Significance
The reconstitution of liposomal growth and division compatible with replication of genetic information are required for the development of model cell growth. The growth dynamics of liposomes and their internal reactions should be synchronized to maintain the whole system. In this report, we demonstrate the concurrent incorporation of nutrients and membranes into RNA-containing liposomes using a modified freeze–thaw technique. The fusion and fission of liposomes, RNA replication, and distribution to daughter liposomes were observed compatibly. Finally, the sustainable proliferation of RNA-containing liposomes was observed by iteratively supplying nutrients for 10 cycles. We propose the use of this culturable liposome as an advanced recursive model of a liposomal protocell.
Keywords: protocell, freeze–thaw, flow cytometry, liposome fusion
Abstract
Although challenging, the construction of a life-like compartment via a bottom–up approach can increase our understanding of life and protocells. The sustainable replication of genome information and the proliferation of phospholipid vesicles are requisites for reconstituting cell growth. However, although the replication of DNA or RNA has been developed in phospholipid vesicles, the sustainable proliferation of phospholipid vesicles has remained difficult to achieve. Here, we demonstrate the sustainable proliferation of liposomes that replicate RNA within them. Nutrients for RNA replication and membranes for liposome proliferation were combined by using a modified freeze–thaw technique. These liposomes showed fusion and fission compatible with RNA replication and distribution to daughter liposomes. The RNAs in daughter liposomes were repeatedly used as templates in the next RNA replication and were distributed to granddaughter liposomes. Liposome proliferation was achieved by 10 cycles of iterative culture operation. Therefore, we propose the use of culturable liposomes as an advanced protocell model with the implication that the concurrent supplement of both the membrane material and the nutrients of inner reactions might have enabled protocells to grow sustainably.
Reconstitution of living cell-like compartments via a bottom–up approach using only well-defined components has remained a challenge in the expansion of our understanding of the possible origin of life, the border between living and nonliving matter, and the use of such compartments in other applications (1–3). Two of the requirements to reconstitute cell growth are the proliferation of compartments (4) and the replication of genetic information (5). These reactions should be sustainable to prevent the extermination of life. Artificial cell models in which phospholipid or fatty acid vesicles encapsulate the replication reactions of genetic information and other biochemical reactions have been proposed (6–10). In addition to replicating RNA/DNA internally, vesicle compartments should grow and divide to proliferate. Fatty acids transform among monomers, small micelles, and enclosed vesicles by changing the pH of the solution (11). The flexibility of fatty acids enables the exchange of fatty acids between vesicles and monomers, reconstituting the model of vesicle “growth” and also “division” into daughter vesicles by shear forces (12, 13). Fatty acid vesicles could continuously grow and divide in a primitive environment, compatible with internal RNA replication (12, 14). However, because phospholipids lack flexible dynamics compared with fatty acids, the coupled growth and division of liposomal artificial cells has been difficult to achieve (15). Because modern cells are composed of phospholipids, which have been proposed to be transited from protocells composed of fatty acids (16), artificial cells composed of phospholipids should be developed to create a valid model. As an attempt to reconstitute liposomal cell growth, phospholipid synthesis (17–20) and liposome division (21) have been reported individually but have not been integrated. Temporal liposome reproduction linked with internal DNA replication has been achieved by supplying membrane precursors and birthing daughter liposomes (22). However, the daughter liposomes were not continuously birthed due to nutrient depletion and gradual changes in lipid composition.
In this study, we aimed to reconstitute a protocell model that can be continuously “cultured.” We fused liposomes containing NTPs and RNA-replicase (nutrient liposomes) with liposomes in which RNA was encapsulated (RNA liposome). We expected that the fusion of the RNA and nutrient liposomes would result in spontaneous fission due to the excess ratio of membrane area to volume (23). Liposome fusion has been achieved using many materials, such as polyethylene glycol (24), amphiphilic molecules (25), cationic ions (26, 27), peptides (28), or lipid-anchored DNA oligonucleotides (29). However, because these systems require the addition of chemical compounds or peptides, the properties of the lipid membranes and/or biochemical reactions will change and may not maintain the same conditions after liposome fusion. To reconstitute sustainable artificial cell growth, lipid composition and inner biochemical reactions should be recursively maintained. Therefore, we developed a method to fuse liposomes without the addition of chemical compounds. The freeze–thaw (F/T) technique has been well-studied as a method to encapsulate drugs into liposomes and to fuse liposomes (30, 31). We modified the F/T method to fuse nutrient liposomes with RNA liposomes.
Using the F/T technique, we observed lipid mixing, followed by the spontaneous fission of liposomes. Simultaneously, we observed the content mixing of liposomes, enabling RNA replication in liposomes. The replicated RNA was distributed to the divided daughter liposomes and used as the template in the next replication reaction. We iteratively repeated this supplementation with nutrient liposomes 10 times and demonstrated the sustainable proliferation of liposomes replicating their RNA. These culturable liposomes suggest that the concurrent supplementation of the membrane material and the nutrients of the inner reactions are a plausible method by which protocells grew on the early earth.
Results
Fusion and Fission of Liposomes.
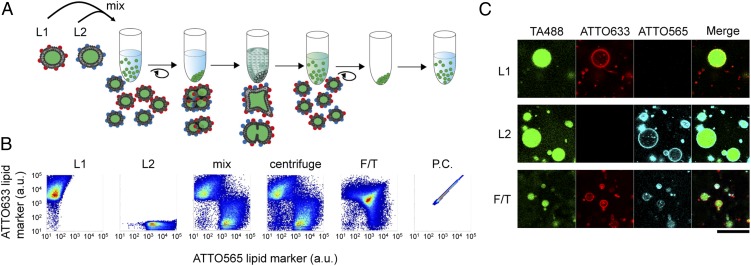
We developed an F/T method to fuse liposomes. We used a water-in-oil (w/o) emulsion transfer method to prepare giant unilamellar vesicles (GUVs) (32) as the model compartments. GUVs resemble living cells in their size and lamellarity and are therefore frequently used as a model of artificial cells (33, 34). Drug delivery research has demonstrated that liposomes can be fused when F/T-ed in liquid nitrogen (30, 31). We confirmed that the liposomes constructed in our experiment could also be fused by F/T (Fig. 1A). We prepared two solutions of liposomes using 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and lipids probed with ATTO 633 (ATTO633; L1) or ATTO 565 (ATTO565; L2). Transferrin protein conjugated with Alexa Fluor 488 (TA488) was encapsulated in both liposomes as a fluorescent content marker. We then mixed those liposome solutions in a concentration ratio of 1:1 and centrifuged the mixture to produce a liposomal pellet to increase the fusion efficiency. Next, we placed the tube into liquid nitrogen without dissolving the pelleted liposomes. We predicted that the inner contents of the liposomes would leak after thawing, and we therefore centrifuged and replaced the supernatant with fresh buffer and measured the liposome fluorescence by flow cytometry (FCM) (Fig. 1B). Liposome fusion would result in mixing of the lipid fluorescent markers, which would be detectable by FCM. We did not observe significant lipid mixing before (L1, L2) or after liposomes were mixed (mix) or even after centrifugation (centrifuge). However, after F/T, many liposomes possessed both fluorescent lipid markers, which suggests that lipids were mixed by F/T. Liposomes probed with both fluorescent lipid markers were constructed and measured as the positive control (P.C.). FCM analysis cannot distinguish fused liposomes from aggregated liposomes because they are both detected as particles emitting both ATTO633 and ATTO565 fluorescence. Therefore, we observed F/T liposomes by confocal microscopy and confirmed that lipids were mixed in liposomal membranes (Fig. 1C). The mixing of inner contents was also confirmed by another approach, as shown in Fig. S1. Briefly, two liposomes encapsulating different fluorescent content markers were constructed and subjected to F/T. Most of the resulting liposomes contained both fluorescent content markers. These results demonstrate that F/T can be used to fuse liposomes.
