Significance
Although much evidence has accrued in research over the past 20 years on the strong causal associations between social relationships and health and longevity, important gaps remain in our understanding of the mechanisms, timing, and duration of these associations. This study integrates social and biological disciplinary perspectives and research to examine how social relationships “get under the skin” to affect physiological well-being as individuals age. By combining data from and harmonizing measurement across four large nationally representative, population-based, contemporary surveys using an innovative longitudinal life course design, this study provides previously unidentified evidence on the biological and life course mechanisms linking social relationship patterns with health. As such, our findings advance explanations of the emergence and progression of diseases across the human life span.
Keywords: social relationships, physiological dysregulation, longevity, life course, biomarker
Abstract
Two decades of research indicate causal associations between social relationships and mortality, but important questions remain as to how social relationships affect health, when effects emerge, and how long they last. Drawing on data from four nationally representative longitudinal samples of the US population, we implemented an innovative life course design to assess the prospective association of both structural and functional dimensions of social relationships (social integration, social support, and social strain) with objectively measured biomarkers of physical health (C-reactive protein, systolic and diastolic blood pressure, waist circumference, and body mass index) within each life stage, including adolescence and young, middle, and late adulthood, and compare such associations across life stages. We found that a higher degree of social integration was associated with lower risk of physiological dysregulation in a dose–response manner in both early and later life. Conversely, lack of social connections was associated with vastly elevated risk in specific life stages. For example, social isolation increased the risk of inflammation by the same magnitude as physical inactivity in adolescence, and the effect of social isolation on hypertension exceeded that of clinical risk factors such as diabetes in old age. Analyses of multiple dimensions of social relationships within multiple samples across the life course produced consistent and robust associations with health. Physiological impacts of structural and functional dimensions of social relationships emerge uniquely in adolescence and midlife and persist into old age.
A defining characteristic of human society is that individual lives are intertwined through social relationships. Full social participation is such a fundamental human need that research since the 1900s has found the lack of social connections increases the odds of death by at least 50% (1, 2). When multidimensional assessments of social relationships were considered, the odds of mortality increased by 91% among the socially isolated (2). The magnitude of this effect is comparable to that of smoking and exceeds those of many other known risk factors of mortality, such as obesity or physical inactivity (2, 3). Although much evidence has accrued on the strong causal associations between social relationships and mortality as well as other health outcomes (4–7), important questions remain as to how social relationships affect health, when these effects emerge, and how long they last (8).
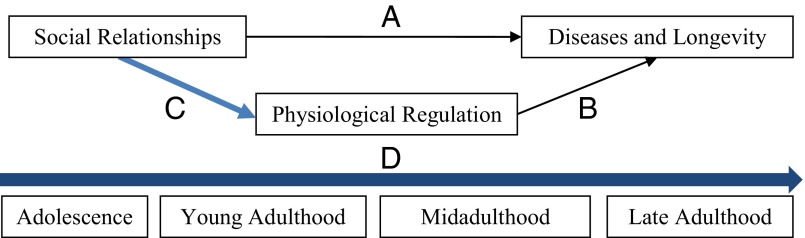
Studies of social, psychological, and behavioral mechanisms underlying the social relationship gradient in health have shed light on the first question (9–11). It is less clear, however, what biological mechanisms may be at play. Recent research on the biology of aging emphasizes the essential role of physiological stress response and regulation across multiple bodily systems in shaping longevity (12, 13). Although social relationship gradients in health and longevity (Fig. 1, path A) and physiological determinants of mortality (Fig. 1, path B) have been widely documented, these separate bodies of research have yet to be fully integrated. We have yet to determine whether social relationship differentials in longevity arise from a biological process in which social experiences “get under the skin” to alter physiological regulatory systems (Fig. 1, path C) (4).
Fig. 1.
A life course model of social relationship gradient in physical health: Mechanism and process. Empirical tests of the link represented in path C were applied in each stage of the life course trajectory D.
