Significance
Heparanase is the predominant enzyme that cleaves heparan sulfate (HS) in mammals, a linear polysaccharide that is normally attached to a core protein, forming HS proteoglycans (HSPGs) that are abundant in the cell surface and extracellular matrix (ECM). Cleavage of HS by heparanase results in structural alterations of the ECM and release of ECM-bound factors that together stimulate cancer metastasis and angiogenesis. Here we provide evidence that heparanase is expressed by B-lymphomas, and heparanase inhibitors restrain tumor growth. Furthermore, we describe, for the first time to our knowledge, the development and characterization of heparanase-neutralizing monoclonal antibodies (mAbs) that inhibit cell invasion and tumor metastasis. Moreover, we show that these mAbs attenuate lymphoma growth by targeting heparanase in the tumor microenvironment.
Keywords: heparanase, lymphoma, neutralizing antibody, tumor growth, metastasis
Abstract
Heparanase is an endoglycosidase that cleaves heparan sulfate side chains of proteoglycans, resulting in disassembly of the extracellular matrix underlying endothelial and epithelial cells and associating with enhanced cell invasion and metastasis. Heparanase expression is induced in carcinomas and sarcomas, often associating with enhanced tumor metastasis and poor prognosis. In contrast, the function of heparanase in hematological malignancies (except myeloma) was not investigated in depth. Here, we provide evidence that heparanase is expressed by human follicular and diffused non-Hodgkin's B-lymphomas, and that heparanase inhibitors restrain the growth of tumor xenografts produced by lymphoma cell lines. Furthermore, we describe, for the first time to our knowledge, the development and characterization of heparanase-neutralizing monoclonal antibodies that inhibit cell invasion and tumor metastasis, the hallmark of heparanase activity. Using luciferase-labeled Raji lymphoma cells, we show that the heparanase-neutralizing monoclonal antibodies profoundly inhibit tumor load in the mouse bones, associating with reduced cell proliferation and angiogenesis. Notably, we found that Raji cells lack intrinsic heparanase activity, but tumor xenografts produced by this cell line exhibit typical heparanase activity, likely contributed by host cells composing the tumor microenvironment. Thus, the neutralizing monoclonal antibodies attenuate lymphoma growth by targeting heparanase in the tumor microenvironment.
Heparanase is an endo-β-d-glucuronidase capable of cleaving heparan sulfate (HS) side chains at a limited number of sites, releasing saccharide products with appreciable size (4–7 kDa) and biological potency. Enzymatic degradation of HS leads to disassembly of the extracellular matrix (ECM) and correlates with the metastatic potential of tumor-derived cells, attributed to enhanced cell dissemination as a consequence of HS cleavage and remodeling of the ECM and basement membrane underlying epithelial and endothelial cells (1, 2). Heparanase expression is induced in human cancer, most often associating with reduced patients’ survival postoperation, increased tumor metastasis, and higher vessel density (3–5). In addition, heparanase up-regulation is associated with tumors larger in size (3, 5). Likewise, heparanase over-expression enhanced (6, 7), whereas local delivery of anti-heparanase siRNA inhibited (8), the growth of tumor xenografts. These results imply that heparanase function is not limited to tumor metastasis but is engaged in progression of the primary lesion, thus critically supporting the intimate involvement of heparanase in tumor progression and encouraging the development of heparanase inhibitors as anticancer therapeutics (9–12). As a consequence, heparanase inhibitors are currently evaluated in phase 1 clinical trials (13).
Heparanase activity is similarly implicated in the progression of multiple myeloma (14–16), but its significance in other hematologic malignancies has not yet been characterized. Lymphomas are a heterogeneous group of cancers that arise from developing lymphocytes and produce tumors predominantly in lymphoid structures (i.e., bone marrow), but also in extranodal tissues. Collectively, lymphomas constitute the fifth most common cancer in North America, with more than 90% of the patients being affected by lymphomas of B-cell origin (17). Despite overall improvements in outcomes of lymphoma, ∼30–40% of patients have disease that is either refractory or relapses after standard therapy (18). Therefore, a better understanding of the molecular pathobiology of lymphomas is needed for the development of new therapeutic approaches. Here, we provide evidence that heparanase is expressed by B-lymphomas and that heparanase inhibitors restrain tumor growth. Furthermore, we describe the development of novel heparanase-neutralizing monoclonal antibodies (mAbs) that attenuate lymphoma growth by targeting heparanase in the tumor microenvironment.
Results
Inhibition of Lymphoma Xenograft Growth by a Potent Heparanase Inhibitor, PG545.
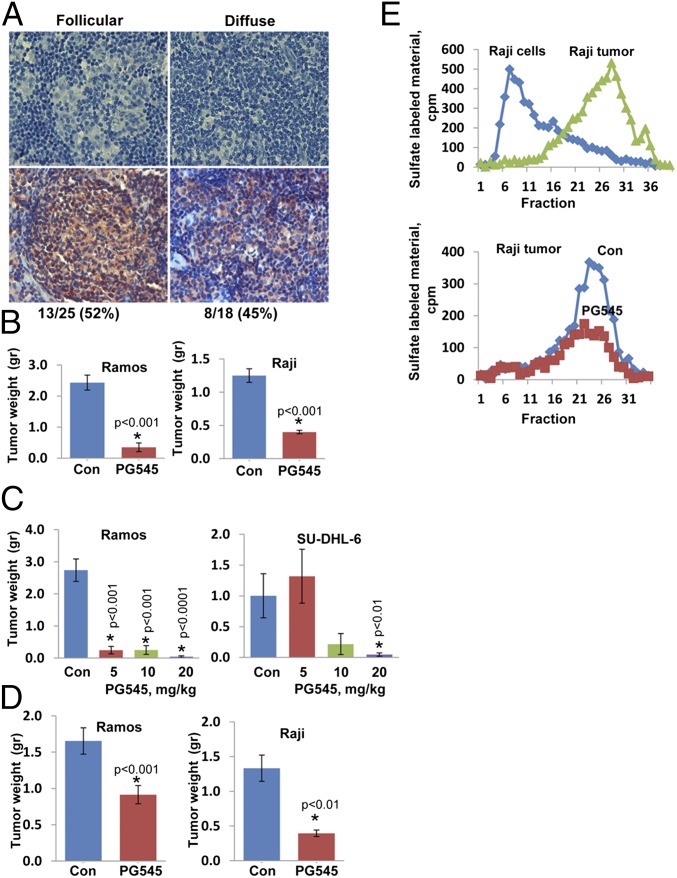
We subjected human lymphoma biopsy specimens to immunostaining and found that heparanase is expressed in both follicular (Fig. 1A, Left) and diffuse (Fig. 1A, Right) lymphomas, representing an indolent and aggressive disease, respectively. To examine the involvement of heparanase in lymphoma in greater detail, we inoculated immunocompromised NOD/SCID mice with human lymphoma cell lines and treated the mice with a potent heparanase inhibitor, PG545 (19). We found that the growth of tumor xenografts produced by Burkitt’s (Ramos, Raji, Daudi), diffuse (OCI-LY-19), and follicular (SU-DHL-6) lymphoma cells was markedly attenuated by PG545 (Fig. 1B and Fig. S1A) in a dose-dependent manner (Fig. 1C). Inhibition of tumor xenograft growth was most effective when PG545 was administrated upon cell inoculation (Fig. 1B and Tables S1 and S2), but significant inhibition was also observed when PG545 was administrated once the lesions became palpable (Fig. 1D).
Fig. 1.
(A) Specimens of human follicular (Left) and diffuse (Right) B-cell lymphomas were subjected for immunostaining applying anti-heparanase Ab. Shown are heparanase-negative (Upper) and heparanase-positive (Lower) lymphoma biopsy specimens. (B) The indicated lymphoma cells (5 × 106) were implanted s.c. in NOD/SCID mice (n = 7), and mice were administered with PG545 (i.p., 20 mg/kg, once weekly, starting 1 d after cell inoculation) or control vehicle (PBS; Con). At termination, tumors were removed, weighed, and embedded in paraffin for histological and immunohistochemical analyses. Mice were similarly inoculated with the indicated lymphoma cells and were treated with the indicated concentration of PG545 (mg/kg once a week; C) or were treated with PG545 (20 mg/kg once a week) once the tumors became palpable (D). Shown are average (±SE) tumor weights (g). (E) Lysates of Raji cells and extracts of tumor xenografts produced by Raji cells were applied onto dishes coated with sulfate-labeled ECM, and heparanase enzymatic activity was determined as described in Materials and Methods (Upper). Note that Raji cells lack heparanase activity, whereas tumor xenografts exhibit typical heparanase activity. Extracts of control (Con) and PG545-treated tumor xenografts produced by Raji cells were applied onto dishes coated with sulfate-labeled ECM, and heparanase activity was determined as earlier (Lower). Note that PG545 inhibits heparanase activity in tumor xenografts produced by Raji cells.