Fig. 1.
Lipid mixing of liposomes by the F/T method. (A) Experimental procedure for liposome fusion by F/T. Two liposome solutions were mixed (L1 and L2), centrifuged, and subjected to F/T using liquid nitrogen. The liposomes were centrifuged again, and the supernatant was replaced with fresh buffer. Fused liposomes possessed both the fluorescent lipid markers ATTO565 and ATTO633. (B) 2D plots of fluorescent lipid markers measured by FCM. Two liposome solutions (L1 and L2) were analyzed before mixing, after mixing (mix), after centrifugation (centrifuge), and after F/T. Liposomes including both fluorescent lipid markers were constructed as a P.C. The horizontal axis shows the fluorescence of ATTO565, and the vertical axis shows the fluorescence of ATTO633. (C) Confocal microscopic images of liposomes before mixing (Upper, L1; Middle, L2) and after F/T (Lower, F/T). Fluorescence of TA488, ATTO633, and ATTO565 and the merged images are shown. (Scale bar, 25 μm.)
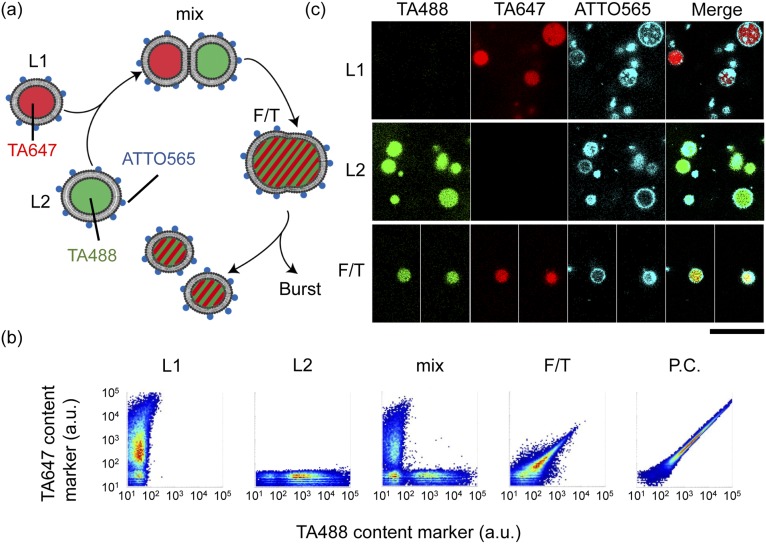
Fig. S1.
Mixing of liposome contents by the F/T method. (A) Experimental scheme of the content mixing assay by F/T. Two liposome solutions encapsulating different fluorescent content markers (L1:TA647 and L2:TA488) were mixed, centrifuged (mix), and subjected to F/T. Both liposomes were labeled with a fluorescent lipid marker (ATTO565). The fused liposomes possessed both TA488 and TA647 content markers. (B) The 2D plots of fluorescence measured by FCM. The horizontal axis shows the fluorescence of the TA488 content marker, and the vertical axis shows the fluorescence of the TA647 content marker. Liposomes containing both fluorescent content markers were constructed as a P.C. (C) Confocal microscopic images of liposomes before mixing (Upper, L1; Middle, L2) and after F/T (Lower, F/T). The fluorescence of TA488, TA647, and ATTO565 and the merged images are shown. (Scale bar, 25 μm.)
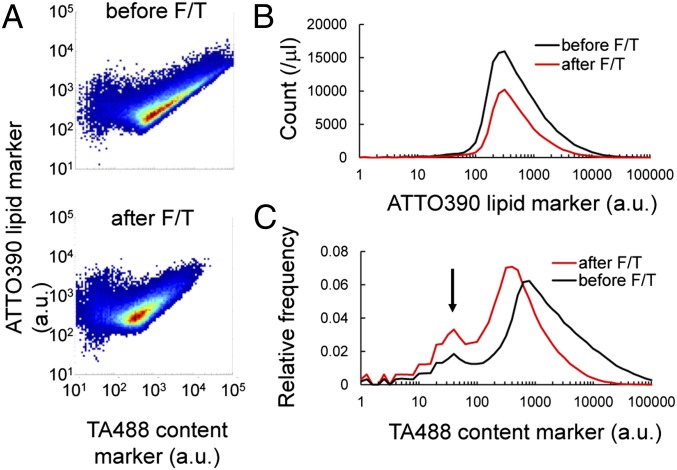
Following liposomal fusion, we expected the fission of liposomes, as has been observed for liposomes fused by other methods (23). To confirm liposome fission, we analyzed the size distribution of liposomes. We prepared liposomes labeled with a fluorescent lipid marker (ATTO390) and an encapsulated fluorescent content marker (TA488). We then subjected the liposome solution to F/T without mixing with the other series of liposome solutions and performed FCM analysis (Fig. 2A). Histograms of the fluorescent lipid marker ATTO390 representing liposomal sizes were constructed (Fig. 2B). The average decreased by 28.4% after F/T, but the peak position did not change. This indicates that fission or rupture frequently occurred in large liposomes. This is consistent with the microscopic images (Fig. 1C). As we describe hereafter, the peak position did not change after the second F/T. Because liposome fusion was demonstrated previously (Fig. 1), the stable size distribution of the liposomes after F/T implies the spontaneous fission of liposomes. Next, we analyzed the effect of the fusion–fission of liposomes by F/T on other parameters. The height of the histograms, which represented liposome concentration (liposomes detected in 1 μL of solution by FCM), decreased 48% after F/T, which indicated that approximately half of the liposomes were ruptured. We then analyzed content leakage by constructing the histograms of the fluorescent content marker TA488 (Fig. 2C). The fluorescence intensity of the main peak decreased by 50%, indicating that F/T resulted in the leakage of half of the inner content. In this analysis, we observed an increase in the minor population (arrow in Fig. 2C) that did not emit significant TA488 fluorescence. This population may not encapsulate sufficient aqueous solution for use as a cell model compartment. We refer to this population as “inactive liposomes” hereafter; this population was considered to have no influence on the conclusion in this study. These results confirmed the successful reconstitution of the growth and fission of liposomes.
Fig. 2.
Size distribution and content leakage of liposomes before (before F/T) and after freeze-thaw (after F/T). (A) 2D plots of liposome fluorescence before and after F/T. The horizontal axis shows TA488 content marker fluorescence, and the vertical axis shows ATTO390 lipid marker fluorescence. (B) Histogram of ATTO390 lipid marker fluorescence. The vertical axis shows the liposome number measured by FCM in 1 μL of liposome solution. (C) Histogram of TA488 content marker fluorescence. The arrow indicates the particles without significant aqueous content. The vertical axis shows the relative frequency.
RNA Replication in Liposomes.
