Significance
Here we show, for the first time to our knowledge, that septum transversum/proepicardium (ST/PE)-derived endothelial cells are required for proper coronary blood vessel morphogenesis. We used different mouse transgenic lines to show that the ST/PE contributes to coronary endothelium and that the endocardium is not the only developmental origin of this tissue. Our results indicate that ST/PE-derived endothelial cells preferentially incorporate into prospective coronary arteries and capillaries but not veins. Deletion of the epicardial and coronary developmental regulator Wilms’ tumor suppressor gene from both the ST/PE and embryonic endothelial cells reveals that ST/PE endothelial cells are required for the establishment of coronary arterio–venous connections through the ventricular wall and thus are necessary for the completion of coronary vascularization.
Keywords: coronary endothelium, septum transversum, proepicardium, Gata4, Wt1
Abstract
Recent reports suggest that mammalian embryonic coronary endothelium (CoE) originates from the sinus venosus and ventricular endocardium. However, the contribution of extracardiac cells to CoE is thought to be minor and nonsignificant for coronary formation. Using classic (Wt1Cre) and previously undescribed (G2-Gata4Cre) transgenic mouse models for the study of coronary vascular development, we show that extracardiac septum transversum/proepicardium (ST/PE)-derived endothelial cells are required for the formation of ventricular coronary arterio–venous vascular connections. Our results indicate that at least 20% of embryonic coronary arterial and capillary endothelial cells derive from the ST/PE compartment. Moreover, we show that conditional deletion of the ST/PE lineage-specific Wilms’ tumor suppressor gene (Wt1) in the ST/PE ofG2-Gata4Cre mice and in the endothelium of Tie2Cre mice disrupts embryonic coronary transmural patterning, leading to embryonic death. Taken together, our results demonstrate that ST/PE-derived endothelial cells contribute significantly to and are required for proper coronary vascular morphogenesis.
The coronary vascular system, whose function is necessary to sustain late embryonic and postnatal cardiac function, is formed by a complex network of blood vessels, including arteries, arterioles, capillaries, venules, and veins (1). Recent reports indicate that various sources of endothelial cells contribute to the mammalian embryonic coronary system (2–4). However, the specific fate and function of these different endothelial cell pools during coronary vascular morphogenesis is the subject of intense controversy (5).
Two endocardial populations have been reported to participate in the building of the embryonic coronary vascular system. The first derives from the sinus venosus endocardium, which sprouts to give rise to the nascent Apelin+ coronary vasculature (2). A careful analysis of this study suggests that the sinus venosus endocardium, which is able to vascularize subepicardial and myocardial heart layers, mainly provides a cellular scaffold for the development of coronary veins (CoV). Accordingly, a second source of coronary endothelium (CoE) has been identified in the ventricular endocardium (Nfatc1+ lineage), which contributes massively to coronary arterial (CoA) endothelium (3, 6).
A third disputed source of CoE is the proepicardium (PE), a structure that comprises epicardial progenitor cells. The PE protrudes from the septum transversum (ST), a folding of the lateral mesoderm that initiates the separation of thoracic and abdominal cavities in mammals (7). Although in vivo cell tracing and in vitro culture of avian PE cells clearly shows that PE cells can differentiate into CoE (8, 9), data from studies in mammals claimed the contribution of PE to CoE is minor (10–12). The so-called “epicardial” Cre constructs used in these studies are based on the expression of genes such as Gata5, Tbx18, or Wilms’ tumor suppressor (Wt1) (Table S1), all of which are expressed by both PE and ST cells, thus confirming that these two tissues form an ontogenetic and histomorphological continuum. Such continuum makes it difficult to distinguish between ST and PE cells based on their gene-expression profile. Interestingly, recent results indicate that the murine PE is constituted of different cell lineages, including a significant number of endothelial progenitors (13). Because the final fate of these cells, the extent of their contribution, and their specific role during coronary blood vessel morphogenesis remain unknown, we aimed at studying these complex aspects of coronary development.
Table S1.
Reported epicardial lineage-derived CoE cells
| Transgenic mouse model | CoE cells from the tagged cell lineages | Reference |
| Gata5Cre | None | Merki et al., 2005 (10) |
| Tbx18Cre | Low, not quantified | Cai et al., 2008 (11) |
| Wt1GFPCre | Low, not quantified | Zhou et al., 2008 (12) |
| Sema3DCre | 6.9% of Sema3DCre+ cells | Katz et al., 2012 (13) |
| ScxCre | 49% of ScxCre+ cells | Katz et al., 2012 (13) |
| Wt1IRESGFPCre | 49.3% of total ventricular CD31+ cells | This work |
| G2-Gata4Cre | 25.1% of total ventricular CD31+ cells | This work |
Quantification of epicardial lineage-derived endothelial cells reported by different studies is shown.
Results
G2-Gata4 Enhancer-Driven Reporter Expression Labels Septum Transversum/Proepicardium Cells.
At embryonic day (E)9.5, G2-Gata4LacZ mice display reporter activity in the septum transversum/proepicardium (ST/PE) but not in heart tissues (myocardium, endocardium) (Fig. 1A). At E11.5, G2-Gata4LacZ expression remains confined mainly to the mesenchyme surrounding the liver (14); weak X-gal staining also is observed in the myocardium of sinus venosus horns (Fig. 1B). Cre recombinase protein is found only in the sinus venosus myocardium and aortic walls (Fig. S1 A and C) and is not present in any other cardiac region (Fig. S1 B–D).
