Significance
Forests dominate carbon fluxes in terrestrial ecosystems. We demonstrate how an intensified climatic influence on tree growth during the last 120 y has increased spatial synchrony in annual ring-width patterns within contrasting (boreal and Mediterranean) Eurasian biomes and on broad spatial scales. Current trends in tree growth synchrony are related to regional changes in climate factors controlling productivity, overriding local and taxonomic imprints on forest carbon dynamics. Enhanced synchrony is becoming a widespread, although regionally dependent, phenomenon related to warmer springs and increased temperature variability in high latitudes and to warmer winters and drier growing seasons in mid-latitudes.
Keywords: tree rings, spatial synchrony, global warming, boreal forests, Mediterranean forests
Abstract
Forests play a key role in the carbon balance of terrestrial ecosystems. One of the main uncertainties in global change predictions lies in how the spatiotemporal dynamics of forest productivity will be affected by climate warming. Here we show an increasing influence of climate on the spatial variability of tree growth during the last 120 y, ultimately leading to unprecedented temporal coherence in ring-width records over wide geographical scales (spatial synchrony). Synchrony in growth patterns across cold-constrained (central Siberia) and drought-constrained (Spain) Eurasian conifer forests have peaked in the early 21st century at subcontinental scales (∼1,000 km). Such enhanced synchrony is similar to that observed in trees co-occurring within a stand. In boreal forests, the combined effects of recent warming and increasing intensity of climate extremes are enhancing synchrony through an earlier start of wood formation and a stronger impact of year-to-year fluctuations of growing-season temperatures on growth. In Mediterranean forests, the impact of warming on synchrony is related mainly to an advanced onset of growth and the strengthening of drought-induced growth limitations. Spatial patterns of enhanced synchrony represent early warning signals of climate change impacts on forest ecosystems at subcontinental scales.
Understanding how climate change affects forests across multiple spatiotemporal scales is important for anticipating its impacts on terrestrial ecosystems. Increases in atmospheric CO2 concentration and shifts in phenology (1–3) could favor tree growth by enhancing photosynthesis and extending the effective growing period, respectively (4). Conversely, recent warming could increase respiration rates and, together with increasing heat and drought stresses, exert negative impacts on forest productivity (5, 6). Given the uncertainty as to what extent enhanced carbon uptake could be offset by the detrimental effects of warming on tree performance, the actual consequences of climate change on forest carbon cycling remain under debate. Notably, climate change has a stronger impact on forests constrained by climatic stressors, such as suboptimal temperatures or water shortage (7). As high-resolution repositories of biological responses to the environment, dendrochronological archives can be used to monitor this impact (8).
The concept of spatial synchrony in tree growth refers to the extent of coincident changes in ring-width patterns among geographically disjunct tree populations (9). Climatic restrictions tend to strengthen growth–climate relationships, resulting in enhanced common ring-width signals (i.e., more synchronous tree growth). Thus, regional bioclimatic patterns can be delineated by identifying groups of trees whose growth is synchronously driven by certain climatic constraints (10, 11). Previous synthesis studies have provided evidence for globally coherent multispecies responses to climate change in natural systems, including forests, with a focus on the role of increasingly warmer temperatures (12, 13). Indeed, climate has changed markedly over the last decades, prompting an array of physiological reactions in trees that could strengthen growth–climate relationships, thereby enhancing spatial synchrony. Such tree responses may be linked to global shifts in the timing of plant activity (2), drought stress in mid-latitudes (6, 14), or an uncoupling of air and soil thermal regimes in the early growing season (15) and direct heat stress (16) in high latitudes, among other factors. Changing tree growth patterns associated with enhanced synchrony in response to warming have been reported at small geographical scales (<150 km) (14–18, but see ref. 19); however, an extended examination of synchrony patterns is currently lacking for large (i.e., subcontinental) areas.
To determine whether climate warming and increased variability (1) lead to more synchronous tree growth, we examined changes in spatial synchrony over the last 120 y across subcontinental areas by using a comprehensive network of 93 ring-width chronologies from six different conifer species across two climatically contrasting Eurasian biomes: boreal forests in central Siberia (n = 45 chronologies) and Mediterranean forests in Spain (n = 48 chronologies) (SI Appendix, Fig. S1 and Table S1). Central Siberia has a severe continental climate with a prolonged cold season, large intra-annual temperature variations, and moderate precipitation. Spain is dominated by a typical Mediterranean climate, with mild (coast) to cool (inland) wet winters and summer droughts. Thus, temperature exerts the main climatic control over productivity in boreal forests, whereas Mediterranean forests are primarily water-limited (SI Appendix, section 1A).
Temporal changes in spatial synchrony (hereinafter, âC) are quantified using a novel mixed model framework (20). This methodology has two fundamental advantages for dendroscience (21) over other alternative approaches useful for interpreting population dynamics in ecology (22) or patterns of environmental synchrony (23): (i) it is capable of dealing with partially overlapping chronologies, yielding valid inferences of spatial synchrony for large areas in which ring-width data are available but covering different time periods, and (ii) it is highly flexible to fit general statistical structures for subdivided groups of chronologies, opening new avenues for interpreting complex spatial patterns through geographic or taxonomic stratification of a target region.