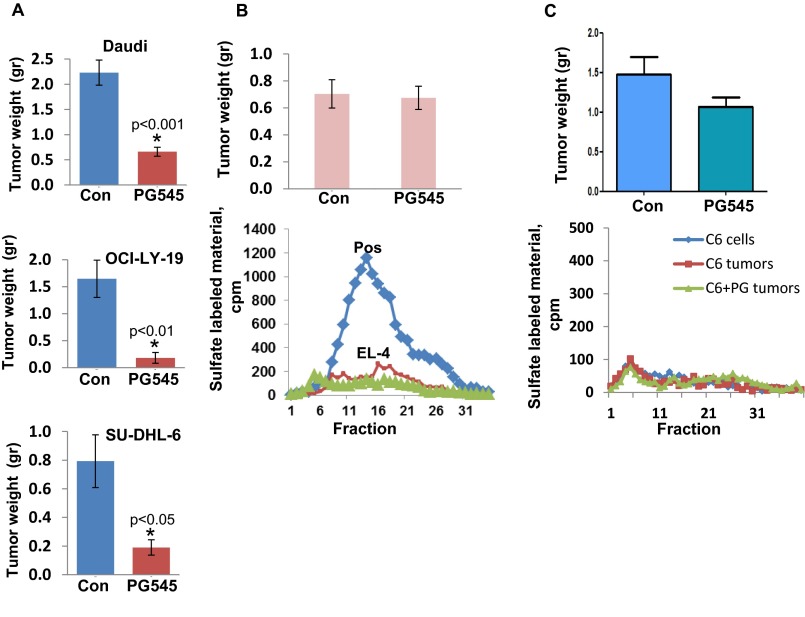
Fig. S1.
(A) The indicated lymphoma cells (5 × 106) were implanted s.c. in NOD/SCID mice (n = 7), and mice were administered PG545 (i.p., 20 mg/kg, once weekly, starting 1 d after cell inoculation), or control vehicle (PBS; Con). At termination, tumors were removed, weighed, and embedded in paraffin for histological and immunohistochemical analyses. Shown are average (±SE) tumor weights (g). El-4 mouse lymphoma (B) and rat C6 glioma (C) cells (1 × 105) were inoculated s.c. into C57BL/6 (El-4) or SCID (C6) mice (n = 7), and mice were treated with PG545 (i.p., 20 mg/kg, once weekly) or control vehicle. At termination, tumor xenografts were harvested, weighed (Upper), and embedded in paraffin for histological examination. Portions of the untreated (red) and treated (green) tumors were lysed for determination of heparanase activity, along with a sample that exhibits high enzymatic activity (Pos, B, Lower). Note that El-4 and C6 cells and tumor xenografts lack heparanase activity, and that PG545 failed to inhibit the growth of these tumors.
Table S1.
Percentage inhibition of lymphoma tumor weight by PG545
| Cell line | Tumor weight, g | P value | Inhibition, % | |
| Con | PG545 | |||
| Ramos | 2.43 ± 0.24 | 0.35 ± 0.14 | <0.001 | 85.6 |
| Raji | 1.25 ± 0.1 | 0.4 ± 0.03 | <0.001 | 67.9 |
| SU-DHL-6 | 0.79 ± 0.18 | 0.19 ± 0.05 | <0.05 | 76 |
| Daudi | 2.23 ± 0.25 | 0.66 ± 0.09 | <0.001 | 70.3 |
| OCI-LY-19 | 1.65 ± 0.35 | 0.18 ± 0.1 | <0.01 | 89.1 |
Table S2.
Percentage inhibition of lymphoma tumor weight by and Ab 1453
| Cell line | Tumor weight, g | P value | Inhibition, % | |
| Con | 1453 | |||
| Ramos | 0.59 ± 0.03 | 0.31 ± 0.07 | <0.001 | 48 |
| Raji | 1.1 ± 0.19 | 0.63 ± 0.11 | <0.05 | 33.7 |
| SU-DHL-6 | 0.84 ± 0.29 | 0.09 ± 0.04 | <0.005 | 88.9 |
| Daudi | 0.67 ± 0.16 | 0.56 ± 0.1 | ns | 15.9 |
| OCI-LY-19 | 0.41 ± 0.02 | 0.24 ± 0.05 | <0.05 | 40.5 |
ns, nonsignificant.
Inhibition of Heparanase Originating from the Tumor Microenvironment Is Sufficient to Restrain Lymphoma Growth.
Despite the lack of heparanase activity in Raji cells (Fig. 1E, Upper, Raji cells) because of gene methylation (20), tumor xenografts produced by Raji cells exhibit typical heparanase activity (Fig. 1E, Upper, Raji tumor), suggesting that heparanase-positive cells derived from the host populate the tumor microenvironment. Inhibition of Raji tumor xenografts by PG545 (Fig. 1) in a manner similar to the other lymphoma cell lines is therefore a result of inhibition of heparanase activity contributed by the tumor microenvironment (Fig. 1E, Lower). This implies that the host contributes a significant amount of heparanase and that inhibition of this fraction is sufficient to attenuate tumor growth. Indeed, tumor xenografts developed by cells that lack heparanase enzymatic activity (i.e., El-4), but fail to attract heparanase-positive cells to the tumor, are not inhibited by PG545 (Fig. S1 B and C).
Inhibition of Lymphoma Xenograft Growth by Heparanase-Neutralizing Polyclonal Ab.
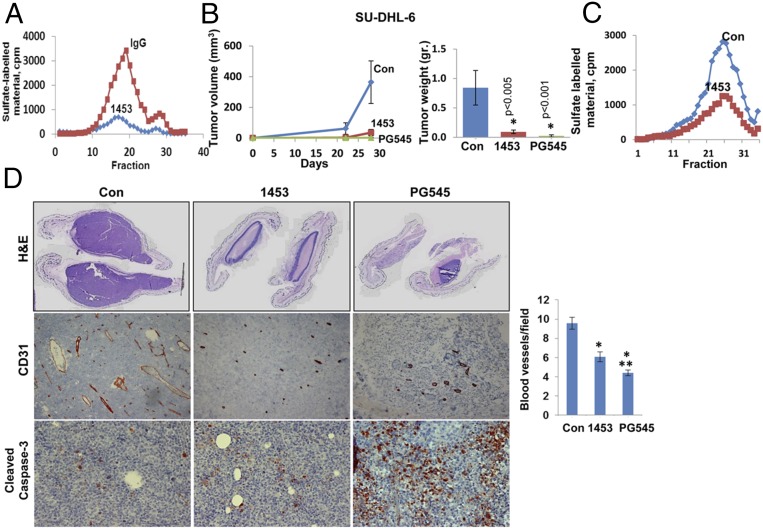
Although PG545 appears as a potent inhibitor that attenuates the progression of several carcinomas (19) and lymphomas (Fig. 1), it also exhibits heparanase-unrelated properties and impedes the signaling of HS-bound growth- and angiogenesis-promoting factors (21). To evaluate the role of heparanase in lymphoma growth in a more specific manner, we used a polyclonal Ab (Ab 1453) that neutralizes heparanase enzymatic activity (Fig. 2A). As expected, cell invasion (Fig. S2A) and tumor metastasis (Fig. S2B) were significantly inhibited by this Ab. Moreover, growth of tumor xenografts produced by U87 glioma cells, shown previously to be strictly dependent on heparanase levels (6), was inhibited by Ab 1453 as a single agent, and even stronger inhibition was obtained when Ab 1453 was combined with SST0001, a heparin mimetic heparanase inhibitor (Fig. S2C) (16). We further found that the growth of tumor xenografts produced by SU-DHL-6 lymphoma cells was markedly inhibited by Ab 1453 (Fig. 2B), associating with decreased heparanase activity in these tumors (Fig. 2C). Ab 1453 similarly inhibited the growth of tumor xenografts produced by Ramos, Raji, and OCI-LY-19 lymphoma cells (Fig. S2D and Tables S1 and S2). Moreover, treatment with Ab 1453 resulted in smaller tumors that were highly necrotic (Fig. 2D, Upper) and exhibited reduced blood vessel density (Fig. 2D, Middle; CD31). Unlike PG545, Ab 1453 does not appear to enhance apoptotic cell death evident by staining for cleaved caspase 3 (Fig. 2D, Lower), suggesting these treatments inhibit tumor growth by different mechanisms. These results imply with greater confidence that heparanase enzymatic activity promotes lymphoma tumor growth.