Fig. 1.
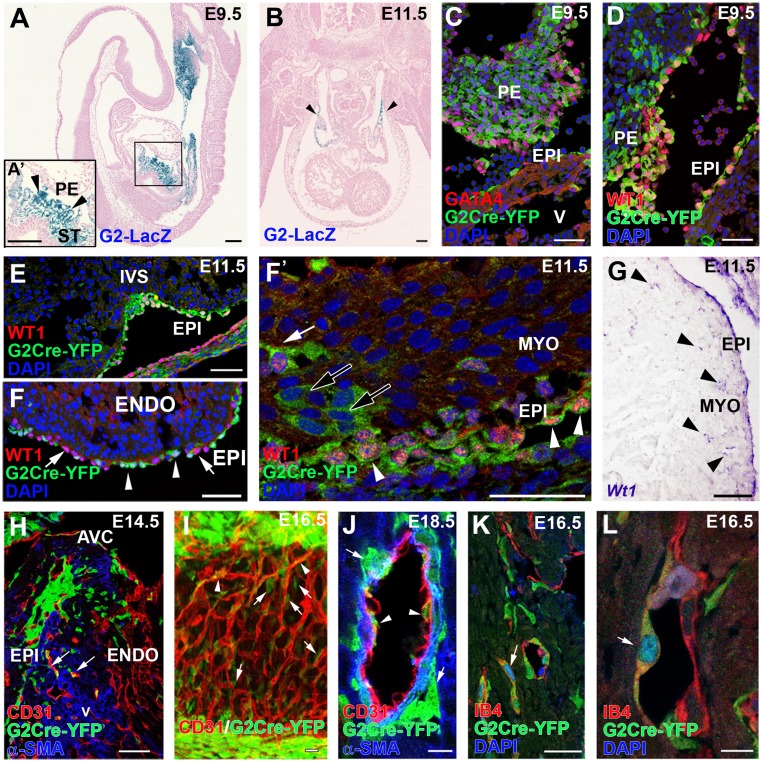
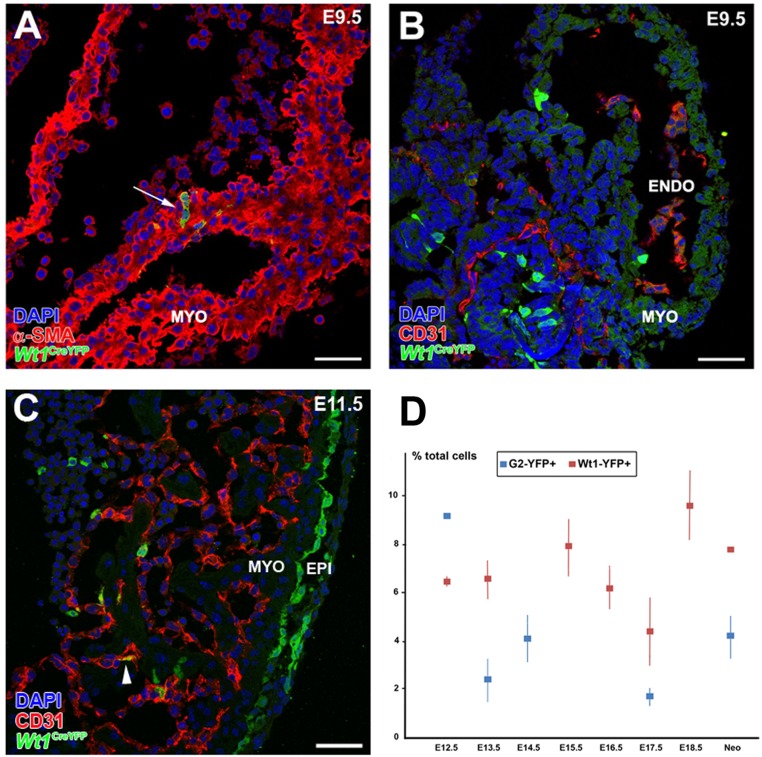
ST/PE G2-Gata4 cells throughout cardiac development. (A and B) G2-Gata4LacZ mice show reporter activity in the septum transversum (including the PE) at E9.5 (A) and inflow myocardium at E11.5 (B, arrowheads). (C and D) Immunohistochemistry of G2-Gata4CreYFP+ samples illustrates the expression of GATA4 (C) and WT1 (D) proteins in G2-Gata4CreYFP+ cells at E9.5 PE. (E–F′) G2-Gata4CreYFP mice show an increasing number of G2-Gata4CreYFP+ cells from the developing epicardium in subepicardial and intramyocardial areas between stages E10.5 and E14.5. The epicardium comprises WT1+/G2-Gata4+ (arrowheads) and WT1+/G2-Gata4− cells (F, arrows). A few EPDCs retain WT1 expression transiently (F and F′, white arrow), but other EPDCs do not (F′, black arrows). (G) Wt1 gene expression is conspicuous in the epicardium but is restricted to a few EPDCs (arrowheads). (H) Progressive expansion of EPDCs through the myocardial walls at E14.5–18.5 parallels G2-Gata4CreYFP+ incorporation in developing coronary blood vessels (arrows). (I and J) 3D reconstructions (I) and tissue section analysis (J) of the developing coronary vasculature allow perivascular cells (arrows in I and J) to be distinguished from G2-Gata4CreYFP+ CoE cells (arrowheads in I and J). (K and L) Identification of active Notch1 signaling by Notch1 intracellular domain (N1ICD) nuclear localization confirms the arterial nature of these vessels (arrows). A, atrium; AVC, atrio–ventricular canal; ENDO, endocardium; EPI, epicardium; IVS, interventricular septum; MYO, myocardium; PE, proepicardium; ST, septum transversum; V, ventricle. (Scale bars: 100 µm in A and B; 50 µm in C–H; 40 µm in I; 10 µm in J; 25 µm in K; 5 µm in L.)
Fig. S1.
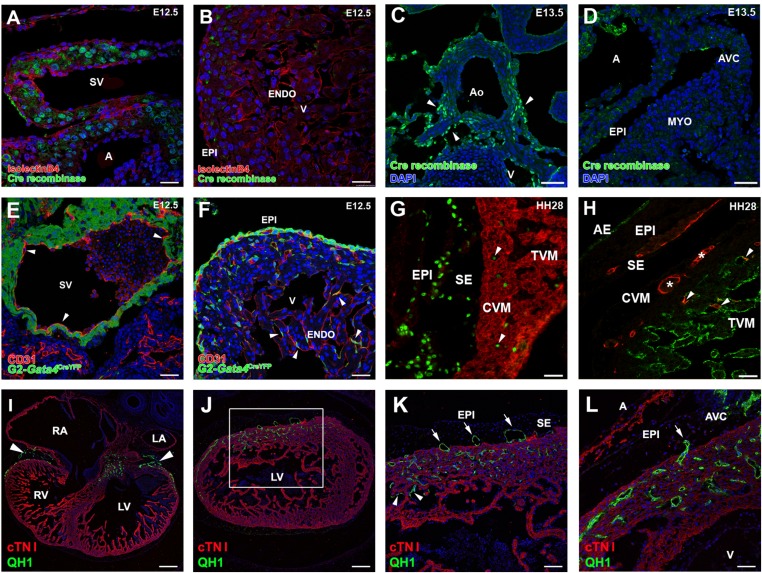
Origin and vascularizing properties of ST/PE-derived endothelial cells. (A–D) Cre recombinase expression in G2CreYFP embryos is evident in the inflow myocardium at E12.5 (A) and in the aortic walls at E13.5 (C, arrowheads) but not in the epicardium at E12.5 (B) or epicardium or myocardial layers at E13.5 (D). (E and F) In G2CreYFP embryos, the sinus venosus endocardium is YFP− (arrowheads in E). Some G2-Gata4CreYFP+/CD31+ cells also can be found incorporated in the ventricular endocardium (arrowheads in F). (G) Incorporation of PE-derived endothelial cells in the ventricular endocardium also occurs in quail-to-chick PE chimeric transplantations. PE derivatives, which express the quail pan-nuclear marker QCPN (green), invade the myosin-expressing ventricular walls (MF20+, red); some of these cells are incorporate in the ventricular endocardium (arrowheads). (H) The endothelial nature of these quail PE-derived cells is confirmed further by their expression of the QH1 endothelial marker (red). After FITC-conjugated Lens culinaris agglutinin counterstaining of the endocardium (green), PE-derived CoE cells (asterisks) can be distinguished easily from those that fused with the endocardium (arrowheads). (I–L) Transplantation of posterior pericardiac mesenchyme without PE tissue (homologous to the mammalian ST) extensively vascularizes the embryonic myocardium (green QH1+ cells in I and J; arrowheads in I mark the accumulation of these cells at the atrioventricular canal). The area boxed in J is magnified in K to show endothelial invasion of the myocardial layers (red, cTNI+) from the subepicardial space (arrows). The same phenomenon is also evident at the atrioventricular canal (arrow in L). Some ST-derived endothelial cells also are incorporated in the endocardium (arrowheads in K). A, atrium; AE, atrial endocardium; AVC, atrioventricular canal; CVM, compact ventricular endocardium; ENDO, endocardium; EPI, epicardium; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; SE, subepicardium; SV, sinus venosus; TVM, trabeculated ventricular myocardium; V, ventricle. (Scale bars: 25 µm in A and B; 50 µm in C–H and L; 150 µm in I and J; 75 µm in K.)
G2-Gata4 Lineage Tracing Identifies the ST/PE Contribution to CoE.
To trace G2-Gata4 ST/PE cells throughout embryonic development, we crossed the G2-Gata4Cre line with Rosa26-YFP reporter mice. The resulting offspring (hereafter, G2CreYFP+) display permanent YFP expression in the ST/PE (Fig. 1 C and D), epicardium, and epicardial-derived cells (EPDCs) (Fig. 1 E and F′). E9.5 G2CreYFP+ ST/PE tissue and some epicardial cells express GATA4 protein (Fig. 1C). WT1 (Wilms’ tumor suppressor-1) protein is ubiquitous in the epicardium (Fig. 1D). Some ST/PE (Fig. 1E) and epicardial (Fig. 1 E, F, and F′) WT1+ cells are G2CreYFP−, suggesting that Wt1 expression may occur in epicardial cells that do not belong to the G2-Gata4 population. WT1 protein is reduced progressively from E11.5 through E12.5 as G2CreYFP+ cells migrate from the subepicardium into the myocardial layers (Fig. 1F′). At these stages, Wt1 gene expression is confined to the epicardium and early EPDCs (Fig. 1G). From E12.5 onwards, numerous ST/PE-derived G2CreYFP+ intramyocardial cells incorporate into developing coronary blood vessels. CD31+ (Fig. 1 H–J) and isolectin B4+ (IB4) endothelial cells intermingled with G2CreYFP- cells in a salt-and-pepper pattern (Fig. 1 I–L and Movie S1), whereas G2CreYFP+/α-smooth muscle actin (α-SMA)+ coronary smooth muscle cells (CoSM) formed continuous, large domains of the medial blood vessel wall. Many G2CreYFP+/IB4+ CoE cells accumulated NOTCH1 intracellular domain (N1ICD) in their nucleus (Fig. 1 K and L).