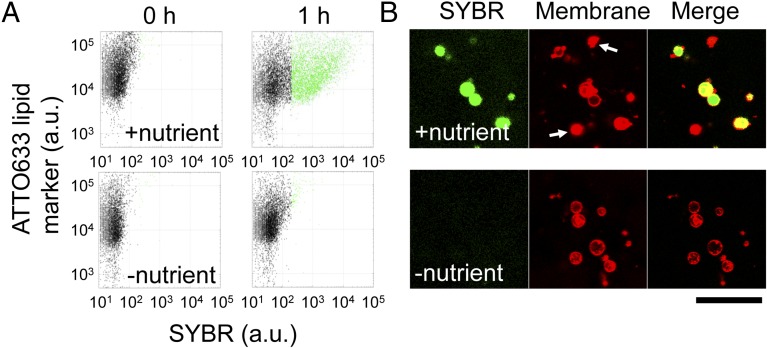
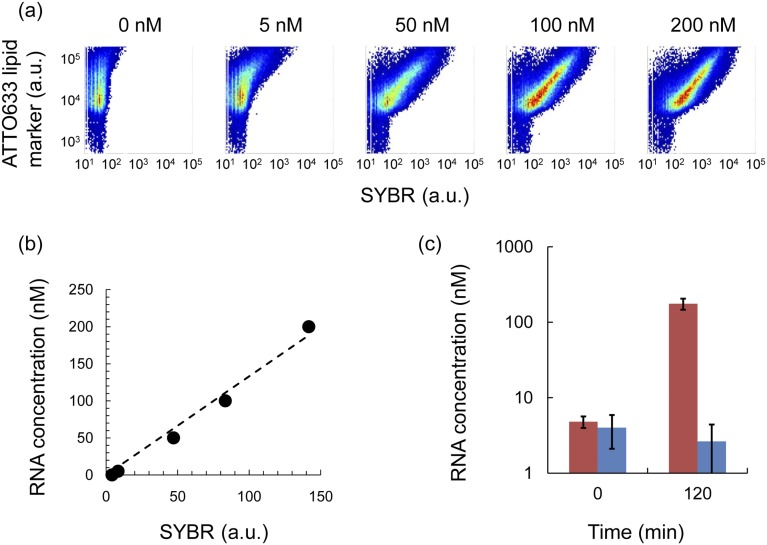
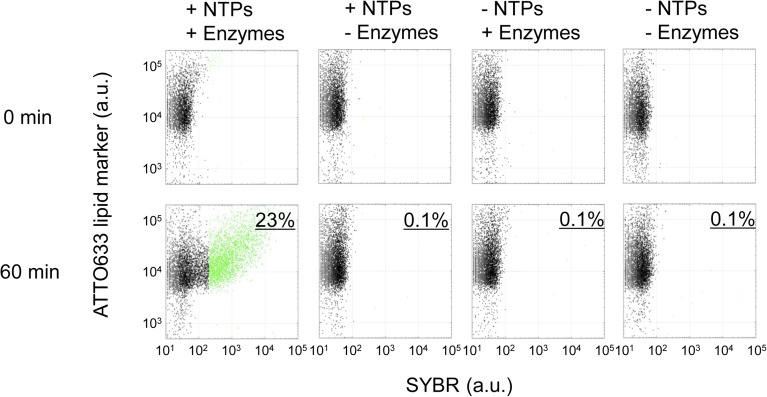
RNA was replicated in liposomes by supplying nutrients and using the F/T method. The reconstitution of the fusion and fission of liposomes must be compatible with the replication of genetic information in liposomes. As a model for an RNA replication system, we used a modified midivariant RNA encoding a modified Qβ-replicase (Rep-RNA) in the replication reaction (35) and supplied NTPs and Qβ-replicase for nutrients. We subjected liposomes encapsulating Rep-RNA (RNA liposome) and liposomes encapsulating the NTPs and Qβ-replicase (nutrient liposome) to F/T at a concentration of 1:1 (Fig. 3A). To remove the leaked Rep-RNA, NTPs, and Qβ-replicase, we centrifuged the liposomes, replaced the outer solution with fresh buffer, and incubated the solution at 37 °C for 1 h to replicate Rep-RNA in liposomes. These steps ensured that Rep-RNA replication proceeded only inside the liposomes. Then, we added SYBR Green II, which permeates the lipid membrane and emits fluorescence when RNA molecules are present in liposomes. We preliminarily confirmed the linear relationship between the Rep-RNA concentration in liposomes and SYBR fluorescence measured by FCM (Fig. S2). We analyzed RNA replication by measuring SYBR fluorescence using FCM (Fig. 3). In this study, we defined liposomes emitting SYBR fluorescence >200 fluorescence intensity (F.I.) as “active liposomes” (green dots in Fig. 3A) in which RNA replicated significantly. Note that liposomes before RNA replication did not emit green fluorescence due to their low initial RNA concentration. After the 1-h RNA replication reaction, 30% of the liposomes were active when mixed with nutrient liposomes (+nutrient), but only 1% were active when nutrient liposomes did not contain NTPs and Qβ-replicase (–nutrient). Nutrient liposomes that lack NTPs or Qβ-replicase also did not generate active liposomes (Fig. S3). We also quantified the RNA in liposomes by sorting the liposomes followed by quantitative reverse transcription PCR (qRT-PCR) and confirmed that RNA was replicated in liposomes (Fig. S2C). Some liposomes, indicated by white arrows in Fig. 3B, did not exhibit SYBR fluorescence and corresponded to liposomes less than 200 F.I. in the FCM analysis (black dots in Fig. 3A). These liposomes were considered to be inactive liposomes that do not replicate RNA internally. These liposomes could have originated from liposomes that lost aqueous solution, as shown before (black arrow in Fig. 2C). These results demonstrate that sufficient nutrients for RNA replication can be supplied from nutrient liposomes to RNA liposomes by F/T.
Fig. 3.
Induction of RNA replication by F/T. Liposomes encapsulating Rep-RNA were mixed with nutrient liposomes, subjected to F/T, and incubated for 1 h at 37 °C. (A) 2D plots of liposome fluorescence before and after RNA replication. Nutrient liposomes that did not contain NTPs and Qβ-replicase were introduced as a negative control (–nutrient). The horizontal axis shows SYBR fluorescence (RNA content), and the vertical axis shows ATTO633 lipid marker fluorescence. (B) Confocal microscopic images of liposomes after a 1-h incubation. The fluorescence of SYBR, the ATTO633 lipid marker (Membrane), and the merged images are shown. (Scale bar, 25 μm.)
Fig. S2.
Probing RNA in liposomes with SYBR. (A) Liposomes were prepared by encapsulating Rep-RNA at concentrations of 0, 5, 50, 100, and 200 nM; probed with SYBR (see details in Materials and Methods); and measured by FCM. The horizontal axis shows SYBR fluorescence (RNA content), and the vertical axis shows the ATTO633 lipid marker fluorescence. (B) The correlation of the RNA concentration and SYBR fluorescence intensity (median) obtained from A was plotted. Analysis was limited to liposomes of significant size with ATTO633 F.I. = 8,000–12,000. Therefore, the RNA concentration in liposomes of major size (the main peak in Fig. 5B) was analyzed. The dashed line represents the linear fit and was used to estimate the RNA concentration in Fig. 5D. SYBR fluorescence intensity was converted to the Rep-RNA concentration according to the following equation: Rep-RNA (nM) = 1.33 × SYBR F.I. (C) The concentration of RNA from 100,000 liposomes (sorted by cell sorter by setting the gate as 20,000–50,000 F.I. of ATTO633 and 20,000–50,000 a.u. of forward-scattered light intensities) was quantified by qRT-PCR. Error bars indicate the SD of three independent experiments. The red bar indicates +nutrient, and blue bar indicates –nutrient conditions.
Fig. S3.
The necessity of NTPs and enzymes for RNA replication. RNA liposomes were subjected to F/T with nutrient liposomes, which encapsulated or did not encapsulate the NTPs and/or Qβ-replicase enzymes (mixing ratio 1:1) and were incubated at 37 °C for 60 min for RNA replication. The horizontal axis in 2D plots shows SYBR fluorescence (RNA content), and the vertical axis shows ATTO633 lipid marker fluorescence. Population ratios of the liposomes, where RNA was replicated by Qβ-replicase after 60 min incubation (green dots), are written on the upper right of plots.
Distribution of RNA to Daughter Liposomes.
