Significance
Several immunotherapies are approved for treating cancer patients, including aCTLA-4 (anti–cytotoxic T-lymphocyte–associated protein 4; ipilimumab) and anti–PD-1 (anti-programmed cell death protein 1; nivolumab; pembrolizumab), but the best clinical results are coming from combination immunotherapy. Our research demonstrates that aOX40 (anti-CD134)/aCTLA-4 immunotherapy can lead to a potentially tumor-promoting Th2-cytokine milieu (IL-4, IL-5, IL-13) when relying on endogenous antigen presentation. However, aOX40/aCTLA-4 treatment combined with a tumor antigen-specific vaccine, DEC-205 (anti–dendritic and epithelial cells, 205 kDa)–HER2 (human epidermal growth factor receptor 2), promoted robust tumor infiltration by effector CD8 T cells and restored Th1 polarization of CD4 T cells, leading to improved overall survival in a mammary carcinoma model. This study has direct relevance for the design of combination therapy trials in patients.
Keywords: CD8 T cell, costimulation, OX40, CTLA-4, anti–DEC-205/HER2
Abstract
Immunotherapy is gathering momentum as a primary therapy for cancer patients. However, monotherapies have limited efficacy in improving outcomes and benefit only a subset of patients. Combination therapies targeting multiple pathways can augment an immune response to improve survival further. Here, we demonstrate that dual aOX40 (anti-CD134)/aCTLA-4 (anti–cytotoxic T-lymphocyte–associated protein 4) immunotherapy generated a potent antigen-specific CD8 T-cell response, enhancing expansion, effector function, and memory T-cell persistence. Importantly, OX40 and CTLA-4 expression on CD8 T cells was critical for promoting their maximal expansion following combination therapy. Animals treated with combination therapy and vaccination using anti–DEC-205 (dendritic and epithelial cells, 205 kDa)–HER2 (human epidermal growth factor receptor 2) had significantly improved survival in a mammary carcinoma model. Vaccination with combination therapy uniquely restricted Th2-cytokine production by CD4 cells, relative to combination therapy alone, and enhanced IFNγ production by CD8 and CD4 cells. We observed an increase in MIP-1α (macrophage inflammatory protein-1α)/CCL3 [chemokine (C-C motif) ligand 3], MIP-1β/CCL4, RANTES (regulated on activation, normal T-cell expressed and excreted)/CCL5, and GM-CSF production by CD8 and CD4 T cells following treatment. Furthermore, this therapy was associated with extensive tumor destruction and T-cell infiltration into the tumor. Notably, in a spontaneous model of prostate adenocarcinoma, vaccination with combination therapy reversed anergy and enhanced the expansion and function of CD8 T cells recognizing a tumor-associated antigen. Collectively, these data demonstrate that the addition of a vaccine with combined aOX40/aCTLA-4 immunotherapy augmented antitumor CD8 T-cell function while limiting Th2 polarization in CD4 cells and improved overall survival.
Promoting a robust CD8 T-cell response is vital for the generation of an effective antitumor immune response. However, immunosuppressive mechanisms used by the tumor result in the exhaustion of tumor-infiltrating lymphocytes (TIL), leading to cytotoxic T-cell anergy and dysfunction. To overcome this dysfunction, investigators have had considerable success using immune checkpoint inhibitors, such as aCTLA-4 (cytotoxic T-lymphocyte–associated protein 4) mAbs, to promote T-cell function. CTLA-4, a negative regulatory molecule on the surface of T cells, is indispensable for preventing the expansion and activation of autoreactive T cells. Inhibition of this surface receptor using antagonist aCTLA-4 mAb–augmented effector CD4 and CD8 T-cell responses and inhibited or reduced the suppressive function of Treg cells (1–5). However, only a subset of patients treated with aCTLA-4 mAb exhibit an objective clinical response (6).
Checkpoint blockade targets T-cell coinhibitory molecules, but other strategies targeting costimulatory molecules, such as the TNF receptor family member OX40 (CD134), have demonstrated success in promoting tumor regression in a wide variety of preclinical models. Treatment with an agonist aOX40 mAb directly stimulated CD4 and CD8 T cells and induced their expansion, differentiation, and up-regulation of prosurvival molecules (7–12). Moreover, OX40 ligation promoted the generation of long-lived memory CD8 T cells and enhanced their function. Recent data indicate that combined aOX40/aCTLA-4 therapy induced a robust effector CD4 and CD8 T-cell response necessary for tumor regression and significantly improved the survival of tumor-bearing hosts relative to therapy with either agent alone (13). Despite this success, the response to combination aOX40/aCTLA-4 treatment was greatly diminished in more established tumors. This therapy may be unable to overcome T-cell anergy in more established immunosuppressive tumor microenvironments, possibly because it is ineffective at specifically targeting and expanding tumor-reactive T cells and relies on limited or defective endogenous priming by dendritic cells (14). Currently, clinically tested vaccines targeting cross-presenting dendritic cells [i.e., anti–DEC-205 (dendritic and epithelial cells, 205 kDa) mAb conjugated to tumor antigens] have demonstrated promise by priming a more robust cytotoxic T-cell response (15–19). The possibility remains that increased Th2-cytokine production by CD4 T cells following combination therapy may reduce its therapeutic efficacy, because inhibition of IL-4 with aOX40/aCTLA-4 treatment significantly improved survival (13). Others have shown that a dominant Th2 cytokine response reduced the efficacy of treatment, whereas a Th1-skewed immune response resulted in more favorable outcomes (20–23).
We hypothesized that dendritic cell-targeted vaccination against a tumor-associated antigen in conjunction with combination aOX40/aCTLA-4 mAb immunotherapy would be sufficient to promote a cytotoxic antitumor T-cell response, redirect a Th2 bias in CD4 T cells, and improve survival in mice with established tumors. Here, we report that vaccination using anti–DEC-205/HER2 (human epidermal growth factor receptor 2) mAb along with combination aOX40/aCTLA-4 mAbs significantly expanded effector CD8 T cells, resulting in a more favorable Th1 cytokine profile and inducing a critical accumulation of effector T cells in the tumor, with increased tumor-free survival relative to either therapy alone. Moreover, combination therapy with vaccination reversed T-cell anergy and promoted a robust effector T-cell response to a tumor-associated antigen in a spontaneous adenocarcinoma model.
Results
Combined aOX40 and aCTLA-4 Therapy Significantly Enhanced Antigen-Specific CD8 T-Cell Expansion, Function, and Persistence.
