |
| 14 | ♀/46 | 3 | aTBAD | 58 | Recurrent pain | Elective |
| 15 | ♂/61 | 4 | aTBAD | 40 | Recurrent pain | Elective |
| 16 | ♂/67 | 4 | cTBAD | 63 | Expansion (5 mm/3 months) | Elective |
| 17 | ♀/69 | 4 | cTBAD | 38 | 11 mm proximal entry tear | Elective |
| 18 | ♂/59 | 3 | cTBAD | 37 | 12 mm distal entry tear | Elective |
| 19 | ♂/45 | 4 | cTBAD | 60 | Expansion (10 mm/30 months) | Elective |
| 20 | ♂/58 | 3 | cTBAD | 36 | TLC/Visceral ischemia | Elective |
| 21 | ♂/68 | 3 | cTBAD | 68 | Recurrent pain | Urgent |
| 22 | ♀/74 | 4 | cTBAD | 71 | Aortobronchial fistula | Urgent |
| 23 | ♂/56 | 3 | cTBAD | 63 | Expansion (15 mm/12 months) | Elective |
♀ – female; ♂ – male; ASA – American Society of Anesthesiologists risk index; aTBAD – acute aortic type B dissection; cTBAD – chronic aortic type B dissection; TLC – true lumen collapse; max. AD – maximal aortic diameter; FL – false lumen.
Procedural data
All operations undertaken before October 2010 were performed in a surgical theatre equipped with an Axiom U imaging system (Siemens Healthcare, Forchheim, Germany). Since October 2009, TEVAR was performed in a hybrid operating room featuring an Artis Zeego multi-axis imaging system (Siemens Healthcare, Forchheim, Germany). The implantation protocol has been previously published [14]. Devices were oversized by 0–10% in correlation to the diameter of the proximal landing zone of the non-dissected aortic arch. Stent-graft deployment was routinely performed with rapid ventricular pacing in zones 0–2 [15]. Correct stent-graft insertion in the true lumen was verified by intraoperative angiography and transesophageal echocardiography. All except 1 (combined spinal and epidural anesthesia) procedures were performed under general anesthesia. In all but 1 patient, who was treated in a percutaneous fashion, a transfemoral cut-down access was used.
Imaging and measurements
CTA scans were preferably performed following a standardized aortic protocol containing 1-mm slice acquisition of the entire aorta with native, arterial, and venous imaging phases, as well as 3-D image reconstructions (Siemens Somatom; Siemens, Erlangen, Germany) [16]. CT measurements were performed on a workstation using 3-D reconstruction software (iNtuition; TeraRecon, Foster City, USA) by 2 readers with multi-year experience in vascular imaging and/or TEVAR (1 radiologist and 1 vascular surgeon) in an independent fashion. In case of divergent measurements, a second common reading with consensual decision was made. All diameter measurements [maximal aortic diameter (max. AD); maximal/minimal true lumen diameter (max./min. TL) no; minimal false lumen diameter (min. FL)] were obtained from axial planes of the preoperative baseline CT scan and the last available FU imaging in a standardized fashion [6]. Conformability of the device was quantified by measuring the distance (D) between the radio-opaque gold band (GB) at the proximal end of the covered stent-graft and the inner curvature (IC) of the aortic arch using parasagittal multiplanar reformations of the postprocedural CTA (Figure 1). Additionally, the length of the longitudinal segment of the unapposed stent-graft (bird-beak) was measured. A total of 20 patients were eligible for the conformability analysis (Table 3). Two patients were excluded due to prior arch-involving TEVAR with a non-CTAG device in other hospitals (patient #18: TAG, W. L. Gore & Associates, Inc., Flagstaff, USA; patient #20: RELAY, Bolton Medical, Inc., Sunrise, USA). In a third patient (patient #21) the endograft was landed in zone 4. In patients with aTBAD, postoperative CT FU was available in all 15 patients (median: 8.4 months; range: 1 day to 34.5 months). In cTBAD cases, radiographic FU was available in all 8 patients (median: 17.9 months, range: 2 days to 34.9 months).
Figure 1.