No G2CreYFP+ cells are found in the sinus venosus endocardium (Fig. S1E), but some G2CreYFP+/CD31+ cells are present in the ventricular endocardium, suggesting an early contribution of ST/PE cells to this tissue (Fig. S1F). Chimeric transplantation of quail PE (Fig. S1 G and H) or ST (Fig. S1 I–L) tissue into chick host hearts confirms that ST/PE endothelial cell incorporation in the developing coronary vessels and CoE is a normal event. To verify the vascular potential of the ST/PE, Scl/Tal1 and Vegfr2 expression was confirmed in ST/PE cells (Fig. S2A), and the vasculogenic potential of ST/PE was tested in vivo and in vitro (Fig. S2 B–F).
Fig. S2.
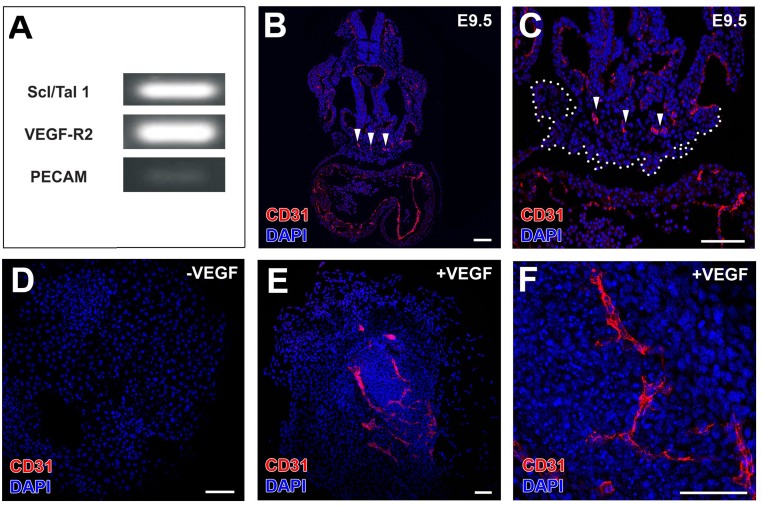
The E9.5 PE holds an intrinsic vascular potential. (A) Semiquantitative RT-PCRs confirm that E9.5 PE cells express vascular progenitor (Scl/Tal1/Vegfr-2) and endothelial (CD31) markers. (B and C) Low CD31 expression in PE is in accordance with the accumulation of differentiated CD31+ CoE cells in the dorsal aspect of the PE (arrowheads in B and C; the whole PE is marked by a dotted line in C). (D–F) In vitro-cultured E9.5 PE differentiate into CD31+ CoE cells only in the presence of VEGF (compare E and F with D). (Scale bars: 100 µm.)
Wt1-Driven GFP Expression Is a Bona Fide Marker for Early Epicardium, EPDCs, and Coronary Blood Vessels.
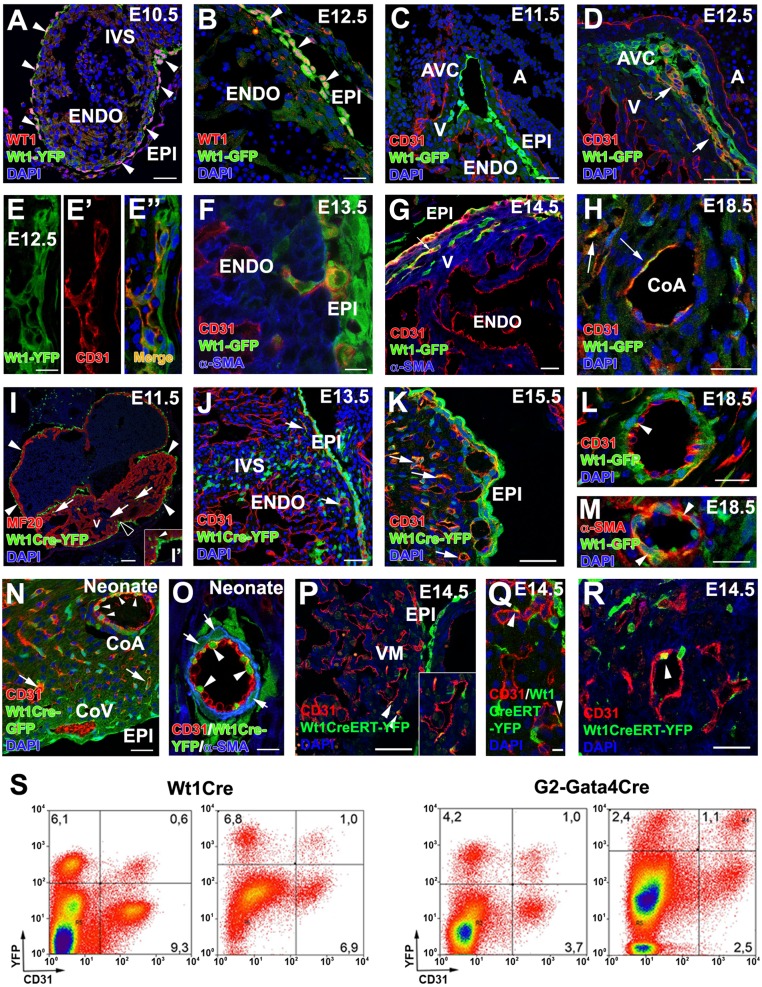
Because Wt1 is also known to be a marker of ST/PE cells (15, 16), we first studied its expression pattern in Wt1GFP knockin mouse embryos. At E10.5, Wt1 protein and Wt1-driven GFP expression overlap in space and time and are restricted to the primitive epicardium (Fig. 2A), confirming that the Wt1GFP knockin mouse faithfully recapitulates native Wt1 gene activity. At E11.5–E12.5, Wt1 expression is detected in epicardial cells and EPDCs, which accumulate at the ventricular, atrio–ventricular, and interventricular subepicardium (Fig. 2 A–C). No CD31 expression is detected in Wt1GFP+ epicardium or EPDCs before E11.5 (Fig. 2C). At E12.5, a primary coronary vascular plexus has formed in the ventricles, and GFP expression is identified in a significant proportion of subepicardial and intramyocardial CD31+ cells of the developing coronary vasculature (Fig. 2 D and E–E′′). Some Wt1GFP+ cells display the typical spindle-shaped morphology of migratory mesenchymal cells, with their major axis oriented orthogonally with respect to the epicardial surface (Fig. 2F). Between E13.5 and 14.5, a few Wt1GFP+/CD31+ cells can be identified in the forming intramyocardial blood vessels (Fig. 2G) with their number decreasing at perinatal stages (E18.5) (Fig. 2H).
Fig. 2.
Wt1-expressing cells and their progeny contribute to CoE. (A and B) WT1 protein is ubiquitously expressed in epicardial cells and EPDCs at E10.5–E12.5, extensively overlapping with Wt1 promoter-driven GFP expression (arrowheads). (C–G) Reporter expression in Wt1GFP embryos can be observed in the CD31+ subepicardial (E–E”) and intramyocardial (F and G) coronary vasculature at E12.5–E14.5 (arrows in D and G), but not at earlier developmental stages (C). (H) A few Wt1GFP+/CD31+ cells are still found at perinatal stages (arrows). (I, I′, and J) Early Wt1CreYFP+ cells form the epicardium (arrowheads in I; the area marked with a black arrowhead is magnified in I′) and the first EPDCs (arrows in J). At E11.5 many Wt1CreYFP+ epicardial cells show morphological EMT features (arrowhead in I′). (J and K) The lineage reporter colocalizes with the vascular marker CD31 in the subepicardial and intramyocardial coronary plexus between E13.5 (J) and E15.5 (K) (arrows). (L–O) Perinatal (L and M) and neonatal (N and O) hearts show Wt1CreYFP+ cells incorporated in the CoE (arrowheads in L and O) and CoSM layers of large CoA (arrowheads in M and arrows in O) as well as in capillaries (arrow in N). (P–R) Wt1CreERT2;Rosa26-YFP embryos induced with tamoxifen at E9.5 show YFP+ cell incorporation in coronary vessels at E.14.5 (arrowheads). (S) Representative cytograms of dissociated ventricles from midgestation embryos and neonates. Numbers indicate percentages of total events. Both the Wt1CreYFP+ and G2CreYFP+ populations include CD31+ in cells. A, atrium; AVC, atrio–ventricular canal; CoA, coronary artery; CoV, coronary vein; ENDO, endocardium; EPI, epicardium; IVS, interventricular septum; V, ventricle. (Scale bars: 100 µm in I; 50 µm in A, C, D, J, M, N, and P; 25 µm in B, F–H, K, and L; 25 µm in R; 10 µm in E–E′′ and O; 5 µm in Q.)