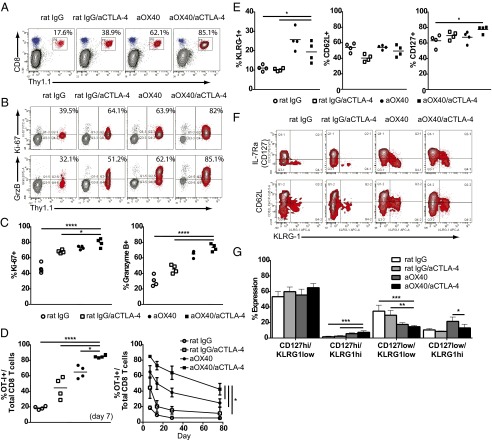
We have shown previously that combining OX40 stimulation with CTLA-4 blockade effectively enhanced tumor regression and survival in several tumor models (13). However, the mechanisms by which combination therapy augments antigen-specific CD8 T-cell responses remain unknown. To address this question, we transferred purified OT-I CD8 T cells into wild-type mice and treated them with control IgG, aCTLA-4, aOX40, or dual aOX40/aCTLA-4 therapy in the presence of antigen. Combination therapy resulted in a significant expansion of circulating antigen-specific T cells (∼85% of all CD8 T cells) compared with either monotherapy alone (38.9% for aCTLA-4 and 62.1% for aOX40) (Fig. 1A). Furthermore, combined aOX40/aCTLA-4 therapy augmented CD8 T-cell proliferation and effector function over controls, as evidenced by increased levels of Ki-67 (cellular marker of proliferation) and granzyme B, respectively (Fig. 1 B and C). Dual therapy also significantly enhanced the frequency of memory T cells: We observed expansion at day 7 that persisted 10 wk later at an elevated frequency compared with either monotherapy alone (Fig. 1D).
Fig. 1.
Combination therapy boosts the expansion, function, and persistence of memory CD8 T cells. Naive OT-I CD8 T cells (3 × 106) were purified by negative selection and adoptively transferred into wild-type C57BL/6 mice. Donor OT-I cells were stimulated with soluble OVA (day 0), along with mono- or combination therapy: 50 µg aOX40 or control rat IgG on days 0 and 1 and 200 µg aCTLA-4 on days 0, 2, and 4. Mice were bled on days 7, 14, 28, and 78, and donor cell phenotype was analyzed by flow cytometry. Donor OT-I cells were gated on live CD8+ Thy1.1+ events. Graphs depict (A and D) the frequency of OT-I+ cells of all CD8 T cells, (B and C) the percentage of Ki-67+ and granzyme B+ OT-I cells, and (E and G) the percentage of KLRG-1+, CD62L+, and CD127+ OT-I cells. Data shown are for one representative mouse (A, B, and F) or all mice in each cohort (C–E and G) at day 7. Graphs depict the mean ± SEM for one of three independent experiments (n = 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To investigate the extent to which combination therapy skewed antigen-specific CD8 T-cell differentiation toward an effector versus memory phenotype, we examined surface expression of KLRG-1 (killer cell lectin-like receptor subfamily G, member 1), CD127, and CD62L. The surface expression of these receptors changes based on differentiation status; central memory cells are typically CD127hi and CD62Lhi, and effector memory cells are CD127lo and CD62L−/lo (24, 25). We observed an increase in KLRG-1 and CD127 expression on CD8 T cells in mice given combination therapy, with no change in CD62L expression (Fig. 1E). CD127hi and KLRG-1hi dual-positive cells were increased, and CD127loKLRG-1lo cells were reduced (Fig. 1 F and G). There was no change in the percentage of CD62L/KLRG-1 dual-positive cells following combination therapy (Fig. 1F). These data demonstrate that combination therapy enhanced the expansion, function, and persistence of antigen-specific memory T cells in vivo.
Maximal Expansion of CD8 T Cells Following Combination Therapy Requires OX40 and CTLA-4 Expression on CD8 T Cells, and the Presence of CD4 T Cells.
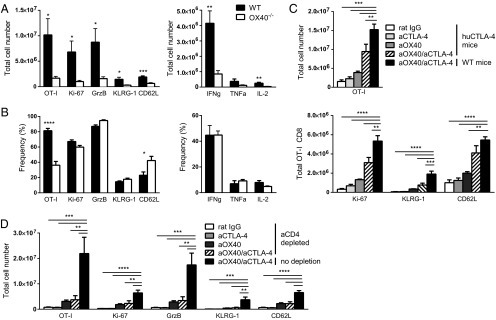
Prior studies have shown that OX40 expression on CD8 T cells contributes to polyclonal expansion and function following aOX40 therapy (7). However, OX40 is constitutively expressed on Treg cells, and engagement on this subset could enhance or limit their suppressive capacity (26–28). Therefore, we investigated whether engagement of OX40 directly on CD8 T cells was necessary for their expansion following combination therapy. The expansion of wild-type or OX40−/− OT-I cells in animals receiving combination therapy was analyzed by flow cytometry. OX40 expression on CD8 T cells was required for optimal expansion, and its expression enhanced the total number of Ki-67+, granzyme B+, IFNγ+, TNFα+, and IL-2+ cells (Fig. 2A); we observed minimal change in the percentage of these populations relative to controls (Fig. 2B). We also confirmed OX40 expression on CD8 T cells from the TIL of mice bearing TUBO mammary carcinomas (Figs. S1B and S2A) and of TRAMP (transgenic adenocarcinoma of the mouse prostate) mice with spontaneous prostate adenocarcinoma (Fig. S3B).
Fig. 2.
OX40 and CTLA-4 expression on CD8 T cells and CD4 T cells is required for maximal CD8 T-cell expansion and function following combination therapy. (A and B) Naive OT-I or OX40−/− OT-I CD8 T cells were purified by negative selection and adoptively transferred into wild-type C57BL/6 mice. (C) Naive wild-type OT-I CD8 T cells were adoptively transferred into humanized CTLA-4 knock-in mice. (D) OT-I CD8 T cells were adoptively transferred into wild-type mice. Donor OT-I cells were stimulated with soluble OVA on day 0 along with mono- or combination therapy: aOX40 or control rat IgG on days 0 and 1 and aCTLA-4 on days 0, 2, and 4. Splenocytes were harvested on day 7, and donor cell phenotype was analyzed by flow cytometry. Graphs depict the mean ± SEM for three independent experiments (n = 8 per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
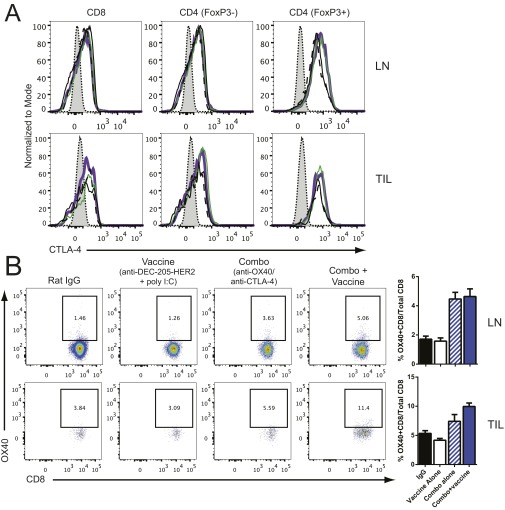
Fig. S1.
