Abstract
Lung cancer remains the most frequent cause of cancer-related death in developed countries. A recent molecular-targeted strategy has contributed to improvement of the remarkable effect of adenocarcinoma of the lung. However, such treatment has not been developed for squamous cell carcinoma (SCC) of the disease. Our recent studies of microRNA (miRNA) expression signatures of human cancers showed that the microRNA-29 family (miR-29a, miR-29b and miR-29c) significantly reduced cancer tissues compared to normal tissues. These findings suggest that miR-29s act as tumor-suppressors by targeting several oncogenic genes. The aim of the study was to investigate the functional significance of miR-29s in lung SCC and to identify miR-29s modulating molecular targets in lung SCC cells. Restoration of all mature members of the miR-29s inhibited cancer cell migration and invasion. Gene expression data combined in silico analysis and luciferase reporter assays demonstrated that the lysyl oxidase-like 2 (LOXL2) gene was a direct regulator of tumor-suppressive miR-29s. Moreover, overexpressed LOXL2 was confirmed in lung SCC clinical specimens, and silencing of LOXL2 inhibited cancer cell migration and invasion in lung SCC cell lines. Our present data suggested that loss of tumor-suppressive miR-29s enhanced cancer cell invasion in lung SCC through direct regulation of oncogenic LOXL2. Elucidation of the novel lung SCC molecular pathways and targets regulated by tumor-suppressive miR-29s will provide new insights into the potential mechanisms of oncogenesis and metastasis of the disease.
Keywords: lung squamous cell carcinoma, microRNA, miR-29a, miR-29b, miR-29c, lysyl oxidase-like 2
Introduction
Lung cancer remains the most frequent cause of cancer-related death in developed countries, and an estimated 1.8 million new cases of lung cancer occurred in 2012 (1). Approximately 80% of lung cancers are classified histopathologically as non-small cell lung cancer (NSCLC), and NSCLC are subdivided into four major histological subtypes: adenocarcinoma, squamous cell carcinoma (SCC), large cell carcinoma, and neuroendocrine cancer (2). NSCLC, as compared to small cell lung cancer, is less sensitive to anticancer drugs and radiation therapy. Recently, molecular target therapies for adenocarcinoma (gefitinib, erlotinib and crizotinib) have shown remarkable therapeutic efficacy; however, no targeted therapeutics are currently approved for treatment of lung SCC (3). Therefore, the lung squamous cell carcinomas need a new treatment option.
The human genome sequence era and the discovery of microRNAs (miRNAs) in human genomes have brought great changes to the study of human cancers. miRNAs are endogenous small non-coding RNA molecules (19–22 bases in length) that regulate protein-coding gene expression by repressing translation or cleaving RNA transcripts in a sequence-specific manner (4,5). Substantial evidence suggests that miRNAs are aberrantly expressed in many human cancers and play significant roles in human oncogenesis and metastasis (6,7). The nature of miRNAs is unique in that one miRNA has the ability to regulate multiple protein-coding RNAs. Bioinformatic predictions indicate that miRNAs regulate 30–60% of the protein-coding genes in the human genome (5,6). Therefore, identification of tumor-suppressive or oncogenic miRNAs and the miRNAs-mediated novel cancer networks are the first step toward elucidating the molecular mechanisms of human cancers.
Based on this foregoing discussion, we sequentially identified tumor-suppressive miRNAs and the miRNA-regulated oncogenic genes in various types of cancers (8–11). A recent study of lung SCC showed that miRNA-1/133a-clustered miRNAs inhibit cancer cell migration and invasion by targeting the CORO1C gene encoding a member of the WD repeat protein family and is involved in a variety of cellular processes (12). Moreover, tumor-suppressive miR-206 inhibited dual signaling networks activated by MET and EGFR in lung SCC cells (13).
Our miRNA expression signatures of human cancers demonstrated that the miR-29 family (miR-29a, miR-29b, and miR-29c) was downregulated in cancer tissues compared to normal tissues (8,9,14–17), suggesting that these miRNAs function as tumor suppressors in lung SCC cells. However, miR-29s-regulating molecular networks have not been sufficiently analyzed in this disease. The aim of this study is to investigate the functional significance of miR-29s in lung SCC and to identify miR-29s-regulating molecular targets in lung SCC cells.
In this study, we found that the restoration of all miR-29s inhibited cancer cell migration and invasion, directly targeting the lysyl oxidase-like 2 (LOXL2) gene. Moreover, overexpression of LOXL2 was detected in lung SCC clinical specimens, and silencing of LOXL2 significantly inhibited cell migration and invasion by cancer cells. The tumor-suppressive miR-29-LOXL2 axis may provide new insights into the potential mechanisms of lung SCC oncogenesis and metastasis.
Materials and methods
Clinical specimens and RNA extraction
A total of 32 lung SCCs and 22 normal lung specimens were collected from patients who underwent pneumonectomy at Kagoshima University Hospital from 2010 to 2013. The patient backgrounds and clinical characteristics are summarized in Table I. Archival formalin-fixed paraffin-embedded (FFPE) samples were used for qRT-PCR analysis and immunohistochemistry.
Table I.
Characteristics of the lung cancer and normal lung cases.
| A, Characteristics of the lung cancer cases | ||
|---|---|---|
|
| ||
| Lung cancer | n | (%) |
| Total number | 32 | |
| Median age (range) | 71 | (50–88) |
| Gender | ||
| Male | 30 | (93.7) |
| Female | 2 | (6.3) |
| Pathological stage | ||
| IA | 4 | (12.5) |
| IB | 8 | (25.0) |
| IIA | 4 | (12.5) |
| IIB | 5 | (15.6) |
| IIIA | 8 | (25.0) |
| IIIB | 1 | (3.1) |
| Unknown | 2 | (6.3) |
| Differentiation | ||
| Well | 8 | (25.0) |
| Moderately | 19 | (59.4) |
| Poorly | 3 | (9.4) |
| Unknown | 2 | (6.3) |
| Pleural invasion | ||
| (+) | 15 | (46.9) |
| (−) | 17 | (53.1) |
| Venous invasion | ||
| (+) | 16 | (50.0) |
| ( −) | 16 | (50.0) |
| Lymphatic invasion | ||
| (+) | 16 | (50.0) |
| ( −) | 16 | (50.0) |
| Recurrence | ||
| (+) | 9 | (28.1) |
| ( −) | 20 | (62.5) |
| Unknown | 3 | (9.4) |
|
| ||
| B, Characteristics of the normal lung cases | ||
|
| ||
| Normal lung | n | (%) |
|
| ||
| Total number | 22 | |
| Median age (range) | 71 | (50–88) |
| Gender | ||
| Male | 22 | |
| Female | 0 | |
Samples were staged according to the International Association for the Study of Lung Cancer TNM classification, and they were histologically graded (18). This study was approved by the Institutional Review Board for Clinical Research of Kagoshima University School of Medicine. Prior written informed consent and approval were provided by each patient.
FFPE tissues were sectioned to a thickness of 10 μm, and 8 tissue sections were used for RNA extraction. Total RNA (including miRNA) was extracted using Recover All™ Total Nucleic Acid Isolation kit (Ambion, Austin, TX, USA) using the manufacturer's protocol. The integrity of the RNA was checked with an RNA 6000 Nano assay kit and a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Cell culture and RNA extraction
We used human lung SCC cell lines (EBC-1 and SK-MES-1) obtained from the Japanese Cancer Research Resources Bank (JCRB) and the American Type Culture Collection (Manassas, VA, USA), respectively. Cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and maintained in a humidified incubator (5% CO2) at 37°C.