We hypothesized that climate warming (1) triggers more synchronous tree growth at subcontinental scales owing to an amplified climatic control of growth, e.g., through higher temperatures in Siberia and decreased water availability in Spain. Our objective was to interpret forest reactions to warming through an alternative approach to model-based assessment or field experimentation. Specifically, this study asked the following questions: (i) is spatial synchrony of tree growth increasing across terrestrial biomes and if so, at what pace?; (ii) how are synchrony patterns related to intraspecific and interspecific responses to climate warming?; and (iii) what are the main climate factors underlying more synchronous forest growth? In ecological theory, it is widely accepted that spatial synchrony influences metapopulation persistence and the likelihood of species extinction (24). As forests are becoming more prone to widespread mortality (25), interpreting long-term synchrony patterns of tree growth may be relevant to identifying broad-scale threshold responses to climate change.
Results and Discussion
Increases in Synchrony and Absolute Growth Trends: Regional Patterns.
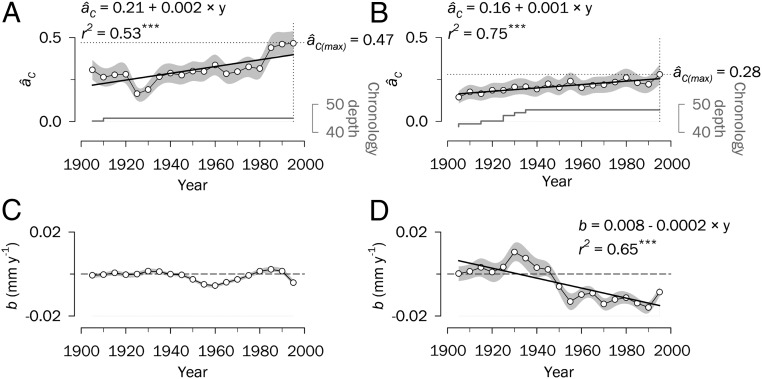
Synchrony trends show that âC has increased during the period 1890–2009 in both study regions (Fig. 1 A and B), but at a twofold higher pace in Siberia than in Spain (b = 0.002 y–1 vs. 0.001 y–1). Whereas the increasing trend is steady over time for Spain, âC has experienced larger fluctuations for Siberia, with an abrupt rise observed in the last three decades. Overall, âC values were significantly higher after 1950 compared with the first half of the 20th century (P = 0.002 for Siberia and P < 0.001 for Spain, one-tailed Student’s t-test), reaching unmatched values after the 1970s in both regions.
Fig. 1.
Synchrony patterns and changes in absolute growth for the period 1890–2009. (A and B) Spatial synchrony, âC, estimated for central Siberia (A) and Spain (B) using ring-width chronologies for successive 30-y periods lagged by 5 y. The dotted lines indicate âC maxima over the study period. Gray lines represent the number of chronologies with an EPS > 0.85 (i.e., chronology depth). (C and D) Temporal trends in the slope (b) of growth in central Siberia (C) and Spain (D) estimated based on raw ring-width data for the same time intervals used for synchrony. The dashed lines indicate no change in growth rate over the study period. Significant linear trends over time are depicted as black lines (***P < 0.001). Shaded areas denote SEs. Values on the x-axes correspond to the middle years of 30-y moving intervals.
Notably, peaks in spatial synchrony were reached in the early 21st century (Fig. 1 A and B). This finding suggests a progressive influence of external forcing mechanisms impacting tree performance through vast geographical areas and overriding local drivers of growth (e.g., topography, nutrient availability, tree competition, management). Regional forest synchrony is currently reaching values comparable to the mean synchrony between co-occurring trees within a stand (mean ± SE, Spain: âC = 0.28 ± 0.06 [region] vs. 0.40 ± 0.13 [stand]; Siberia: âC = 0.47 ± 0.07 [region] vs. 0.49 ± 0.10 [stand]). The lower mean âC in Spain over the study period (0.21 ± 0.02 vs. 0.32 ± 0.03 in Siberia) contrasts with the lower average distance between stands in Spain compared with Siberia (mean ± SD, 346 ± 195 km vs. 575 ± 331 km) (SI Appendix, Fig. S1). In any case, synchrony patterns in Siberia and Spain are statistically independent (SI Appendix, Tables S2 and S3), indicating that the increasing synchrony in tree growth is a widespread ecological phenomenon, although regionally dependent. Synchrony estimates could be sensitive to the number of available chronologies, a number that has decreased progressively in the most recent years (SI Appendix, Table S1); however, there is a close agreement in âC between the complete set of chronologies and a subset that extends beyond the year 2000 (Siberia, r = 0.90; Spain, r = 0.87; P < 0.001 in both cases). An analysis of anomalously wide or narrow rings (i.e., “pointer years”), originating from extreme external events (26), is in agreement with the absolute peaks in synchrony seen in both regions at the turn of this century (SI Appendix, section 2A and Fig. S2).
To further explore how increasing synchrony is related to regional growth patterns, we used raw ring-width records and assessed shifts in growth rates over time. We noted differential paces of tree growth in Siberia and Spain for the period 1890–2009. A clear growth slowdown was seen in Spain (b = –0.00024 mm y–2), but no significant trend appeared in Siberia (Fig. 1 C and D). Such temporal changes could be affected by variations in the population age/size structure (mean ± SD chronology length, 303 ± 82 y for Siberia and 247 ± 129 y for Spain). To check for age-independent growth declines, we assessed growth dynamics only for old-growth forest stands (>200 y old; n = 43 chronologies for Siberia, n = 30 chronologies for Spain). This approach minimizes the temporary dynamics associated with stand development and succession. We observed a good agreement when comparing the results from this subset with the growth patterns found for the complete dataset (r = 0.99, P < 0.001 for Siberia; r = 0.87, P < 0.001 for Spain; slowdown for Spain, b = –0.00014 mm y–2), indicating that age-dependent declines cannot explain temporal changes in growth. The growth reduction in Mediterranean forests may be caused by increasing water limitations (6), but the lack of a positive growth response to warming in boreal forests is puzzling. Various hypotheses have been proposed to explain this phenomenon (27), but temperature-induced drought or direct heat stress are plausible constraints of forest productivity in high latitudes (16).