Fig. 2.
(A) Purified recombinant active heparanase (200 ng) was preincubated with control rabbit IgG (2 μg/mL; ■) or Ab 1453 (2 μg/mL; ◆) for 2 h in serum-free RPMI medium on ice. The mixture was then applied onto dishes coated with 35S-labeled ECM, and heparanase enzymatic activity was determined as described in Materials and Methods. (B) SU-DHL-6 cells (5 × 106) were implanted s.c. in SCID mice (n = 7), and mice were administered with Ab 1453 (250 µg/mouse; every other day), PG545 (20 mg/kg; once a week), or control vehicle (PBS), and tumor growth was inspected over time (B, Left). At termination, tumors were harvested, weighed (B, Right), and cut into two portions; one portion was lysed and subjected to determination of heparanase activity (C), whereas the other portion was fixed in formalin and subjected to histological (D, Upper) and immunohistochemical analyses applying anti CD31 (D, Middle) and anticleaved caspase 3 (D, Lower) Abs. Blood vessel density was quantified by counting the number of CD31-positive vessels per field and is presented graphically at the Middle Right panel. At least 25 fields of each treatment were counted. (Magnifications: Upper, ×1; Middle and Lower, ×20.) *P = 4.5 × 10−6 and 1.2 × 10−8 for Con versus 1453 and Con versus PG545, respectively; **P = 0.01 for 1453 versus PG545.
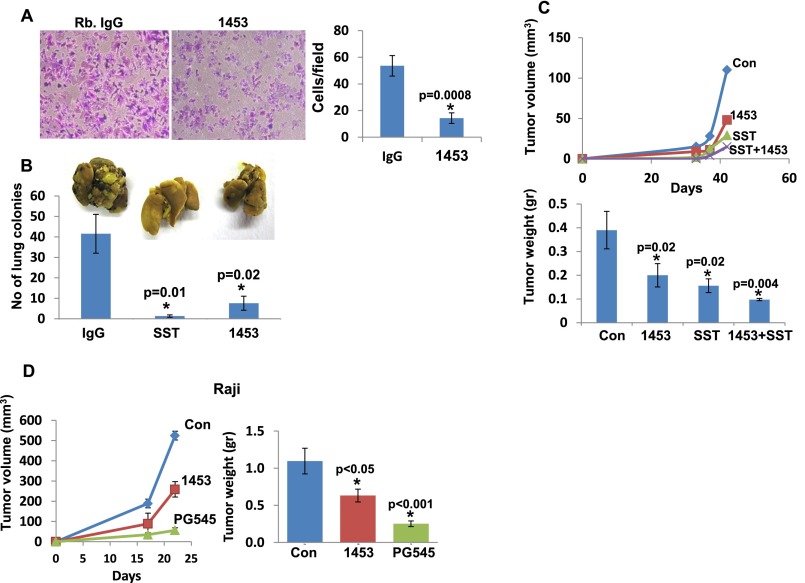
Fig. S2.
Anti-heparanase neutralizing polyclonal Ab 1453 attenuates cell invasion, metastasis, and tumor growth. (A) Cell invasion. U87 glioma cells (2 × 105) were plated onto Matrigel-coated 8-μm Transwell filters in the presence of rabbit IgG or Ab 1453 (2 μg). Invading cells adhering to the lower side of the membrane were visualized after 6 h (*P = 0.0008). (B) Tumor metastasis. Mouse B16 melanoma cells (1.5 × 105) were inoculated into the tail vein of C57BL/6 mice (n = 7), together with rabbit IgG (300 µg), Ab 1453 (300 µg), or SST0001 (400 µg). Lungs were harvested after 18 d, fixed in Bouin's solution, and photographed (Inset), and the number of metastatic lesions was counted (P = 0.01 and 0.02 for Con vs. SST0001 and Con vs. Ab 1453, respectively). (C) Tumor growth. U87 glioma cells (5 × 106/0.1 mL) were inoculated s.c. at the right flank of 5-wk-old female SCID mice (n = 7). Mice were administrated with Ab 1453 (250 µg/mouse every other day), SST0001 (400 µg/mouse twice daily), or both compounds starting 2 d after cell inoculation. Control mice were administrated with PBS. Xenograft size was determined twice a week by externally measuring tumors in two dimensions, using a caliper, and tumor volume was calculated (Upper). At the end of the experiment, xenografts were removed, weighed (Lower), and fixed in formalin. Note the reduced tumor development after heparanase inhibition (P = 0.02, 0.02, and 0.004 for control vs. 1453, control vs. SST0001, and control vs. 1453+SST0001, respectively). (D) Lymphoma tumor growth. Raji cells (5 × 106) were implanted s.c. in SCID mice (n = 6), and mice were administrated with Ab 1453 (250 µg/mouse; every other day), PG545 (20 mg/kg; once a week), or control vehicle (PBS), and tumor growth was inspected over time (Left). At termination, tumors were harvested, weighed (Right), and fixed in formalin for subsequent histological examination.
Development of Heparanase-Neutralizing mAbs.