Total RNA was isolated using Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer's protocol. The integrity of the RNA was checked with an RNA 6000 Nano assay kit and a 2100 Bioanalyzer (Agilent Technologies).
Quantitative reverse transcription PCR (qRT-PCR)
The procedure for PCR quantification was described previously (9–11). TaqMan probes and primers for LOXL2 (P/N: Hs00158757_m1; Applied Biosystems, Foster City, CA, USA) were assay-on-demand gene expression products. Stem-loop RT-PCRs for miR-29a (P/N: 002112; Applied Biosystems), miR-29b (P/N: 000413), and miR-29c (P/N: 000587) were used to quantify the expression levels of miRNAs according to the manufacturer's protocol. To normalize the data for quantification of mRNA and miRNAs, we used human GUSB (P/N: Hs99999908_m1; Applied Biosystems) and RNU48 (P/N: 001006; Applied Biosystems), respectively.
Transfections with mature miRNA and small interfering RNA (siRNA) into cell lines
The following mature miRNA species were used in the present study: Pre-miR™ miRNA precursors (hsa-miR-29a-3p, P/N: AM 12499; hsa-miR-29b-3p, P/N: AM 10103; hsa-miR-29c-3p, P/N: AM 10518; and negative control miRNA, P/N: AM 17111), Stealth Select RNAi siRNA, si-LOXL2 (P/N: HSS106124, P/N: HSS106125 and P/N: HSS180848; Invitrogen, Carlsbad, CA, USA), and negative-control siRNA (D-001810-10; Thermo Fisher Scientific, Waltham, MA, USA). RNAs were incubated with OPTI-MEM and Lipofectamine RNAiMAX Reagent (both from Invitrogen) as described previously (9–11).
Cell proliferation, migration, and invasion assays
Cells were transfected with 10 nM miRNAs by reverse transfection and plated in 96-well plates at 8×103 cells/well. After 96 h, cell proliferation was determined with the XTT assay using the Cell Proliferation kit II (Roche Molecular Biochemicals, Mannheim, Germany) as described previously (9–11).
Cell migration activity was evaluated with wound healing assays. Cells were plated in 6-well plates at 8×105 cells/well, and after 48 h of transfection, the cell monolayer was scraped using a P-20 micropipette tip. The initial gap length (0 h) and the residual gap length 24 h after wounding were calculated from photomicrographs as described previously (9–11).
Cell invasion assays were performed using modified Boyden chambers, consisting of Transwell-precoated Matrigel membrane filter inserts with 8 μm pores in 24-well tissue culture plates (BD Biosciences, Bedford, MA, USA). After 72 h of transfection, cells were plated in 24-well plates at 1×105 cells/well. Minimum essential medium containing 10% FBS in the lower chamber served as the chemoattractant, as described previously (9–11). All experiments were performed in triplicate.
Western blotting
After 96 h of transfection, protein lysates (50 μg) were separated on NuPAGE on 4–12% Bis-Tris gels (Invitrogen) and transferred to polyvinylidene fluoride membranes. Immunoblotting was performed with diluted anti-LOXL2 antibodies (1:1,000; ab96233; Abcam, Cambridge, UK) and anti-GAPDH antibodies (1:5,000; MAB374; Chemicon, Temecula, CA, USA). The membrane was washed and then incubated with an anti-rabbit-IgG, HRP-linked antibody (#7074; Cell Signaling Technology, Danvers, MA, USA). Specific complexes were visualized with an echochemiluminescence (ECL) detection system (GE Healthcare, Little Chalfont, UK) as described previously (9–11).
Plasmid construction and dual-luciferase reporter assay
A partial wild-type sequence of the LOXL2 3′-UTR or those with a deleted miR-29 target site (position 555–561 or position 757–763 of the LOXL2 3′-UTR) was inserted between the XhoI-PmeI restriction sites in the 3′-UTR of the hRluc gene in the psiCHECK-2 vector (C8021; Promega, Madison, WI, USA).
The synthesized DNA was cloned into the psiCHECK-2 vector. EBC-1 cells and SK-MES-1 cells were transfected with a 50 ng vector, 10 nM miRNAs, and 1 μl of Lipofectamine 2000 in 100 μl of Opti-MEM (both from Invitrogen). The activities of Firefly and Renilla luciferases in cell lysates were determined with a dual-luciferase assay system (E1910; Promega). Normalized data were calculated as the quotient of Renilla/Firefly luciferase activities.
Immunohistochemistry
We stained the tissue array (LC2083; US Biomax, Rockville, MD, USA). The tissues were immunostained following the manufacturer's protocol with an UltraVision Detection System (Thermo Fisher Scientific). The primary rabbit polyclonal antibodies against LOXL2 (ab96233) were diluted 1:1,000. The slides were treated with biotinylated goat anti-rabbit antibodies. Diaminobenzidine hydrogen peroxidase was the chromogen, and counterstaining was done with 0.5% hematoxylin.
Identification of putative miR-29 target genes
To identify putative miR-29-regulated genes, we used the TargetScan database (http://www.targetscan.org/). We investigated the expression status of putative targets of miR-29 using lung SCC clinical expression data from the GEO database (accession no. GSE 11117). Additionally, we performed gene expression analysis using miR-29a transfected EBC-1 cells. Oligo-microarray procedures and data mining methods were described in previous studies (9,10).
Statistical analysis
Relationships between two or three variables and numerical values were analyzed using the Mann-Whitney U test or Bonferroni-adjusted Mann-Whitney U test. Expert StatView version 4 was used in these analyses.