Increases in Synchrony and Absolute Growth Trends: Taxonomic Patterns.
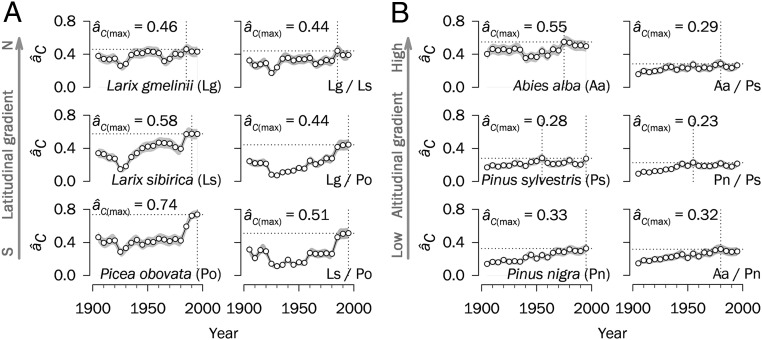
At the species level, âC values peaked at the turn of this century in most cases (Fig. 2). Overall, species-specific changes in synchrony were in good agreement with regional trends, although Larix gmelinii in Siberia and Pinus sylvestris in Spain exhibited less obvious (but also significant) increments in âC over the past 120 y. Notably, the spatial synchrony between pairs of species also increased with time (Fig. 2); however, âC values across species pairs tended to be lower than those for individual species, indicating that although interspecific variability in growth responses certainly exists and is important (e.g., deciduous vs. evergreen conifers in Siberia), the strength of these interspecific differences is diminishing with time, resulting in more synchronous tree growth.
Fig. 2.
Temporal trends in spatial synchrony for single and paired species for central Siberia (A) and Spain (B). Synchrony estimates (âC) in single species (first and third columns) and species pairs (second and fourth columns) are calculated based on ring-width chronologies using successive 30-y periods lagged by 5 y. The dotted lines indicate âC maxima over the study period in each case. SEs are shown as shaded areas. Values on the x-axes correspond to the middle years of 30-y moving intervals.
Boreal forests in Siberia, limited mainly by low temperatures (15, 16), show high synchrony irrespective of species. In contrast, the primarily water-limited forests in Spain (14, 19) have lower synchrony. It is likely that temperature exerts a greater influence than precipitation on the spatial signals imprinted in tree rings (10), owing to the greater spatial homogeneity of temperature in Siberia compared with the more complex rainfall pattern countrywide in Spain (SI Appendix, Fig. S3). In fact, Abies alba has the largest âC among the three Spanish species, which could be explained by its limited spatial distribution and narrow niche amplitude at mesic sites in northeastern Spain (mean ± SD distance between stands, 124 ± 68 km) (SI Appendix, Fig. S1).
To further test for spatial consistency in synchrony patterns (22), we examined how the correlations between pairs of chronologies vary with distance among forest stands. The spatial synchrony spreads over distances >900 km in Siberia and >600 km in Spain (SI Appendix, Fig. S3). More importantly, synchrony was higher in the second half of the 20th century than in the first half for all species and regions at most distances (SI Appendix, Fig. S4). The intercept (but not the slope) of the linear relationship between r values and distances increased for all evergreen conifers. Larix spp. demonstrated significant slope changes, indicating that greater synchrony occurred after 1950 than before 1950 proportionally with distance. Taken together, our results suggest that external forcing factors superimpose on species-specific and local controls, triggering more synchronous tree growth.
We also investigated shifts in absolute growth trends over time at the species level (SI Appendix, Fig. S5). We found a growth slowdown for all species in Spain, but a steady growth rate in Siberia. Notably, growth fluctuations in Siberia became more consistent across species after the 1950s (mean correlation of growth trends between pairs of species before 1950, r = –0.41; after 1950, r = 0.53). A gradual growth decline in Spain, reflecting increasingly limiting conditions over time, and a more systematic pattern of growth changes in Siberia from 1950 onward seems to concur with the rise in synchrony observed at different regional paces (sustained in Spain, abrupt in the last decades in Siberia) over the last 120 y.
Processes Driving Increases in Synchrony.
Increases in synchrony are usually attributed to the influence of climate on tree growth, given that no other environmental driver is likely to act on the same range in time and space (8); however, other global drivers also might trigger the observed increase in synchrony through their effect on growth. First, increasing atmospheric CO2 concentrations can be expected to augment water use efficiency, thereby boosting forest productivity (4). Second, nitrogen deposition could further increase growth through higher rates of carbon sequestration in sites with unfertile or poorly developed soils (28). Third, a decrease in solar radiation caused by anthropogenic aerosol emissions from 1950 (global dimming; ref. 29) may counteract the effect of CO2 fertilization or rising N deposition, negatively impacting photosynthesis, especially in high latitudes (30). Fourth, changes in stand structure due to rural abandonment, limited forest management, and encroachment usually increase competition for resources and could reduce tree growth (31). A detailed examination of these drivers (SI Appendix, section 2B) led us to discard them as major causes of growth synchrony as opposed to climate.