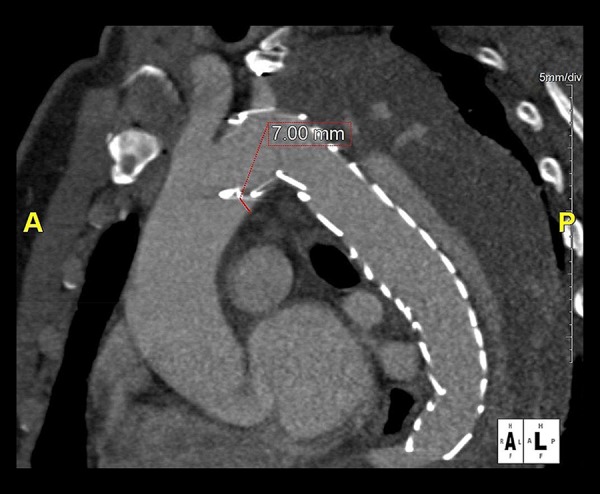
Displayed is a parasagittal multiplanar reformation of the postprocedural CT of the patient with the type I endoleak. After previous left subclavian artery revascularization the patient had undergone TEVAR for chronic aortic dissection Stanford type B (patient #19; Tables 2 and 3). The conformability analysis showed a 7 mm distance between the gold band of the endograft and the inner curvature of a type III aortic arch.
Table 3.
Case-by-case presentation of procedural details (N=23).
| Patient N. | Arch type | PLZ | Device N. | Device size (mm) | GM-IC-D (mm) | Bird-beak length (mm) | Primary LSA revascularization | CSD |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 2 | 3 | 21–100/26–100/26–100 | 0 | 0 | 1 | 1 |
| 2 | 2 | 2 | 2 | 34–150/34–200 | 6 | 8 | 0 | 1 |
| 3 | 3 | 3 | 4 | 31–100*/34–100*/37–100*/34–150 | 0 | 0 | 0 | 1 |
| 4 | 2 | 2 | 2 | 34–150/34–200 | 3 | 6 | 0 | 0 |
| 5 | 1 | 3 | 3 | 28–100/28–100/28–150 | 0 | 0 | 0 | 0 |
| 6 | 2 | 3 | 1 | 31–150 | 0 | 0 | 0 | 1 |
| 7 | 2 | 1 | 1 | 37–150 | 10 | 15 | 0 | 0 |
| 8 | 2 | 2 | 1 | 34–150 | 0 | 0 | 0 | 1 |
| 9 | 2 | 3 | 1 | 37–200 | 0 | 0 | 0 | 1 |
| 10 | 2 | 2 | 1 | 37–200 | 5 | 6 | 1 | 0 |
| 11 | 2 | 3 | 1 | 31–150 | 0 | 0 | 0 | 0 |
| 12 | 3 | 2 | 1 | 37–200 | 2 | 2 | 0 | 0 |
| 13 | 3 | 2 | 1 | 31–150 | 0 | 0 | 0 | 0 |
| 14 | 2 | 2 | 1 | 31–150 | 0 | 0 | 1 | 1 |
| 15 | 2 | 2 | 2 | 40–150/40–200 | 2 | 5 | 1 | 1 |
| 16 | 3 | 2 | 1 | 37–200 | 0 | 0 | 1 | 1 |
| 17 | 2 | 2 | 1 | 31–150 | 0 | 0 | 1 | 1 |
| 18 | 2 | 4 | 1 | 34–150 | NA | NA | 0 | 1 |
| 19 | 2 | 2 | 1 | 37–200 | 7 | 9 | 1 | 1 |
| 20 | 2 | 4 | 2 | 34–100/34–150 | NA | NA | 0 | 1 |
| 21 | 2 | 4 | 2 | 37–200/37–150 | NA | NA | 0 | 1 |
| 22 | 3 | 3 | 1 | 28–150 | 0 | 0 | 0 | 0 |
| 23 | 2 | 2 | 1 | 40–200 | 8 | 14 | 1 | 0 |
GM-IC-D – gold marker inner curvature distance; PLZ – proximal landing zone; LSA – left subclavian artery; CSD – cerebrospinal fluid drainage,
GORE TAG thoracic endoprosthesis;
NA – not applicable (patients #18 and #20 received TEVAR for aTBAD in an external hospital and underwent distal TEVAR extension in our institution; in patient #21 the endograft was anchored in zone 4).