TUBO cells (5 × 105) were s.c. injected into the flank of BALB/c mice. Mice were treated with IgG or aOX40 (200 µg) on days 10 and 14 and with aCTLA-4 (200 µg) on days on 10, 12, and 14 with or without 5 µg anti–DEC-205/HER2 vaccine plus 50 µg poly(I:C) on days 10 and 1. Lymph nodes (LN) and tumors (TIL) were harvested and processed on day 21 for analysis by flow cytometry. (A) CTLA-4 expression on CD8, CD4 effectors (CD4+FoxP3− T cells), and Tregs (CD4+FoxP3+ T cells) in the lymph node and TIL. The unstained control is shown in gray; the IgG control is shown as a solid black line; vaccine alone is shown as a dashed black line; combination therapy alone is shown as a green line; combination therapy with vaccine is shown as a purple line. (B) OX40 expression on CD8 T cells. (Left) Representative flow cytometry dot plots showing the frequency of OX40+ CD8 T cells to total CD8 T cells. (Right) Cumulative data for all groups.
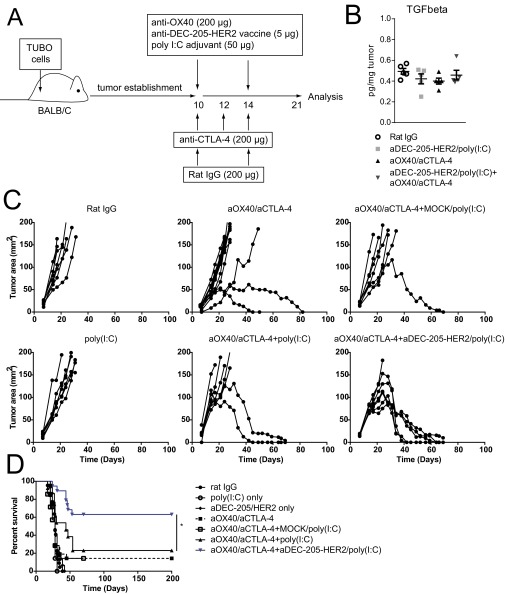
Fig. S2.
TUBO cells (5 × 105) were s.c. injected into the flank of BALB/c mice. Mice were treated with IgG or aOX40 (200 µg) on days 10 and 14 and with aCTLA-4 (200 µg) on days 10, 12, and 14, with or without 5 µg anti–DEC-205/HER2 vaccine plus 50 µg poly(I:C) on days 10 and 14. (A) Model of treatment. (B) Tumors were harvested and processed on day 21. TGF-β levels were determined by multiplex ELISA. (C) Tumor growth curves for the respective treatments. (D) Survival analysis for the respective treatments. n = 7–14 per group. *P < 0.05.
Fig. S3.
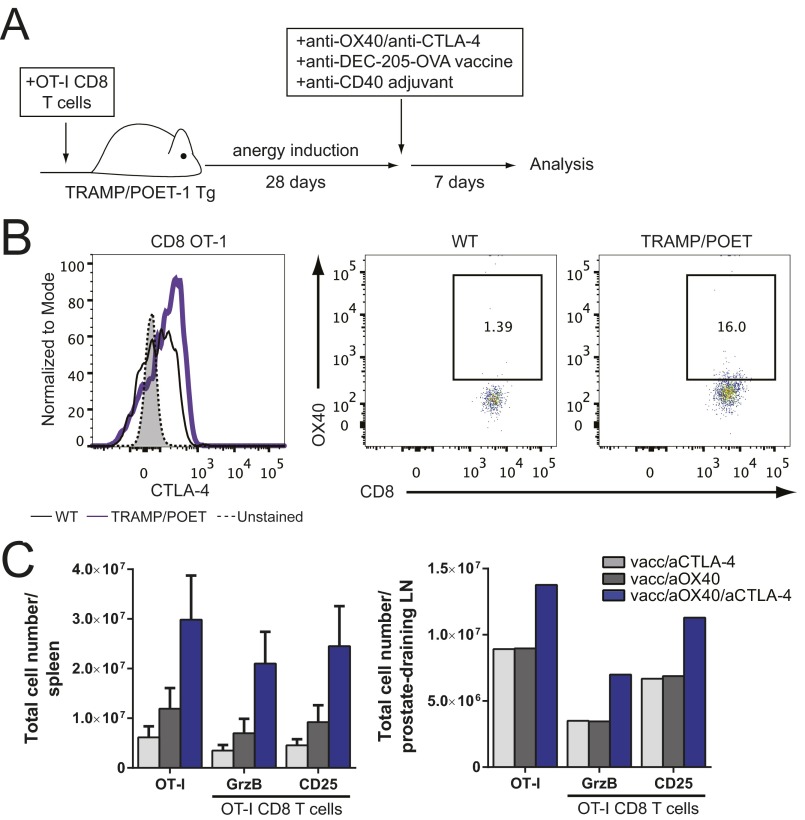
(A and C) Naive OT-I CD8 T cells (5 × 105) were purified by negative selection and adoptively transferred into TRAMP/POET mice (day 0). Mice were stimulated with 5 µg anti–DEC-205–OVA and 50 µg anti-CD40 on day 28, along with mono- or combination therapy: 50 µg aOX40 or control rat IgG on days 28 and 29, and 200 µg aCTLA-4 on days 28, 30, and 3. (A) Model of treatment. (B) Naive OT-I CD8 T cells (2.5 × 106) were purified by negative selection and adoptively transferred into TRAMP/POET or wild-type C57BL/6 mice. Three days later, lymph nodes were processed, and OX40 and CTLA-4 expression was analyzed by flow cytometry on donor OT-1 cells. For CTLA-4 expression on donor OT-1 cells, the unstained control is shown in gray; the wild-type control is shown as a solid black line; TRAMP/POET mice are shown as a purple line. For OX40 expression (Middle and Right), images represent OT-I+ (Thy1.1+) CD8 T cells versus OX40 expression; the numbers shown are the percentage of OX40+ OT-I cells. (C) Splenocytes and prostate-draining lymph nodes (pooled for each group) were analyzed on day 35, and donor cell phenotype was determined by flow cytometry. Donor OT-I cells were gated on live CD8+ Thy1.1+ events. Graphs depict the mean ± SEM for one of three independent experiments (n = 4 or 5 per group).