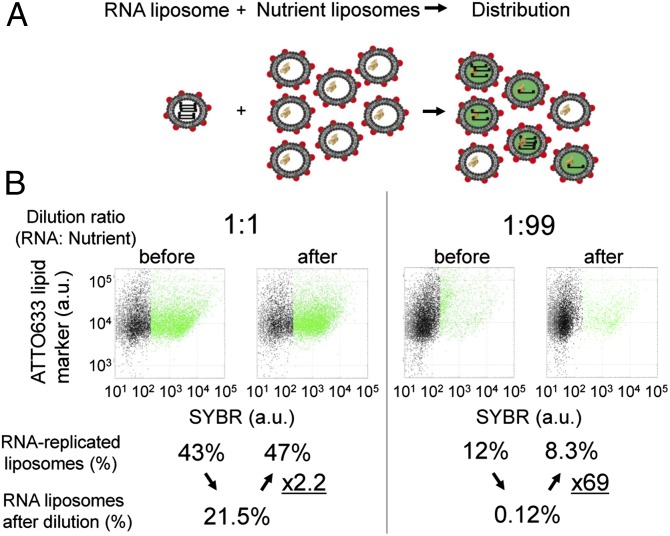
Next, we tested whether the replicated RNA could be distributed to the daughter liposomes. We mixed a constant volume of RNA liposome solutions with nutrient liposomes at ratios of 1:1 and 1:99 (Fig. 4). After the first F/T and RNA replication, the distribution of the RNA molecules to more than two daughter liposomes by the second F/T and RNA replication reaction would result in an increase in the number of liposomes with significant SYBR fluorescence (active liposomes). After the first F/T and RNA replication for 2 h, the proportions of active, RNA-replicating liposomes (green dots in Fig. 4B) were 43% and 12% at mixing conditions of 1:1 and 1:99, respectively. These liposome solutions were again diluted with each mixing ratio and subjected to the second F/T. Thus, 21.5% and 0.12% of the liposomes could have amplified the RNA molecules in the second replication reaction if the RNA was not distributed. However, RNA-replicating liposome populations increased from 21.5% to 47% and 0.12% to 8.3% at mixing conditions of 1:1 and 1:99, respectively. These fold increases imply that single mother liposomes distributed RNA to 2.2 and 69 daughter liposomes at mixing ratios of 1:1 and 1:99, respectively. Excess nutrient liposomes compared with RNA liposomes may have caused the RNA molecules to distribute by single molecules (Fig. 4A), therefore resulting in the higher fold increase at the 1:99 mixing ratio. This indicates that at least 69 daughter liposomes can be birthed from a single mother liposome. This may be caused not only by fusion–fission but together with spreading the inner contents via a transient pore, therefore exchanging the nutrients and RNAs among liposomes nearby.
Fig. 4.
Distribution of RNA to other liposomes by F/T. (A) Schematic of the RNA distribution experiment. RNA liposomes were mixed with an excess ratio of nutrient liposomes, subjected to F/T, and incubated for 2 h at 37 °C. The fold increase in the population ratio of RNA-replicating liposomes represents the distribution rate. (B) 2D plots of liposome fluorescence before and after RNA replication following F/T with mixing ratios of RNA:nutrient of 1:1 and 1:99. The horizontal axis shows the fluorescence of SYBR (RNA content), and the vertical axis shows the fluorescence of the ATTO633 lipid marker. Green dots represent the liposomes actively replicating RNA, which exhibit SYBR fluorescence >200 F.I. The population ratios of green dots among all dots in each plot are shown below the plots. Estimated ratios after F/T and RNA replication, assuming no distribution of RNA, are also shown.
Continuous Cultivation of Active Liposomes.
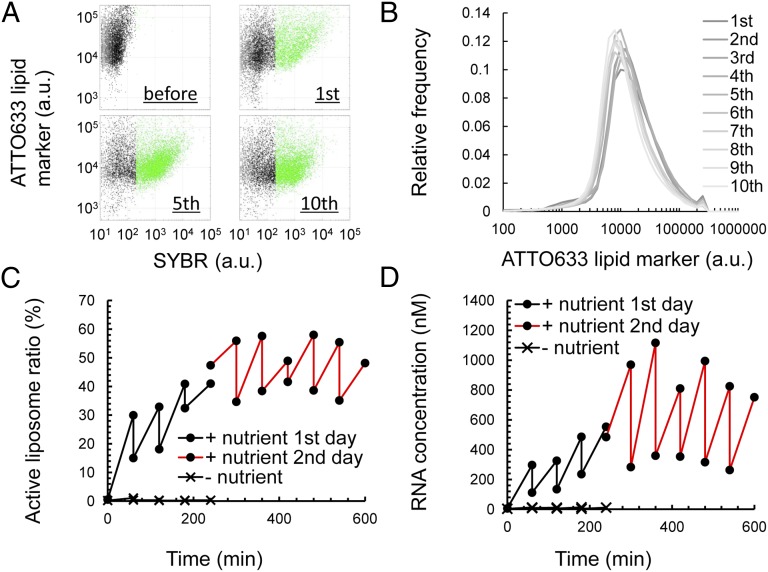
Thus far, we have demonstrated liposome fusion and fission, RNA replication, and the distribution of RNA to daughter liposomes. Next, we tested whether this system was “sustainable” by repeating the F/T (Fig. 5). We constructed RNA liposomes encapsulating Rep-RNA as the original protocells and nutrient liposomes encapsulating NTPs and Qβ-replicase. Both liposomes were probed with the fluorescent lipid marker ATTO633. We mixed the two liposome solutions at a 1:1 volume ratio, performed F/T, and incubated the liposomes to permit RNA replication. We then repeated the cycle of dilution of the resulting liposome solution with fresh nutrient liposome solution at a 1:1 ratio, F/T, and incubation for 1 h of RNA replication 10 times iteratively (Fig. 5). When we analyzed the liposomes by FCM, SYBR fluorescence was consistently observed until the 10th F/T operation (the horizontal axis in Fig. 5A). Thus, RNA replication was not terminated and was sustained by supplying nutrients. This result also indicates that the proliferation of liposomes and the replication of RNA are sustainable for 10 cycles despite the dilution with nutrient liposomes and rupture and leakage by F/T. We performed the first to fourth F/T cycles and the fifth freezing on the first day and then stored the liposome solution at –80 °C for 10 d. We began again from the fifth thawing, fifth RNA replication, and the 6th to 10th F/T cycles. These results demonstrate that active liposomes can be stored with standard laboratory equipment.
Fig. 5.
Repetitive culturing of RNA-replicating liposomes. (A) 2D plots of fluorescence measured by FCM. Liposomes (before) were F/T-ed with nutrient liposomes and incubated for 1 h (first). F/T with nutrient liposomes was repeated for 10 cycles. The plots of liposomes incubated after the 5th and 10th F/T are also shown. The horizontal axis shows SYBR fluorescence (RNA content), and the vertical axis shows ATTO633 lipid marker fluorescence. (B) Histogram of ATTO633 lipid marker fluorescence after each 1-h incubation period following F/T. (C) The population ratios of active liposomes (green dots in A) were plotted against the total incubation time. The black and red lines represent data from the first- or second-day experiments, respectively. RNA liposomes were subjected to F/T with liposomes that do not contain NTPs and Qβ-replicase (–nutrient) for five cycles as a negative control. (D) RNA concentrations in liposomes were plotted against the total incubation time. Note that the analysis was limited to liposomes with a lipid content of ATTO633 = 8,000–12,000 F.I.
We first analyzed the sustainability of liposome size (Fig. 5B). The histograms of fluorescent lipid markers did not change significantly during the experiment, which indicates that a stable liposome size was maintained despite repetitive fusion, fission, and rupture by 10 F/T cycles. The majority of the liposomes are expected to contain aqueous volumes of ∼10 fL based on our previous reports (36, 37). The lamellarity of the liposomes was not significantly changed by the repetition of F/T (Fig. S4).
Fig. S4.