We then examined whether enhanced synchrony is driven by regional climate trends. Although the Earth’s surface has experienced conspicuous warming in the last century, the pace of temperature changes differs regionally (1). Interestingly, the steady increase in synchrony among Spanish forests seems in line with the gradual warming observed over the 20th century. In turn, temperatures at high Eurasian latitudes have shown a consistent upward trend since the 1970s, which coincides with the steep increase in synchrony observed in Siberia (SI Appendix, Fig. S6).
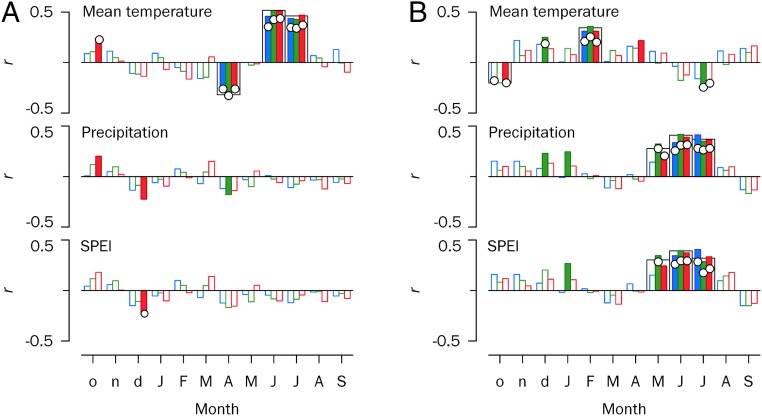
Our analyses of climatic factors causing changes in growth and of climate drivers underlying âC fluctuations were restricted to data collected after 1930, in concert with the availability of reliable instrumental records (32). Despite species-specific ecological features, the main climate variables related to growth were regionally consistent across taxa (Fig. 3). As expected, we found strong positive associations between growth and June–July temperatures in Siberia (i.e., cold-limited growth) (15, 33) and between May–July precipitation and the Standardized Precipitation Evapotranspiration Index (SPEI) in Spain (i.e., drought-constrained growth) (19). Moreover, growth was correlated with early spring (April) temperatures in Siberia (negatively) and with late winter (February) temperatures in Spain (positively) (Fig. 3). Such contrasting regional responses to temperature in the early growing season may be a consequence of varying physiological processes underlying the mechanisms of dormancy breaking. After chilling requirements are met in winter, cambial reactivation can occur earlier in Siberia under higher April temperatures (34), hastening the onset of xylogenesis (35); however, newly formed tissues can be damaged if followed by severe frosts (33), causing hydraulic dysfunction (i.e., freeze–thaw events) (36). A warm spring also may activate the photosynthetic machinery in crowns that are functionally uncoupled from roots (35), and sudden early-spring temperature drops may impair photosynthesis in boreal evergreen conifers (37). These mechanisms may restrict radial growth in high latitudes (38). In contrast, in Mediterranean environments, early cambial reactivation triggered by warm winters usually increases carbon uptake and enhances wood formation (39).
Fig. 3.
Growth–climate relationships at the species and regional levels for central Siberia (A) and Spain (B). Tree growth responses to climate are based on bootstrapped correlations (indicated by bars) and response function partial regression coefficients between tree-ring indices for species or regions and monthly mean temperature, precipitation, and SPEI for 1930–2009. Significant correlation and partial regression coefficients (P < 0.05) are indicated by filled bars and white circles, respectively. Species are represented in blue (Larix gmelinii, Abies alba), green (Larix sibirica, Pinus nigra), and red (Picea obovata, Pinus sylvestris). Wide bars denote significant relationships at the regional level. Lowercase and uppercase letters on the x-axes correspond to the years before and during tree-ring formation, respectively.
We characterized the spatial structures of these growth–climate relationships through cross-correlation analyses. The spatial association was significant for most climate factors at sites >900 km apart in Siberia and >600 km apart in Spain (SI Appendix, Fig. S7), confirming the existence of consistent subcontinental forest responses to climate (11). This result identifies climate as a primary mechanism of synchrony in our tree-ring network, and suggests that increases in forest synchrony could be driven by concomitant increases in regional climate synchrony. We found that this actually was not the case, however (SI Appendix, section 2C and Fig. S8).
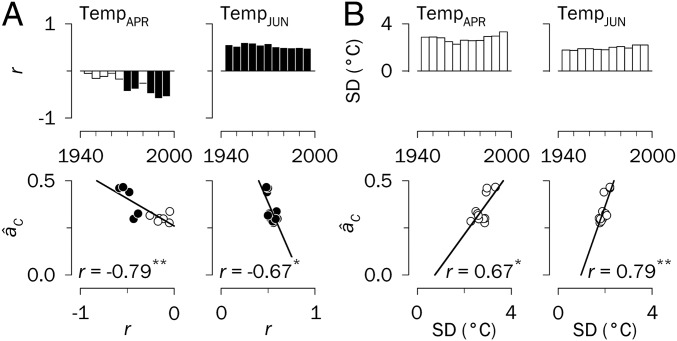
Alternatively, we evaluated whether enhanced synchrony is related to climate trends and variability. For this purpose, we first assessed regional changes in growth–climate relationships through moving correlations. Most relevant changes span the period from the onset of the growing season through early summer, matching the period of early wood formation. For Siberia, the negative dependence of growth on warmer April temperature increased over time (Fig. 4A); for Spain, high February temperatures and high May–June precipitation and SPEI became more important for growth (Fig. 5). In this regard, we found that âC values were significantly associated with the correlation coefficients of the varying relationship between growth and early growing season temperature. Notably, this association was negative for April in Siberia (Fig. 4A) and positive for February in Spain (Fig. 5), indicating divergent regional growth dynamics. In both cases, we interpret this result as a sign of earlier cambial reactivation promoting more synchronous growth over time.