Previous studies demonstrated that CTLA-4 expression on CD4 T cells is required to augment CD8 T-cell function indirectly (1, 2). To assess the role of CTLA-4 expression on CD8 T cells, we used humanized CTLA-4 knock-in (huCTLA-4) mice engineered to express only the extracellular portion of the human CTLA-4 receptor, thereby preventing them from responding to mouse aCTLA-4 mAb (2). Animals were treated with combination therapy following adoptive transfer of OT-I cells; as expected, huCTLA-4 mice treated with mouse aCTLA-4 mAb had no change in OT-I expansion relative to IgG controls (Fig. 2C). Although we still observed an additive effect of combination therapy over monotherapy alone in huCTLA-4 mice, OT-1 expansion and proliferation were blunted compared with the wild-type controls given combination therapy (Fig. 2C). We also confirmed the expression of CTLA-4 on CD8 T cells from the TIL of tumor-bearing mice (Fig. S1). These data indicate that mAb binding to the CTLA-4 receptor on CD4 T cells, or other accessory cells, mediates only a portion of the CD8 T-cell expansion induced by combination therapy and that CD8 T-cell–specific CTLA-4 blockade is critical in the context of combination therapy to induce maximal efficacy.
It has been shown that CD4 T cells are required for optimal efficacy of aOX40 or aCTLA-4 mAb (1, 2, 29). To assess whether CD4 T cells are necessary to achieve maximal CD8 T-cell expansion following combination therapy, we depleted CD4 T cells before treatment. Antigen-specific CD8 T-cell expansion was significantly reduced in CD4-depleted animals compared with control mice, with an approximately fivefold difference in total cell numbers (Fig. 2D). Together, these data indicate that OX40 and CTLA-4 expression on CD8 T cells, in addition to CD4 T-cell help, is necessary to promote maximal CD8 T-cell expansion and effector function following combination therapy.
Combination Therapy with Vaccination-Induced Tumor Regression and Enhanced Survival in Tumor-Bearing Mice.
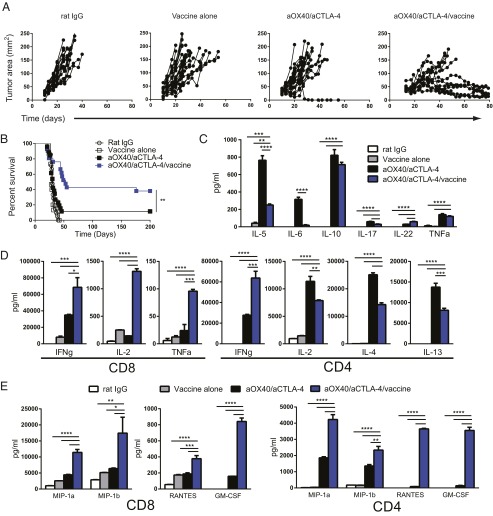
It has been shown that combination therapy drives Th2 cytokine production and that IL-4 expression limits the efficacy of treatment (13). Moreover, this treatment relies on endogenously primed T cells and is likely to be limited by suboptimal dendritic cell priming against tumor antigens (14). We hypothesized that the presence of a vaccine to drive cross-presentation of a tumor-associated antigen would enhance T-cell priming and diminish the Th2 bias of combination therapy. HER2 is a tumor-associated antigen that is overexpressed in a subset of breast cancers and is associated with disease recurrence and poor prognosis (30). Mice bearing established HER2-expressing TUBO mammary carcinomas were immunized with anti–DEC-205/HER2 mAb and adjuvant [poly(I:C)] and were treated with combination therapy (Fig. S2A). Combination therapy plus HER2 vaccination significantly enhanced tumor regression (Fig. 3A) and survival (Fig. 3B) over combination therapy or vaccine and adjuvant. There was no difference among between groups treated with anti–DEC-205–Mock/poly(I:C) plus aOX40/aCTLA-4 or with poly(I:C) plus aOX40/aCTLA-4 relative to combination aOX40/aCTLA-4 alone (Fig. S2 C and D). We observed that although combination therapy drove Th2 cytokine production from CD4 T cells, combination therapy plus HER2 vaccination significantly reduced the production of IL-4, IL-5, and IL-13 (Fig. 3 C and D). Furthermore, combination therapy with vaccination significantly increased IFNγ, TNFα, and IL-2 production by CD8 T cells (Fig. 3D); there was no difference in TGF-β expression in the tumor following treatment (Fig. S2B). Production of MIP-1α (macrophage regulatory protein-1α)/CCL3 [chemokine (C-C motif) ligand 3], MIP-1β/CCL4, RANTES (regulated on activation, normal T-cell expressed and excreted)/CCL5, and GM-CSF also was significantly enhanced by both CD4 and CD8 T cells in mice receiving combination therapy with HER2 vaccination (Fig. 3E).
Fig. 3.
Combination therapy with vaccination improves survival and inhibits Th2-polarization in CD4 T cells in a mammary carcinoma model. TUBO mammary carcinoma cells were implanted on day 0 into the flank of female BALB/c mice. Tumor-bearing mice were given control rat IgG on days 10 and 14, vaccine [anti–DEC-205/HER2/poly(I:C)] on days 10 and 14, combination aOX40 on days 10 and 14/aCTLA-4 on days 10, 12, and 14, or both vaccine and combination immunotherapy. (A and B) Tumor growth (A) and survival (B) were monitored over time. Graphs depict the mean ± SEM for three independent experiments combined (n = 15–25 per group). (C–E) CD4 (C) and CD8 (D and E) T cells were purified and sorted from the lymph nodes of tumor-bearing mice 7 d after the end of therapy (day 21). Cells were stimulated in vitro with anti-CD3 for 4 h, and supernatants were collected. Cytokine and chemokine levels were determined by multiplex ELISA. Graphs depict the mean ± SEM for two independent experiments (n = 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
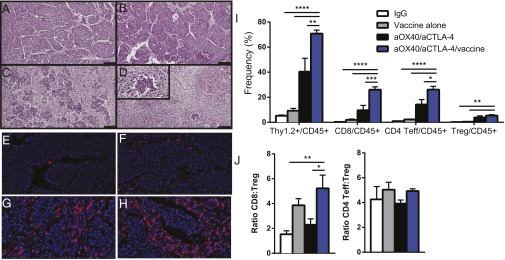
To understand the impact of this therapy on the tumor microenvironment, we examined tumor immune infiltration following treatment. Immunohistochemistry revealed that vaccination alone was insufficient to drive tumor destruction and T-cell infiltration. However, mice treated with combination immunotherapy plus HER2 vaccination had extensive tumor destruction and increased CD3+ lymphocyte infiltration throughout the tumors relative to mice treated with combination therapy or controls (Fig. 4 A–H). We further quantified this finding using flow cytometry and observed a significant increase in the frequency of tumor-infiltrating CD4 and CD8 T cells in mice receiving combination therapy with anti–DEC-205/HER2 vaccination (Fig. 4I). This increase coincided with a significant increase in the CD8:Treg ratio (Fig. 4J), which has been associated with the sensitivity of a tumor to a given therapy and also with improved overall survival in patients with breast cancer and other cancer subtypes (3, 31–33). Collectively, these data indicate that directing the CD8 T-cell response using anti–DEC-205/HER2 vaccination enhances the efficacy of combination therapy, reverses Th2 cytokine production by CD4 T cells, and enhances effector CD8 T-cell infiltration into the tumor.