Wt1 Lineage Cells Incorporate in Coronary Blood Vessels.
To confirm further the ST/PE contribution to the developing coronary vasculature, we selected a Wt1Cre mouse line that has been used previously to study PE and coronary development (15, 16). Crossing these mice with the Rosa26-YFP reporter line allows the tracing of the Wt1 cell lineage (hereafter, Wt1CreYFP+). At E9.0–9.5, no Wt1CreYFP+ cells are seen in heart, except for a few isolated cardiomyocytes (Wt1CreYFP+/α-SMA+) that form part of the cardiac chamber walls (Fig. S3A). None of these cells express the vascular marker CD31 (Fig. S3B). Between E10.5 and E11.5 almost all epicardial cells and the majority of subepicardial EPDCs are Wt1CreYFP+ (Fig. 2I). A fraction of Wt1CreYFP+ epicardial cells apparently were detaching from the epicardial lining (Fig. 2F). Active epicardial epithelial-to-mesenchymal transition (EMT) was confirmed by time-lapse analysis of Wt1CreYFP+ whole-heart explants (Movie S2). At these stages, a minor number of Wt1CreYFP+/CD31+ cells could be identified in the endocardial layer (Fig. S3C). From E12.5 onwards, subepicardial and intramyocardial Wt1CreYFP+ cells increase (Fig. 2 J and K). Wt1CreYFP+ cells are CD31+ endothelial (Fig. 2L) and αSMA+ smooth muscle (Fig. 2M) cells of the intramyocardial coronary vessels (prospective CoA). Neonatal arterial CoE was found to be a mosaic of Wt1CreYFP+ and Wt1CreYFP- cells. Perivascular cells closer to the CoE expressed α-SMA, but only a fraction of them was Wt1CreYFP- (Fig. 2 N and O). To confirm that Wt1+ ST/PE cells contribute to the formation of coronary vessels, Wt1CreERT2 mice were crossed with the Rosa26-YFP line, and recombination was induced with tamoxifen at PE stages (E9.0). At E14.5 all embryos showed a reduced but evident contribution to the developing CoE (Fig. 2 P–R).
Fig. S3.
Wt1 lineage-derived cardiomyocytes in the E9.0 mouse heart. (A and B) A minority of Wt1CreYFP+ cells can be found in the developing heart before or during PE cell transference to the myocardial surface (E9.5). These Wt1CreYFP+ cells express myocardial markers (α-SMA; arrow in A) but not vascular markers (CD31+) (B). (C) At early epicardial stages (E10.5–11.5) some rare endocardial Wt1CreYFP+/CD31+ cells could be recorded (arrowhead). (D) The total percentage of cardiac Wt1CreYFP+ and G2CreYFP+ cells throughout the course of development is shown. ENDO, endocardium; EPI, epicardium; MYO, myocardium. (Scale bars: 50 µm.)
Wt1 and G2-Gata4 ST/PE Cell Populations Contribute Differentially to the CoE.
To quantify further the contribution of PE cells to the embryonic CoE, we analyzed G2CreYFP and Wt1CreYFP dissociated ventricles by FACS. CD31 was used as pan-endothelial marker. From midgestation to birth, Wt1CreYFP+ cells account for 6.5 of total ventricular cells at E12.5 and 8% in neonates. At E12.5, the percentage of G2CreYFP+ cells is 9.1%; this percentage decreases to 4% of total ventricular cells by the end of gestation (Fig. S3D). Interestingly, the cytometric analysis reveals that the percentage of CD31+/G2CreYFP+ cells is higher than that of CD31+/Wt1CreYFP+ cells: By E17.5, 22.7 ± 4.2% of all CD31+ cells are G2CreYFP+ (n = 4), and this percentage reaches 35.7 ± 5.0% in neonates (n = 3). Only 11.3 ± 1.9% of CD31+ cardiac cells are Wt1CreYFP+ by E18.5 (n = 6) (Fig. 2S). This differential contribution of the two lineages to the CoE was confirmed by quantitative image analysis in E18.5 G2CreYFP and Wt1CreYFP embryos. When only the compact ventricular layer (i.e., excluding the endocardium, epicardium, and trabeculae) is considered, 49.3 ± 13.9% of CD31+ cells are G2CreYFP+, and only 25.1 ± 4.1% are Wt1CreYFP+. The percentages of ST/PE-derived endothelial cells obtained after the image analysis are higher than those from the cytometry analysis because of the exclusion of the endocardial cells, but the 1:2 proportion between Wt1CreYFP+ and G2CreYFP+ cells remains evident. Thus, the more stringent estimate of these data indicates that at least 20% of embryonic CoE are ST/PE derivatives.
Wt1 Expression in G2-Gata4 Cells Is Required for CoA Development.
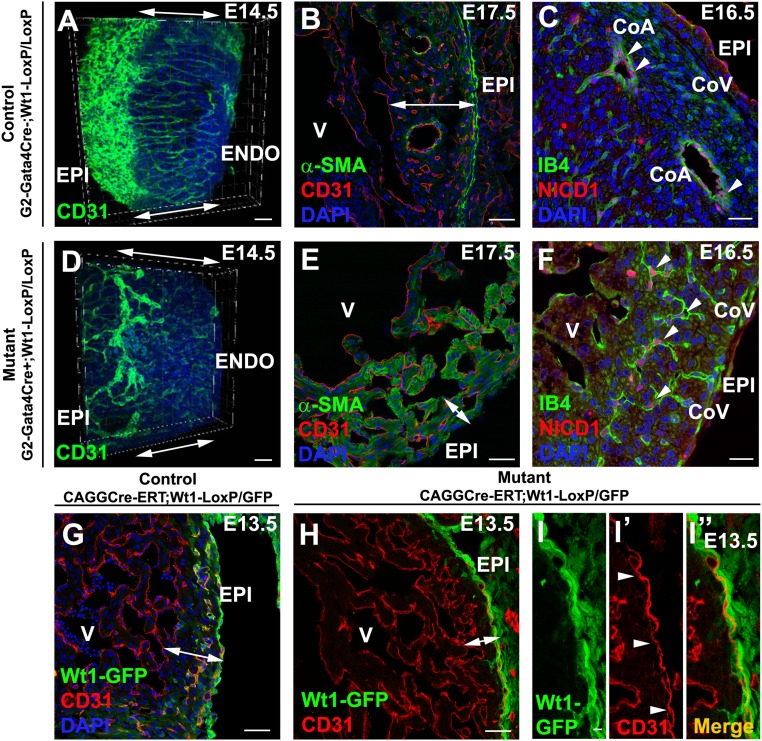
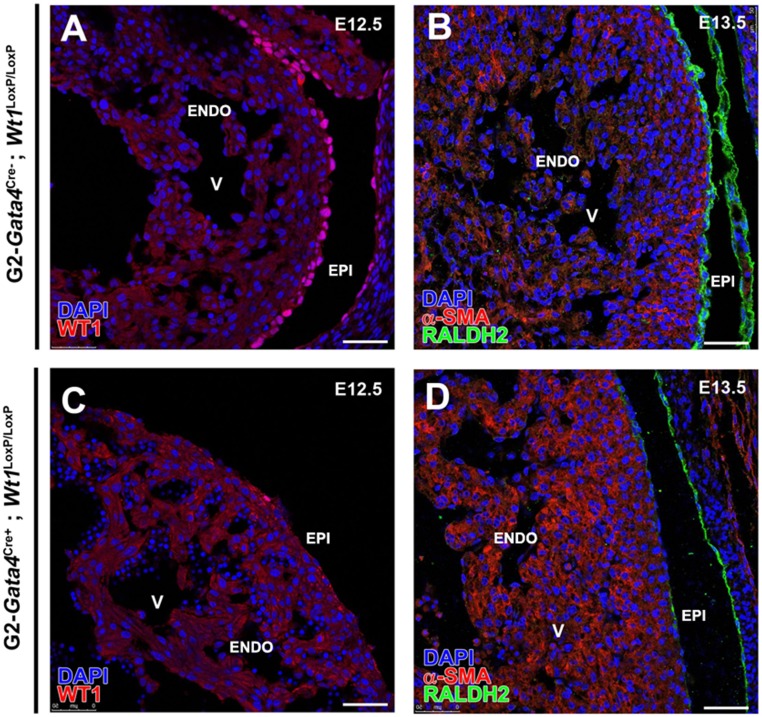
G2-Gata4Cre–driven Wt1 deletion (G2Cre;Wt1LoxP/LoxP) severely impairs the development of coronary vasculature, causing embryonic lethality around E15.5 (Fig. 3 A–F). 3D reconstruction of CoE (CD31+) shows that mutant embryos develop tortuous and sinusoidal irregular vessels that fail to progress transmurally and do not complete ventricular myocardium invasion (Fig. 3 A–D and Movies S3 and S4). Normal CoA are missing in the mutants, which also display a thin ventricular compact myocardium (Fig. 3 B and E). Effective Wt1 deletion in these embryos was confirmed by the absence of WT1 protein and the marked decrease of RALDH2, a known Wt1 target (17) (Fig. S4). However, the epicardium remains intact in the mutants (Fig. 3F). Prompted by the recorded N1ICD nuclear accumulation in G2CreYFP+ CoE, we tested whether this endothelial compartment is affected after G2-mediated Wt1 deletion (G2-Gata4Cre;Wt1LoxP/LoxP). N1ICD+ cells are frequent in capillaries and putative CoA endothelium; however, mutant N1ICD+ CoE does not form normal transmural coronary blood vessels, failing to connect to the deeper vascular elements of the developing coronary vasculature (Fig. 3 C–F).