Fig. 4.
Climate factors underlying changes in synchrony in central Siberia, 1930–2009. (Upper) Moving correlation analyses (r values) between regional tree-ring indices and April and June mean temperatures (Temp) (A) and standard deviations (SD) of April and June mean temperatures (B) displayed in bar diagrams for successive 30-y periods lagged by 5 y. Significant correlations are indicated by filled bars in A (P < 0.05). (Lower) Relationships between synchrony estimates (âC) and r values (A) and SD of climate variables (B) presented in scatterplots. Only monthly climate factors significantly correlated with âC are displayed (*P < 0.05; **P < 0.01). The filled circles correspond to the periods with significant growth–climate relationships, as indicated by the moving correlation analyses. Years displayed on the x-axes correspond to the middle years of 30-y moving intervals.
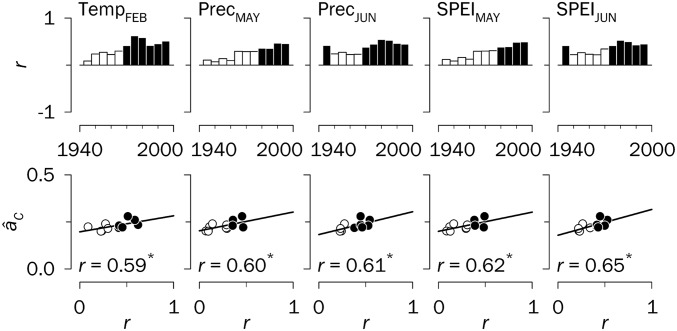
Fig. 5.
Climate factors underlying changes in synchrony in Spain, 1930–2009. (Upper) Moving correlation analyses (r values) between regional tree-ring indices and February mean temperatures (Temp), and May and June precipitation (Prec) and SPEI in May and June displayed in bar diagrams for successive 30-y periods lagged by 5 y. (Lower) Relationships between synchrony estimates (âC) and r values presented in scatterplots. More details are provided in Fig. 4.
Rising temperatures may induce earlier cambial reactivation (40), resulting in both a higher rate of xylem cell production and a longer duration of cambial growth, which in principle should increase ring width (41). This was not the case for Siberia, however, where an earlier onset of cambial activity may be associated with an increased risk of frost damage to the cambium (40). In this region, tree growth has remained limited by low summer temperatures (Fig. 4A and SI Appendix, Fig. S9), exerting growth desynchronization in June (Fig. 4A), but this constraint has decreased after the 1970s because of the positive warming trend (SI Appendix, Fig. S6). This effect is counterbalanced by a reinforcement of the negative impact of high April temperatures on growth as a driver of synchrony (Fig. 4A). In Spain we also observed a diminishing growth dependence on July precipitation and SPEI (SI Appendix, Fig. S10) (19), which suggests reduced cambial activity in summer (36) in response to warming-induced drought (SI Appendix, Fig. S11) and a growth shift toward spring (May–June) (Fig. 5), thereby enhancing synchrony.
Synchrony patterns might not be linked exclusively to long-term climate trends, but also to changes in climate extremes over time (26), as suggested by the reported independence of spatial correlations of record-breaking monthly temperature time series from those of the temperatures themselves (42). In general, significant linear trends in climate variability (i.e., standard deviations of monthly climate factors; SI Appendix, Fig. S11) were observed for relevant growth periods (Fig. 3). We found that a more synchronous growth pattern in Siberia was related to a larger temperature variability in April and June (Fig. 4B), but not in peak summer (July) (SI Appendix, Fig. S9). In contrast, climate variability was unrelated to changes in synchrony in Spain (SI Appendix, Fig. S10).
Finally, we explored the complementarity of climate trends and variability in explaining regional synchrony through stepwise linear regression (Table 1). Initially, the impact of climate trends on synchrony was considered using the subset of monthly climate factors whose temporal relationships with growth exhibit a significant association with âC values (Figs. 4A and 5). These models provide a good explanation of synchrony changes (especially for Spain), identifying recent warming as a significant driver of synchrony across the studied Eurasian forests; however, when climate variability is also considered (Fig. 4B), the model fitting is further improved in Siberia, up to r2 values similar to those for Spain. In fact, climate variability for April and June in Siberia and climate trends for February temperature and June water availability (precipitation and SPEI) in Spain explain 75% of the observed regional synchrony patterns. Unraveling the exact effects of climate warming and extremes on forest synchrony would require both a greater spatial saturation of site chronologies and more detailed instrumental records covering large geographic areas.
Table 1.