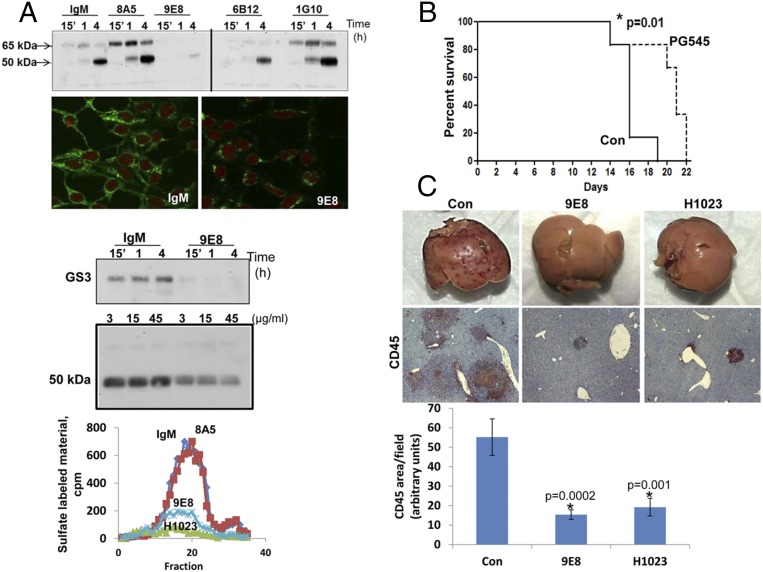
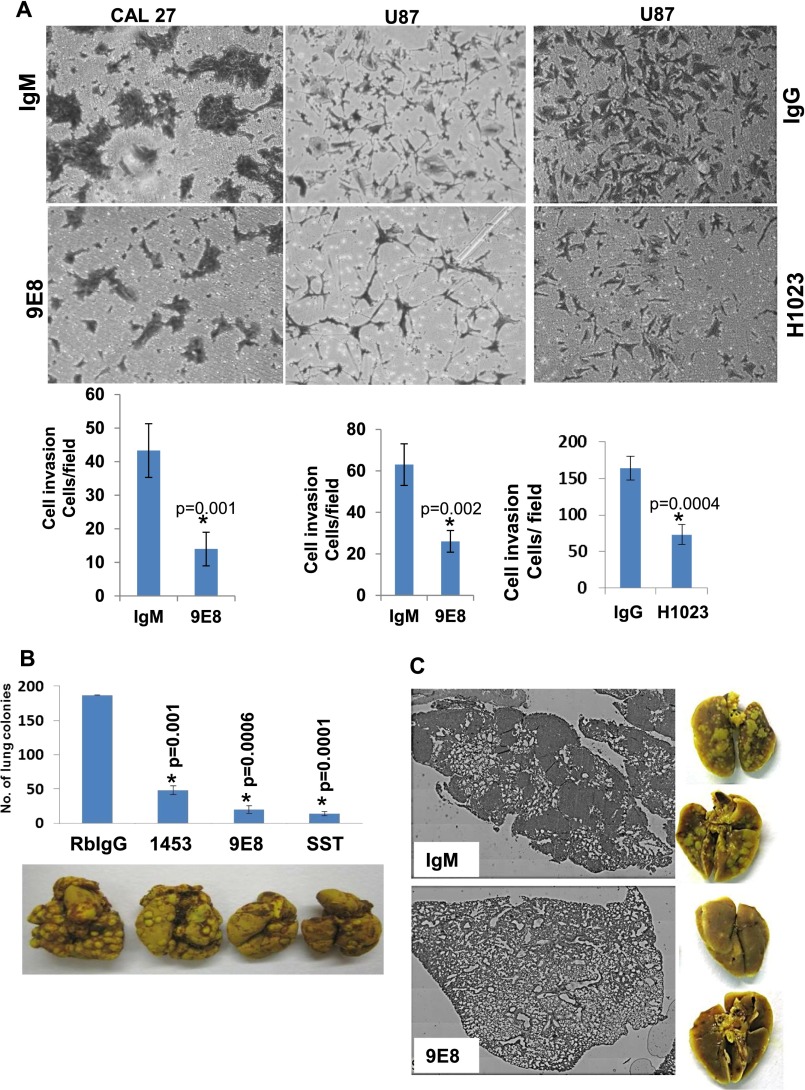
To target heparanase activity even more specifically, we developed a panel of mAbs directed against the KKDC peptide (Lys158-Asn171), which comprises the substrate (HS)-binding domain of heparanase (22). To validate that the resulting Abs target the heparin/HS binding domain, we examined cellular uptake of latent 65-kDa heparanase in the absence or presence of the various newly generated mAbs. This system appeared most relevant because previous studies have shown that heparanase uptake is HS-dependent (22, 23). Heparanase uptake and processing, evident by accumulation of the 50-kDa active subunit, were readily observed in HEK293 cells incubated with control mouse IgM or anti-heparanase mAb 8A5, 6B12, or 1G10 (Fig. 3A). In striking contrast, heparanase uptake was markedly decreased in the presence of mAb 9E8 (Fig. 3A, Upper). Similarly, uptake of the constitutively active heparanase (GS3) (24) was inhibited profoundly by mAb 9E8 (Fig. 3A, Third, GS3). Furthermore, incubation of cells overexpressing heparanase with mAb 9E8 resulted in a marked decrease in the intracellular content of heparanase evident by immunofluorescent staining (Fig. 3A, Second, IgM and 9E8) and immunoblotting (Fig. 3A, Fourth, 50 kDa), in agreement with the notion that intracellular processing of heparanase into its active (50+8 kDa) form occurs after uptake of the secreted 65-kDa latent protein (3, 23). Importantly, mAb 9E8 neutralizes heparanase enzymatic activity to a magnitude comparable to that observed with polyclonal Ab 1453 (Fig. 3A, Lower) and inhibits cell invasion and tumor metastasis, the hallmarks of heparanase activity (Fig. S3 A–C). We further used a model of spontaneous metastasis and found that the survival of mice inoculated with highly metastatic ESb mouse T-lymphoma cells is prolonged by PG545 (Fig. 3B) associating with reduced liver metastasis. Moreover, liver metastasis of ESb cells was inhibited four- to fivefold by mAb 9E8 or mAb H1023 (Fig. 3C). The latter mAb was developed by Eli Lilly (Fig. S4) and found to neutralize heparanase enzymatic activity (Fig. 3A, Lower) and cell invasion (Fig. S3A, Right) to an extent similar to mAb 9E8. The availability of highly specific heparanase-neutralizing mAbs enables us to conclude that heparanase enzymatic activity is required for cell invasion and tumor metastasis.
Fig. 3.
(A) HEK 293 cells were incubated with latent 65-kDa (Upper) or GS3 constitutively active heparanase (1 µg/mL; Third, GS3) and the indicated mAbs (10 µg/mL). The cell medium was aspirated at the time designated, and cell lysates were subjected to immunoblotting applying anti-heparanase Ab (Upper and Third, GS3). Note an almost complete inhibition of heparanase uptake by mAb 9E8. Heparanase-transfected 293 cells were grown with control mouse IgM or mAb 9E8 for 2 d. Cells were then fixed with methanol and subjected to immunofluorescent staining applying anti-heparanase Ab (Second, IgM and 9E8) or lysed and subjected to immunoblotting applying anti-heparanase Ab (Fourth, 50 kDa). Purified recombinant active heparanase (200 ng) was preincubated with control mouse IgM (◆), mAb 8A5 (■), mAb 9E8 (x), or mAb H1023 (▲), all at 2 μg/mL, for 2 h in serum-free RPMI medium on ice. The mixture was then applied onto 35S-labeled ECM-coated dishes, and heparanase activity was determined as described earlier (Lower). (B and C) ESb mouse T-lymphoma cells (5 × 105) were inoculated s.c. in NOD/SCID mice (n = 5) and treated with PG545 (20 mg/kg, once a week) (B) or the indicated mAb (500 µg/mouse every other day) (C). Survival of mice treated with PG545 versus vehicle (Con) is presented in B. (C) Livers of mice treated with mAb 9E8 or H1023 were removed after 3 wk, photographed (Upper), and 5-μm sections of formalin-fixed, paraffin-embedded liver tissue were subjected to immunostaining, applying anti-CD45 Ab (Middle). (Lower) Quantification of the extent of CD45 staining is shown graphically. (Original magnifications: Upper, ×1; Middle, ×20.)
Fig. S3.
Heparanase neutralizing mAbs attenuate cell invasion and tumor metastasis. (A) Cell invasion. Cal-27 tongue carcinoma (Left) and U87 glioma cells (Middle) (2 × 105) were plated onto Matrigel-coated filters in the presence of mouse IgM/IgG (Upper), mAb 9E8 (2 μg; Middle), or mAb H1023 (2 μg; Middle). Invading cells adhering to the lower side of the membrane were visualized after 6 h. (Lower) Number of invading cells per high power field is shown graphically. (B and C) Tumor metastasis. (B) Mouse B16 melanoma cells (2 × 105) were inoculated into the tail vein of C57BL/6 mice (n = 7), together with rabbit IgG (300 µg; Con), Ab 1453 (250 µg), mAb 9E8 (500 µg), or SST0001 (400 µg). Lungs were harvested after 18 d and photographed (Lower), and the number of metastatic lesions was counted (Upper; P = 0.001, 0.0006, 0.0001 for Con vs. Ab 1453, Con vs. mAb 9E8, and Con vs. SST0001, respectively). (C) LM2-4 human breast carcinoma cells (2 × 106) were inoculated into the tail vein of NOD/SCID mice (n = 5), together with PBS or mAb 9E8 (500 µg/mouse). Lungs were harvested after 18 d and photographed (C, Right). The respective lung tissue sections are presented (C, Left).
Fig. S4.
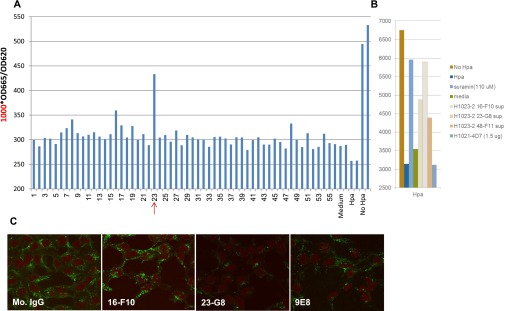
Generation of H1023 heparanase-neutralizing mAb. Mice were immunized with human heparanase, and the resulting hybridomas were screened for their ability to bind heparanase by ELISA. Supernatant of the indicated hybridoma was evaluated for heparanase inhibition, using a high-throughput heparanase activity assay (Cisbio Bioassays HTRF). The HTRF heparanase assay is based on HS substrate labeled with both biotin and Eu3+ Cryptate. Active heparanase enzyme cleaves the substrate, causing the loss of energy transfer, and thus a reduction in emissions. High readings therefore represent low heparanase activity. Hybridoma 23 emerged to neutralize heparanase activity (A), and heparanase neutralization was recapitulated by clone H1023 23-G8 (B) used for subsequent experiments. (C) Heparanase-transfected 293 cells were grown with control mouse IgM or the indicated mAb for 2 d. Cells were then fixed with methanol and subjected to immunofluorescent staining applying anti-heparanase Ab.