Definitions and statistical analysis
Acute and chronic dissections were differentiated according to the time frame from onset of symptoms until intervention (acute: first 2 weeks; chronic: after 6 weeks) [17]. Primary technical success (successful access/deployment, absence of type I/III endoleak, absence of conversion to open repair, and/or death within the first 24 hours) can include the use of additional components, stents, angioplasty, and adjunctive surgical procedures [18,19]. Proximal landing zone was defined based on the classification by Mitchell et al. (Table 3) [15]. Arch type morphology was classified in Type I, II, or III (Table 3) [20]. The status of the false lumen after TEVAR (incomplete, complete, or no FL thrombosis) was assessed as proposed by Tsai et al. [21]. In aTBAD, aortic remodeling was defined as sealed entry, thrombosed FL with shrinkage along the stent graft, and absence of AD expansion within the thoracic aorta [2]. Bird-beak configuration was defined as a lack of apposition of the proximal stent-graft to the aortic wall along the lesser curve [22].
PASW Statistics (version 18.0; IBM Corporation, Somers, NY, USA) was used for statistical analysis. Patient and disease characteristics are described as percentage or median (range). Diameter changes are expressed as median, lower and upper quartile. The Mann-Whitney U test was used to describe the influence of the proximal landing zone on GM-IC-D. The Wilcoxon signed-rank test was used for diameter analysis. Values of p<0.05 were considered to indicate statistical significance.
Results
Procedural results
The median number of devices implanted was 1 (range: 1 to 4; Table 3). The primary technical success rate was 91.3% (21/23). In 1 cTBAD patient (patient #19), a primary type Ia endoleak was detected. The patient was experiencing ongoing diameter expansion (10 mm/30 months) and is currently scheduled for open surgery. No further endoleak was observed. In an aTBAD patient (patient #7), accidental partial coverage of the left common carotid artery occurred, which was treated by carotid-carotidal bypass grafting. Thus, the secondary technical success rate was 95.6% (22/23). There was no retrograde Stanford type A dissection or primary conversion. The left subclavian artery (LSA) was over-stented intentionally in 65.2% of patients (15/23) to achieve adequate proximal fixation. Previous LSA revascularization was performed in 8 of 15 patients (53.3%; Table 3). In 2 patients simultaneous stenting of the renal artery (N=2, patient #14 and #23) was performed due to visceral malperfusion. The median stay in the intensive care unit was 4.5 days (range: 1 day to 17 days). The median hospital stay was 16 days (range: 6 days to 38 days).
Mortality and morbidity
In-hospital mortality was 8.6% (2/23); both deaths were procedure-related. The first patient (patient #4) received emergency TEVAR for a ruptured aTBAD. The operation was performed under cardiopulmonary resuscitation. The patient died of severe cerebral edema after prolonged reanimation 2 days after the procedure. The second patient (patient #22) died of respiratory failure 7 days after TEVAR. Three patients died during FU; thus, the overall mortality rate was 21.7% (5/23). The first patient died of multi-organ failure after secondary conversion (patient #5). In the remaining 2 patients, the causes of death were stroke and gastrointestinal bleeding (N=1, each).
The in-hospital morbidity rate was 26.1% (6/23). Two patients suffered from neurological events (fatal cerebral edema and transient spinal cord ischemia, N=1, each), and 2 patients experienced respiratory failure (deadly in 1 patient). Additionally, there was 1 patient with acute renal failure requiring transient dialysis and 1 case with chylus fistula in which sclerotherapy was performed.
Endograft conformability
The median GB-IC-D was 0 mm (range: 0 mm to 10 mm). The GB-IC-D was 0 mm in 60.0% (12/20), between 1 mm and 5 mm in 20.0% (4/20), and between 6 mm and 10 mm in 20.0% of cases (4/20). In the patient with the type I endoleak, a GB-IC-D of 7 mm was observed (patient #19; bird-beak length: 9 mm; Figure 1). Zone 2 landing was significantly associated with GB-IC-D formation compared to zone 3 (P=0.036). The aortic arch type did not influence endograft wall apposition (P=0.211).