Results
Expression levels of miR-29a, miR-29b and miR-29c in lung SCC clinical specimens
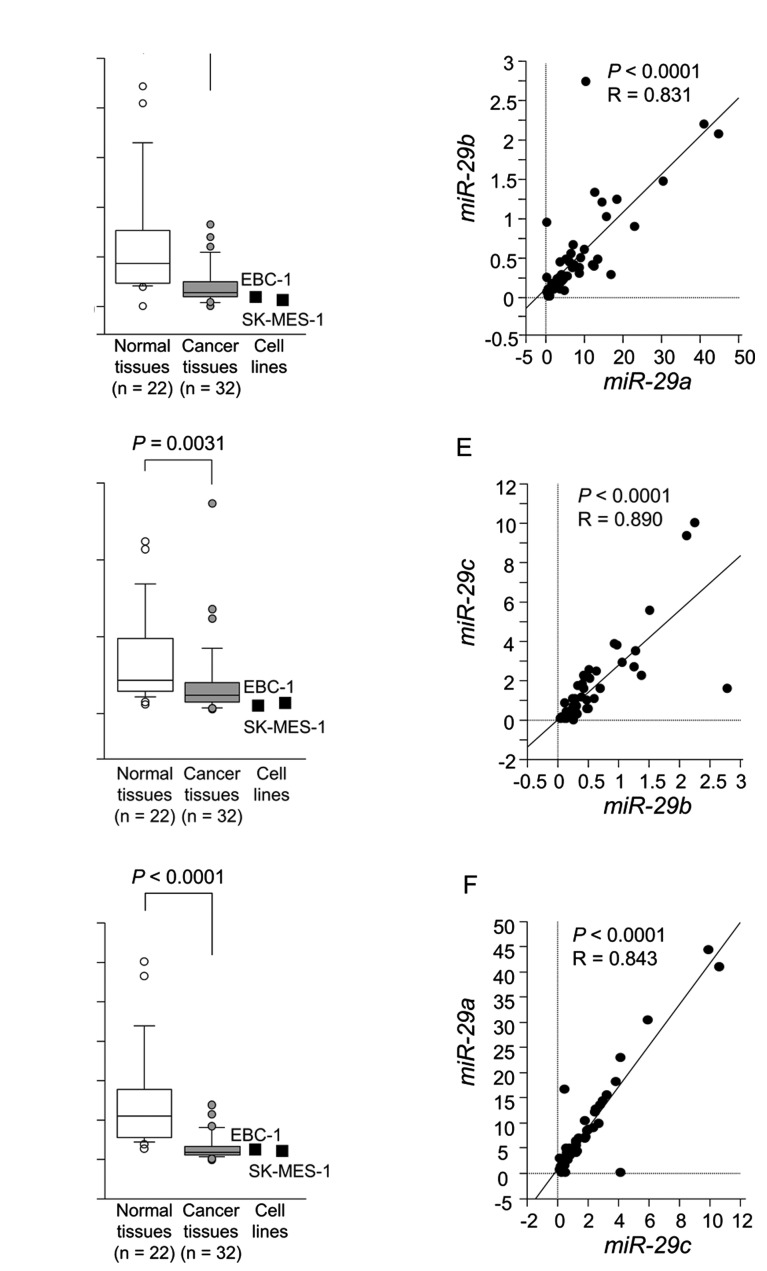
The expression levels of miR-29a, miR-29b and miR-29c were significantly reduced in tumor tissues compared to corresponding noncancerous tissues (P<0.0001, P=0.0031 and P<0.0001, respectively) (Fig. 1A–C). Spearman's rank test showed a positive correlation between the expression of miR-29a and that of miR-29b (R=0.836 and P<0.0001) (Fig. 1D). The expression of miR-29a was positively correlated with that of miR-29c (R=0.878 and P<0.0001) (Fig. 1E). Similarly, the expression of miR-29b was positively correlated with that of miR-29c (R=0.744 and P<0.0001) (Fig. 1F).
Figure 1.
The expression levels of miR-29a, miR-29b and miR-29c in clinical specimens and cell lines. Real-time PCR showed that the expression levels of (A) miR-29a, (B) miR-29b and (C) miR-29c were significantly lower in lung SCC tissues than in normal lung tissues (P<0.0001, P=0.0031 and P<0.0001, respectively). RNU48 was used as an internal control. Correlations between (D) miR-29a-miR-29b, (E) miR-29b-miR-29c and (F) miR-29c-miR-29a were determined in lung SCC clinical specimens.
Effects of miR-29a, miR-29b and miR-29c restoration on the proliferation, migration and invasion in lung SCC cell lines
To examine the functional roles of the miR-29 family (miR-29a, miR-29b and miR-29c), we performed gain-of-function studies using miRNA transfection into lung SCC cell lines (EBC-1 and SK-MES-1).
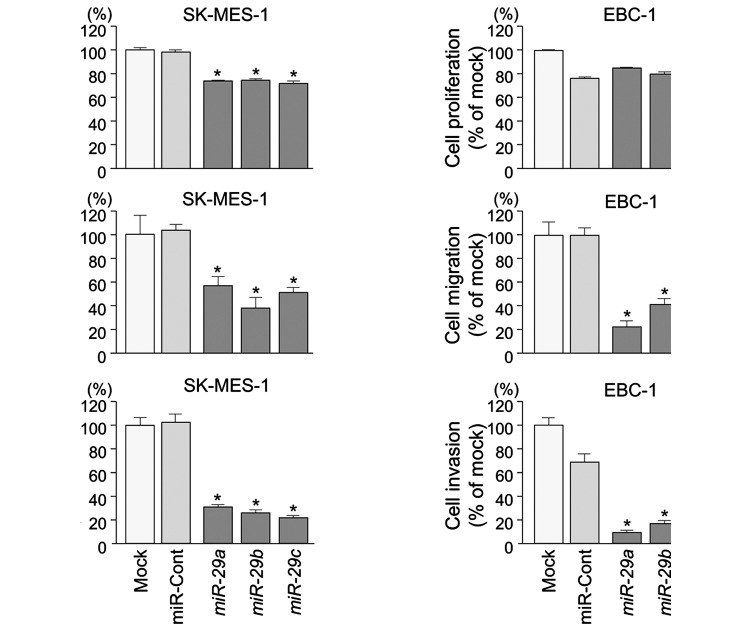
XTT assays revealed significant inhibition of cell proliferation in SK-MES-1 cells transfected with miR-29s in comparison with mock or control transfectants (P<0.0001) (Fig. 2A). Otherwise, in EBC-1 cells transfected with miR-29s, there was no significant inhibition of cell proliferation in comparison with control transfectants (Fig. 2A).
Figure 2.
Effects of miR-29a, miR-29b and miR-29c transfection on SK-MES-1 and EBC-1 cells. (A) Cell proliferation was determined with XTT assays 96 h after transfection with 10 nM miR-29s, miR-control, or mock transfection. (B) Cell migration activity was determined by migration assay 48 h after transfection. (C) Cell invasion activity was determined by Matrigel invasion assay 72 h after transfection. *P<0.0001.
Wound healing assays showed significant inhibition of cell migration activity after transfection with miR-29s (P<0.0001) (Fig. 2B).
Similarly, Matrigel invasion assays revealed that transfection with miR-29 reduced cell invasion activities (P<0.0001) (Fig. 2C).
Identification of candidate target genes of miR-29s in lung SCC
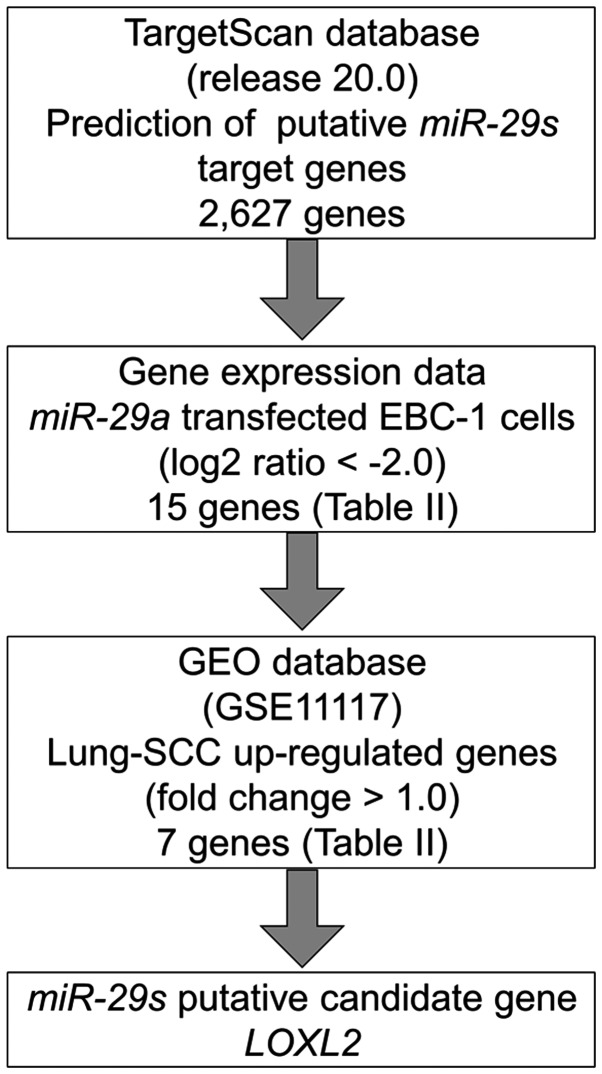
To identify molecular targets of miR-29s, we used a combination of in silico analysis and lung SCC gene expression data. In total, 2,627 genes were putative targets of miR-29s according to the TargetScan database. Next, we pared down the list of genes based on two kinds of gene expression data: downregulated genes (Log2 ratio <-2.0) following miR-29a-transfected EBC-1 cells and upregulated genes determined by the gene expression data set of lung SCC clinical specimens according to the GEO database (accession no. GSE 11117). From this selection, 7 candidate genes were identified as targets of the miR-29s (Table II). Among these genes, we focused on the LOXL2 gene and examined the LOXL2 function and characteristics in further analyses. Our strategy for selecting miR-29 target genes is shown in Fig. 3.