Multiple linear stepwise regressions explaining synchrony (âC) patterns from changing growth–climate relationships (correlation coefficients; r) and climate variability (standard deviations of climate factors; SD) over 1930–2009
| Synchrony | Initial variable | Initial r2 | Initial MSE | Final stepwise model | Final r2 | Final MSE |
| Siberia | Correlation with climate factors (rTempAPR, rTempJUN) | |||||
| rTempAPR | 0.63** | 0.020 | âC = 0.26 ‒ 0.29 rTempAPR | 0.63** | 0.020 | |
| Correlation with climate factors and variability (rTempAPR, rTempJUN, SDTempAPR, SDTempJUN) | ||||||
| SDTempJUN | 0.63** | 0.020 | âC = ‒0.47 + 0.28 SDTempJUN + 0.10 SDTempAPR | 0.75*** | 0.013 | |
| Spain | Correlation with climate factors (rTempFEB, rPrecMAY, rPrecJUN, rSPEIMAY, rSPEIJUN) | |||||
| rSPEIJUN | 0.43* | 0.003 | âC = 0.18 + 1.03 rSPEIJUN ‒ 0.93 rPrecJUN + 0.08 rTempFEB | 0.75*** | 0.002 |
This study illustrates how early signals of climate change impacts on forests can be traced back through the interpretation of synchrony patterns stored in tree rings. Here we demonstrate that climate change is modulating spatial synchrony in forest growth over disparate Eurasian regions, increasing the strength of the common signal shared by trees under warmer conditions to levels comparable to the mean synchrony between co-occurring trees within a stand. Even reporting on different Pinaceae, including evergreen and deciduous conifers, our results are consistent across species. The mechanisms behind this pattern require further examination, but they seem to be dependent on the increasing importance of regional-scale climate signals in tree rings overriding local growth drivers. The observed enhanced synchrony may anticipate declines in tree vigor and growth that are critical for the functioning and maintenance of forest ecosystem services under a warmer future climate.
Materials and Methods
Tree-Ring Indices and Absolute Growth Trends.
Site chronologies of tree-ring indices were obtained by cross-dating the tree-ring width (TRW) series (SI Appendix, section 1B) and posterior detrending and autocorrelation removal with the Friedman supersmoother spline and autoregressive modeling (43). This procedure eliminates the juvenile growth trend and generates stationary (mean = 1) and residual TRW chronologies of dimensionless indices that preserve a common variance encompassing interannual time scales (high-frequency variability potentially related to climate). The adequacy of sample size for capturing the hypothetical population signal was assessed by the expressed population signal (EPS), with a threshold value of 0.85 used to identify the “critical year” at which the site chronology became reliable (44). The study period was 1890–2009, i.e., the period when the impact of climate change on tree performance became noticeable (45). The common time spans across chronologies were 1924–1990 in central Siberia and 1950–1988 in Spain.
Temporal trends in tree growth were characterized by the estimation of growth rate changes using raw TRW records. To this end, the slope of the linear regression of TRW on time was calculated for each chronology for successive 30-y periods lagged by 5 y. The slopes were then averaged for a particular region (or species) and for each period, and a simple linear function was fitted after visual inspection. The slope of this function was interpreted as rate of regional (species) growth change. Comparison of growth rates between regions (species) was performed through ANOVA heterogeneity of slopes.
Mixed Modeling.
To investigate spatial synchrony patterns in indexed chronologies, we made extensive use of variance-covariance (VCOV) mixed modeling (20) (SI Appendix, section 1C). To this end, chronologies were grouped into potentially homogeneous subsets (20). First, two groups were formed corresponding to each region. Next, chronologies were classified at the regional level into three groups according to membership in a particular species. A number of VCOV models accommodating between- and within-group variability were tested (SI Appendix, section 1C) and compared using Akaike and Bayesian information criteria, which favor parsimonious models (46). Then estimates of spatial synchrony (or mean interchronology correlation, âC) were derived for chronologies belonging to either the same region (species) or different regions (pairs of species) through the best VCOV model (SI Appendix, section 1D and Tables S2 and S3). The parameter âC is related to the mean interseries correlation defined at the intrasite level in dendroscience (44). In both cases, VCOV structures were tested for successive 30-y segments lagged by 5 y. The 30-y duration was established by plotting the SE of âC against segments of varying duration (from 10 to 50 y) for each region. The segment length corresponding to the inflection point of the curves was selected as a balance between number of years and precision achieved (SI Appendix, Fig. S12).
Development of Regional and Species Master Chronologies.
We estimated TRW indices at the regional and species levels by best linear unbiased prediction (BLUP), thus obtaining regional and species master chronologies underlying the network of site chronologies (SI Appendix, section 1E). BLUP estimates were derived from the best VCOV model fitted to the whole study period using either a regional or a species classification as grouping criterion. BLUPs were used as input for climate analysis.
Spatial Patterns in Synchrony.
To assess how far the spatial patterns in forest synchrony extend, we calculated the geographical distance between pairs of chronologies, which were used (i) to obtain correlograms (47) testing for significant correlations between chronologies located within ranges of 300 km apart (SI Appendix, section 1F) and (ii) to regress correlations between pairs of chronologies on distances. The linear regressions were calculated at the species level for the periods 1890–1949 and 1950–2009, and their slopes were tested through ANOVA heterogeneity of slopes. Regression slopes were interpreted as indicators of spatial synchrony.
Relationships with Climate.
We quantified the effects of temperature, precipitation, and SPEI (48) on the interannual variability of TRW indices through bootstrapping correlations and response function analyses (8) relating regional and species master chronologies to monthly climatic data (SI Appendix, section 1G). We analyzed growth–climate relationships from the previous October to the current September, i.e., the year of tree-ring formation. In addition, we obtained correlograms for temperature and precipitation testing for significant correlations between sites located within 300 km apart (SI Appendix, section 1F) (47). We also performed cross-correlation analyses to test for causality of synchrony patterns in relevant climate factors as drivers of forest synchrony over progressively larger distances (SI Appendix, section 1F) (47).