Heparanase-Neutralizing mAbs Restrain Lymphoma Growth.
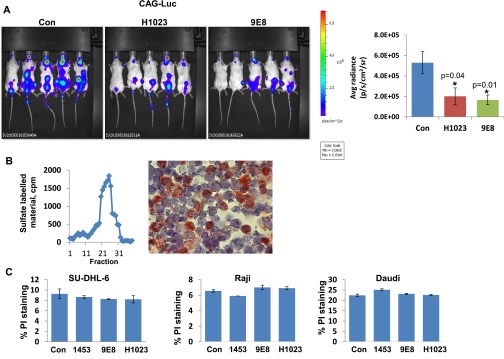
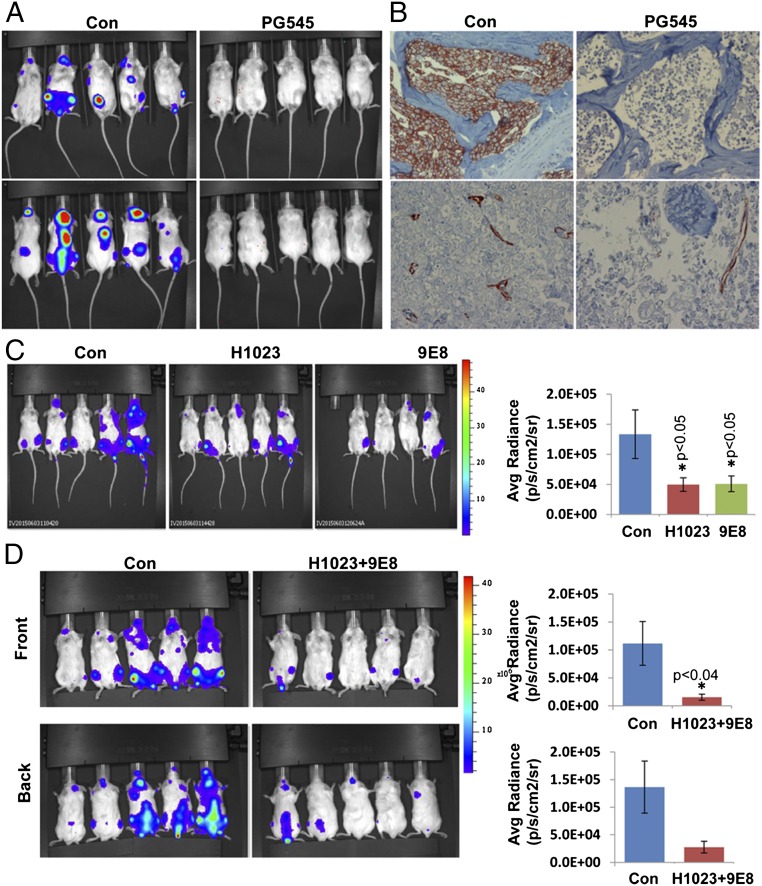
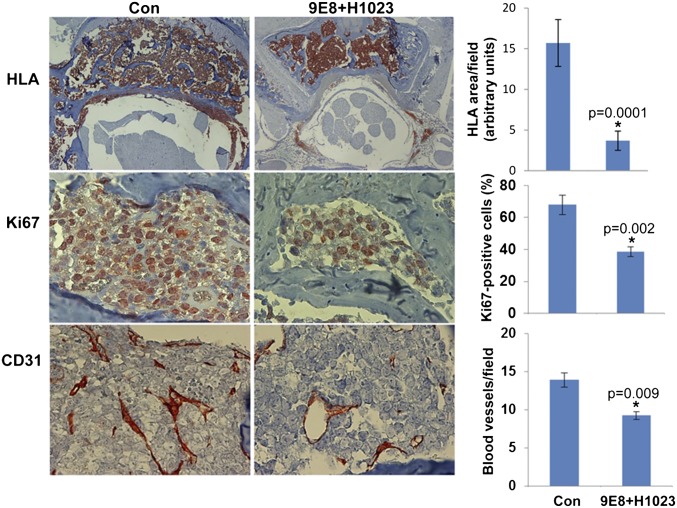
Next, we examined the ability of our neutralizing mAbs 9E8 and H1023 to restrain tumor growth, applying luciferase-labeled cells and in vivo imaging system (IVIS) bioluminescent imaging that enables accurate quantification of tumor load. To this end, we have first used CAG myeloma cells shown previously to strictly depend on heparanase activity for tumor expansion (16, 25). After tail vein inoculation, CAG-luciferase cells home to and expand in the bone marrow (Fig. S5A, Con). The bone marrow exhibits high heparanase activity (Fig. S5B, Left) and a high percentage of heparanase-positive cells (Fig. S5B, Right), and thus appears as a supportive environment that can be targeted by the neutralizing mAbs. Notably, IVIS imaging and analysis revealed a significant, two- to threefold decrease in tumor burden after treatment with mAb 9E8 or H1023 (Fig. S5A). Similar homing and expansion in the bone marrow was observed after tail vein inoculation of Raji-luciferase cells (Fig. 4A, Con), expansion that was markedly reduced by PG545 (Fig. 4 A and B, PG545) associating with decreased blood vessel density (Fig. 4B, Lower). Most important, Raji cell growth was decreased two- to threefold by mAbs 9E8 or H1023 as single agents (Fig. 4C), and an even greater, sevenfold decrease in tumor growth was obtained when the two mAbs were combined (Fig. 4D). Decreased tumor growth by the combined treatment with mAb 9E8 and H1023 evident by IVIS analysis was further confirmed by immunostaining of the mice backbones with anti-human HLA (Fig. 5, Upper). This was associated with reduced tumor cell proliferation (Fig. 5, Middle) and tumor angiogenesis (Fig. 5, Lower). These results strongly indicate that lymphoma growth can be restrained by specific neutralization of heparanase enzymatic activity within the tumor microenvironment.
Fig. S5.
(A) CAG myeloma. NOD/SCID mice (n = 5) were inoculated (i.v.) with CAG-luciferase cells (5 × 106), and mice were treated with mAb 9E8 (600 µg/mouse every other day) or mAb H1023 (500 µg/mouse every other day). Tumor growth was evaluated by IVIS imaging (Left). (Right) Quantification of luciferase signals is shown graphically. Note two- to threefold decrease in tumor growth by the heparanase-neutralizing mAbs. (B) Bone marrow was harvested from C57BL/6 mice, cells were collected by centrifugation, and heparanase activity was determined as described in Materials and Methods (Left). Corresponding bones were fixed in formalin and, after decalcification, were subjected to immunostaining applying anti-heparanase Ab (Right). (C) The indicated cell line (0.25 × 106) was treated with Abs 1453, 9E8, or H1023 (50 µg/mL) for 24 h. Cells were then washed once with FACS buffer (PBS containing 2% FCS), incubated with propidium iodide (PI; 1 µg/mL) for 10 min, and analyzed using FACS Cyan ADP system (Beckman Coulter, Inc.). PI-positivity indicates a disturbed membrane and cell death. Note that anti-heparanase Abs do not elicit lymphoma cell death.
Fig. 4.
(A) Raji-luciferase cells (1 × 106) were injected into the tail vein of NOD/SCID mice (n = 5). Mice were treated with PG545 (20 mg/kg once a week) starting 1 d after cell inoculation, and IVIS imaging was applied to quantify tumor load. Homing of Raji cells to the bone marrow was further confirmed by immunostaining of decalcified backbone sections with anti-human HLA that labels human cells (B, Upper). Slides were similarly stained with anti-CD31 Ab that marks blood vessels (B, Lower). (C and D) NOD/SCID mice (n = 5) were inoculated (i.v.) with Raji-luciferase cells, and mice were treated with mAb 9E8 (600 µg/mouse every other day) or mAb H1023 (500 µg/mouse every other day) as single agents (C) or both together (D), and tumor growth was evaluated by IVIS imaging (Left). (Right) Quantification of luciferase signals is shown graphically.