Table II.
Downregulated genes in miR-29a transfectant.
| Entrez gene ID | Gene symbol | Description | EBC-1 miR-29 transfectant Log2 ratio | miR-29a conserved site | miR-29a poorly conserved site | GSE1117 fold-change |
|---|---|---|---|---|---|---|
| 4017 | LOXL2 | Lysyl oxidase-like 2 | −4.05 | 1 | 1 | 2.10 |
| 3038 | HAS3 | Hyaluronan synthase 3 | −3.37 | 3 | 4.19 | |
| 9535 | GMFG | Glia maturation factor γ | −3.10 | 2 | ND | |
| 3655 | ITGA6 | Integrin α6 | −2.62 | 1 | 2.78 | |
| 634 | CEACAM1 | Carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein) | −2.58 | 1 | 1 | 1.41 |
| 871 | SERPINH1 | Serpin peptidase inhibitor, clade H (heat shock protein 47), member 1 (collagen binding protein 1) | −2.57 | 1 | ND | |
| 22801 | ITGA11 | Integrin α11 | −2.42 | 1 | 1.50 | |
| 80381 | CD276 | CD276 molecule | −2.35 | 1 | ND | |
| 50848 | F11R | F11 receptor | −2.26 | 1 | 1 | ND |
| 8894 | EIF2S2 | Eukaryotic translation initiation factor 2, subunit 2β, 38 kDa | −2.17 | 1 | 1 | 1.70 |
| 91584 | PLXNA4 | Plexin A4 | −2.15 | 1 | 1 | ND |
| 55920 | RCC2 | Regulator of chromosome condensation 2 | −2.11 | 1 | ND | |
| 9076 | CLDN1 | Claudin 1 | −2.10 | 1 | ND | |
| 2118 | ETV4 | Ets variant 4 | −2.09 | 1 | 4.84 | |
| 284119 | PTRF | Polymerase I and transcript release factor | −2.05 | 1 | −1.86 |
ND, no data.
Figure 3.
Flow chart of the strategy for identification of miR-29 target genes. In total, 2,627 genes were putative targets of miR-29s according to the TargetScan database. We merged the expression analysis data of downregulated genes in miR-29a-transfected EBC-1 cells (Log2 ratio <-2.0). Upregulated genes were determined according to the gene expression data set of lung SCC clinical specimens according to the GEO database (accession no. GSE 11117). From this selection, 7 candidate genes were identified as targets of the miR-29s.
LOXL2 is directly regulated by miR-29s in lung SCC cells
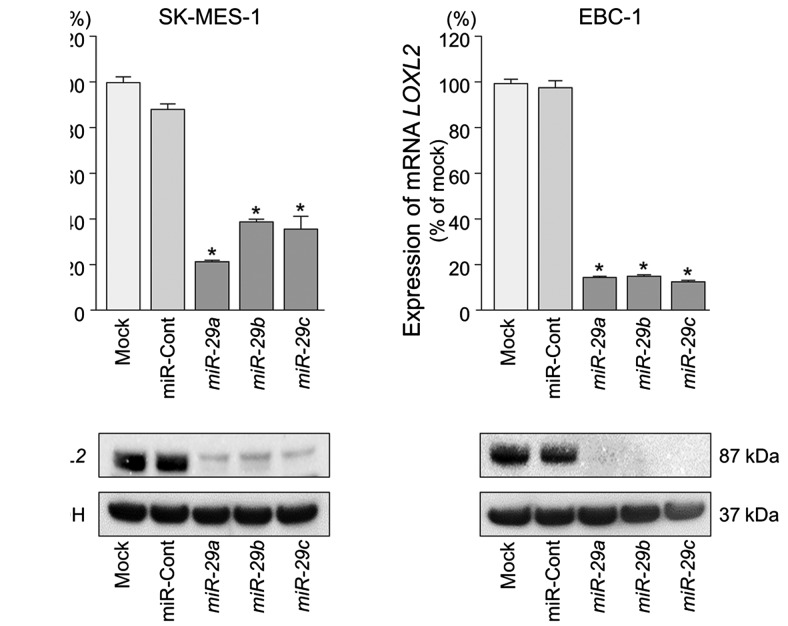
We performed qRT-PCR and western blotting to confirm LOXL2 downregulation following restoration of miR-29s expression in lung SCC cell lines. The mRNA and protein expression levels of LOXL2 were significantly repressed in miR-29s transfectants in comparison with mock or miR-control transfectants (P<0.001) (Fig. 4).
Figure 4.
Direct regulation of LOXL2 by miR-29s in SK-MES-1 and EBC-1 cells. (A) LOXL2 mRNA expression was evaluated by qRT-PCR 72 h after transfection with miR-29s. GUSB was used as an internal control. (B) LOXL2 protein expression was evaluated by western blotting 96 h after transfection with miR-29s. GAPDH was used as a loading control.
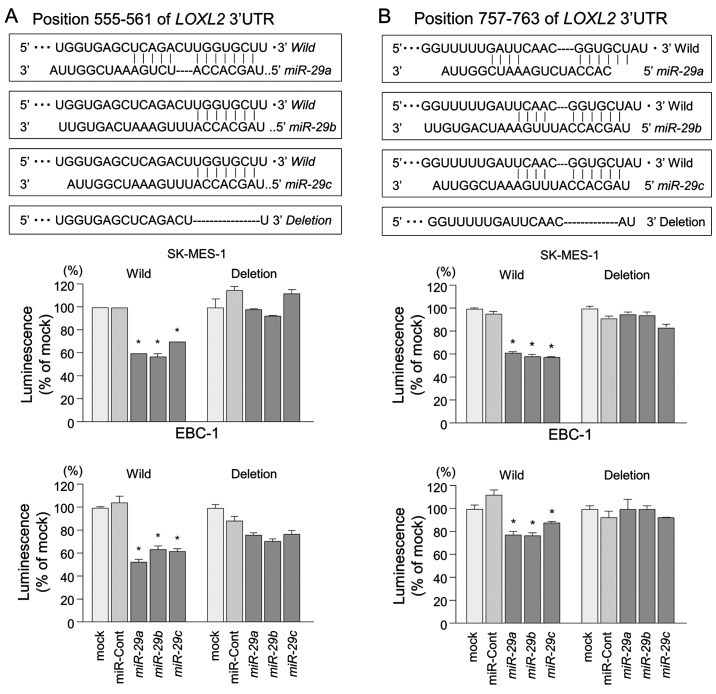
The TargetScan database identified two putative target sites in the 3′-UTR of LOXL2 (Fig. 5, upper part). A lucif-erase reporter assay confirmed that the 3′-UTR of LOXL2 was indeed an actual target of miR-29s. Luciferase activity was significantly decreased in two miR-29 target sites (positions 555–561 and 757–763 in the 3′-UTR of LOXL2) (Fig. 5, lower part).