Aortic diameter assessment
The preoperative-postoperative diameter comparison revealed no significant difference in max. thoracic AD in patients with aTBAD (Table 4). The max. TL diameter significantly increased after stent-grafting (P=0.017). A significant increase in minimal TL diameter was detected in aTBAD patients (P=0.003). In contrast, maximum FL diameter underwent shrinkage (P=0.025; Table 4). Aortic remodeling was seen in 53.3% (8/15). Complete FL thrombosis was seen in 66.7% (10/15) and partial FL thrombosis in 33.3% (5/15).
Table 4.
Diameter changes of the thoracic aorta in patients with acute aortic dissection type Stanford B (N=15). Data is expressed as median, 25. and 75. percentile.
| aTBAD | Preoperative diameter (mm) | Postoperative diameter (mm) | |||||
|---|---|---|---|---|---|---|---|
| 25% | Median | 75% | 25% | Median | 75% | P | |
| Max. AD | 39.00 | 41.00 | 53.00 | 35.00 | 45.00 | 54.00 | 0.864 |
| Max. TL | 18.00 | 22.00 | 34.00 | 29.00 | 32.00 | 36.00 | 0.017 |
| Max. FL | 20.00 | 24.00 | 31.00 | 0.00 | 16.00 | 31.00 | 0.025 |
| Min. TL | 5.00 | 10.00 | 17.00 | 17.00 | 22.00 | 25.00 | 0.003 |
aTBAD – acute aortic type B dissection; AD – aortic diameter; TL – true lumen diameter; FL – false lumen diameter; Max. – maximal; Min. – minimal.
There was no change regarding the max. thoracic AD in patients with cTBAD (P=0.397) and in max. TL diameter (P=0.203). There was an enlargement of the min. TL diameter (P=0.012). Max. FL diameter was reduced after TEVAR (P=0.036; (Table 5). Complete FL thrombosis was seen in 37.5% (3/8) and partial FL thrombosis in 62.5% (5/8) of cases.
Table 5.
Diameter changes of the thoracic aorta in patients with chronic aortic dissection type Stanford B (N=8). Data is expressed as median, 25. and 75. percentile.
| cTBAD | Preoperative diameter (mm) | Postoperative diameter (mm) | |||||
|---|---|---|---|---|---|---|---|
| 25% | Median | 75% | 25% | Median | 75% | P | |
| Max. AD | 37.25 | 61.50 | 66.75 | 42.75 | 54.50 | 71.50 | 0.397 |
| Max. TL | 18.00 | 26.50 | 32.75 | 26.50 | 32.50 | 35.00 | 0.203 |
| Max. FL | 25.00 | 32.00 | 43.75 | 16.25 | 25.00 | 35.75 | 0.036 |
| Min. TL | 7.00 | 10.00 | 10.75 | 12.50 | 18.50 | 26.00 | 0.012 |
cTBAD – chronic aortic type B dissection; AD – aortic diameter; TL – true lumen diameter; FL – false lumen diameter; Max. – maximal; Min. – minimal.
Discussion
This study demonstrates that TEVAR of TBAD using the CTAG endoprosthesis can be performed with excellent technical outcome. Furthermore, the C-TAG conformed favorably in our analyzed patient collective with challenging arch morphology and hemodynamics (Figure 2). This is reflected by a GM-IC-D of less than 5 mm in 80% of cases and a median distance of 0 mm. Aortic arch zone 2 – the predominant proximal landing zone in TBAD cases – is especially challenging for endograft alignment, as this region was significantly associated with GM-IC-D formation between the endograft and the inner aortic arch wall (P=0.036).
Figure 2.