Fig. 5.
NOD/SCID mice (n = 5) were inoculated (i.v.) with Raji-luciferase cells, and mice were treated with mAb 9E8 (600 µg/mouse every other day) and mAb H1023 (500 µg/mouse every other day). At termination, the backbones were collected, fixed, decalcified, and embedded in paraffin. Five-micrometer sections were subjected to immunostaining applying anti-human HLA (Upper), anti-Ki67 (Middle), and anti-CD31 (Lower) Abs. (Upper Right) Staining intensity of HLA was quantified by the Image-Pro Plus software and is shown graphically. (Middle Right) Ki67-positive cells were counted at each treatment, and the results are presented graphically as percentage of total cells per field. (Lower Right) Blood vessel density was quantified by counting the number of CD31-positive vessels per field, presented graphically. At least 25 fields at each treatment were counted. (Original magnifications: ×80.)
Discussion
Attempts to inhibit heparanase enzymatic activity were already initiated in the early days of heparanase research, in parallel with the emerging clinical relevance of this activity (26). Since then, a variety of inhibitory molecules have been developed, including peptides, small molecules, and modified nonanticoagulant species of heparin, as well as several other polyanionic molecules such as laminaran sulfate, suramin, PI-88, and PG545 (13). Similarly, anti-heparanase polyclonal Abs were developed and demonstrated to neutralize heparanase enzymatic activity and to inhibit cell invasion (27), proteinuria (28), and neointima formation (29). Neutralizing anti-heparanase mAbs, however, have not been reported so far. Our earlier study (22) was undertaken to identify functional domains of heparanase that would serve as targets for rational drug development. Based on published consensus sequences that mediate the interaction between polypeptides and heparin, we have identified three potential heparin-binding domains of heparanase (22). Particular attention was given to the Lys158-Asp171 domain, as a peptide corresponding to this sequence (termed KKDC) physically interacts with heparin and HS with high affinity and inhibits heparanase enzymatic activity (22). Furthermore, a deletion construct lacking this domain exhibits no enzymatic activity (22), and polyclonal Ab 733 directed to this region inhibits heparanase activity (30). We have followed this rationale and generated a panel of mAbs directed against the KKDC peptide, attempting to recapitulate the performance of Ab 733, targeting the interaction of heparanase with its HS substrate.
Here, we report, for the first time to our knowledge, the development of mAbs 9E8 and H1023, which neutralize heparanase enzymatic activity. As expected, mAb 9E8, directed against the KKDC peptide, substantially decreased the uptake of latent and active heparanase (Fig. 3A), an HS-dependent cellular mechanism thought to limit extracellular retention of the enzyme, thereby preventing adverse outcomes (23). In contrast, the epitope of mAb H1023, generated against the full-length heparanase protein, appears to be 3D and could not be identified by linear peptide mapping. Notably, however, the two Abs exhibit similar performance characteristics in vitro and in vivo. Decreased intracellular content of the 50-kDa processed heparanase in cells incubated with mAb 9E8 (Fig. 3A) or H1023 (Fig. S4C) implies that the Abs not only neutralize the enzyme extracellularly but also affect heparanase levels inside the cell. Both Abs markedly inhibit cellular invasion and tumor metastasis (Fig. S3), the hallmarks of heparanase function. Moreover, mAbs 9E8 and H1023 inhibited the spontaneous metastasis of ESb lymphoma cells from the s.c. primary lesion to the liver (Fig. 3C). Treatment with mAb 9E8 or mAb H1023, as a single agent, attenuated the growth of CAG myeloma (Fig. S5A) and Raji (Fig. 4C) lymphoma tumors, and even greater inhibition was observed by combining the two mAbs together (Fig. 4D). This is in agreement with the notion that combining two different mAbs increases the inhibitory outcome (31).
Not surprisingly, Ab 1453, mAb 9E8, or mAb H1023 are not cytotoxic to lymphoma (Fig. S5C). This implies that the mAbs do not exert a direct effect on tumor cells but, rather, affect the tumor microenvironment. This is best demonstrated in Raji cells that lack intrinsic heparanase activity (Fig. 1E) (20), whereas tumor xenografts produced by these cells exhibit typical heparanase activity. The ability of mAbs 9E8 and H1023 to attenuate the growth of these tumors (Figs. 4 and 5) is thus a result of neutralization of heparanase contributed by the tumor microenvironment. The microenvironment of B-cell lymphomas contains variable numbers of immune cells (i.e., T-cells, macrophages, neutrophils), stromal cells (i.e., fibroblasts), and endothelial cells (17, 32, 33), many of which have been reported to express heparanase (34, 35). The significance of the tumor microenvironment in tumorigenesis is well documented and accepted (36). In B lymphomas, a complex cross-talk between the lymphoma cells and their respective microenvironment is bidirectional, and multiple secreted factors and cell surface molecules contribute to the activation of signaling pathways in both the lymphoma and stromal cells (32), many of which are targets for the development of lymphoma therapeutics (37). Heparanase activity is thought to release a multitude of HS-bound cytokines, chemokines, and growth-promoting factors sequestered in the ECM and convert them into bioavailable and biologically active mediators that promote cell proliferation, survival, and motility (38–40). Thus, heparanase inhibitors are thought to inhibit the combined outcome of many of the above targets. Decreased blood vessel density in Raji lymphoma treated with mAbs 9E8 and H1023 (Fig. 5) critically stamp the proangiogenic capacity of heparanase (3). This is most likely a result of reduced release by heparanase of angiogenic-promoting factors sequestered in the bone marrow ECM (41). Similar inhibition of angiogenesis was evident in tumors produced by SU-DHL-6 cells and treated with heparanase-neutralizing polyclonal Ab 1453 (Fig. 2), suggesting that simultaneous targeting of multiple epitopes of heparanase by a polyclonal Ab is more effective in restraining tumor growth (Fig. 2B) that cannot be explained solely by reduced tumor angiogenesis (Fig. 2D). The improved capacity of mAbs 9E8 and H1023 to restrain tumor growth once applied together versus each mAb alone (Fig. 4) supports this notion and encourages the identification and targeting of additional functional domains of heparanase by means of neutralizing mAbs or small molecule inhibitors. Importantly, evidence accumulating in recent years shows that targeting the tumor microenvironment (i.e., VEGF) unexpectedly results in accelerated metastasis and more aggressive disease (42). In contrast, heparanase targeting uniquely inhibits both tumor growth and metastasis, thus possibly offering new opportunities and a safer mode to obstruct the tumor microenvironment.
Taken together, mAbs 9E8 and H1023 are expected to exert high specificity, enabling solely the targeting of heparanase enzymatic activity, and hence revealing its involvement and therapeutic significance in tumor progression, inflammation (43), type 1 diabetes (44), and diabetic nephropathy (45), as a single agent or in combination with approved therapies.
Materials and Methods
Generation of anti-heparanase mAbs and evaluation of heparanase activity were performed essentially as described (6, 30, 46). Standard cellular and molecular biology techniques of cellular transfections, immunoblotting, and immunostaining were performed as described (6, 22–24).
Luciferase-labeled Raji lymphoma (1 × 106) cells were injected into the tail vein of NOD/SCID mice (n = 5). Mice were treated with PG545 (20 mg/kg, once weekly), mAb 9E8 (600 µg/mouse), and/or mAb H1023 (500 µg/mouse) every other day starting 1 d after cell inoculation and tumor growth was examined by IVIS imaging. Models of s.c. tumor xenografts and spontaneous metastasis are described in detailed in SI Materials and Methods.
SI Materials and Methods
Generation of mAbs.