Fig. 4.
Combination therapy with vaccination induces robust tumor destruction and effector T-cell infiltration into the tumor. (A–H) TUBO mammary carcinoma cells were implanted into the flanks of female BALB/c mice on day 0. Tumor-bearing mice were given control rat IgG on days 10 and 14 (A and E), vaccine [anti–DEC-205/HER2/poly(I:C)] on days 10 and 14 (B and F), combination immunotherapy with aOX40 on days 10 and 14/aCTLA-4 on days 10, 12, and 14 (C and G), or both vaccine and combination immunotherapy (D and H). Tumors were harvested on day 21 and were analyzed by immunohistochemistry. Images depict sections of paraffin-embedded slides stained using H&E (A–D) or anti-CD3 plus DAPI (E–H). (I and J) Tumors were harvested and analyzed by flow cytometry. Graphs depict the mean ± SEM for one of two independent experiments (n = 4 or 5 per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Combination Therapy with Vaccination Reversed T-Cell Anergy and Augmented Effector Function to a Tumor-Associated Antigen in Mice with Spontaneous Prostate Cancer.
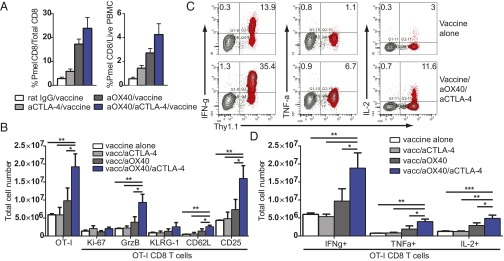
To determine whether combination therapy with vaccination was more effective at inducing tumor-specific CD8 T-cell expansion, we used the B16F10 model with adoptive transfer of tumor-specific Pmel (premelanosome protein) CD8 T cells (hereafter, “Pmels”), which are insufficient on their own to overcome peripheral tolerance and induce tumor regression (34). B16F10 tumor-bearing mice given Pmels were immunized with gp100 peptide and adjuvant along with combination therapy. Peripheral blood was analyzed by flow cytometry to assess the expansion of tumor-specific Pmels. We observed an increase in Pmel expansion in animals treated with combination therapy and vaccination, specifically an increase in the proportion of Pmels to CD8 T cells and Pmels to peripheral blood mononuclear cells (Fig. 5A).
Fig. 5.
Combination therapy with vaccination reverses CD8 T-cell anergy and restores T-cell function in a spontaneous adenocarcinoma model. (A) Naive Pmel TCR transgenic CD8 T cells were purified by negative selection and adoptively transferred on day 6 into B16F10 tumor-bearing wild-type C57BL/6 mice. Mice were treated with IgG or aOX40 on days 7 and 11; with aCTLA-4 on days 7, 9, and 11; or with monotherapy or combination therapy and vaccine (gp100/anti-CD40) on day 7. Blood was analyzed by flow cytometry on day 14. (B–D) Naive OT-I CD8 T cells (5 × 105) were purified by negative selection and adoptively transferred into TRAMP/POET-1 mice on day 0. Mice were stimulated with 5 µg anti–DEC-205–OVA and 50 µg anti-CD40 on day 28 along with mono- or combination therapy (50 µg aOX40 or control rat IgG on days 28 and 29 and 200 µg aCTLA-4 on days 28, 30, and 32). Splenocytes were analyzed on day 35, and donor cell phenotype was determined by flow cytometry. Donor OT-I cells were gated on live CD8+ Thy1.1+ events. Graphs depict (B) the total number of OT-I+ cells; the total number of Ki-67+, granzyme B+ (GrzB), KLRG-1+, CD62L+, and CD25+ OT-I; (C and D) and the percentage (C) and total number (D) of IFNγ-, TNFα-, and IL-2--producing OT-I cells. Data are shown for one representative mouse (C) or for all mice in each cohort for donor OT-I cells (B and D). Graphs depict the mean ± SEM for one of three independent experiments (n = 4 or 5 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
It has been shown that antigen-specific T-cell anergy is an early event in tumor development and poses a significant barrier to therapeutic vaccination. To test the efficacy of combination therapy with vaccination on T-cell anergy in a spontaneous tumor model, we used TRAMP/POET-1 (probasin ovalbumin expressing transgenic) mice. TRAMP mice express SV40 T antigen under control of the rat probasin promoter, resulting in antigen expression in the prostate epithelium upon sexual maturity. These mice develop prostate intraepithelial neoplasia (PIN) by roughly age 12 wk and adenocarcinoma by age 24 wk (35). We crossed TRAMP mice with POET-1 mice in which the probasin promoter was used to drive prostatic expression of membrane-bound ovalbumin (OVA) (36). OT-I CD8 T cells were adoptively transferred into TRAMP/POET-1 mice and rested for 28 d to induce anergy (37). Mice were treated with combination therapy and anti–DEC-205–OVA vaccination (model shown in Fig. S3A). TRAMP/POET-1 mice treated with combination therapy plus vaccination had a significant boost in CD8 T-cell expansion, proliferation, and effector function in the spleen (Fig. 5 B–D) and lymph nodes (Fig. S3C). Combination therapy with vaccination induced a significantly greater number of IFNγ-, TNFα-, and IL-2–producing CD8 T cells compared with vaccination and either monotherapy (Fig. 5 C and D). These data indicate that combined aOX40/aCTLA-4 therapy with vaccination was uniquely capable of reversing CD8 T-cell anergy to a tumor-associated antigen in vivo and promoting robust expansion and function of these cells.
Discussion
Although aOX40 or aCTLA-4 monotherapy improves an antitumor immune response, monotherapy alone is insufficient to eliminate tumor burden in the majority of patients. Our previous studies demonstrated that combined aOX40/aCTLA-4 therapy significantly increased the survival of animals with TRAMP-C1 prostate or MCA-205 sarcoma tumors compared with monotherapy alone (13). We elaborate on these data to show that combination therapy significantly boosted an antigen-specific CD8 T-cell response that persisted for many weeks following treatment (Fig. 1). Our previous studies also suggested that combined therapy affected T-cell differentiation, in terms of transcriptional regulation. In the current study, we demonstrate an increase in dual CD127hiKLRG-1hi antigen-specific CD8 T cells and a decrease in CD127loKLRG-1lo cells (Fig. 1E). For virus-specific CD8 T cells, CD127hiKLRG-1hi CD8 T cells typically represent memory cells, whereas CD127loKLRG-1lo cells represent early effectors (24, 38). Combination therapy may preferentially skew a CD8 T-cell population toward a memory phenotype or potentially could target those cells specifically. The precise reason for this effect is currently unclear, but this skewing may prove beneficial when targeting a rare population of tumor-associated antigen-specific CD8 T cells in vivo.