Figure 5.
Direct regulation of LOXL2 by miR-29s in lung SCC cells. A luciferase reporter assay using vectors encoding putative miR-29 target sites at positions (A) 555–561 and (B) 757–763 for both wild-types and deletion types, respectively. Renilla luciferase values were normalized to Firefly luciferase values. *P<0.0001.
Effects of downregulating LOXL2 on cell proliferation, migration, and invasion in lung SCC cell lines
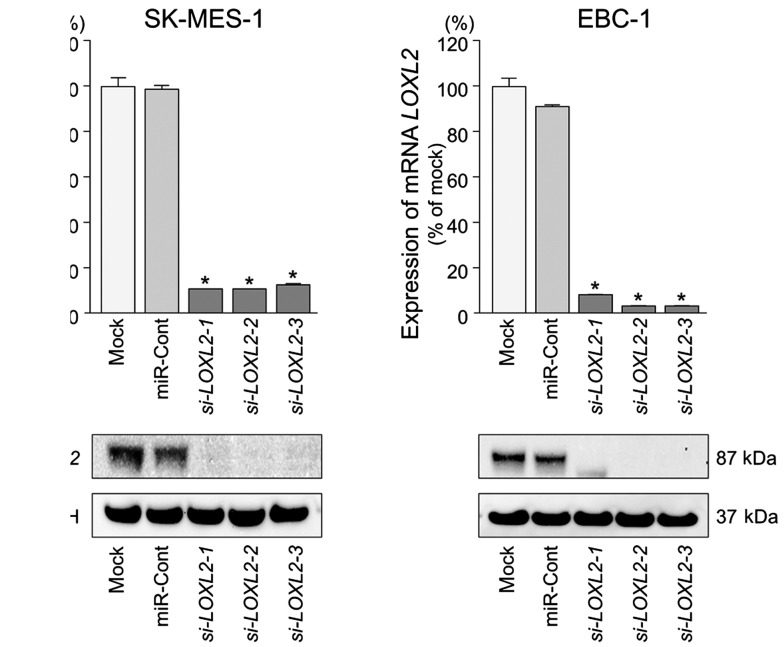
To investigate the functional role of LOXL2 in lung SCC cells, we performed loss-of-function studies using si-LOXL2 transfectants. First, we evaluated the knockdown efficiency of si-LOXL2 transfection in lung SCC cells. Western blotting and qRT-PCR indicated that si-LOXL2 effectively downregulated LOXL2 expression in lung SCC cells (P<0.0001) (Fig. 6).
Figure 6.
Silencing of LOXL2 by using si-LOXL2 in lung SCC cells. Silencing of LOXL2 mRNA and protein expression by si-LOXL2 transfection and the effects of the silencing of LOXL2 on SK-MES-1 and EBC-1 cell activities. (A) LOXL2 mRNA expression was evaluated by qRT-PCR 72 h after transfection with miR-29s. GUSB was used as an internal control. *P<0.0001. (B) LOXL2 protein expression was evaluated by western blotting 96 h after transfection. GAPDH was used as a loading control.
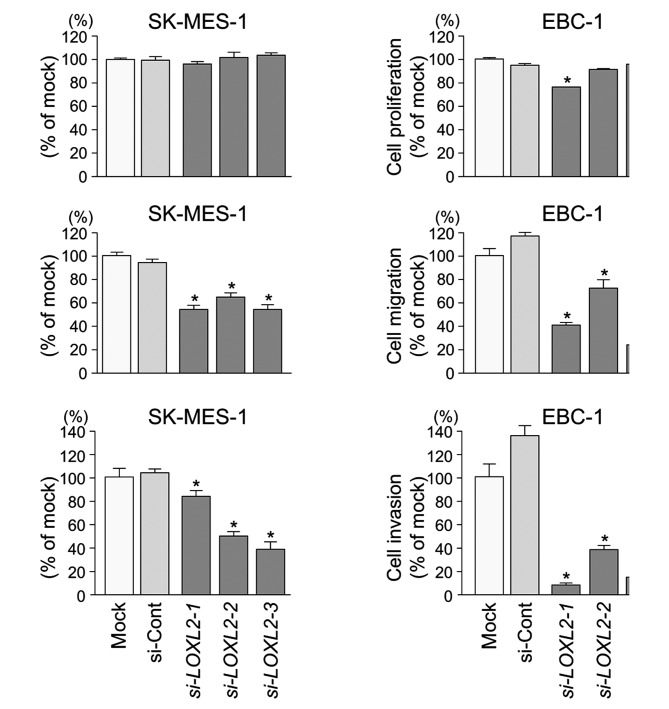
XTT assays demonstrated that cell proliferation was not inhibited in si-LOXL2 transfectants in comparison with mock or si-control transfectants in lung SCC cells (Fig. 7A).
Figure 7.
Effects of si-LOXL2 transfection on lung SCC cell lines. (A) Cell proliferation was determined using XTT assays 96 h after transfection with 10 nM si-LOXL2, miR-control, or mock transfection. (B) Cell migration activity was determined by migration assay 48 h after transfection. (C) Cell invasion activity was determined by Matrigel invasion assay 72 h after transfection. *P<0.0001.
Wound healing assays showed significant inhibition of cell migration in si-LOXL2 transfectants in comparison with mock or si-control transfectants in lung SCC cells (P<0.0001) (Fig. 7B).
Similarly, Matrigel invasion assays revealed that the number of invading cells was significantly decreased when lung SCC cells were transfected with si-LOXL2 (P<0.0001) (Fig. 7C).
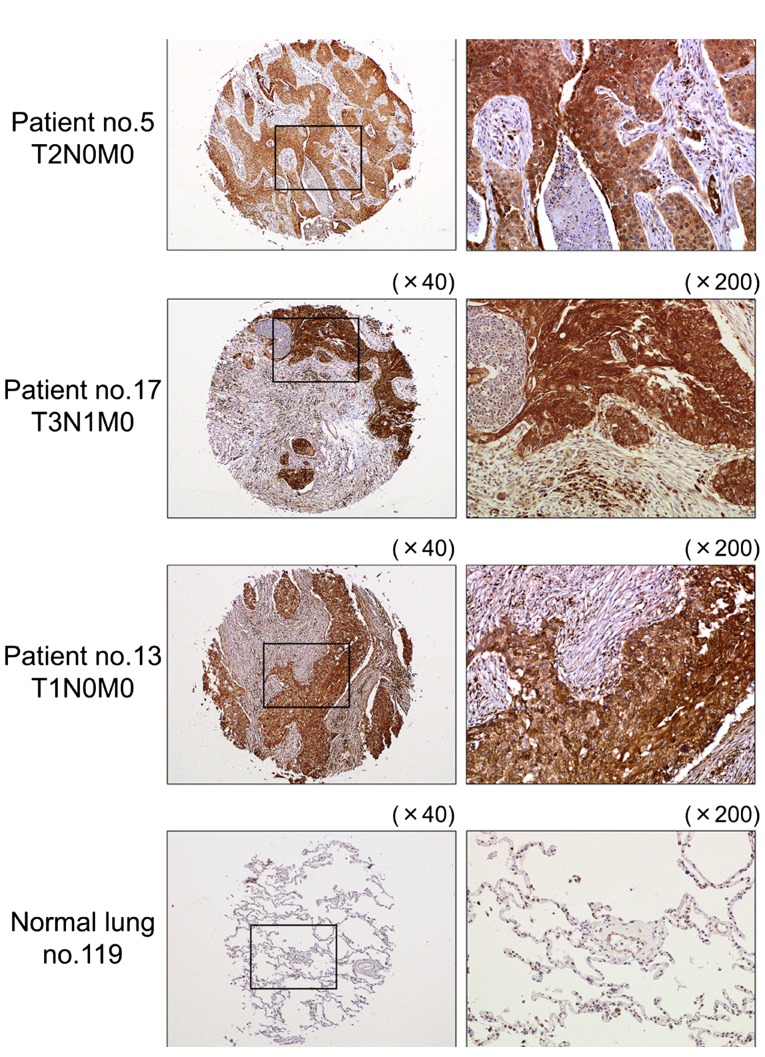
Immunohistochemical staining of LOXL2 in lung SCC clinical specimens
We confirmed the expression status of LOXL2 in lung SCC clinical specimens using immunohistochemical staining. Fifty specimens were checked in this study: 32 of 40 lung SCC specimens stained moderately or strongly, and 9 of 10 normal lung specimens stained weakly or negatively for LOXL2 (Table III and Fig. 8).