Laboratory research on rats using experimental designs demonstrated that social isolation and hypervigilance increase the incidence of mammary tumors (14, 15) and compromise innate immune response to stress (16). In humans, deficits in social relationships such as social isolation or low social support can similarly lead to chronic activation of immune, neuroendocrine, and metabolic systems that lie in the pathways, leading to cardiovascular, neoplastic, and other common aging-related diseases (5, 8, 17, 18). Previous nonexperimental studies using observational data from human subjects tentatively support this proposition by documenting associations between social relationship measures such as social integration and support with biomarkers of inflammation (5, 8, 18), metabolic syndrome (18, 19), and cumulative dysregulation indicated by allostatic load (20). However, because these associations are largely based on cross-sectional data, they cannot be assumed to represent underlying causal relationships. Additional prospective longitudinal studies are needed to better address bias due to potential confounding factors and reverse causality, further explaining path C.
Examining how social and biological processes unfold and interact as individuals age is a critical step in advancing scientific explanations of the emergence and progression of diseases. A life course perspective, represented by the horizontal arrow D as a developmental trajectory, has not been fully brought to bear on this question. This perspective may offer considerable leverage by linking physical risks to social exposures that occur over time across multiple developmental stages from early to late life. The vast majority of biosocial research to date on this association has focused on older adults for whom morbidity and mortality rates are high (8, 21, 22). However, early life social experiences may be biologically embedded at that time, shown by an increasing body of research linking childhood disadvantage and maltreatment to increased likelihood of exaggerated biological stress response and, in turn, higher risks of inflammation and cardiovascular disease throughout adulthood (23–26).
Relationship deficits—such as social isolation, lack of support, or high strain—are alternative forms of social adversity that can create chronic stress by continuous exposure to chains of risk that accumulate over the life course (27, 28). Individuals who experienced early adversity are subject to multiple and longer durations of stress exposures and more prone to inflammatory and stress-related diseases as they age. At the same time, the emergence of chronic diseases usually takes many decades due to the long latency after the initial risk exposures (29). Therefore, extensive longitudinal data and analyses are imperative to understanding how the connection of social relationships and longevity unfolds over the human life span. Little empirical research exists that depicts this lifelong process partly because data that extend sufficiently over long periods of the life course, as depicted in trajectory D, are exceedingly rare.
This study addresses the aforementioned questions, integrates previous research on social relationships and health across disciplines, and tests a new longitudinal model of how social relationships matter for physiological health across the human life span. We make three unique contributions that shed new light on path C across trajectory D. First, using data from an array of nationally representative longitudinal samples of the US population, we implement an innovative life course design that begins at the earliest developmental stage (adolescence) in which physiological consequences of key social relationship patterns begin to manifest and traces subsequent life stages (young, middle, late adulthood) to depict the life-long process of stress response cascades that such relationship patterns initiate. The data come from The National Longitudinal Study of Adolescent to Adult Health (Add Health) to capture adolescence and young adulthood, the National Survey of Midlife Development in the United States (MIDUS) for middle adulthood, and both the Health and Retirement Study (HRS) and the National Social Life, Health, and Aging Project (NSHAP) for late adulthood. The use of multiple large, population-based samples in an integrative design allows us to assess linkages between social relationships and health for each life stage. It also offers an unprecedented fuller view of age variations in such linkages than any previous study of a particular sample or single life stage alone. Second, this study uses comprehensive and refined measurements of social relationships that encompass two primary dimensions that may differentially influence physical health at different stages of the life course. It assesses measures of social integration to capture the structural–quantitative dimension and measures of social support and strain to capture the functional–qualitative dimension, using age-appropriate conceptualizations of these domains for each life stage. Third, the study examines multiple objectively measured biomarkers or endophenotypes including inflammation (C-reactive protein, CRP), cardiovascular function (hypertension), and energy metabolism (overall obesity and abdominal obesity) to capture key physiological mechanisms underlying common diseases of aging and longevity (12).