BALB/c mice were immunized with the KKDC peptide that makes up the substrate (HS)-binding domain (Lys158-Asn171) of heparanase, coupled to KLH, and resulting hybridomas were screened for their ability to bind BSA-KKDC or heparanase by ELISA, essentially as described (46, 47). Positive hybridomas were selected, cloned, and examined for heparanase neutralization, using dishes coated with sulfate-labeled, naturally deposited ECM substrate. Hybridoma subclass was determined by isotyping kit according to the manufacturer’s (Serotec) instructions. mAb 9E8 was characterized as IgM and was purified by affinity chromatography on mannan binding protein, according to the manufacturer’s (Pierce Biotechnology) instructions. Anti-human heparanase mAb H1023 (IgG) was generated in BALB/c mice immunized with human heparanase at Eli Lilly and Company laboratories essentially as described earlier, and tested for binding to heparanase by ELISA. Positives supernatants were tested for blocking heparanase enzymatic activity using a high-throughput heparanase activity assay (Cisbio Bioassays HTRF). Hybridoma H1023 and its subclone 23-G8 were found to neutralize heparanase enzymatic activity (Fig. S4).
Cells and Cell Culture.
Daudi, Ramos, Raji, and SU-DHL-6 human B lymphoma cells were purchased from the American Type Culture Collection. OCI-LY-19 cell line was purchased from DSMZ. ESb and El-4 mouse lymphoma cells have been described previously (48). Cells were grown in RPMI medium (Biological Industries) supplemented with 10% (vol/vol) FCS and antibiotics. Raji cells were infected with luciferase gene construct, and a cell clone exhibiting high luciferase activity was selected for in vivo experiments. B16-BL6 mouse melanoma, rat C6 glioma, and U87 human glioma cells have been described previously.
Luciferase-labeled CAG myeloma cells were kindly provided by Gera Neufeld, Rappaport Faculty of Medicine. LM2-4 human breast carcinoma cells were kindly provided by Robert Kerbel, University of Toronto, Toronto (49).
Abs and Reagents.
Anti-heparanase polyclonal Ab 1453 has been described previously (30). Rat anti-mouse CD31 was from Dianova, Rat anti-mouse CD45 was purchased from BioLegends, anti-human HLA and anti-Ki67 mAbs were purchased from Abcam, and anticleaved caspase 3 was purchased from Cell Signaling. The heparanase and angiogenesis inhibitor PG545 was kindly provided by Progen Pharmaceuticals. The heparanase inhibitor SST0001 (Roneparstat; nonanticoagulant glycol-split heparin) was kindly provided by Sigma Tau Research Switzerland.
Cell Lysates, Heparanase Activity, and Protein Blotting.
Preparation of cell lysates, protein blotting, and measurement of heparanase enzymatic activity were carried out as described (6, 22–24). For inhibition studies, highly purified constitutively active GS3 heparanase (200 ng) (24) was preincubated with the indicated Ab (2 μg/mL) for 30 min on ice under neutral pH conditions (pH 7.2) before being added to 35S-labeled ECM, used as a naturally produced substrate for heparanase. Likewise, cell lysates or tumor extracts were incubated (12 h; 37 °C; pH 6.0), with the labeled ECM and sulfate labeled degradation fragments released into the incubation medium analyzed by gel filtration on a Sepharose CL-6B column, as described (6, 22–24). Degradation fragments of HS side chains are eluted from Sepharose 6B at 0.5 < Kav < 0.8 (peak II, fractions 17–30). Nearly intact sulfate-labeled HSPGs are eluted just after the void volume (Kav < 0.2, peak I, fractions 1–10). These high-molecular-weight products are released by proteases that cleave the HSPG core protein. Each experiment was performed at least three times, and the variation in elution positions (Kav values) did not exceed ± 15%.
Cell Invasion.
Matrigel invasion assay was performed using modified Boyden chambers with polycarbonate Nucleopore membrane (Corning), essentially as described (6). Briefly, filters (6.5 mm in diameter, 8 µm pore-size) were coated with Matrigel (30 µL). Cells (2 × 105) in 100 µL serum-free medium were seeded in triplicate on the upper part of each chamber, and the lower compartment was filled with 600 µL medium supplemented with 10% FCS. After incubation for 5 h at 37 °C in a 5% CO2 incubator, noninvading cells on the upper surface of the filter were wiped with a cotton swab, and migrated cells on the lower surface of the filter were fixed, stained with 0.5% crystal violet (Sigma), and counted by examination of at least seven microscopic fields.
Experimental Metastasis.
B16 BL6 mouse melanoma (2 × 105) or LM2-4 human breast cancer cells (2 × 106) were injected into the tail vein of C57/BL mice or SCID/Beige mice (respectively), together with the indicated compound, essentially as described. Lungs were harvested 18 d thereafter, and metastasis occurrence was determined after fixation by gross inspection and histology.
Tumorigenicity.
Luciferase-labeled CAG myeloma (5 × 106) or Raji lymphoma (1 × 106) cells were injected into the tail vein of NOD/SCID mice (n = 5). Mice were treated with PG545 (20 mg/kg, once weekly), mAb 9E8 (600 µg/mouse), and/or mAb H1023 (500 µg/mouse) every other day, starting 1 d after cell inoculation, and tumor growth was examined by IVIS imaging. At termination, mice were killed and their backbones were excised, fixed in formalin, and after decalcification with 10% EDTA solution, were embedded in paraffin and subjected to histological and immunohistochemical analyses essentially as described. A dose of 25–30 mg/kg (i.e., 500–600 µg mAb/mouse) is typical in cancer-restraining experiments (50).
Lymphoma cells were also inoculated s.c. at the right flank of 6-wk-old NOD/SCID mice (human cell lines) or C57BL/6 mice (El-4 mouse lymphoma cells) (n = 7). Mice were treated with heparanase inhibitors (PG545; SST0001) or neutralizing Abs, and xenograft size was determined by externally measuring the tumors in two dimensions, using a caliper. At the end of the experiment, mice were killed, and tumor xenografts were removed, weighed, and fixed for histological and immunohistochemical analyses. ESb mouse T-lymphoma cells (5 × 105) were similarly inoculated s.c. into SCID mice, and spontaneous metastasis to the liver (48) was examined after 3 wk of treatment with PG545 or the neutralizing mAbs, as described earlier. All animal experiments were approved by the Animal Care Committee of the Technion, Haifa, Israel.
IVIS Imaging.
Bioluminescent imaging of the luciferase-expressing tumors was performed with a highly sensitive, cooled CCD camera mounted in a light-tight specimen box (IVIS; Xenogen Corp.). Briefly, mice were injected intraperitoneally with d-luciferin substrate at 150 mg/kg, anesthetized, and placed onto a stage inside the light-tight camera box, with continuous exposure to isoflurane (EZAnesthesia). Light emitted from the bioluminescent cells was detected by the IVIS camera system, with images quantified for tumor burden using a log-scale color range set at 5 × 104 to 1 × 107 and measurement of total photon counts per second, using Living Image software (Xenogen).
Statistics.
Data are presented as mean ± SE. Statistical significance was analyzed by one-way ANOVA (Tukey post-test) or two-tailed Student's t test. The value of P < 0.05 is considered significant.
Acknowledgments
We thank Dr. Paul Kussie and Dr. Jacqueline Doody (Eli Lilly and Company) for supporting the development of the H1023 anti-heparanase mAb. We thank Dr. Alessandro Noseda (Sigma-Tau Research Switzerland) for the kind gift of SST0001 and for his continuous help in supporting the heparanase research project. This study was supported by research grants awarded to I.V. by Sigma-Tau Research Switzerland; the Israel Science Foundation (Grant 601/14); the National Cancer Institute, NIH (Grant CA106456); the United States-Israel Binational Science Foundation; the Israel Cancer Research Fund; and the Rappaport Family Institute Fund. I.V. is a research professor at the Israel Cancer Research Fund.
Footnotes
Conflict of interest statement: I.V. received grant support from Sigma-Tau Research Switzerland. SST0001 is a proprietary drug of Sigma-Tau Research Switzerland. E.H. is an employee of Progen Pharmaceuticals. PG545 is a proprietary drug of Progen Pharmaceuticals. Y.Z. and M.N. are employees of Eli Lilly and Company.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519453113/-/DCSupplemental.