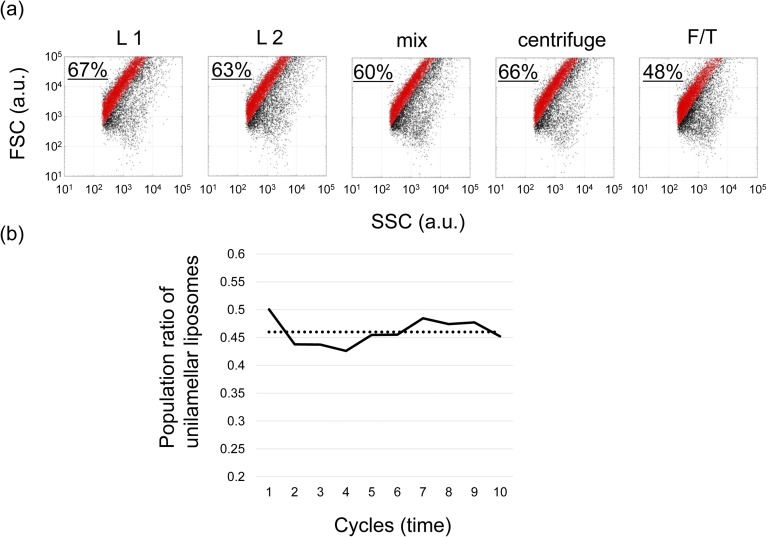
Lamellarity of liposomes. (A) 2D plots of scattered light intensities of liposomes described in Fig. 1. Horizontal axis shows the value of side-scattered (SSC) and vertical axis shows the value of forward-scattered light intensities (FSC). These enable the estimation of the population of unilamellar and spherical liposomes (3, 4). Two solutions of liposomes encapsulating different fluorescent lipid markers (L1 and L2) were mixed, centrifuged, and F/T-ed. The population ratio of unilamellar and spherical liposomes among the total liposomes are shown in each plot. The population ratio of unilamellar and spherical liposomes decreased from 60–67% to 48% after the first F/T. The decrease in the ratio of unilamellar vesicles at the first F/T can be attributed in part to the increase of the ratio of the inactive liposomes, which were supposedly generated by lacking aqueous solution in liposomes (Fig. 3B, white arrows). (B) Population ratio of unilamellar and spherical liposomes, plotted here against the culturing cycles, was calculated using the data obtained at Fig. 5. The dotted line represents the average ratio (46%).
We then analyzed the sustainability of active liposome proliferation (Fig. 5C). Active liposomes representing liposomes encapsulating significant amounts of RNA increased after 1 h of replication. Dilution with nutrient liposomes and subsequent F/T generated the zigzag plot in Fig. 5C. The active ratio after the fifth F/T (left end of red line) did not decrease from the previous data point before the F/T cycle (right end of black line). Thus, the RNA was less diluted by leakage and/or more daughter liposomes were born than in other cycles after the liposomes were frozen for 10 d. The reasons for this effect are unclear. When nutrient liposomes did not contain NTPs and Qβ-replicase, the active liposomes were exterminated (–nutrient). Therefore, we observed the sustainable proliferation of RNA-encapsulating liposomes.
We then analyzed the sustainability of RNA replication (Fig. 5D). The average SYBR fluorescence of all liposomes of significant size was converted to the actual Rep-RNA concentration using the equation obtained from the standard curve in Fig. S2. RNA was repeatedly replicated except when nutrient liposomes did not encapsulate NTPs and Qβ-replicase (–nutrient). Therefore, we observed sustainable RNA replication in this system.
Here, we analyzed these data at the molecular and population levels by taking into account the leakage, distribution, and fold replication of RNA. The RNA liposomes initially encapsulated 30 Rep-RNA molecules at a size of 10 fL, following a Poisson distribution. When the first F/T was performed, 50% of the RNA leaked (Fig. 2C), distributed (Fig. S1), and gave birth to 2.2-fold daughter liposomes (Fig. 4). Taking this leakage and the distribution to nutrient liposomes together, the initial RNA concentration before the first replication was expected to decrease fourfold from 5 nM to 1.25 nM. When these liposomes were incubated for 1 h, 297 nM RNA was synthesized (Fig. 5D), suggesting that more than 200-fold RNA was replicated. Next, we analyzed the population of liposomes replicating RNA internally (Fig. 5C). If all liposomes were filled with RNA, all liposomes should replicate RNA. However, because 1.25 nM RNA is below the detection threshold of FCM, the ratio of liposomes with significant SYBR fluorescence was 0.2% at time 0. After replication for 1 h, RNA replication was measured by FCM over the threshold, and the active liposome ratio thus increased to 30% (Fig. 5C). However, even though all liposomes were expected to encapsulate RNA, the active liposome ratio did not reach 100%, possibly because of the presence of inactive liposomes that lacked a significant volume of aqueous solution for the replication reaction to occur (Fig. 2C, black arrow) or liposomes that did not receive the RNAs/nutrients for sufficient RNA replication. Indeed, there were two distinct populations, one that emitted SYBR fluorescence and one that did not, in the FCM analysis (Fig. 5A).
Following the second F/T, RNA was again leaked and distributed, and thus a fourfold dilution of RNA was expected for the next initial RNA concentration. As a result, 112 nM, roughly a threefold dilution of the RNA concentration before F/T (297 nM), was measured as the next initial RNA concentration (Fig. 5D). After 1 h of replication, a threefold increase in RNA (326 nM) was measured. From the second F/T, an approximately threefold dilution and threefold replication were consistently observed until the 10th cycle (Fig. 5D). We then considered why the replication was lower than the first incubation (which was >200-fold). In nutrient liposomes, 6.25 mM NTPs each were encapsulated. These NTPs were also expected to be distributed following the fourfold dilution, as mentioned above. Thus, when we assume 1.56 mM NTPs each were used, the replication of 2,041 bp RNA would terminate at 3,060 nM due to NTP depletion. Because the RNA concentration was on the same order as this upper limitation (the latter half of Fig. 5D), we believe the replication was suppressed by the depletion of NTPs in our experiment. Whereas a fourfold dilution was expected from 50% leakage and 1:1 dilution with nutrient liposomes, a slightly lower threefold dilution was consistently observed. One possible explanation for this discrepancy was that the degree of content leakage decreased after the second F/T due to the loss of large liposomes (Fig. 2B). Next, we analyzed the population of liposomes replicating RNA internally (Fig. 5C). From the second F/T cycle, the ratio gradually increased to 50% and maintained the 50% maximum during the latter half of the cycle experiments (Fig. 5C). This result indicates that inactive liposomes possibly lacking aqueous solution did not exceed 50% and did not dominate the active liposomes.
Together, these data confirm the sustainability of the liposome compartment, RNA replication, RNA distribution, and active liposome proliferation.