Fig. 3.
G2-Gata4 and conditional systemic Wt1 deletion disrupt CoA formation. (A–F) G2-Gata4Cre;Wt1LoxP/LoxP embryonic CoE structures are dysmorphic and fail to contact the endocardium (compare double-headed arrows in A and D and compare Movie S3 with Movie S4). The mutants show dramatic reduction of compact ventricular myocardium thickness (compare double-headed arrows in B and E). N1ICD immunohistochemistry identifies dysmorphic intramyocardial vessels as developing CoA (arrowheads in C and F), whereas subepicardial vascular structures are N1ICD− (prospective CoV). (G and H) Tamoxifen-induced (E10.5) systemic Wt1 deletion sharply reduces the number of developing intramyocardial blood vessels. (I–I′′) Subepicardial blood vessels (arrowheads) form normally. CoA, coronary artery; CoV, coronary vein; ENDO, endocardium; EPI, epicardium; V, ventricle. (Scale bars: 50 µm in A and B; 25 µm in C; 50 µm in D, E, G–I; 25 µm in F.)
Fig. S4.
G2Cre-driven Wt1 deletion. Effective deletion of Wt1 in the G2-Gata4 lineage is confirmed by the absence of WT1 protein (compare A and C) and by the decrease in the RALDH2 enzyme, a known direct Wt1 target (compare B and D). ENDO, endocardium; EPI, epicardium; V, ventricle. (Scale bars: 50 µm.)
Wt1 Systemic Deletion Results in a Severe Coronary Phenotype.
Because the E10.5 epicardial Wt1-expressing population encompasses all epicardial G2-Gata4 cells, we decided to cross tamoxifen-inducible CAGGCreERT2 and Wt1LoxP/GFP mouse lines to generate systemic Wt1 mutants at early epicardial stages. CAGG-Cre–mediated Wt1 deletion at E10.5 results in embryonic death by E13.5. These embryos display a sharp impairment of EPDC migration into the compact ventricular myocardium and disruption of intramyocardial coronary vessel morphogenesis, as revealed by the GFP copy carried by mutant mice (Fig. 3 G–I′′). However, the subepicardial coronary vasculature is still formed. This phenotype is more severe than that of G2Cre;Wt1LoxP/LoxP mutants (compare Fig. 3 A–F and Fig. 3 G–I′′).
Conditional Wt1 Endothelial Deletion Reproduces G2-Gata4Cre;Wt1LoxP/LoxP Coronary Defects.
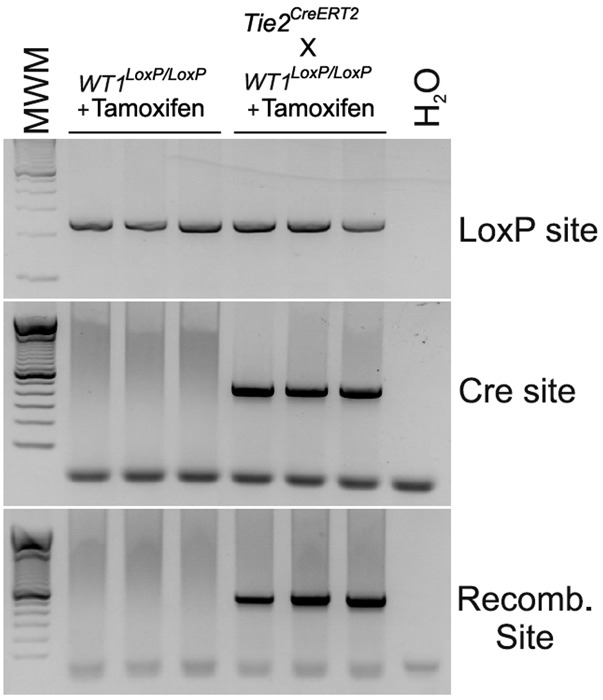
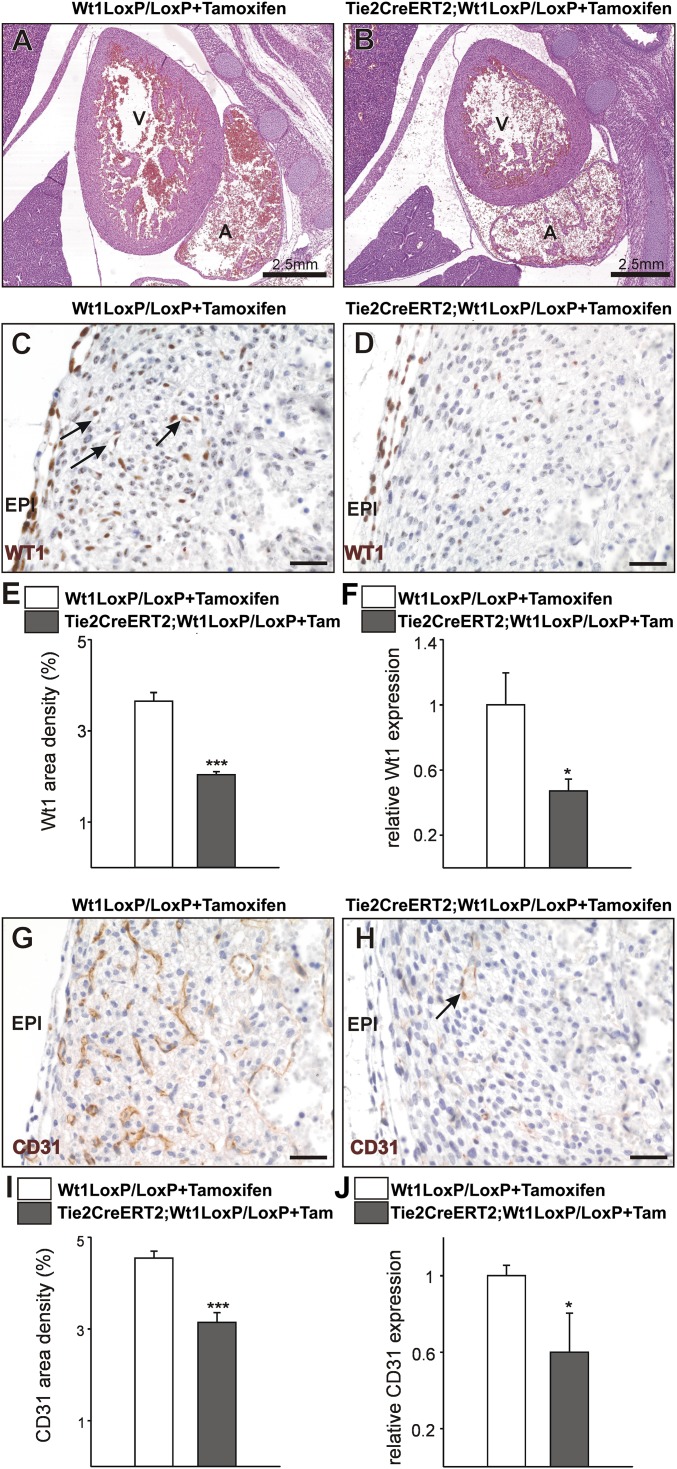
To prove that coronary vascular defects in G2-Gata4Cre;Wt1LoxP/LoxP mutants are not caused by disrupted epicardial signaling, we crossed Tie2CreERT2 (18) and Wt1LoxP/LoxP mice to create endothelial cell-specific Wt1 knockouts (19). Tamoxifen was injected at E10; the specificity of tamoxifen-induced recombination is shown in Fig. S5. Heart morphology is similar in wild-type and mutant animals (E16.5), which do not show compact myocardial thinning (Fig. 4 A and B). WT1 protein is found in some coronary vascular structures and isolated EPDCs of wild-type animals but is much reduced in Tie2CreERT2;Wt1LoxP/LoxP mutants (Fig. 4 C–E). Wt1 gene expression also is down-regulated significantly in mutant embryos (Fig. 4F). CD31 immunostaining of wild-type and mutant samples reveals a marked reduction in the number of coronary vessels, especially transmural vessels connecting endocardial and epicardial elements of the coronary vasculature (Fig. 4 G and H). Reduced coronary vascularization in mutant hearts was confirmed by the analysis of the area occupied by CD31+ cells (Fig. 4I) and by down-regulation of the CD31 gene (Fig. 4J).