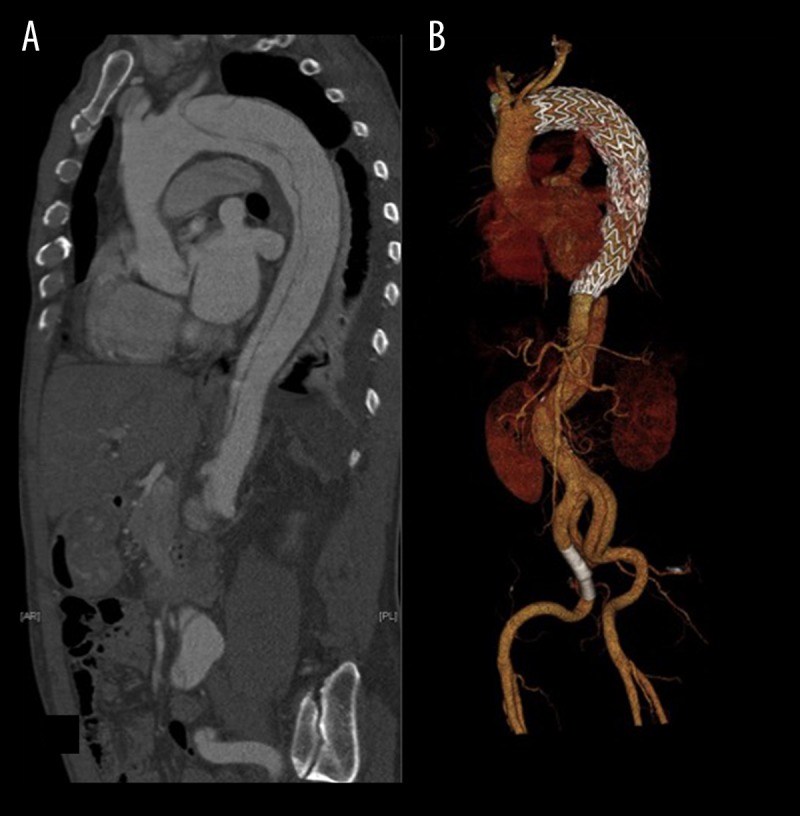
Displayed are a preoperative sagittal reconstruction (A) of a CT-angiography in 61-year-old patient, who was treated for acute complicated aortic dissection Stanford type B (patient #15; Tables 2 and 3) and a 3D-reconstruction (B) of a follow-up CT scan in the same patient, showing favorable conformability of the endograft within the aortic arch.
Commercially available stent-grafts, which do not conform to the contours of the aortic arch, may show misalignment of the inner curvature of the arch and therefore may end up with bird-beaking. Poor endograft apposition increases the risk of technical failure, especially type I endoleak and retrograde dissection [23]. Dong et al. investigated the clinical significance of stent-graft-induced entry tears after endovascular repair for TBAD [24]. Among their 650 cases, the incidence of endograft-induced entries was 3.4% with a mortality rate of 26.1%. The authors suggested that the stress produced by the endograft plays an important role in the occurrence of stent-graft-induced entries. Interestingly, over-sizing was not significantly associated with stent-graft-induced entry tears. At our institution, an over-sizing of at most 10% is used for the treatment of TBAD patients in order to prevent retrograde dissection of the arch [25]. The modifications of the CTAG compared to the former TAG device account for an increased compression resistance and an enhanced conformability of the device within the aortic arch. In our clinical practice the CTAG device is used in aTBAD and cTBAD cases for several reasons. The device offers a broad over-sizing window, reaching from 6% to 33%. This allows for an expanded treatment range, including tapered configurations, which is advantageous in dissection cases with significant size discrepancy in the aortic diameters of the proximal and distal landing zones. Due to the design of the device, the radial force of the stent-graft adapts to the underlying pathology. Thus, the same device exerts less radial force in a smaller over-sizing condition. The interaction between radial fit and design permits favorable conformability of the endograft within the aortic arch [26,27]. According to the CTAG registry investigators, a GM-IC-D of ≤2 mm shows that the device had conformed to the arch anatomy [28]. With regard to this definition, the device conformed in this study in about two-thirds of patients with TBAD (13/20). These patients predominantly had steep type 2 or 3 arches (19/20; Table 3), which represent a special challenge for the apposition of the endograft to the aortic wall.