Table III.
Immunohistochemistry status and characteristics of the lung cancer and normal lung cases.
| A, Immunohistochemistry status and characteristic of the lung cancer cases | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Patient no. | Grade | T | N | M | Pathological stage | Immunohistochemistry |
| 1 | 1 | 3 | 1 | 0 | IIIA | (++) |
| 2 | 2 | 2 | 1 | 0 | IIA | (++) |
| 3 | 2 | 2 | 0 | 0 | IB | (+) |
| 4 | 2 | 2 | 0 | 0 | IB | (+++) |
| 5 | 2 | 2 | 0 | 0 | IB | (+++) |
| 6 | 2 | 2 | 1 | 0 | IIB | (++) |
| 7 | 2 | 3 | 2 | 0 | IIIA | (+++) |
| 8 | 2 | 2 | 0 | 0 | I | (++) |
| 9 | 2 | 2 | 2 | 0 | IIIA | (+) |
| 10 | 2 | 2 | 2 | 0 | IIIA | (+) |
| 11 | 2 | 2 | 2 | 0 | IIIA | (+) |
| 12 | 2 | 3 | 0 | 0 | IIB | (+) |
| 13 | 2 | 1 | 0 | 0 | IA | (+++) |
| 14 | 2 | 2 | 0 | 0 | I | (++) |
| 15 | 2 | 2 | 1 | 0 | IIB | (++) |
| 16 | 2 | 2 | 1 | 0 | IB | (++) |
| 17 | 2 | 3 | 1 | 0 | IIIA | (+++) |
| 18 | 2 | 2 | 0 | 0 | IB | (++) |
| 19 | 2 | 3 | 1 | 0 | IIIA | (+++) |
| 20 | 2 | 2 | 0 | 0 | IB | (++) |
| 21 | 3 | 2 | 0 | 0 | IB | (+) |
| 22 | 3 | 2 | 1 | 0 | II | (++) |
| 23 | 3 | 2 | 0 | 0 | IB | (++) |
| 24 | 3 | 2 | 0 | 0 | IB | (++) |
| 25 | 3 | 2 | 0 | 0 | IB | (+++) |
| 27 | 3 | 3 | 2 | 0 | IIIA | (++) |
| 28 | 3 | 2 | 0 | 0 | IB | (+++) |
| 29 | 2 | 3 | 1 | 0 | IIIA | (+++) |
| 30 | 3 | 3 | 1 | 0 | IIIA | (++) |
| 31 | 3 | 2 | 0 | 0 | IB | (++) |
| 32 | 3 | 3 | 1 | 0 | IIIA | (++) |
| 33 | 3 | 2 | 1 | 0 | IIA | (++) |
| 34 | 3 | 1 | 2 | 0 | IIIA | (++) |
| 35 | 3 | 2 | 2 | 0 | IIIA | (+) |
| 36 | 3 | 2 | 0 | 0 | I | (+) |
| 37 | 2 | 0 | 0 | IB | (+++) | |
| 38 | 3 | 2 | 0 | 0 | IB | (++) |
| 39 | 3 | 2 | 0 | 0 | IB | (+++) |
| 40 | 3 | 1 | 0 | 0 | IA | (+++) |
| 41 | 3 | 2 | 0 | 0 | IB | (++) |
|
| ||||||
| B, Immunohistochemistry status of normal lung cases | ||||||
|
| ||||||
| Patient no. | Immunohistochemistry | |||||
|
| ||||||
| 111 | (+) | |||||
| 112 | (+) | |||||
| 113 | (−) | |||||
| 114 | (++) | |||||
| 115 | (−) | |||||
| 116 | (+) | |||||
| 117 | (+) | |||||
| 118 | (−) | |||||
| 119 | (−) | |||||
| 120 | (−) | |||||
Figure 8.
Immunohistochemical staining of LOXL2 in lung SCC specimens. Differences in LOXL2 expression are observed in cancer lesions and adjacent non-cancerous tissues in the same fields. Normal lung specimens stained negatively.
Discussion
Recent studies have suggested that the interaction of cancer cells with their microenvironment has influenced the initiation, development, and metastasis of tumors (19,20). Overexpression of extracellular matrix (ECM) components has frequently been observed in cancer lesions and aberrantly expressed ECM-mediated signals have triggered cancer cell metastasis (21,22). Our past studies demonstrated that miR-29s and miR-218 directly regulated laminin-integrin signaling and thereby activated cancer cell migration and invasion (23–25). Other studies indicated that miR-29s modulated ECM components such as collagen, laminin and elastin (26). Therefore, the identification of ECM-regulated tumor-suppressive miRNAs may provide a better appreciation of novel pathways and how their interrelations are involved in cancer metastasis.
Our present data showed that all members of the miR-29 family were significantly reduced in lung SCC specimens. Our previous studies also showed the downregulation of miR-29s in renal cell carcinoma, cervical cell carcinoma, and head and neck squamous cell carcinoma (24,25,27,28) and are consistent with present data on lung SCC. However, the molecular mechanisms underlying the dysregulated expression of the miR-29s in lung SCC cells are still unclear. The genomic structure of the miR-29 family consists of two clusters in the human genome: miR-29b-1 and miR-29a in 7q32 and miR-29b-2 and miR-29c in 1q32 (26). Several studies indicated the molecular mechanisms of the silencing of miR-29s (26). Previous studies demonstrated that the promoter regions of miR-29b-1/miR-29a showed that two putative E-box (MYC-binding) sites, a Gli-binding site and four NF-κB-binding sites, were contained within this region, and c-Myc and NF-κB suppressed miR-29 expression at transcriptional levels (29). A recent study showed that cancer cells with high c-Myc, low miR-29b, and low FHIT expression had a shorter overall survival and relapse-free survival in NSCLC patients (30). In breast cancer, GATA3 is a transcription factor that specifies and maintains luminal epithelial cell differentiation in the mammary gland (31). Loss of GATA3 is involved in breast cancer pathogenesis (31). The miR-29a/miR-29b-1 promoter region contains three GATA3-binding sites, and GATA3 induced miR-29b expression, which inhibits metastasis by targeting metastatically involved genes (31). Moreover, recent data have suggested that TGF-β inhibited the expression of miR-29s and promoted the expression of ECM components (32,33). However, the silencing of molecular mechanisms of miR-29s in lung SCC are still unclear; detailed examination will be necessary to better understand these processes.