Fig. S5.
Tie2-CreERT-driven Wt1 deletion. Western blotting shows the specificity of tamoxifen-induced recombination in Wt1LoxP/LoxP mice versus Tie2CreERT2;Wt1LoxP/LoxP mutant mice.
Fig. 4.
Endothelial Wt1 expression is required for coronary vessel formation. (A and B) H&E-stained sections from control Wt1LoxP/LoxP+Tamoxifen (A) and mutant TieCreERT2;Wt1LoxP/LoxP+Tamoxifen (B) E16.5 embryos. (C–F) The WT1+ cell contribution to coronary vessels (arrows in C) is reduced in the mutants (D and E), and Wt1 gene expression in E16.5 mutant hearts is reduced (F). (G and H) E16.5 mutant embryonic hearts show a decrease in compact ventricular wall coronary CD31+ cells (arrow in H; compare with G). (I and J) Quantification of the area occupied by CD31+ cells (I) and CD31 gene expression (J) in E16.5 hearts. A, atrium; EPI, epicardium; V, ventricle. (Scale bars: 50 µm.) Data are mean ± SEM; *P < 0.05, ***P < 0.001.
Discussion
To understand the morphogenesis of the coronary vascular system fully, we need to uncover the molecular and cellular mechanisms that integrate different endothelial compartments (i.e., the embryonic arterial and venous coronary vasculature) into a single, continuous vascular bed and to identify the origin of coronary endothelial progenitors (1). In this regard, Katz and collaborators (13) have proven that the PE comprises several cell compartments (identified by either Scleraxis or Semaphorin3D gene expression) that contribute differentially to CoE. However, the specific developmental function of ST/PE-derived CoE during coronary morphogenesis has remained unknown.
Our results indicate that the G2-Gata4 enhancer, to our knowledge used here for the first time to study coronary vascular development, is as a bona fide marker of ST/PE cells, being active only at the extracardiac ST/PE location (E9.5) and not in heart tissues proper. G2-Gata4-driven reporter expression first is confined to the epicardium and its mesenchymal derivatives (1, 20–22) and then expands to the CoE.
Wt1CreYFP+ ST/PE cells also contribute to the CoE, although they are less abundant than G2-Gata4CreYFP+ cells, suggesting an early specification of this endothelial lineage. This finding also explains why other studies, relying mostly on genetic tracking of the Wt1 cell lineage, deemed ST/PE contribution to the CoE to be negligible, especially when inducible mouse Cre lines are used (e.g., ERT2). The extent of the induced recombination depends on the stage of development considered, the dose and procedure for delivering tamoxifen, and the genetic construct itself. It is possible that the differences in the relative abundance of Wt1CreYFP+ and G2-Gata4CreYFP+ cells in the developing heart could relate to the reported de novo expression of WT1 protein in cardiac vessels following myocardial infarction (23, 24). Whether this Wt1 activation is linked to the developmental origin of these cells (25) or instead represents an ectopic activation of the gene in response to hypoxia (26) has been discussed extensively (27). However, the lack of activation of the G2 enhancer within heart tissues strongly suggests an ST/PE, extracardiac origin for G2-Gata4CreYFP+ cells, whose endothelial differentiation potential is higher than that shown by Wt1CreYFP+ cells. Our most restrictive estimate of G2-Gata4CreYFP+ and Wt1CreYFP+ cell incorporation in the CoE (a minimum of 20%) is compatible with the reported variable endocardial contribution to coronary vasculature, ranging roughly from 70–40% of embryonic CoE cells (3).
Our cell-tracing analysis shows a preferential incorporation of ST/PE endothelial cells to intramyocardial coronary blood vessels (prospective CoA, arterioles, and capillaries) and suggests a different developmental origin for CoA and CoV. This concept is supported further by the CoA (but not CoV) endothelial phenotype found in mouse embryos with deficient epicardial NOTCH1 signaling (15) and the anomalies found in the prospective intramyocardial CoA endothelium (N1ICD+) of the Wt1 mutants we have described in this study. Such anomalies involve the disruption of the transmural connection between prospective intramyocardial arteries and prospective subepicardial veins. Coronary anomalies recorded in Tie2CreERT2;Wt1LoxP/LoxP mutants, which display a reduction in both WT1+ and CD31+ cells in the ventricular myocardial walls and an anomalous organization of embryonic coronary vessels, confirm that the G2-Gata4–mediated loss of Wt1 in ST/PE cells primarily affects the CoE.
In summary, our work unambiguously shows that the ST/PE significantly contributes cells to the CoE that are necessary for proper coronary vascular morphogenesis (Fig. 5) and suggest that the ST/PE-derived component of CoE is mechanistically related to CoA rather than to CoV, revealing a functional role for these cells in transmural arterio–venous patterning of the coronary vascular tree, most likely via the segregation of NOTCH+ and NOTCH− endothelial domains. This study also supports the idea that CoE is a developmental mosaic formed from different sources of endothelial cells and opens new perspectives on the understanding of congenital coronary anomalies and adult coronary endothelium malfunction.
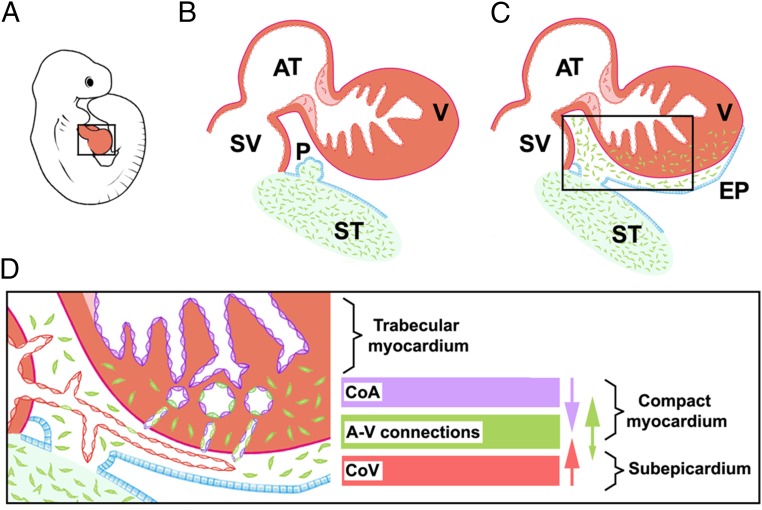
Fig. 5.
A model of the EPDC contribution to the CoE. ST/PE-derived endothelial cells (green) give rise to the epicardium and EPDCs (A–C) and are incorporated in intramyocardial CoA and capillary endothelium (D). Major transmural endothelial cell flows are indicated by arrow color; the arrow size estimates the frequency of the events. A, atrium; A-V, arterio–venous; CoA, coronary arteries; CoV: coronary veins; EPI: epicardium; PE: proepicardium; ST, septum transversum; SV, sinus venosus; V, ventricle.
Methods
Additional methods are described in SI Methods.
Mouse Lines and Embryo Extraction.