Discussion
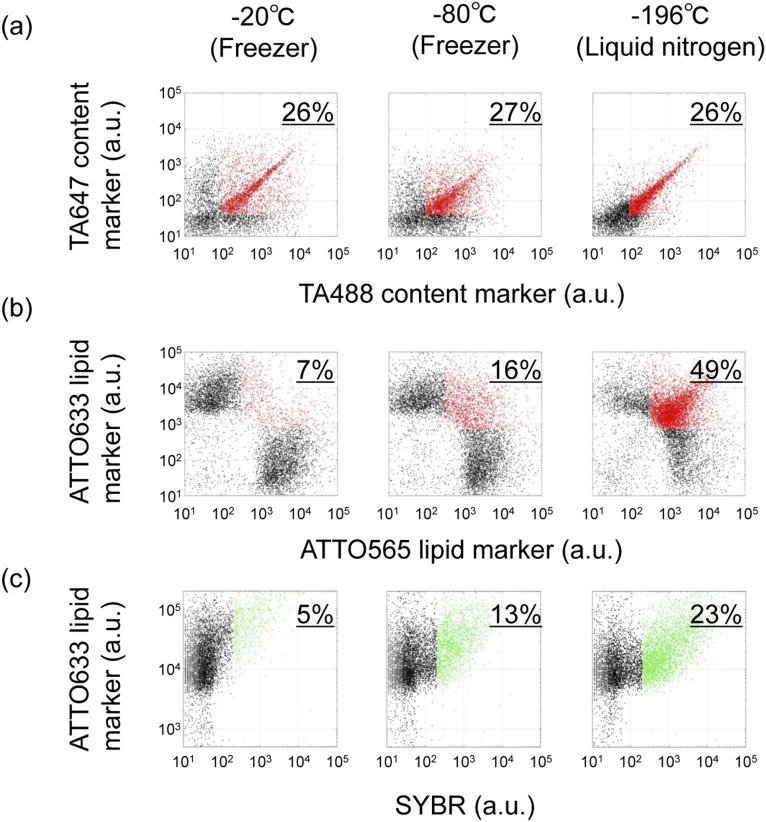
We reconstituted culturable liposomes that follow fusion–fission and are compatible with a model of RNA replication and RNA diffusion to daughter liposomes. This system requires the supply of nutrient liposomes by the F/T method and thus does not self-grow or self-replicate via the expression of genome information. However, we demonstrated the sustainable proliferation of liposomes with 10 instances of iterative culturing. Although phospholipid membranes are thought to lack the flexible dynamics to grow and divide compared with fatty acids, we demonstrated that concentration and freezing with the supply of nutrient liposomes allowed the RNA liposomes to undergo sustainable proliferation. In addition, we showed that freezing at –80 °C or –20 °C could also cause content mixing, lipid mixing, and RNA replication by supplying nutrients (Fig. S5). All three temperatures showed similar levels of content mixing. However, lipid mixing and RNA replication were highest in liquid nitrogen and lowest at –20 °C. We clarified that the fusion–fission of liposomes compatible with RNA replication was robust at a wide range of freezing temperatures, including the plausible temperature on primitive earth (38). Therefore, with the sustainable proliferation of liposomes compatible with RNA replication, we propose that the concurrent supplementation of the membrane material and the nutrients of the inner reactions are a plausible method by which protocells were able to grow on the early earth (39, 40).
Fig. S5.
Content/lipid mixing and RNA replication after F/T in three different temperatures. Liposomes were subjected to F/T by freezing at –20 °C, –80 °C, or by liquid nitrogen. (A) Content mixings were measured using two liposome solutions as described in Fig. S1. The horizontal axis in 2D plots shows the fluorescence intensity of TA488, and the vertical axis shows the fluorescence of TA647. The population ratios of liposomes possessing both content markers (red dots) are written on the upper right of each plot. (B) Lipid mixings were measured using two liposome solutions as described in Fig. 1. The horizontal axis in 2D plots shows the fluorescence of ATTO565, and the vertical axis shows the fluorescence of ATTO633. The population ratios of liposomes possessing both lipid markers (red dots) are written on the upper right of each plot. (C) RNA replication was measured using two liposome solutions as described in Fig. 3. Following F/T at three temperatures, liposome solutions were incubated at 37 °C for RNA replication (60 min). The horizontal axis in 2D plots shows SYBR fluorescence (RNA content), and the vertical axis shows ATTO633 lipid marker fluorescence. The population ratios of liposomes in which RNA was significantly replicated (green dots) are written on the upper right of each plot.
The fusion of liposomes by F/T is thought to be driven by dehydration and ice growth, leading to the disruption of the lipid bilayer (30). Therefore, half of the liposomes were ruptured, and the residual liposomes were used for fusion–fission and RNA replications. Although the details of the volume dependency of fusion, fission, and rupture of liposomes are not clearly understood, a stable size distribution of liposomes during the repetition of F/T was observed. Centrifugation before F/T concentrated the liposomes, which may have enhanced the fusion and exchange rate of inner content. The centrifuged liposomes were able to exchange those inner contents by fusion–fission as shown with lipid mixing (Fig. 1) and possibly by spreading through the transient pore to give birth to more than 69 daughter liposomes (Fig. 4). Observations of the frozen sample before thawing are needed to understand these fusion–fission events in greater detail (30). The majority of the liposomes were expected to contain an aqueous volume of ∼10 fL and have a diameter of 2.6 µm, which is similar to the size of living cells and suggests their potential as a good cell model. The volume and diameter will change slightly if we take into account that approximately half of the liposome population is unilamellar (Fig. S4).
Using this system, if we prepare multiple series of nutrient liposomes and fuse these liposomes step by step, multistep reactions in liposomes could be easily reconstituted. Because we can supply lipid membranes, small chemical compounds, and macromolecules (Qβ-replicase), this system would enable the supply of ribosomes or cell-free translation systems (41). As a next step, the encapsulation of cell-free translation systems in liposomes will enable the reconstitution of functionally active liposomes (34, 42) possessing replication activity driven by their own genome products (9). Then, we can introduce additional beneficial functions one by one and culture the artificial cells by iterative F/T steps. To increase the complexity of genomic information, genotype–phenotype linkages should be maintained. In our experiment, if RNA liposomes and nutrient liposomes were mixed at the ratio of 1:1, multiple copies of genomes were encapsulated in single liposomes, giving rise to a weakened genotype–phenotype linkage. To maintain a high selection pressure for individual genomes, we could avoid RNA mixing when excess ratios of nutrient liposomes were supplied (e.g., 1:99). On the other hand, genome mixing could increase the chance for recombination among mutated genes to increase the genetic diversity because the Qβ-replicase has high recombination activity (43). Future experiments should find the appropriate number of genomes per liposome in the balance of selection pressure and genetic diversity. Thus, these culturable artificial cells would enrich the fittest genotypes among variants, which is the essence of “natural selection” and would enable Darwinian evolution of the liposomal artificial cells.
Materials and Methods
Details of the materials, liposome preparation, FCM analysis, confocal microscopy, and qRT-PCR are provided in SI Materials and Methods.
F/T Method for Liposome Fusion–Fission.
The concentrations of liposomes were estimated from the detected liposome number by FCM (FACSAria2; BD Biosciences) (10 μL/min at a flow rate of 1.0). Liposome solutions were mixed at the concentration or volume ratios presented in the text and then centrifuged at 18,000 × g for 5 min at 4 °C. Liposome solutions were then incubated at –196 °C (using liquid nitrogen) for 30 min in Fig. 1 and Figs. S1 and S5 A and B or 1 min in other experiments. Then, liposome solutions were thawed at room temperature for 10 min, resuspended, and centrifuged at 18,000 × g for 5 min at 4 °C. The supernatant was gently replaced with the same volume of new outer solution to wash out leaked materials from liposomes.
Rep-RNA Replication Inside Liposomes.
The RNA encoding modified β-subunits of Qβ-replicase with enhanced replicability (35) was used as the template (2,041 bp) (Rep-RNA). The replication reaction of Rep-RNA in liposomes was performed at 37 °C using Rep-RNA and Qβ-replicase purified in our laboratory (44). Nutrients in liposomes required for RNA replication were set to fivefold higher concentrations than in the previous report (44) because we considered the leakage and diffusion of inner contents by F/T. RNA liposomes contained 125 mM Tris–HCl (pH 8.0), 25 mM MgCl2, 0.01% BSA, 500 mM sucrose, 350 mM potassium glutamate, 50 mM Hepes–KOH (pH 7.6), and 5 nM Rep-RNA. Nutrient liposomes contained the same buffer, but replacing Rep-RNA with 6.25 mM of each NTP and 100 nM of purified Qβ-replicase.
Measuring RNA in Liposomes by SYBR.