In this study, the restoration of miR-29s into cancer cells significantly inhibited migration and invasion; thus miR-29-mediated novel targets deeply contribute to meta-static pathways. To better understand lung SCC metastasis, we searched putative miR-29-regulated genes by using gene expression analysis combined with in silico analysis. Finally, 15 putative candidate genes were listed in this analysis. Among these genes, integrin α6 (ITGA6) and serpin peptidase inhibitor, clade H, member 1 (SERPINH1) have already been reported by our group as miR-29-regulated genes in head and neck squamous cell carcinoma and cervical cancer (24,28). Moreover, another group showed that miR-29c may be involved in the regulation of cell proliferation through targeting regulator of chromosome condensation 2 (RCC2) in gastric carcinoma (34). The target gene list provided by this analysis is effective for miR-29-regulated target analysis.
Here, we focused on the LOXL2 gene and validated the direct regulation of miR-29s in lung SCC cells. Furthermore, overexpression of LOXL2 was detected in lung SCC clinical specimens, and silencing of LOXL2 expression in lung SCC cells inhibited cancer cell migration and invasion, indicating that LOXL2 acts as an oncogene in the disease. Interestingly, our latest data on renal cell carcinoma also showed that LOXL2 was a direct regulator of tumor-suppressive miR-29s (27). The lysyl oxidase (LOX) protein family comprises LOX and four LOX-like proteins (LOXL1-LOXL4). These proteins are copper- and quinone-dependent amino oxidases (35). Basically, the function of the LOX family is the covalent cross-linking of collagen and/or elastin in the ECM (36–38). Several studies suggested that LOXL2-mediated cancer progression cause ECM modification and increased ECM deposition, and subsequent tissue stiffness derives malignant progression through activation of ECM-integrin or ECM-growth factor signaling (39–42). It has been reported that overexpression of LOXL2 in a number of cancers and high expression levels of LOXL2 are correlated with cancer cell invasion, lymph node metastasis, and poor overall patient survival in patients with gastric cancer, breast cancer and squamous cell carcinomas (43–45).
It is well known that metastasis is associated with the aberrant activation of epithelial-mesenchymal transition (EMT)-related transcriptional factors and TGF-β signaling, which endows cancer cells with elevated capabilities to invade and disseminate to distant sites (46). Previous studies have shown that LOXL2 is a direct transcriptional target of HIF1 (46). Moreover, nuclear LOXL2 interacts with transcription factor SNAIL1 and represses E-cadherin as well as inducing EMT (47,48). These findings suggest that hypoxia conditions and overexpression of LOXL2 trigger the ability for metastasis acquisition of the cancer cells. Several studies indicated that targeting LOXL2 with antibodies inhibited primary and metastatic xenograft models of cancers via suppression of SRC/FAK signaling or the production of growth factors and cytokines and TGF-β pathways (49,50). Interestingly, recent data indicated that expression of the LOX family was induced by TGF-β (51,52). In contrast, TGF-β inhibited the expression of miR-29s and promoted the expression of ECM components (53,54). The present data suggest that the miR-29-LOXL2 axis regulates the cancer cell microenvironment and activates metastatic pathways. Therefore, the miR-29-regulated meta-static pathway is a potential target in the development of novel therapies to treat pathological lung SCC.
In conclusion, downregulation of miR-29s was frequently observed in lung SCC clinical specimens, and all members of the miR-29 family act as tumor-suppressive miRNAs in this disease. LOXL2 was a direct regulator of miR-29s in lung SCC cells. Overexpression of LOXL2 was detected in lung SCC clinical specimens, and functional assays showed that LOXL2 promoted cancer cell invasion and migration, indicating this gene as an oncogene in lung SCC cells. The identification of novel molecular pathways mediated by the miR-29-LOXL2 axis may lead to a better understanding of lung SCC and the development of new therapeutic strategies to treat this disease.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669–692. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: Recent developments. Lancet. 2013;382:709–719. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 6.Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- 7.Iorio MV, Croce CM. MicroRNAs in cancer: Small molecules with a huge impact. J Clin Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumoto I, Hanazawa T, Kinoshita T, Kikkawa N, Koshizuka K, Goto Y, Nishikawa R, Chiyomaru T, Enokida H, Nakagawa M, et al. MicroRNA expression signature of oral squamous cell carcinoma: Functional role of microRNA-26a/b in the modulation of novel cancer pathways. Br J Cancer. 2015;112:891–900. doi: 10.1038/bjc.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukumoto I, Kinoshita T, Hanazawa T, Kikkawa N, Chiyomaru T, Enokida H, Yamamoto N, Goto Y, Nishikawa R, Nakagawa M, et al. Identification of tumour suppressive microRNA-451a in hypopharyngeal squamous cell carcinoma based on microRNA expression signature. Br J Cancer. 2014;111:386–394. doi: 10.1038/bjc.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita R, Seki N, Chiyomaru T, Inoguchi S, Ishihara T, Goto Y, Nishikawa R, Mataki H, Tatarano S, Itesako T, et al. Tumour-suppressive microRNA-144-5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br J Cancer. 2015;113:282–289. doi: 10.1038/bjc.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto Y, Kojima S, Nishikawa R, Enokida H, Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T, Seki N. The microRNA-23b/27b/24-1 cluster is a disease progression marker and tumor suppressor in prostate cancer. Oncotarget. 2014;5:7748–7759. doi: 10.18632/oncotarget.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mataki H, Enokida H, Chiyomaru T, Mizuno K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T, Nakagawa M, et al. Downregulation of the microRNA-1/133a cluster enhances cancer cell migration and invasion in lung-squamous cell carcinoma via regulation of Coronin1C. J Hum Genet. 2015;60:53–61. doi: 10.1038/jhg.2014.111. [DOI] [PubMed] [Google Scholar]
- 13.Mataki H, Seki N, Chiyomaru T, Enokida H, Goto Y, Kumamoto T, Machida K, Mizuno K, Nakagawa M, Inoue H. Tumor-suppressive microRNA-206 as a dual inhibitor of MET and EGFR oncogenic signaling in lung squamous cell carcinoma. Int J Oncol. 2015;46:1039–1050. doi: 10.3892/ijo.2014.2802. [DOI] [PubMed] [Google Scholar]
- 14.Goto Y, Kurozumi A, Enokida H, Ichikawa T, Seki N. Functional significance of aberrantly expressed microRNAs in prostate cancer. Int J Urol. 2015;22:242–252. doi: 10.1111/iju.12700. [DOI] [PubMed] [Google Scholar]
- 15.Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol. 2013;10:396–404. doi: 10.1038/nrurol.2013.113. [DOI] [PubMed] [Google Scholar]
- 16.Kikkawa N, Hanazawa T, Fujimura L, Nohata N, Suzuki H, Chazono H, Sakurai D, Horiguchi S, Okamoto Y, Seki N. miR-489 is a tumour-suppressive miRNA target PTPN11 in hypopharyngeal squamous cell carcinoma (HSCC) Br J Cancer. 2010;103:877–884. doi: 10.1038/sj.bjc.6605811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuse M, Kojima S, Enokida H, Chiyomaru T, Yoshino H, Nohata N, Kinoshita T, Sakamoto S, Naya Y, Nakagawa M, et al. Tumor suppressive microRNAs (miR-222 and miR-31) regulate molecular pathways based on microRNA expression signature in prostate cancer. J Hum Genet. 2012;57:691–699. doi: 10.1038/jhg.2012.95. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P. International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions: The International Association for the Study of Lung Cancer lung cancer staging project: Proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067–1077. doi: 10.1097/JTO.0b013e31815bdc0d. [DOI] [PubMed] [Google Scholar]