The conformability results presented herein show that zone 2 landing is significantly associated with GM-IC-D formation compared to zone 3. Hsu et al. described zones 2 and 3 as a “fulcrum” over which the stent-graft is bent along the inner curvature [29]. Endograft apposition may be especially challenging in this area because it depends on the interaction between spring-back force, device flexibility, radial force, and the aortic wall. Additionally, the differences in aortic diameter between the intact proximal landing zone (zone 2) and the adjacent TL (zone 3) may lead to a straightening tendency of the stent-graft, resulting in GM-IC-D formation in zone 2.
It is important to note that the CTAG opens up bidirectionally from its center, extending simultaneously towards both ends. For optimal deployment, a stiff double-curved wire is placed in the ascending aorta. Pushing the stiff wire into the ascending aorta results in alignment of the endograft along the outer curvature of the aortic arch and the proximal descending aorta. By this maneuver the device is stabilized during deployment within its intended position. Furthermore, positioning of the endograft in the arch results in stored energy; therefore, gentle retraction is recommended and necessary during stent-graft release in order to prevent an unwanted forward movement of the device [27]. To achieve optimal conditions during deployment, a standardized implantation strategy is recommended. Our protocol includes transesophageal echo and rapid ventricular pacing for zone 0–2 deployment [30].
The impact of LSA coverage on bird-beak/endoleak formation has been previously emphasized [22,31]. Ueda et al. reported that a bird-beak configuration increases the risk for endoleak formation [22]. In their study, the risk of endoleak formation (combined for type I and II) depended on bird-beak length, reaching a risk of more than 50% at a length of 10 mm and more than 80% at a length of 15 mm. In the present study, we observed a bird-beak length of more than 10 mm in 2 patients (patient #7 and #23; Table 3), but this had no impact on the patients’ clinical outcome.
The type Ia endoleak rate observed in the present study was 4.3% (1/23), which is comparable to the results achieved with other devices in TBAD patients. In 2009 Sze et al. reported a 20% type I endoleak rate for the TAG device in TBAD patients [31]. Zipfel et al. detailed a 7% endoleak type I rate within the RESTORE registry using the RELAY device for dissection cases [32]. A recently published multicenter trial describing the safety and efficacy of the CTAG for patients with complicated aTBAD reported a similar type I endoleak rate of 4% (2/50) [33]. Of note, the type Ia endoleak in this study (patient #19; bird-beak length: 9 mm) was not caused by the bird-beak, but by incomplete sealing of the endograft at the outer curvature proximal to the ostium of the LSA. The patient had undergone primary LSA revascularization by carotid-subclavian bypass in order to achieve a sufficient proximal neck length of 2 cm. However, the device was deployed in zone 2 and flared into the ostium of the LSA. FU imaging revealed ongoing expansion of the distal aortic arch due to the antegrade false lumen perfusion by the endoleak.
At present, clinical and morphological FU data on dissection patients treated with the CTGA are limited. A recent systematic review by Patterson et al. reported that aortic remodeling is most often seen within the first postoperative year [34]. In aTBAD cases we observed total TL thrombosis in two-thirds of patients after a median imaging FU of 8.4 months. Furthermore, a significant reduction in FL diameter in conjunction with an increase of TL diameter after TEVAR is common and was also observed in this study (Tables 4, 5) [34].
Limitations of this study include its retrospective character, the short FU, the heterogeneity of the treated pathologies, and the limited number of subjects analyzed.
Conclusions
This study shows that the CTAG device conforms well to the aortic arch geometry in TBAD patients. Aortic arch zone 2 deployment is a special challenge to endograft alignment properties. Clinical results are promising. Further studies are needed to assess the clinical performance of the device in larger patient cohorts and the impact of distance formation on patient outcome needs to be addressed by long-term FU.
Footnotes
Disclosures
Dr. Dittmar Böckler states that he is a consultant for W.L. GORE & Associates, receiving speaker honoraria as well as educational and research grants. Dr. Dittmar Böckler also states that he is a consultant for Siemens AG. All other authors state that there is no conflict of interest to declare.