OX40 ligation can induce the formation of long-lived memory CD4 T cells and boost an effector CD8 T-cell response through both direct and indirect ligation of the respective T-cell subsets. Here, we demonstrate that, in the context of dual aOX40/aCTLA-4 therapy, OX40 deficiency on CD8 T cells significantly reduced their ability to become IFNγ-producing, granzyme B+ effector cells. OX40 expression on CD8 T cells is required to boost CD8 T-cell expansion and survival following dual therapy, as has been demonstrated previously in the context of OX40 agonism by itself (7, 9, 39). These data demonstrate the need for OX40 engagement on CD8 T cells in the context of combined therapy, indicating that, although antibody-dependent, cell-mediated cytotoxicity is one mechanism by which aOX40 improves tumor eradication (40), OX40 expression on CD8 T cells is still an essential component of the efficacy of aOX40 therapies.
We also investigated the requirement for CTLA-4 expression on CD8 T cells following dual therapy, because previous work has demonstrated that CTLA-4 blockade indirectly augments effector CD8 T-cell function through cell-extrinsic effects on CD4 T cells (1, 4, 41). Surprisingly, in the context of combined OX40 agonism/CTLA-4 blockade, the expression of CTLA-4 on CD8 T cells is required to promote maximal CD8 T-cell expansion, because we observed twofold greater CD8 T-cell expansion in mice treated with dual aOX40/aCTLA-4 therapy than mice treated with aOX40 alone. In contrast, Pedicord et al. (1) established that in studies combining CTLA-4 blockade with Listeria infection, CTLA-4 expression on CD8 T cells was dispensable for the boost in CD8 T-cell expansion following treatment with aCTLA-4 mAb. However, Listeria infection provides other potent costimulatory molecules, such as CD40, and the production of proinflammatory cytokines, which may overshadow any effect of CTLA-4 expression on CD8 T cells. Along these lines, Gattinoni et al. (41) demonstrated that CTLA-4 expression on CD8 T cells had a modest impact on tumor growth in the B16 melanoma model. It appears that in the context of a strong costimulatory signal, as provided by OX40 ligation, and in the absence of other inflammatory signals to drive T-cell differentiation, CTLA-4 blockade provides an added boost in CD8 T-cell expansion and effector function.
The efficacy of combination therapy relies on endogenous antigen presentation to T cells to drive an antitumor immune response. However, it has been shown that dendritic cell function is diminished in tumor-bearing hosts, and the increase in Th2 polarization in CD4 T cells limited the efficacy of dual immunotherapy (13, 14, 42). Targeting cross-presenting dendritic cells using anti–DEC-205/HER2 with combination therapy reduced the generation of Th2-polarized CD4 T cells, promoted a robust cytotoxic CD8 T-cell response and infiltration into the tumor, and enhanced overall survival (Figs. 3 and 4). One probable mechanism for the efficacy of vaccination plus combination therapy is the promotion of better T-cell receptor (TCR) stimulation and Th1-polarized T-cell priming. Evidence for this notion comes from studies showing that lower-affinity stimulation, which is thought to be the majority of endogenous antigen presentation in the periphery because of central and peripheral tolerance, tends to promote Th2 CD4 T-cell responses preferentially (43, 44). In particular, anti–DEC-205/HER2 vaccination was shown to contain epitopes specifically capable of eliciting a strong CD4 T-cell response (17). In the absence of a specific antigen, administration of aOX40/aCTLA-4 therapy likely promotes the expansion of T cells receiving suboptimal TCR stimulation from endogenous peptides on dendritic cells, hence the increase in Th2 cytokine production. By providing tumor-specific antigen to cross-presenting dendritic cells, we were able to promote a robust Th1 response, as evidenced by increased IFNγ, TNFα, and IL-2 production by CD8 T cells (Fig. 3). Furthermore, we observed an increase in MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5 production by CD4 and CD8 T cells following combination therapy plus HER2 vaccination (Fig. 3). CCL3 and CCL4 are important for recruiting dendritic cells and T cells and promoting T-cell homing to sites of infection or inflammation (45, 46). Moreover, CCL3, CCL4, and CCL5 are preferentially expressed in tumors with T-cell infiltration in melanoma patients (47). The boost in these chemokines by combination aOX40/aCTLA-4 therapy with HER2 vaccination may drive the recruitment of TIL. Further studies in our laboratory are aimed at determining whether these chemokines are necessary for the recruitment of CD8 effector T cells into the tumor.
Despite an increase in antitumor immunity, the effectiveness of combination therapy alone was reduced in mice with established tumors (13). Moreover, overcoming tumor tolerance and T-cell anergy remains a concern for creating more effective therapeutic modalities. Prior studies demonstrate that aOX40 mAb in the absence of exogenous antigen is insufficient to overcome tolerance; overcoming T-cell tolerance requires the administration of both aOX40 mAb and antigen (37). We hypothesized that combination therapy with vaccination would be sufficient to overcome tumor tolerance and induce the expansion of CD8 T cells recognizing a tumor-associated antigen. Our data demonstrate that combination aOX40/aCTLA-4 mAb with peptide vaccination specifically induced the robust expansion of tumor-specific Pmel CD8 T cells (Fig. 5A). Using a model of T-cell anergy combined with a spontaneous prostate cancer model, we have demonstrated that treatment with aOX40/aCTLA-4 and vaccination can overcome T-cell anergy to a tumor-associated antigen to promote a potent expansion of effector cells and significantly augment the number of Th1 cytokine-producing CD8 T cells (Fig. 5). Based on these data, it seems likely that aOX40/aCTLA-4 therapy will be more effective for patients in the presence of a vaccination strategy designed to overcome immune tolerance to the tumor. Indeed, recent studies suggest that checkpoint blockade using PD-1 or CTLA-4 blockade is more effective in tumors with a higher somatic mutation load (48, 49). However, the mutational burden in prostate cancer is low relative to other cancer types and is more often associated with chromosomal rearrangements or deletions (50–53). Our data support these studies, because combination therapy in the TRAMP model of spontaneous prostate adenocarcinoma was effective only when administered with a vaccine against a tumor-associated antigen. Moreover, because the induction of Th2 cytokines by aOX40/aCTLA-4 limited the effectiveness of this therapy, using a vaccine strategy to target cross-presenting dendritic cells specifically should preferentially skew the response away from a Th2 T-cell response and toward a Th1 CD4 T-cell response, which is more favorable for tumor regression and patient survival (54–57). The use of anti–DEC-205 mAb conjugated to NY-ESO-1 in patients with solid tumors (NCT01522820) finished testing with promising results (19) and currently is being tested clinically in patients with acute myeloid leukemia (NCT01834248). Preclinical and clinical studies support our data on vaccination with anti–DEC-205 antibodies. It will be important for future clinical studies investigating combined aOX40/aCTLA-4 therapy to address whether Th2-biased T cells are induced in patients subsequent to treatment, the extent to which they limit the efficacy of immunotherapy, and whether the inclusion of a dendritic cell–targeted vaccination with combined immunotherapy can restore Th1 polarization to enhance the efficacy of treatment.