References
- 1.Parish CR, Freeman C, Hulett MD. Heparanase: A key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471(3):M99–M108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 2.Vlodavsky I, Friedmann Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest. 2001;108(3):341–347. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38(12):2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: Structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13(20):2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- 5.Vreys V, David G. Mammalian heparanase: What is the message? J Cell Mol Med. 2007;11(3):427–452. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvatz G, Barash U, Nativ O, Ilan N, Vlodavsky I. Post-transcriptional regulation of heparanase gene expression by a 3′ AU-rich element. FASEB J. 2010;24(12):4969–4976. doi: 10.1096/fj.10-156372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen I, et al. Heparanase promotes growth, angiogenesis and survival of primary breast tumors. Int J Cancer. 2006;118(7):1609–1617. doi: 10.1002/ijc.21552. [DOI] [PubMed] [Google Scholar]
- 8.Lerner I, et al. Function of heparanase in prostate tumorigenesis: Potential for therapy. Clin Cancer Res. 2008;14(3):668–676. doi: 10.1158/1078-0432.CCR-07-1866. [DOI] [PubMed] [Google Scholar]
- 9.Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol Haemost Thromb. 2008;36(3-4):195–203. doi: 10.1159/000175157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dredge K, et al. The PG500 series: Novel heparan sulfate mimetics as potent angiogenesis and heparanase inhibitors for cancer therapy. Invest New Drugs. 2010;28(3):276–283. doi: 10.1007/s10637-009-9245-5. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie EA. Heparanase: A target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151(1):1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao HQ, Liu H, Navarro E, Kussie P, Zhu Z. Development of heparanase inhibitors for anti-cancer therapy. Curr Med Chem. 2006;13(18):2101–2111. doi: 10.2174/092986706777935230. [DOI] [PubMed] [Google Scholar]
- 13.Hammond E, Khurana A, Shridhar V, Dredge K. The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics. Front Oncol. 2014;4:195. doi: 10.3389/fonc.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly T, et al. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003;63(24):8749–8756. [PubMed] [Google Scholar]
- 15.Mahtouk K, et al. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109(11):4914–4923. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchie JP, et al. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin Cancer Res. 2011;17(6):1382–1393. doi: 10.1158/1078-0432.CCR-10-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14(8):517–534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 18.Ansell SM. Non-Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin Proc. 2015;90(8):1152–1163. doi: 10.1016/j.mayocp.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Dredge K, et al. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br J Cancer. 2011;104(4):635–642. doi: 10.1038/bjc.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shteper PJ, et al. Role of promoter methylation in regulation of the mammalian heparanase gene. Oncogene. 2003;22(49):7737–7749. doi: 10.1038/sj.onc.1207056. [DOI] [PubMed] [Google Scholar]
- 21.Ferro V, et al. Discovery of PG545: A highly potent and simultaneous inhibitor of angiogenesis, tumor growth, and metastasis. J Med Chem. 2012;55(8):3804–3813. doi: 10.1021/jm201708h. [DOI] [PubMed] [Google Scholar]
- 22.Levy-Adam F, et al. Identification and characterization of heparin/heparan sulfate binding domains of the endoglycosidase heparanase. J Biol Chem. 2005;280(21):20457–20466. doi: 10.1074/jbc.M414546200. [DOI] [PubMed] [Google Scholar]
- 23.Gingis-Velitski S, et al. Heparanase uptake is mediated by cell membrane heparan sulfate proteoglycans. J Biol Chem. 2004;279(42):44084–44092. doi: 10.1074/jbc.M402131200. [DOI] [PubMed] [Google Scholar]
- 24.Fux L, et al. Structure-function approach identifies a COOH-terminal domain that mediates heparanase signaling. Cancer Res. 2009;69(5):1758–1767. doi: 10.1158/0008-5472.CAN-08-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanderson RD, Yang Y. Syndecan-1: A dynamic regulator of the myeloma microenvironment. Clin Exp Metastasis. 2008;25(2):149–159. doi: 10.1007/s10585-007-9125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar-Ner M, et al. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987;70(2):551–557. [PubMed] [Google Scholar]
- 27.He X, et al. Hypoxia increases heparanase-dependent tumor cell invasion, which can be inhibited by antiheparanase antibodies. Cancer Res. 2004;64(11):3928–3933. doi: 10.1158/0008-5472.CAN-03-2718. [DOI] [PubMed] [Google Scholar]
- 28.Levidiotis V, Freeman C, Tikellis C, Cooper ME, Power DA. Heparanase inhibition reduces proteinuria in a model of accelerated anti-glomerular basement membrane antibody disease. Nephrology (Carlton) 2005;10(2):167–173. doi: 10.1111/j.1440-1797.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 29.Myler HA, Lipke EA, Rice EE, West JL. Novel heparanase-inhibiting antibody reduces neointima formation. J Biochem. 2006;139(3):339–345. doi: 10.1093/jb/mvj061. [DOI] [PubMed] [Google Scholar]
- 30.Zetser A, et al. Processing and activation of latent heparanase occurs in lysosomes. J Cell Sci. 2004;117(Pt 11):2249–2258. doi: 10.1242/jcs.01068. [DOI] [PubMed] [Google Scholar]
- 31.Spangler JB, et al. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci USA. 2010;107(30):13252–13257. doi: 10.1073/pnas.0913476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blonska M, Agarwal NK, Vega F. Shaping of the tumor microenvironment: Stromal cells and vessels. Semin Cancer Biol. 2015;34:3–13. doi: 10.1016/j.semcancer.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shain KH, Dalton WS, Tao J. The tumor microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene. 2015;34(36):4673–4682. doi: 10.1038/onc.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matzner Y, et al. Degradation of heparan sulfate in the subendothelial extracellular matrix by a readily released heparanase from human neutrophils. Possible role in invasion through basement membranes. J Clin Invest. 1985;76(4):1306–1313. doi: 10.1172/JCI112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlodavsky I, et al. Expression of heparanase by platelets and circulating cells of the immune system: Possible involvement in diapedesis and extravasation. Invasion Metastasis. 1992;12(2):112–127. [PubMed] [Google Scholar]
- 36.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Mehta-Shah N, Younes A. Novel targeted therapies in diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):126–137. doi: 10.1053/j.seminhematol.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Ferro V. Heparan sulfate inhibitors and their therapeutic implications in inflammatory illnesses. Expert Opin Ther Targets. 2013;17(8):965–975. doi: 10.1517/14728222.2013.811491. [DOI] [PubMed] [Google Scholar]
- 39.Lindahl U, Kjellén L. Pathophysiology of heparan sulphate: Many diseases, few drugs. J Intern Med. 2013;273(6):555–571. doi: 10.1111/joim.12061. [DOI] [PubMed] [Google Scholar]
- 40.Xu D, Esko JD. Demystifying heparan sulfate-protein interactions. Annu Rev Biochem. 2014;83:129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elkin M, et al. Heparanase as mediator of angiogenesis: Mode of action. FASEB J. 2001;15(9):1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- 42.Ebos JM. Prodding the Beast: Assessing the Impact of Treatment-Induced Metastasis. Cancer Res. 2015;75(17):3427–3435. doi: 10.1158/0008-5472.CAN-15-0308. [DOI] [PubMed] [Google Scholar]
- 43.Lerner I, et al. Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest. 2011;121(5):1709–1721. doi: 10.1172/JCI43792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. J Clin Invest. 2012;122(1):132–141. doi: 10.1172/JCI46177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil N, et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61(1):208–216. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gingis-Velitski S, Ishai-Michaeli R, Vlodavsky I, Ilan N. Anti-heparanase monoclonal antibody enhances heparanase enzymatic activity and facilitates wound healing. FASEB J. 2007;21(14):3986–3993. doi: 10.1096/fj.07-8866com. [DOI] [PubMed] [Google Scholar]
- 47.Shafat I, et al. An ELISA method for the detection and quantification of human heparanase. Biochem Biophys Res Commun. 2006;341(4):958–963. doi: 10.1016/j.bbrc.2006.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vlodavsky I, Fuks Z, Bar-Ner M, Ariav Y, Schirrmacher V. Lymphoma cell-mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: Relationship to tumor cell metastasis. Cancer Res. 1983;43(6):2704–2711. [PubMed] [Google Scholar]
- 49.Munoz R, et al. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66(7):3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 50.Van Looy T, et al. Therapeutic efficacy assessment of CK6, a monoclonal KIT antibody, in a panel of gastrointestinal stromal tumor xenograft models. Transl Oncol. 2015;8(2):112–118. doi: 10.1016/j.tranon.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]