Source of support: Departmental sources
References
- 1.Kasprzak B. Therapeutic strategies and techniques for treatment of acute and subacute type B aortic dissection. Gefässchirurgie. 2015;20:428–34. [Google Scholar]
- 2.Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407–16. doi: 10.1161/CIRCINTERVENTIONS.113.000463. [DOI] [PubMed] [Google Scholar]
- 3.Hanna JM, Andersen ND, Ganapathi AM, et al. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2014;59:96–106. doi: 10.1016/j.jvs.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Brunkwall J, Lammer J, Verhoeven E, Taylor P. ADSORB: A study on the efficacy of endovascular grafting in uncomplicated acute dissection of the descending aorta. Eur J Vasc Endovasc Surg. 2012;44:31–36. doi: 10.1016/j.ejvs.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Divchev D, Najjar T, Tillwich F, et al. Predicting long-term outcomes of acute aortic dissection: a focus on gender. Expert Rev Cardiovasc Ther. 2015;13:325–31. doi: 10.1586/14779072.2015.1004313. [DOI] [PubMed] [Google Scholar]
- 6.Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: Insight from the European registry on endovascular aortic repair complications. Circulation. 2009;120:S276–81. doi: 10.1161/CIRCULATIONAHA.108.835926. [DOI] [PubMed] [Google Scholar]
- 7.Böckler D, Schumacher H, Ganten M, et al. Complications after endovascular repair of acute symptomatic and chronic expanding Stanford type B aortic dissections. J Thorac Cardiovasc Surg. 2006;132:361–68. doi: 10.1016/j.jtcvs.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 8.Eggebrecht H, Schmermund A, Voigtlander T. Clinical safety and efficacy of a next-generation stent-graft device for thoracic endovascular aortic repair. Expert Rev Cardiovasc Ther. 2013;11(1):27–30. doi: 10.1586/erc.12.164. [DOI] [PubMed] [Google Scholar]
- 9.Canaud L, Cathala P, Joyeux F, et al. Improvement in conformability of the latest generation of thoracic stent grafts. J Vasc Surg. 2013;57:1084–89. doi: 10.1016/j.jvs.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Kasirajan K. New C-TAG device and overcome of compression events. J Cardiovasc Surg (Torino) 2012;53:169–72. [PubMed] [Google Scholar]
- 11.Jordan WD, Jr, Rovin J, Moainie S, et al. Results of a prospective multicenter trial of CTAG thoracic endograft. J Vasc Surg. 2015;61:589–95. doi: 10.1016/j.jvs.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Farber MA, Giglia JS, Starnes BW, et al. Evaluation of the redesigned conformable GORE TAG thoracic endoprosthesis for traumatic aortic transection. J Vasc Surg. 2013;58:651–58. doi: 10.1016/j.jvs.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Georg Y, Schwein A, Lejay A, et al. Practical experience with the TAG and conformable TAG devices: lessons learned in about 100 cases. J Cardiovasc Surg (Torino) 2013;54:605–15. [PubMed] [Google Scholar]
- 14.Geisbüsch P, Kotelis D, Weber TF, et al. Early and midterm results after endovascular stent graft repair of penetrating aortic ulcers. J Vasc Surg. 2008;48:1361–68. doi: 10.1016/j.jvs.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell RS, Ishimaru S, Ehrlich MP, et al. First International Summit on Thoracic Aortic Endografting: roundtable on thoracic aortic dissection as an indication for endografting. J Endovasc Ther. 2002;9(Suppl 2):II98–105. [PubMed] [Google Scholar]
- 16.Bischoff MS, Geisbüsch P, Kotelis D, et al. Clinical significance of type II endoleaks after thoracic endovascular aortic repair. J Vasc Surg. 2013;58:643–50. doi: 10.1016/j.jvs.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61:1661–78. doi: 10.1016/j.jacc.2012.11.072. [DOI] [PubMed] [Google Scholar]