The procedures for animal experiments were approved by the Committee on the Ethics of Animal Experiments of the University of Malaga (procedure code 2009-0037), and also followed the guidelines of the French Coordination Committee on Cancer Research and local Home Office regulations. The animals used in our research program were handled in compliance with the institutional and European Union guidelines for animal care and welfare.
All embryos were staged from the time point of vaginal plug, which was designated as E0.5. Embryos were excised and washed in PBS before further processing.
Tissue Sampling for Fluorescent Reporter and Immunohistochemical Analysis.
Embryos were fixed in 2–4% fresh paraformaldehyde solution in PBS for 2–8 h, washed in PBS, cryoprotected in sucrose solutions, embedded in optimum cutting temperature (OCT) embedding compound (Tissue-Tek), and frozen in liquid N2-cooled isopentane. Immunofluorescence staining was performed as described elsewhere (see SI Methods for a detailed protocol). All images were captured on a Leica SP5 confocal microscope.
Quantitative and Semiquantitative RT-PCR of Mouse Tissue.
Total RNA was isolated from embryonic hearts using the TRIzol reagent (Invitrogen). First-strand cDNA synthesis was performed with 0.5 µg of total RNA using oligo(dT) and random primers and superscript III reverse transcriptase (Invitrogen).
SI Methods
Mouse Lines, Embryo Extraction, and Processing.
The procedures for animal experimentation were approved by the Committee on the Ethics of Animal Experiments of the University of Malaga (procedure code 2009-0037), and also followed the guidelines of the French Coordination Committee on Cancer Research and local Home Office regulations.
The mWt1/IRES/GFP-Cre (Wt1Cre) mouse was developed using a BAC recombineering strategy to insert an IRES/EGFP-CRE cassette into the 3′ UTR of a full-length mouse Wt1 gene. The endogenous GFP expression has been shown to be nondetectable (16). The resultant recombinant BAC clone was used to generate several independent transgenic mouse lines that express Cre recombinase in the epicardial lineage beginning at the PE stage. These Wt1Cre transgenic mice have been used in previous studies to trace or delete specific genes in Wt1-expressing cells (15, 16). Homozygote (Wt1Cre+/+) mice were crossed with B6.129 × 1-Gt (ROSA)26Sortm1(EYFP)Cos/J mice (Rosa26-YFP in short) to generate permanent reporter expression in Wt1-expressing cells (Wt1CreYFP). The Wt1GFP knockin line (28, 29), in which exon 1 of one Wt1 allele has been replaced by the GFP sequence, also was used as an independent reporter for active Wt1 transcription. Wt1CreERT2 mice have been used previously to study epicardial development (12). G2-Gata4Cre and G2-Gata4LacZ mice were generated by cloning an 817-bp fragment containing the conserved region CR2 of the previously identified mouse G2-Gata4 enhancer (14) into the Cre expression vector to generate a G2-Gata4Cre transgene. Heterozygote (G2-Gata4Cre+/−) mice were crossed with Rosa26-YFP mice. Generation of LoxP-flanked Wt1 (Wt1LoxP/LoxP) mice was performed as described (30). In utero deletion of Wt1 was performed by crossing the CAGGCreERT2 and Wt1LoxP/GFP transgenic lines so that mutant embryos (Wt1−/−) carry one GFP copy in the Wt1 locus. Pregnant females were gavaged with one dose of tamoxifen (8 mg/40 g body weight) at E10.5, and embryos were collected at E13.5. Wt1loxP/loxP 9 and Tie2CreERT2 (18) animals were crossed to generate Tie2CreERT2;Wt1loxP/loxP mice. Beginning at day 10 of gestation, pregnant Tie2CreERT2;Wt1loxP/loxP animals were injected i.p. for 5 d with tamoxifen dissolved in sunflower oil in a dose of 33 mg⋅kg⋅−1d−1. Deletion of Wt1 was investigated by recombination PCR using the following primers as described (19):
5′-TGGGTTCCAACCGTACCAAAGA-3′ (forward);
5′-GTACGCGCGAACACTGACTA-3′ (reverse).
To exclude acute tamoxifen toxicity effects, Tie2CreERT2;Wt1loxP/loxP embryos were compared with Wt1loxP/loxP littermates.
All samples were sectioned on a cryostat (10 μm), and cryosections were stored at −20 °C until use.
To investigate WT1 and CD31 expression in the embryonic hearts, samples were fixed overnight at 4 °C in paraformaldehyde [4% (wt/vol) in PBS] and further processed for paraffin embedding and sectioning (3-µm sections of entire embryos were taken at 50-µm intervals). For immunohistology, after heat-mediated antigen retrieval and quenching of endogenous peroxidase activity, antigens were detected after antibody application using the EnVision Peroxidase/DAB Detection System from Dako. Omission of the first antibody served as a negative control. Additionally, some slides were incubated with IgG Isotype controls (1:100; rabbit monoclonal; clone SP137; Abcam). The following antibodies were used: WT1 (1:100; rabbit monoclonal; ab52933; Abcam) and CD31 (1:100; rabbit polyclonal; ab28364; Abcam). Slides were viewed under an epifluorescence microscope (DMLB; Leica) connected to a digital camera (Spot RT Slider; Diagnostic Instruments). Sections were routinely counterstained with hematoxylin (Sigma). Area densities (analyzed on at least five different sections per embryo) for all immunohistological stainings were determined using ImageJ software (NIH).
Mouse PE in Vitro Culture.
PE from E9.5 mouse embryos were dissected in DMEM (Gibco) using tungsten needles and fine forceps. PE cell clusters were cultured on 0.1% gelatin-coated coverslips. DMEM supplemented with 1% FBS (Gibco), 50 ng/mL VEGF (R&D), and 100 ng/mL FGF2 (R&D) was used as regular culture medium. Explants were cultured for 24–48 h and then were washed in Earle’s balanced salt solution (EBSS) (Gibco), fixed overnight in cold Dent’s fixative (methanol:DMSO, 4:1), washed in cold 70% ethanol, hydrated, and rinsed in PBS. Double WT1/CD31 immunohistochemistry was performed by serial incubation of the tissue in the primary anti-WT1 (MAB4234; Millipore) and anti-CD31 (550274; BD Pharmingen) antibodies, extensive washes in PBS, and final incubation with the adequate secondary antibodies (FITC-conjugated anti-mouse IgG and Cy5-conjugated anti-rat IgG, respectively). Cell nuclei were counterstained with DAPI, and the samples were analyzed using an SP5 Leica confocal laser microscope.
Quail-to-Chick Chimeras.
Donor quail embryos were incubated until stages HH16-17, excised, and washed in sterile EBSS (GIBCO). PE or small pieces of the posterior pericardiac mesoderm were dissected carefully using tungsten needles, small iridectomy forceps, and scissors, transplanted into the hearts of chicken embryo hosts, and incubated for 60 h. Chimeras were reincubated for 3 d. Details on the transplantation procedure and identification of donor cells can be found elsewhere (21).
Immunofluorescence.
The primary antibodies used were monoclonal rabbit anti-mouse WT1 (MAB4234; Millipore), monoclonal rat anti-mouse CD31 (550274; BD Pharmingen), mouse monoclonal anti-mouse α-SMA (A2574; Sigma), polyclonal chicken anti-GFP (13970; Abcam), polyclonal rabbit anti-mouse Cre recombinase (69050; Novagen), polyclonal rabbit anti-mouse cleaved NOTCH1 (N1ICD) (2421; Cell Signaling), polyclonal rabbit anti-mouse RALDH2 (75674; Abcam), monoclonal mouse anti-mouse GATA4 (25310; Santa Cruz), monoclonal mouse anti-mouse MF20 (MH20; Developmental Studies Hybridoma Bank), and TRITC-conjugated isolectin B4 (L5264; Sigma). Nuclei were counterstained with DAPI (Sigma). Single immunofluorescence was performed by incubating the sections with the primary antibody overnight at 4 °C, washing in Tris-PBS, and incubation with the corresponding fluorochrome-conjugated secondary antibody for 1 h at room temperature. For double CD31/α-SMA immunostaining, sections were incubated serially in anti-CD31 and anti–α-SMA antibodies overnight before incubation with the proper fluorochrome-conjugated secondary antibodies. For WT1 and N1ICD immunostaining, citrate buffer antigen retrieval was used; because of the thermosensitivity of GFP and YFP, these proteins were retrieved using an anti-GFP antibody. Negative controls were performed by incubation with nonimmune rat, mouse, or rabbit IgGs instead of the primary antibody.