We assessed the influence of climate change on regional synchrony through a two-step procedure. First, we performed a moving correlation analysis involving regional chronologies and monthly climate factors for successive 30-y periods lagged by 5 y and then related changes in temporal dependence of growth on climate (i.e., r values of the moving correlation analysis) to variations in âC over time through simple correlations. Second, we examined a possible role of climate variability in explaining changes in synchrony. To this end, we calculated the SD for monthly climate factors of successive 30-y periods lagged by 5 y. We also investigated the relationships between the SD of climate variables and âC through simple correlations. The analyses of both climate trends and variability determining synchrony was restricted to the subset of climate factors showing a significant impact on growth for the entire period 1930–2009, as a prerequisite for identifying climate drivers of âC fluctuations. To determine the predictive ability of climate factors and their variability on the explanation of synchrony patterns, we built linear stepwise models at the regional level using the r values of the moving correlation analysis (step 1) and the SD changes over time (step 2) as independent variables, with a threshold value of P = 0.15 as the criterion for the inclusion or removal of variables.
Supplementary Material
Acknowledgments
T.A.S. is supported by ERANET-MUNDUS (Euro-Russian Academic Network-Mundus) (European Commission). V.R.d.D. is supported by the Ramón y Cajal Program (Contract RYC-2012-10970). Support for this work was provided by European Union projects ForMAT (Sensitivity of tree growth to climate change and growth modelling from past to future) (Contract ENV4-CT97-0641), ISONET (400 years of annual reconstructions of European climate variability using a high resolution isotopic network) (Contract EV K2-2001-00237), and MILLENNIUM (European climate of the last millennium) (Contract GOCE 017008); the Russian Science Foundation (Project 14-14-00295); the Catalan Government (Project 2014 SGR 1141); Studying Tree Responses to Extreme Events: A Synthesis (STReESS) COST (European Cooperation in Science and Technology) Action FP1106; and Spanish Projects CGL2011-26654 and CGL2013-48843-C2-1-R (Spanish Ministry of Economy and Competitiveness).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514717113/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change . In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, et al., editors. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 2.Menzel A, et al. European phenological response to climate change matches the warming pattern. Glob Change Biol. 2006;12:1969–1976. [Google Scholar]
- 3.Reyes-Fox M, et al. Elevated CO2 further lengthens growing season under warming conditions. Nature. 2014;510(7504):259–262. doi: 10.1038/nature13207. [DOI] [PubMed] [Google Scholar]
- 4.McMahon SM, Parker GG, Miller DR. Evidence for a recent increase in forest growth. Proc Natl Acad Sci USA. 2010;107(8):3611–3615. doi: 10.1073/pnas.0912376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva LCR, Anand M, Leithead MD. Recent widespread tree growth decline despite increasing atmospheric CO2. PLoS One. 2010;5(7):e11543. doi: 10.1371/journal.pone.0011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams AP, et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat Clim Chang. 2013;3:292–297. [Google Scholar]
- 7.Hampe A, Petit RJ. Conserving biodiversity under climate change: The rear edge matters. Ecol Lett. 2005;8(5):461–467. doi: 10.1111/j.1461-0248.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- 8.Fritts HC. Tree Rings and Climate. Blackburn Press; Caldwell, NJ: 2001. [Google Scholar]
- 9.Liebhold A, Koenig WD, Bjørnstad ON. Spatial synchrony in population dynamics. Annu Rev Ecol Evol Syst. 2004;35:467–490. [Google Scholar]
- 10.Wettstein JJ, Littell JS, Wallace JM, Gedalof Z. Coherent region-, species-, and frequency-dependent local climate signals in Northern Hemisphere tree-ring widths. J Climate. 2011;24:5998–6012. [Google Scholar]
- 11.Babst F, et al. Site- and species-specific responses of forest growth to climate across the European continent. Glob Ecol Biogeogr. 2013;22:706–717. [Google Scholar]
- 12.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416(6879):389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 13.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 14.Macias M, Andreu L, Bosch O, Camarero JJ, Gutiérrez E. Increasing aridity is enhancing silver fir (Abies alba Mill.) water stress in its southwestern distribution limit. Clim Change. 2006;79:289–313. [Google Scholar]
- 15.Vaganov EA, Hughes MK, Kirdyanov AV, Schweingruber FH, Silkin PP. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature. 1999;400:149–151. [Google Scholar]
- 16.Lloyd AH, Bunn AG. Responses of the circumpolar boreal forest to 20th century climate variability. Environ Res Lett. 2007;2:045013. [Google Scholar]
- 17.Latte N, Lebourgeois F, Claessens H. Increased tree-growth synchronization of beech (Fagus sylvatica L.) in response to climate change in northwestern Europe. Dendrochronologia. 2015;33:69–77. [Google Scholar]
- 18.Tardif J, Camarero JJ, Ribas M, Gutiérrez E. Spatiotemporal variability in tree growth in the central Pyrenees: Climatic and site influences. Ecol Monogr. 2003;73:241–257. [Google Scholar]