SYBR Green II was used to stain Rep-RNA in liposomes. Before measuring fluorescence by FCM or microscopy, SYBR Green II was added to the outer solution of liposomes to a final concentration of 1/10,000 the original and incubated for 10 min on ice. The Rep-RNA concentration (nM) in limited size liposomes (8,000–12,000 F.I. of ATTO633 measured by FCM) was calculated using the standard curve obtained in Fig. S2 by converting the SYBR fluorescence intensity as follows: Rep-RNA (nM) = 1.33 × SYBR F.I.
SI Materials and Methods
Materials.
POPC was purchased from Avanti Polar Lipids. Liquid paraffin (0.86–0.89 g/mL at 20 °C) was purchased from Wako. Transferrin Alexa Fluor 488 conjugate (TA488) and Transferrin Alexa Fluor 647 (TA647) were purchased from Invitrogen. We purchased 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine labeled with ATTO 633 (ATTO633), ATTO 565 (ATTO565), and ATTO 390 (ATTO390) from ATTO-TEC. SYBR Green II RNA Gel Stain was purchased from Lonza. SYBR premix Ex Taq II for quantitative PCR and PrimeScript reverse transcriptase was purchased from Takara, and an RNA purification micro kit was purchased from QIAGEN. RNase inhibitor (RNasin) was purchased from Promega.
Primers.
The following primers were used: primer 1, 5′-CCACGGTCAGGACTGACACTCGTAGCGCTGAGAGATCAACCGTTGCTAAG-3′; primer 2, 5′-CCACGGTCAGGACTGACACTCGTAG-3′; and primer 3, 5′-GGTATCGGTGGCATTCTACGCGA-3′.
Liposome Preparation.
Liposomes were prepared using the w/o emulsion-transfer method (28) by modifying the protocol reported previously by our group (33). POPC was dissolved in chloroform at 0.1 mg/μL, mixed with liquid paraffin to produce a 5 mg/mL lipid solution, and heated at 80 °C for 30 min to completely dissolve the lipids and evaporate the chloroform. Then, 400 μL of this solution was transferred to a glass tube. When the fluorescent lipid markers were used, 4 μL of 0.1 mg/μL ATTO390, ATTO565, and/or ATTO633 were added to the solution. The inner solution was prepared in 50 mM Hepes–KOH (pH 7.6), 500 mM sucrose, and 350 mM potassium glutamate. When fluorescent content markers were used, 1.5 μM TA488 and/or TA647 were also added to the inner solution. For Rep-RNA replication experiments, the inner solution components were as described in Rep-RNA Replication Inside Liposomes. Then, 20 μL of inner solution was mixed with 400 μL of lipid solution, vortexed for 40 s to form a w/o emulsion, and equilibrated on ice for 10 min. Next, 400 μL of emulsion was gently placed on top of 200 μL of bottom solution containing 50 mM Hepes–KOH (pH 7.6), 1 M glucose, and 350 mM potassium glutamate in a new tube. When this tube was centrifuged at 18,000 × g (14,000 rpm) for 30 min at 4 °C, the emulsions passed through the oil/water interface and formed liposomes. The precipitated liposomes were collected through a hole opened at the bottom of the tube. Then, the liposomes were centrifuged at 18,000 × g (14,000 rpm) for 5 min at 4 °C. The supernatant was removed and the sediment (liposomes) was resuspended in 30 μL of outer solution [50 mM Hepes–KOH (pH 7.6), 1 M glucose].
FCM Analysis.
The six fluorescent signals from TA488, TA647, ATTO390, ATTO565, ATTO633, and SYBR Green II were measured by FCM. We obtained 100,000 data for each measurement. TA488 and SYBR Green II were excited with a 488-nm semiconductor laser, and the emission was detected with a 530 ± 15-nm band-pass filter (FITC-A). ATTO565 was excited with a 488-nm semiconductor laser, and the emission was detected with a 585 ± 21-nm band-pass filter (PE-A). TA647 and ATTO633 were excited with a HeNe laser (633 nm), and emission was detected with a 660 ± 10-nm band-pass filter (APC-A). ATTO390 was excited with a 375-nm near-UV laser, and emission was detected with a 450 ± 20-nm band-pass filter. When we used TA488 and ATTO565 in the same experiment, compensation parameters were set to 20.50% and 0.43% for PE-A minus FITC-A and FITC-A minus PE-A, respectively.
Confocal Microscopy.
The fluorescence images of TA488, TA647, ATTO565, ATTO633, and SYBR Green II on the liposomal membranes or in the liposomes were obtained using a confocal laser scanning microscope (Leica TCS SP8; Leica Microsystems) with a 100× oil immersion objective. The intensity of TA488 and SYBR Green II were measured using an Argon laser (488 nm excitation) and a hybrid detector set to 490–550-nm or 500–560-nm ranges, respectively. ATTO565 was excited by a DPSS 561-nm laser, and the hybrid detector was set to 590–620 nm. Both TA647 and ATTO633 were excited by a HeNe 633-nm laser, and the hybrid detector was set to 650–710 nm.
Quantification of RNA Molecules Inside Liposomes by qRT-PCR.
qRT-PCR was performed to quantify the amount of RNA in liposomes. The major-sized liposomes (100,000 events) were sorted by a cell sorter (20,000–50,000 F.I. of ATTO633 and 20,000–50,000 a.u. of forward-scattered light intensities). Then, RNA in liposomes was purified by an RNA purification micro kit (Qiagen). Reverse transcription was performed using a reaction mix (100 nM primer 1, dNTP 0.5 mM each, PrimeScript buffer, 1 unit per μL of RNasin, and 20 units per μL of PrimeScript Reverse Transcriptase). Quantitative PCR was performed using SYBR premix Ex Taq II, primers 2 and 3 (400 nM, respectively). The reaction was carried out at 95 °C for 30 s, followed by 25 cycles of 95 °C for 5 s and 63 °C for 30 s using a real-time PCR system (Mx3005P, Agilent Technologies).
Acknowledgments
This work was supported in part by Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research 15K17454 and 25840127.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516893113/-/DCSupplemental.