- 18.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg. 2010;52:1022–33. 33e15. doi: 10.1016/j.jvs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–60. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 20.Casserly IP, Kapadia SR. Advances in percutaneous valvular intervention. Expert Rev Cardiovasc Ther. 2005;3:143–58. doi: 10.1586/14779072.3.1.143. [DOI] [PubMed] [Google Scholar]
- 21.Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357:349–59. doi: 10.1056/NEJMoa063232. [DOI] [PubMed] [Google Scholar]
- 22.Ueda T, Fleischmann D, Dake MD, et al. Incomplete endograft apposition to the aortic arch: bird-beak configuration increases risk of endoleak formation after thoracic endovascular aortic repair. Radiology. 2010;255:645–52. doi: 10.1148/radiol.10091468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotelis D, Brenke C, Worz S, et al. Aortic morphometry at endograft position as assessed by 3D image analysis affects risk of type I endoleak formation after TEVAR. Langenbecks Arch Surg. 2015;400:523–29. doi: 10.1007/s00423-015-1291-1. [DOI] [PubMed] [Google Scholar]
- 24.Dong Z, Fu W, Wang Y, et al. Stent graft-induced new entry after endovascular repair for Stanford type B aortic dissection. J Vasc Surg. 2010;52:1450–57. doi: 10.1016/j.jvs.2010.05.121. [DOI] [PubMed] [Google Scholar]
- 25.Canaud L, Ozdemir BA, Patterson BO, et al. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg. 2014;260:389–95. doi: 10.1097/SLA.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 26.Morasch MD. Conformable GORE TAG thoracic endoprosthesis. Expert Rev Cardiovasc Ther. 2014;12:949–54. doi: 10.1586/14779072.2014.928592. [DOI] [PubMed] [Google Scholar]
- 27.Böckler D, Müller-Eschner M, Tengg-Kobligk H, Bischoff MS. Conformability in aortic type B dissection. Enovascular Today. 2014;2014:8–11. [Google Scholar]
- 28.Böckler D, Brunkwall J, Taylor P, et al. Investigators: obotCr. Thoracic Endovascular Aortic Repair (TEVAR) of Aortic arch pathologies with the conformable TAG – early and 2 year results from a European multicentre registry. Eur J Vasc Endovasc Surg. 2015 doi: 10.1016/j.ejvs.2016.02.006. [under revision] [DOI] [PubMed] [Google Scholar]
- 29.Hsu HL, Chen CK, Chen PL, et al. The impact of bird-beak configuration on aortic remodeling of distal arch pathology after thoracic endovascular aortic repair with the Zenith Pro-Form TX2 thoracic endograft. J Vasc Surg. 2014;59:80–88. doi: 10.1016/j.jvs.2013.07.098. [DOI] [PubMed] [Google Scholar]
- 30.Kotelis D, Bischoff MS, Jobst B, et al. Morphological risk factors of stroke during thoracic endovascular aortic repair. Langenbecks Arch Surg. 2012;397:1267–73. doi: 10.1007/s00423-012-0997-6. [DOI] [PubMed] [Google Scholar]
- 31.Sze DY, van den Bosch MA, Dake MD, et al. Factors portending endoleak formation after thoracic aortic stent-graft repair of complicated aortic dissection. Circ Cardiovasc Interv. 2009;2(2):105–12. doi: 10.1161/CIRCINTERVENTIONS.108.819722. [DOI] [PubMed] [Google Scholar]
- 32.Zipfel B, Czerny M, Funovics M, et al. Endovascular treatment of patients with types A and B thoracic aortic dissection using Relay thoracic stent-grafts: Results from the RESTORE Patient Registry. J Endovasc Ther. 2011;18:131–43. doi: 10.1583/10-3233MR.1. [DOI] [PubMed] [Google Scholar]
- 33.Cambria RP, Conrad MF, Matsumoto AH, et al. Multicenter clinical trial of the conformable stent graft for the treatment of acute, complicated type B dissection. J Vasc Surg. 2015;62:271–78. doi: 10.1016/j.jvs.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Patterson BO, Cobb RJ, Karthikesalingam A, et al. A systematic review of aortic remodeling after endovascular repair of type B aortic dissection: methods and outcomes. Ann Thorac Surg. 2014;97:588–95. doi: 10.1016/j.athoracsur.2013.07.128. [DOI] [PubMed] [Google Scholar]