- 19.Andreu L, et al. Climate increases regional tree growth variability in Iberian pine forests. Glob Change Biol. 2007;13:804–815. [Google Scholar]
- 20.Shestakova TA, Aguilera M, Ferrio JP, Gutiérrez E, Voltas J. Unravelling spatiotemporal tree-ring signals in Mediterranean oaks: A variance-covariance modelling approach of carbon and oxygen isotope ratios. Tree Physiol. 2014;34(8):819–838. doi: 10.1093/treephys/tpu037. [DOI] [PubMed] [Google Scholar]
- 21.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Wiley; New York: 2002. [Google Scholar]
- 22.Koenig WD, Knops JMH. Scale of mast-seeding and tree-ring growth. Nature. 1998;396:225–226. [Google Scholar]
- 23.Jones PD, Osborn TJ, Briffa KR. Estimating sampling errors in large-scale temperature averages. J Climate. 1997;10:2548–2568. [Google Scholar]
- 24.Earn DJD, Levin SA, Rohani P. Coherence and conservation. Science. 2000;290(5495):1360–1364. doi: 10.1126/science.290.5495.1360. [DOI] [PubMed] [Google Scholar]
- 25.Allen CD, Breshears DD, McDowell NG. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere. 2015;6:129. [Google Scholar]
- 26.Babst F, Carrer M, Urbinati C, Neuwirth B, Frank D. 500 years of regional forest growth variability and links to climatic extreme events in Europe. Environ Res Lett. 2012;7:1–11. [Google Scholar]
- 27.D’Arrigo R, Wilson R, Liepert B, Cherubini P. On the “divergence problem” in northern forests: A review of the tree ring evidence and possible causes. Global Planet Change. 2008;60:289–305. [Google Scholar]
- 28.Thomas RQ, Canham CD, Weathers KC, Goodale CL. Increased tree carbon storage in response to nitrogen deposition in the US. Nat Geosci. 2010;3:13–17. [Google Scholar]
- 29.Liepert B. Observed reductions of surface solar radiation at sites in the United States and worldwide from 1961 to 1990. Geophys Res Lett. 2002;29:1421. [Google Scholar]
- 30.Stine AR, Huybers P. Arctic tree rings as recorders of variations in light availability. Nat Commun. 2014;5:3836. doi: 10.1038/ncomms4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linares JC, Camarero JJ, Carreira JA. Competition modulates the adaptation capacity of forests to climatic stress: Insights from recent growth decline and death in relict stands of the Mediterranean fir Abies pinsapo. J Ecol. 2010;98:592–603. [Google Scholar]
- 32.Esper J, et al. Trends and uncertainties in Siberian indicators of 20th century warming. Glob Change Biol. 2010;16:386–398. [Google Scholar]
- 33.Knorre AA, Kirdyanov AV, Vaganov EA. Climatically induced interannual variability in aboveground production in forest-tundra and northern taiga of central Siberia. Oecologia. 2006;147(1):86–95. doi: 10.1007/s00442-005-0248-4. [DOI] [PubMed] [Google Scholar]
- 34.Oquist G, Huner NP. Photosynthesis of overwintering evergreen plants. Annu Rev Plant Biol. 2003;54:329–355. doi: 10.1146/annurev.arplant.54.072402.115741. [DOI] [PubMed] [Google Scholar]
- 35.Linkosalo T. Regularities and patterns in the spring phenology of some boreal trees. Silva Fenn. 1999;33:237–245. [Google Scholar]
- 36.Voltas J, et al. A retrospective, dual-isotope approach reveals individual predispositions to winter drought-induced tree dieback in the southernmost distribution limit of Scots pine. Plant Cell Environ. 2013;36(8):1435–1448. doi: 10.1111/pce.12072. [DOI] [PubMed] [Google Scholar]
- 37.Heide OM. Day length and thermal time responses of bud burst during dormancy release in some northern deciduous trees. Physiol Plant. 1993;88:531–540. doi: 10.1111/j.1399-3054.1993.tb01368.x. [DOI] [PubMed] [Google Scholar]
- 38.Panyushkina IP, Vaganov EA, Shishov VV. Spatial-temporal variation of radial tree growth in relation to climate in the north of middle Siberia. Dendrochronologia. 1996;14:115–126. [Google Scholar]
- 39.Bigelow SW, Papaik MJ, Caum C, North MP. Faster growth in warmer winters for large trees in a Mediterranean-climate ecosystem. Clim Change. 2014;123:215–224. [Google Scholar]
- 40.Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol Plant. 2013;147(1):46–54. doi: 10.1111/j.1399-3054.2012.01663.x. [DOI] [PubMed] [Google Scholar]
- 41.Rossi S, Girard MJ, Morin H. Lengthening of the duration of xylogenesis engenders disproportionate increases in xylem production. Glob Change Biol. 2014;20(7):2261–2271. doi: 10.1111/gcb.12470. [DOI] [PubMed] [Google Scholar]
- 42.Kostinski A, Anderson A. Spatial patterns of record-setting temperatures. J Environ Stat. 2014;6:1–13. [Google Scholar]
- 43.Friedman JH. 1984 A Variable Span Smoother (Stanford University, Stanford, CA), Laboratory for Computational Statistics Technical Report 5. [Google Scholar]
- 44.Wigley TML, Briffa KR, Jones PD. On the average value of correlated time series, with applications in dendroclimatology and hydrometerology. J Clim Appl Meteorol. 1984;23:201–213. [Google Scholar]
- 45.Pretzsch H, Biber P, Schütze G, Uhl E, Rötzer T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat Commun. 2014;5:4967. doi: 10.1038/ncomms5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burnham KP, Anderson DR. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach. Springer; New York: 2002. [Google Scholar]
- 47.Koenig WD, Knops JMH. Testing for spatial autocorrelation in ecological studies. Ecography. 1998;21:423–429. [Google Scholar]
- 48.Vicente-Serrano SM, Beguería S, López-Moreno JI. A multi-scalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index. J Climate. 2010;23:1696–1718. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.