References
- 1.Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature. 2001;409(6818):387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- 2.Monnard PA, Luptak A, Deamer DW. Models of primitive cellular life: Polymerases and templates in liposomes. Philos Trans R Soc Lond B Biol Sci. 2007;362(1486):1741–1750. doi: 10.1098/rstb.2007.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane MD, Seelig B. Advances in the directed evolution of proteins. Curr Opin Chem Biol. 2014;22:129–136. doi: 10.1016/j.cbpa.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stano P, Luisi PL. Achievements and open questions in the self-reproduction of vesicles and synthetic minimal cells. Chem Commun (Camb) 2010;46(21):3639–3653. doi: 10.1039/b913997d. [DOI] [PubMed] [Google Scholar]
- 5.Mills DR, Peterson RL, Spiegelman S. An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule. Proc Natl Acad Sci USA. 1967;58(1):217–224. doi: 10.1073/pnas.58.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noireaux V, Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc Natl Acad Sci USA. 2004;101(51):17669–17674. doi: 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobori S, Ichihashi N, Kazuta Y, Yomo T. A controllable gene expression system in liposomes that includes a positive feedback loop. Mol Biosyst. 2013;9(6):1282–1285. doi: 10.1039/c3mb70032a. [DOI] [PubMed] [Google Scholar]
- 8.Fenz SF, Sengupta K. Giant vesicles as cell models. Integr Biol (Camb) 2012;4(9):982–995. doi: 10.1039/c2ib00188h. [DOI] [PubMed] [Google Scholar]
- 9.Kita H, et al. Replication of genetic information with self-encoded replicase in liposomes. ChemBioChem. 2008;9(15):2403–2410. doi: 10.1002/cbic.200800360. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti AC, Breaker RR, Joyce GF, Deamer DW. Production of RNA by a polymerase protein encapsulated within phospholipid vesicles. J Mol Evol. 1994;39(6):555–559. doi: 10.1007/BF00160400. [DOI] [PubMed] [Google Scholar]
- 11.Blain JC, Szostak JW. Progress toward synthetic cells. Annu Rev Biochem. 2014;83:615–640. doi: 10.1146/annurev-biochem-080411-124036. [DOI] [PubMed] [Google Scholar]
- 12.Zhu TF, Szostak JW. Coupled growth and division of model protocell membranes. J Am Chem Soc. 2009;131(15):5705–5713. doi: 10.1021/ja900919c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budin I, Debnath A, Szostak JW. Concentration-driven growth of model protocell membranes. J Am Chem Soc. 2012;134(51):20812–20819. doi: 10.1021/ja310382d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanczyc MM, Fujikawa SM, Szostak JW. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science. 2003;302(5645):618–622. doi: 10.1126/science.1089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noireaux V, Maeda YT, Libchaber A. Development of an artificial cell, from self-organization to computation and self-reproduction. Proc Natl Acad Sci USA. 2011;108(9):3473–3480. doi: 10.1073/pnas.1017075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budin I, Szostak JW. Physical effects underlying the transition from primitive to modern cell membranes. Proc Natl Acad Sci USA. 2011;108(13):5249–5254. doi: 10.1073/pnas.1100498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takakura K, Toyota T, Sugawara T. A novel system of self-reproducing giant vesicles. J Am Chem Soc. 2003;125(27):8134–8140. doi: 10.1021/ja029379a. [DOI] [PubMed] [Google Scholar]
- 18.Hardy MD, et al. Self-reproducing catalyst drives repeated phospholipid synthesis and membrane growth. Proc Natl Acad Sci USA. 2015;112(27):8187–8192. doi: 10.1073/pnas.1506704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brea RJ, Cole CM, Devaraj NK. In situ vesicle formation by native chemical ligation. Angew Chem Int Ed Engl. 2014;53(51):14102–14105. doi: 10.1002/anie.201408538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidli PK, Schurtenberger P, Luisi PL. Liposome-mediated enzymatic synthesis of phosphatidylcholine as an approach to self-replicating liposomes. J Am Chem Soc. 1991;113(21):8127–8130. [Google Scholar]
- 21.Osawa M, Erickson HP. Liposome division by a simple bacterial division machinery. Proc Natl Acad Sci USA. 2013;110(27):11000–11004. doi: 10.1073/pnas.1222254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurihara K, et al. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat Chem. 2011;3(10):775–781. doi: 10.1038/nchem.1127. [DOI] [PubMed] [Google Scholar]
- 23.Terasawa H, Nishimura K, Suzuki H, Matsuura T, Yomo T. Coupling of the fusion and budding of giant phospholipid vesicles containing macromolecules. Proc Natl Acad Sci USA. 2012;109(16):5942–5947. doi: 10.1073/pnas.1120327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boni LT, Stewart TP, Alderfer JL, Hui SW. Lipid-polyethylene glycol interactions: I. Induction of fusion between liposomes. J Membr Biol. 1981;62(1-2):65–70. doi: 10.1007/BF01870200. [DOI] [PubMed] [Google Scholar]
- 25.Sunami T, et al. Detection of association and fusion of giant vesicles using a fluorescence-activated cell sorter. Langmuir. 2010;26(19):15098–15103. doi: 10.1021/la102689v. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Yamazaki M. Membrane fusion of giant unilamellar vesicles of neutral phospholipid membranes induced by La3+ Langmuir. 2004;20(13):5160–5164. doi: 10.1021/la049681s. [DOI] [PubMed] [Google Scholar]
- 27.Tarahovsky YS, Yagolnik EA, Muzafarov EN, Abdrasilov BS, Kim YA. Calcium-dependent aggregation and fusion of phosphatidylcholine liposomes induced by complexes of flavonoids with divalent iron. Biochim Biophys Acta. 2012;1818(3):695–702. doi: 10.1016/j.bbamem.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Toraya S, et al. Morphological behavior of lipid bilayers induced by melittin near the phase transition temperature. Biophys J. 2005;89(5):3214–3222. doi: 10.1529/biophysj.105.059311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan YH, van Lengerich B, Boxer SG. Effects of linker sequences on vesicle fusion mediated by lipid-anchored DNA oligonucleotides. Proc Natl Acad Sci USA. 2009;106(4):979–984. doi: 10.1073/pnas.0812356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa AP, Xu X, Burgess DJ. Freeze-anneal-thaw cycling of unilamellar liposomes: Effect on encapsulation efficiency. Pharm Res. 2014;31(1):97–103. doi: 10.1007/s11095-013-1135-z. [DOI] [PubMed] [Google Scholar]
- 31.Anzai K, Yoshida M, Kirino Y. Change in intravesicular volume of liposomes by freeze-thaw treatment as studied by the Esr stopped-flow technique. Biochim Biophys Acta. 1990;1021(1):21–26. [Google Scholar]
- 32.Pautot S, Frisken BJ, Weitz DA. Production of unilamellar vesicles using an inverted emulsion. Langmuir. 2003;19(7):2870–2879. [Google Scholar]
- 33.Matosevic S. Synthesizing artificial cells from giant unilamellar vesicles: State-of-the art in the development of microfluidic technology. BioEssays. 2012;34(11):992–1001. doi: 10.1002/bies.201200105. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura K, et al. Cell-free protein synthesis inside giant unilamellar vesicles analyzed by flow cytometry. Langmuir. 2012;28(22):8426–8432. doi: 10.1021/la3001703. [DOI] [PubMed] [Google Scholar]
- 35.Ichihashi N, et al. Darwinian evolution in a translation-coupled RNA replication system within a cell-like compartment. Nat Commun. 2013;4:2494. doi: 10.1038/ncomms3494. [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, et al. Liposome display for in vitro selection and evolution of membrane proteins. Nat Protoc. 2014;9(7):1578–1591. doi: 10.1038/nprot.2014.107. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura K, et al. Population analysis of structural properties of giant liposomes by flow cytometry. Langmuir. 2009;25(18):10439–10443. doi: 10.1021/la902237y. [DOI] [PubMed] [Google Scholar]
- 38.Zahnle K, Schaefer L, Fegley B. Earth’s earliest atmospheres. Cold Spring Harb Perspect Biol. 2010;2(10):a004895. doi: 10.1101/cshperspect.a004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oberholzer T, Wick R, Luisi PL, Biebricher CK. Enzymatic RNA replication in self-reproducing vesicles: An approach to a minimal cell. Biochem Biophys Res Commun. 1995;207(1):250–257. doi: 10.1006/bbrc.1995.1180. [DOI] [PubMed] [Google Scholar]
- 40.Mansy SS, et al. Template-directed synthesis of a genetic polymer in a model protocell. Nature. 2008;454(7200):122–125. doi: 10.1038/nature07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazuta Y, Matsuura T, Ichihashi N, Yomo T. Synthesis of milligram quantities of proteins using a reconstituted in vitro protein synthesis system. J Biosci Bioeng. 2014;118(5):554–557. doi: 10.1016/j.jbiosc.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Sunami T, Matsuura T, Suzuki H, Yomo T. Synthesis of functional proteins within liposomes. Methods Mol Biol. 2010;607:243–256. doi: 10.1007/978-1-60327-331-2_20. [DOI] [PubMed] [Google Scholar]
- 43.Chetverin AB, Chetverina HV, Demidenko AA, Ugarov VI. Nonhomologous RNA recombination in a cell-free system: Evidence for a transesterification mechanism guided by secondary structure. Cell. 1997;88(4):503–513. doi: 10.1016/S0092-8674(00)81890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kita H, et al. Functional Qbeta replicase genetically fusing essential subunits EF-Ts and EF-Tu with β-subunit. J Biosci Bioeng. 2006;101(5):421–426. doi: 10.1263/jbb.101.421. [DOI] [PubMed] [Google Scholar]