Materials and Methods
Mice.
Wild-type C57BL/6 or BALB/c mice were purchased from Jackson Laboratories. OT-I Thy1.1 TCR transgenic mice, OX40−/− OT-I TCR transgenic mice, Pmel TCR transgenic/Rag-1−/− transgenic mice, TRAMP mice, POET-1 mice, and humanized CTLA-4 knock-in mice were bred and maintained under specific pathogen-free conditions in the Providence Portland Medical Center vivarium. Humanized CTLA-4 knock-in mice were a gift from Jim Allison (University of Texas, MD Anderson Cancer Center, Houston). Experimental procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals (58) and were approved by the Institutional Animal Care and Use Committee at Providence Cancer Center.
FACS Analysis.
For FACS analysis, cells were incubated for 30 min at 4 °C with Thy1.1 PE-Cy7, Thy1.1 eFluor 450, CD45 APC, CD8 eFluor 605, CD8 BV785, KLRG-1 APC, FoxP3 eFluor 450, CD25 Alexa Fluor 700, CD25 PE, OX40 PE, Fixable Viability Dye eFluor 780 and eFluor506, CD62L PerCP Cy5.5, CD127 PE-Cy7, Vb13 APC, or CD4 V500. All antibodies were obtained from eBioscience, BD Biosciences, BioLegend, or Life Technologies. For intracellular staining, cells were fixed and permeabilized with the Foxp3 Staining Buffer kit (eBioscience) according to the manufacturer’s instructions. Cells were incubated for 30 min at 4 °C with IL-2 PE, TNF-α PE-Cy7, IFN-γ APC, Ki-67 FITC, granzyme B PE, and Foxp3 eF450. Cells were collected and analyzed using the LSR II flow cytometer using Diva (BD Biosciences) or FlowJo (Tree Star) software. For the multiplex cytokine/chemokine ELISA, CD4 or CD8 T cells were purified by cell sorting (B220/CD11b/MHC II−), and 2 × 106 cells per well were stimulated with medium or plate-bound anti-CD3 (2 μg/mL) in 24-well plates. Supernatants were collected after 4 h, and cytokine expression was determined using FlowCytomix Th1/Th2/Th17 and chemokine kits according to the manufacturer’s instructions (eBioscience).
Adoptive Transfer and Purification of OT-I T Cells.
Single-cell suspensions were prepared from spleens of OT-I Thy1.1 TCR transgenic or Pmel TCR transgenic/Rag-1−/− mice. Cell suspensions were depleted of CD4+, CD11b+, CD45R+, DX5+, and Ter-119+ cells using the Dynabeads CD8 T-cell negative isolation kit (Life Technologies), and cells were purified by negative selection according to the manufacturer’s instructions. On day 7, 3 × 106 wild-type OT-I T cells or 5 × 105 Pmel CD8 cells in PBS were i.v. injected into recipient C57BL/6 mice or B16F10 tumor-bearing C57BL/6 mice, respectively. For the anergy model, 5 × 105 wild-type OT-I T cells were i.v. injected into TRAMP/POET recipients.
Lymphocyte Isolation and Analysis.
Spleens were harvested and processed to obtain single-cell suspensions. ACK lysing buffer was used to lyse red blood cells. Cells were rinsed with Roswell Park Memorial Institute [RPMI] 1640 medium containing 10% (vol/vol) FBS (10% cRPMI) supplemented with 1 M Hepes, nonessential amino acids, sodium pyruvate, and penicillin-streptomycin glutamine. Murine peripheral blood lymphocytes from the superficial temporal vein were collected into tubes containing 50 μL heparin. One milliliter of flow cytometry wash buffer (0.5% FBS, 0.5 mM EDTA, and 0.02% NaN3 in PBS) was added, and the cells were vortexed and layered on top of 700 μL of Ficoll-Paque medium (GE Healthcare) before centrifugation. Lymphocytes were collected from the interface and washed with wash buffer before staining. To measure antigen-specific cytokine production, lymphocytes were incubated in 10% cRPMI with 5 μg/mL of the OVA (SIINFEKL) peptide (Anaspec) and 2.5 μL/mL of Golgi-Plug solution containing brefeldin A (BD Biosciences) for 5 h at 37 °C. After washing, cells were stained and fixed as described above.
Tumor Challenge and Antibody Administration.
TUBO cells (5 × 105), an HER2-expressing cell line derived from a spontaneous mammary gland tumor from a BALB-neuT mouse (59), were injected into the right flank of BALB/C mice. Tumor growth (area) was assessed every 2 to 3 d with microcalipers, and mice were killed when tumors reached >200 mm2. B16 F10 melanoma cells (3.5 × 105) were injected in to the right flank of C57BL/6 mice. Animals were treated with 200 μg rat IgG (Sigma), 200 μg aOX40 (clone OX86; BioXCell), and/or 200 μg aCTLA-4 (clone 9D9; BioXCell) mAb. All mAbs were verified to be endotoxin free and were injected i.p. (aCTLA-4) or s.c. (IgG or aOX40) into recipient mice. Murine anti–DEC-205–OVA and anti–DEC-205/HER2 mAbs were provided by Celldex Therapeutics, Inc., and 5 μg were injected s.c. along with 50 μg anti-CD40 (clone FGK4.5; BioXCell) or 50 μg poly(I:C) (InvivoGen) as an adjuvant. For the TUBO mammary carcinoma model, treatment with IgG, OX40, and vaccine/adjuvant was on days 10 and 14 after tumor implantation; aCTLA-4 was administered on days 10, 12, and 14. For the Pmel/B16F10 model, only 50 μg of aOX40 per dose was used and was administered to tumor-bearing mice on days 8 and 9 after tumor implantation, along with gp100 peptide and anti-CD40 or control IgG; aCTLA-4 was administered on days 8, 10, and 12. In the TRAMP/POET model, mice were vaccinated with 5 µg anti–DEC-205-OVA, 50 µg anti-CD40 (on day 28 after adoptive transfer) along with mono- or combination therapy (50 µg aOX40 or control rat IgG on days 28 and 29 and 200 µg aCTLA-4 on days 28, 30, and 32).