- 19.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paszek MJ, Weaver VM. The tension mounts: Mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 22.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita T, Hanazawa T, Nohata N, Kikkawa N, Enokida H, Yoshino H, Yamasaki T, Hidaka H, Nakagawa M, Okamoto Y, et al. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma. Oncotarget. 2012;3:1386–1400. doi: 10.18632/oncotarget.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita T, Nohata N, Hanazawa T, Kikkawa N, Yamamoto N, Yoshino H, Itesako T, Enokida H, Nakagawa M, Okamoto Y, et al. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma. Br J Cancer. 2013;109:2636–2645. doi: 10.1038/bjc.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishikawa R, Goto Y, Kojima S, Enokida H, Chiyomaru T, Kinoshita T, Sakamoto S, Fuse M, Nakagawa M, Naya Y, et al. Tumor-suppressive microRNA-29s inhibit cancer cell migration and invasion via targeting LAMC1 in prostate cancer. Int J Oncol. 2014;45:401–410. doi: 10.3892/ijo.2014.2437. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Zhang X, Li H, Yu J, Ren X. The role of miRNA-29 family in cancer. Eur J Cell Biol. 2013;92:123–128. doi: 10.1016/j.ejcb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa R, Chiyomaru T, Enokida H, Inoguchi S, Ishihara T, Matsushita R, Goto Y, Fukumoto I, Nakagawa M, Seki N. Tumour-suppressive microRNA-29s directly regulate LOXL2 expression and inhibit cancer cell migration and invasion in renal cell carcinoma. FEBS Lett. 2015;589:2136–2145. doi: 10.1016/j.febslet.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto N, Kinoshita T, Nohata N, Yoshino H, Itesako T, Fujimura L, Mitsuhashi A, Usui H, Enokida H, Nakagawa M, et al. Tumor-suppressive microRNA-29a inhibits cancer cell migration and invasion via targeting HSP47 in cervical squamous cell carcinoma. Int J Oncol. 2013;43:1855–1863. doi: 10.3892/ijo.2013.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 2010;110:1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu DW, Hsu NY, Wang YC, Lee MC, Cheng YW, Chen CY, Lee H. c-Myc suppresses microRNA-29b to promote tumor aggressiveness and poor outcomes in non-small cell lung cancer by targeting FHIT. Oncogene. 2015;34:2072–2082. doi: 10.1038/onc.2014.152. [DOI] [PubMed] [Google Scholar]
- 31.Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z. GATA3 suppresses metastasis and modulates the tumour micro-environment by regulating microRNA-29b expression. Nat Cell Biol. 2013;15:201–213. doi: 10.1038/ncb2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurer B, Stanczyk J, Jüngel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 33.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo M, Nakada C, Tsukamoto Y, Noguchi T, Uchida T, Hijiya N, Matsuura K, Moriyama M. MiR-29c is down-regulated in gastric carcinomas and regulates cell proliferation by targeting RCC2. Mol Cancer. 2013;12:15. doi: 10.1186/1476-4598-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;12:540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 36.Kagan HM, Li W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 37.Vadasz Z, Kessler O, Akiri G, Gengrinovitch S, Kagan HM, Baruch Y, Izhak OB, Neufeld G. Abnormal deposition of collagen around hepatocytes in Wilson's disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J Hepatol. 2005;43:499–507. doi: 10.1016/j.jhep.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 38.Kim YM, Kim EC, Kim Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep. 2011;38:145–149. doi: 10.1007/s11033-010-0088-0. [DOI] [PubMed] [Google Scholar]
- 39.Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kauppila S, Stenbäck F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186:262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Mackie EJ, Chiquet-Ehrismann R, Pearson CA, Inaguma Y, Taya K, Kawarada Y, Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci USA. 1987;84:4621–4625. doi: 10.1073/pnas.84.13.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu GG, Risteli L, Mäkinen M, Risteli J, Kauppila A, Stenbäck F. Immunohistochemical study of type I collagen and type I pN-collagen in benign and malignant ovarian neoplasms. Cancer. 1995;75:1010–1017. doi: 10.1002/1097-0142(19950215)75:4<1010::AID-CNCR2820750417>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Kasashima H, Yashiro M, Kinoshita H, Fukuoka T, Morisaki T, Masuda G, Sakurai K, Kubo N, Ohira M, Hirakawa K. Lysyl oxidase-like 2 (LOXL2) from stromal fibroblasts stimulates the progression of gastric cancer. Cancer Lett. 2014;354:438–446. doi: 10.1016/j.canlet.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Ahn SG, Dong SM, Oshima A, Kim WH, Lee HM, Lee SA, Kwon SH, Lee JH, Lee JM, Jeong J, et al. LOXL2 expression is associated with invasiveness and negatively influences survival in breast cancer patients. Breast Cancer Res Treat. 2013;141:89–99. doi: 10.1007/s10549-013-2662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peinado H, Moreno-Bueno G, Hardisson D, Pérez-Gómez E, Santos V, Mendiola M, de Diego JI, Nistal M, Quintanilla M, Portillo F, et al. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- 46.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schietke R, Warnecke C, Wacker I, Schödel J, Mole DR, Campean V, Amann K, Goppelt-Struebe M, Behrens J, Eckardt KU, et al. The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: Insights into cellular transformation processes mediated by HIF-1. J Biol Chem. 2010;285:6658–6669. doi: 10.1074/jbc.M109.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moon HJ, Finney J, Xu L, Moore D, Welch DR, Mure M. MCF-7 cells expressing nuclear associated lysyl oxidase-like 2 (LOXL2) exhibit an epithelial-to-mesenchymal transition (EMT) phenotype and are highly invasive in vitro. J Biol Chem. 2013;288:30000–30008. doi: 10.1074/jbc.C113.502310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX, et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- 50.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 51.Voloshenyuk TG, Landesman ES, Khoutorova E, Hart AD, Gardner JD. Induction of cardiac fibroblast lysyl oxidase by TGF-β1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine. 2011;55:90–97. doi: 10.1016/j.cyto.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 52.Roy R, Polgar P, Wang Y, Goldstein RH, Taylor L, Kagan HM. Regulation of lysyl oxidase and cyclooxygenase expression in human lung fibroblasts: Interactions among TGF-beta, IL-1 beta, and prostaglandin E. J Cell Biochem. 1996;62:411–417. doi: 10.1002/(SICI)1097-4644(199609)62:3<411::AID-JCB11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 53.Tan J, Tong BD, Wu YJ, Xiong W. MicroRNA-29 mediates TGFβ1-induced extracellular matrix synthesis by targeting wnt/β-catenin pathway in human orbital fibroblasts. Int J Clin Exp Pathol. 2014;7:7571–7577. [PMC free article] [PubMed] [Google Scholar]
- 54.Yang T, Liang Y, Lin Q, Liu J, Luo F, Li X, Zhou H, Zhuang S, Zhang H. miR-29 mediates TGFβ1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway in human lung fibroblasts. J Cell Biochem. 2013;114:1336–1342. doi: 10.1002/jcb.24474. [DOI] [PubMed] [Google Scholar]