X-Gal staining.
β-Galactosidase expression in G2-Gata4LacZ transgenic embryos or tissues was detected by X-gal staining. Transverse and sagittal sections from X-gal–stained embryos and tissues were prepared and counterstained with Neutral Fast Red.
In situ hybridization.
Embryos were isolated in ice-cold PBS and were fixed overnight in 4% paraformaldehyde. In situ hybridization was performed as previously described (16).
Quantitative and Semiquantitative RT-PCR.
RNA pellets were dissolved in diethyl pyrocarbonate-treated H2O. One microliter of the reverse-transcriptase reaction product was taken for real-time RT-PCR amplification (ABI Prism 7000; Applied Biosystems) using a commercial SYBR Green kit (Eurogentec). Total RNA from PE cells was extracted using the NucleoSpin RNA XS kit (Macherey-Nagel) and submitted to reverse transcription using the oligo(dT) (First Strand cDNA Synthesis Kit/AMV; Roche). Ribosomal18S RNA was amplified as a reference gene. Analysis of gene expression was carried out using the QuantiTect Primer Assays (Qiagen).
Flow cytometry (FACS).
Hearts from Wt1CreYFP and G2-Gata4CreYFP mice were dissociated for 15 min at 37 °C in a prewarmed collagenase solution (0.1% in HBSS, 3 mM CaCl2) (Sigma) and were homogenized by repeated pipetting. The cell suspension was washed in PBS plus 2% FBS and 10 mM Hepes. The cells then were incubated for 20 min on ice with Alexa 660-conjugated rat anti-mouse CD31 (17-0311; eBioscience). After washing, the cell suspension was analyzed in a MoFlo cell sorter. Damaged cells were excluded from the analysis by propidium iodide staining. The SD of replicates is indicated. Quadrant limits were established with fluorochrome-conjugated isotypes.
Wt1CreYFP whole-mount and time-lapse analysis.
For time-lapse analysis, E11.5 Wt1CreYFP- embryos were dissected in PBS supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL). Hearts were isolated and transferred to Hepes-buffered DMEM-F12 medium supplemented with 2% FBS and penicillin-streptomycin. An Alexa 660-conjugated rat anti-mouse CD31 antibody (eBiosciences; 17-0311) was injected into the hearts through the outflow tract using a microinjector (Picospritzer II, Science Products GmbH). Hearts then were embedded into a 1.5-mg/mL collagen gel (BD Bioscience) in a glass-bottomed culture dish (MatTek). For CD31 whole-mount immunofluorescence, embryonic hearts were fixed in 4% paraformaldehyde and incubated in Alexa 660-conjugated rat anti-mouse CD31 (170311; eBiosciences). Images were captured in a Leica SP5 laser confocal microscope every 10 min for 12 h. During image capture the culture chamber was maintained at 37 °C in a 5% CO2 humidified atmosphere.
Supplementary Material
Acknowledgments
We thank Dr. John Burch and Dr. Brian Black for providing Wt1Cre and G2-Gata4LacZ mice, respectively; Dr. José Luis de la Pompa and Dr. Robert Kelly for fruitful discussions; and Dr. John Pearson (Andalusian Center for Nanomedicine and Biotechnology) and Mr. David Navas (University of Malaga) for technical support. This research was supported by Ministry of Economy and Competitiveness Grants BFU2012-35799 and ISCIII-RD12/0019-0022 (to J.M.P.-P.) and BFU2011-25304 (to R.M.-C.); European Commission Seventh Framework Program-Marie Curie-Innovative Training Network Action PITN-GA-2011-289600 (to J.M.P.-P.); Junta de Andalucía CTS-7564 (to R.M.-C. and J.M.P.-P.); Instituto de Salud Carlos III-Fondos FEDER Grant PI14/00814 (to A.R.); the French Government National Research Agency through the Laboratory of Excellence SIGNALIFE program (reference ANR-11-LABX-0028-01) (K.D.W.); the Association pour la Recherche sur le Cancer Grant R13026AA-ARC-WAGNER (to K.D.W.); and Fondation de France Grant FDF-U1081-WAGNER (to K.D.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509834113/-/DCSupplemental.
References
- 1.Pérez-Pomares JM, de la Pompa JL. Signaling during epicardium and coronary vessel development. Circ Res. 2011;109(12):1429–1442. doi: 10.1161/CIRCRESAHA.111.245589. [DOI] [PubMed] [Google Scholar]
- 2.Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464(7288):549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu B, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151(5):1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian X, et al. Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart. Science. 2014;345(6192):90–94. doi: 10.1126/science.1251487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian X, Pu WT, Zhou B. Cellular origin and developmental program of coronary angiogenesis. Circ Res. 2015;116(3):515–530. doi: 10.1161/CIRCRESAHA.116.305097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Zhou B. Accelerated coronary angiogenesis by vegfr1-knockout endocardial cells. PLoS One. 2013;8(7):e70570. doi: 10.1371/journal.pone.0070570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Männer J, Pérez-Pomares JM, Macías D, Muñoz-Chápuli R. The origin, formation and developmental significance of the epicardium: A review. Cells Tissues Organs. 2001;169(2):89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- 8.Guadix JA, Carmona R, Muñoz-Chápuli R, Pérez-Pomares JM. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev Dyn. 2006;235(4):1014–1026. doi: 10.1002/dvdy.20685. [DOI] [PubMed] [Google Scholar]
- 9.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: Discontinuous formation of coronary vessels. Proc Natl Acad Sci USA. 1992;89(20):9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merki E, et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci USA. 2005;102(51):18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai CL, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou B, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz TC, et al. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell. 2012;22(3):639–650. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas A, et al. Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development. 2005;132(15):3405–3417. doi: 10.1242/dev.01913. [DOI] [PubMed] [Google Scholar]
- 15.del Monte G, et al. Differential Notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ Res. 2011;108(7):824–836. doi: 10.1161/CIRCRESAHA.110.229062. [DOI] [PubMed] [Google Scholar]
- 16.Wessels A, et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol. 2012;366(2):111–124. doi: 10.1016/j.ydbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guadix JA, et al. Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development. 2011;138(6):1093–1097. doi: 10.1242/dev.044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forde A, Constien R, Gröne HJ, Hämmerling G, Arnold B. Temporal Cre-mediated recombination exclusively in endothelial cells using Tie2 regulatory elements. Genesis. 2002;33(4):191–197. doi: 10.1002/gene.10117. [DOI] [PubMed] [Google Scholar]
- 19.Wagner K-D, et al. The Wilms’ tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat Commun. 2014;5:5852. doi: 10.1038/ncomms6852. [DOI] [PubMed] [Google Scholar]
- 20.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82(10):1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Pomares JM, Macías D, García-Garrido L, Muñoz-Chápuli R. The origin of the subepicardial mesenchyme in the avian embryo: An immunohistochemical and quail-chick chimera study. Dev Biol. 1998;200(1):57–68. doi: 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- 22.Männer J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255(2):212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Wagner KD, et al. The Wilms’ tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J. 2002;16(9):1117–1119. doi: 10.1096/fj.01-0986fje. [DOI] [PubMed] [Google Scholar]
- 24.Duim SN, Kurakula K, Goumans M-J, Kruithof BPT. Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J Mol Cell Cardiol. 2015;81:127–135. doi: 10.1016/j.yjmcc.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Wagner N, et al. Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms’ tumor transcription factor Wt1. Genes Dev. 2005;19(21):2631–2642. doi: 10.1101/gad.346405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner KD, et al. Oxygen-regulated expression of the Wilms’ tumor suppressor Wt1 involves hypoxia-inducible factor-1 (HIF-1) FASEB J. 2003;17(10):1364–1366. doi: 10.1096/fj.02-1065fje. [DOI] [PubMed] [Google Scholar]
- 27.Rudat C, Kispert A. Wt1 and epicardial fate mapping. Circ Res. 2012;111(2):165–169. doi: 10.1161/CIRCRESAHA.112.273946. [DOI] [PubMed] [Google Scholar]
- 28.Hosen N, et al. The Wilms’ tumor gene WT1-GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis. Leukemia. 2007;21(8):1783–1791. doi: 10.1038/sj.leu.2404752. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Estrada OM, et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2010;42(1):89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.