TIL Isolation.
TILs were harvested on day 21 by dissection of tissue into small fragments followed by digestion in 1 mg/mL collagenase and 5 mg/mL DNase in PBS. After filtration through nylon mesh, lymphocytes were stained as described above and were analyzed by flow cytometry. For immunohistochemistry, tumors were fixed in a 10% (wt/vol) zinc solution for 24 h and then were embedded in paraffin blocks. Paraffin blocks were sectioned, placed onto slides, and dried overnight. Slides were deparaffinized in xylene and stained with H&E. For fluorescence microscopy, slides were blocked with polyclonal IgG and PeroxAbolish (Biocare Medical) before staining with rabbit anti-mouse CD3 (SP7) (Spring Bioscience) followed by the anti-rabbit secondary SuperPicTure Polymer Detection Kit (Life Technologies). The signal was amplified using TSA-Cyanine 5 (PerkinElmer). Slides were mounted in Vectashield mounting medium with DAPI (Vector Laboratories) and visualized using the Vectra Microscopy imaging system (PerkinElmer). Representative regions were captured at 200× magnification using Vectra Software and were analyzed using Inform Image Analysis software (PerkinElmer).
Statistical Analysis.
Statistical significance was determined by unpaired Student t test, one-way ANOVA, or Kaplan–Meier survival where appropriate using GraphPad Prism software (GraphPad).
Acknowledgments
We thank Zefora Alderman and the Earle A. Chiles Research Institute Vivarium for excellent technical assistance; Tibor Keler (Celldex Therapeutics) for providing the anti–DEC-205 mAb vaccines; and Drs. Michael Gough, Andrew Weinberg, and Walter Urba for helpful discussions and critical reading of the manuscript. This work was supported by the Providence Portland Medical Foundation, the Safeway Foundation, Susan G. Komen Grant CCR15329664, NIH Pathway to Independence Award 5R00CA136678, and American Cancer Society 2014 Roaring Fork Valley Postdoctoral Fellowship PF-14-249-01-LIB (to S.N.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. L.Z. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510518113/-/DCSupplemental.
References
- 1.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA. 2011;108(1):266–271. doi: 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11(4):483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 5.Sutmuller RP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179(11):7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 8.Redmond WL, Ruby CE, Weinberg AD. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit Rev Immunol. 2009;29(3):187–201. doi: 10.1615/critrevimmunol.v29.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175(6):3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 10.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22(5):621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180(11):7240–7248. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara MJ, Kasiewicz MJ, Linch SN, Dubay C, Redmond WL. Common gamma chain (γc) cytokines differentially potentiate TNFR family signaling in antigen-activated CD8(+) T cells. J Immunother Cancer. 2014;2:28. doi: 10.1186/s40425-014-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2(2):142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170(1):101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 15.Mahnke K, et al. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res. 2005;65(15):7007–7012. doi: 10.1158/0008-5472.CAN-05-0938. [DOI] [PubMed] [Google Scholar]
- 16.Iwai Y, et al. An IFN-gamma-IL-18 signaling loop accelerates memory CD8+ T cell proliferation. PLoS One. 2008;3(6):e2404. doi: 10.1371/journal.pone.0002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, et al. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012;14(2):R39. doi: 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol. 2011;186(3):1325–1331. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhodapkar MV, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014;6(232):232ra51. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiao SL, et al. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res. 2015;3(5):518–525. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochi A, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209(9):1671–1687. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatsumi T, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196(5):619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 24.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinrichs CS, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA. 2009;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taraban VY, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32(12):3617–3627. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 27.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: Comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167(12):6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K, et al. Combination therapy of established tumors by antibodies targeting immune activating and suppressing molecules. J Immunol. 2010;184(10):5493–5501. doi: 10.4049/jimmunol.0903033. [DOI] [PubMed] [Google Scholar]
- 29.Song A, Song J, Tang X, Croft M. Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. Eur J Immunol. 2007;37(5):1224–1232. doi: 10.1002/eji.200636957. [DOI] [PubMed] [Google Scholar]
- 30.Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353(16):1652–1654. doi: 10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 31.Liu F, et al. CD8⁺ cytotoxic T cell and FOXP3⁺ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130(2):645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 32.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preston CC, et al. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8(11):e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan-Lefko PJ, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55(3):219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 36.Lees JR, et al. Deletion is neither sufficient nor necessary for the induction of peripheral tolerance in mature CD8+ T cells. Immunology. 2006;117(2):248–261. doi: 10.1111/j.1365-2567.2005.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redmond WL, Gough MJ, Weinberg AD. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur J Immunol. 2009;39(8):2184–2194. doi: 10.1002/eji.200939348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obar JJ, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187(10):4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172(8):4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 40.Bulliard Y, et al. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92(6):475–480. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 41.Gattinoni L, et al. CTLA-4 dysregulation of self/tumor-reactive CD8+ T-cell function is CD4+ T-cell dependent. Blood. 2006;108(12):3818–3823. doi: 10.1182/blood-2006-07-034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinzon-Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: A mechanism for immunosuppression. Immunol Cell Biol. 2005;83(5):451–461. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 43.Milner JD, Fazilleau N, McHeyzer-Williams M, Paul W. Cutting edge: Lack of high affinity competition for peptide in polyclonal CD4+ responses unmasks IL-4 production. J Immunol. 2010;184(12):6569–6573. doi: 10.4049/jimmunol.1000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao X, Grant C, Constant S, Bottomly K. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J Immunol. 1997;158(9):4237–4244. [PubMed] [Google Scholar]
- 45.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440(7086):890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 46.Charmoy M, et al. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010;6(2):e1000755. doi: 10.1371/journal.ppat.1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harlin H, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gubin MM, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Champiat S, Ferté C, Lebel-Binay S, Eggermont A, Soria JC. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. OncoImmunology. 2014;3(1):e27817. doi: 10.4161/onci.27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alexandrov LB, et al. Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grasso CS, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berger MF, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470(7333):214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar A, et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci USA. 2011;108(41):17087–17092. doi: 10.1073/pnas.1108745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goto S, et al. Analysis of Th1 and Th2 cytokine production by peripheral blood mononuclear cells as a parameter of immunological dysfunction in advanced cancer patients. Cancer Immunol Immunother. 1999;48(8):435–442. doi: 10.1007/s002620050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tosolini M, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 56.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Monte L, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed. [Google Scholar]
- 59.Rovero S, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165(9):5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]