Results
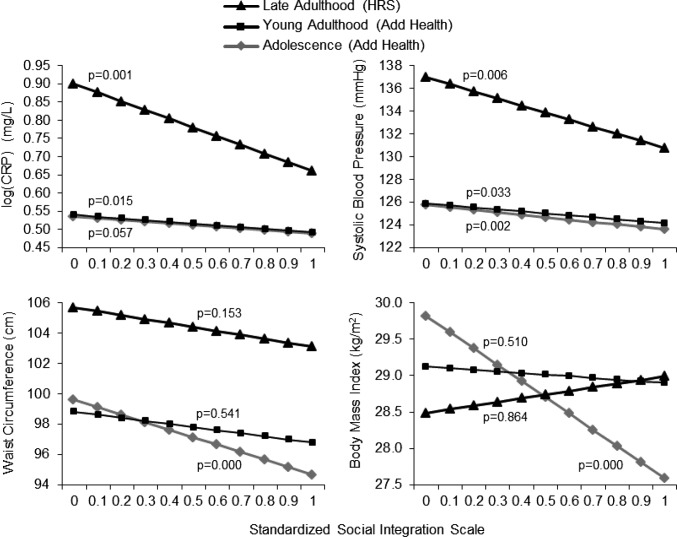
Our results indicate that social integration is related to better physiological functioning and lower risks of physical disorders in a dose–response fashion across life stage samples. Fig. 2 illustrates that individuals with a higher degree of social connectedness at prior points in time have lower predicted values of all four markers. Descriptive statistics on biomarkers, sample characteristics, and covariates are shown in Tables S1–S3, respectively. Adjusting for age, sex, and race, the negative associations of social integration with poor physical functioning were statistically significant for log-transformed CRP [log(CRP)] and systolic blood pressure (BP) in all three life stages shown, including adolescence and young and late adulthood. Additional analyses with diastolic BP revealed qualitatively similar results, so we presented results of systolic BP because of its clinical relevance for subsequent vascular and other chronic disease events and mortality as individuals age (30). Social integration gradients in waist circumference (WC) and body mass index (BMI) were also evident across samples, but the associations were significant only in adolescence. The association between social integration index and BMI estimated in the late adulthood HRS sample was in the opposite direction as expected but did not reach statistical significance to warrant substantive explanations. The results from the NSHAP analyses were similar to those from the HRS and were thus omitted from Fig. 2. We focused instead on the longitudinal change model results from NSHAP below.
Fig. 2.
Prospective associations of social integration with biomarkers of physiological functioning over the life course. Results based on ordinary least squares (OLS) models of biomarkers at follow-up regressed on baseline social integration, adjusting for age, sex, and race. HRS and NSHAP findings are similar, so we present the larger HRS sample results.
Table S1.
Sample characteristics: Weighted descriptive statistics of biomarkers
| Young adulthood | Young to midadulthood | Late adulthood | |||
| Add Health; age, 24–32 | MIDUS; age, 34–74 | HRS; age, 50–98 | NSHAP; age, 57–91 | ||
| Wave/survey year | IV, 2008–09 | II, 2004–06 | V, 2006 | I, 2005–06 | II, 2010 |
| CRP, mg/dL, mean (SD) | 3.8 (5.4) | 2.6 (3.8) | 2.2 (3.7) | 2.6 (4.0) | — |
| <1, normal | 34.5% | 40.6% | 46.8% | 42.3% | — |
| 1–3, low inflammation | 29.1% | 32.2% | 34.2% | 31.9% | — |
| 3–10, high inflammation | 27.5% | 24.2% | 16.5% | 22.5% | — |
| >10, very high inflammation | 9.0% | 3.0% | 2.6% | 3.3% | — |
| N | 6,747 | 863 | 4,323 | 1,153 | — |
| BP, mm Hg | |||||
| Systolic BP (SBP), mean (SD) | 125.2 (14.4) | 130.3 (17.3) | 130.6 (19.9) | 135.9 (19.4) | 137.5 (22.0) |
| Diastolic BP (DBP), mean (SD) | 79.6 (10.8) | 75.3 (10.3) | 80.1 (11.2) | 81.0 (11.1) | 80.2 (12.7) |
| Hypertension, SBP ≥ 140/DBP ≥ 90 | 27.1% | 50.2% | 66.6% | 70.0% | 73.5% |
| N | 7,561 | 863 | 4,323 | 1,516 | 1,516 |
| WC, cm, mean (SD) | 98.0 (18.1) | 96.6 (17.0) | 100.5 (14.4) | 97.6 (15.0) | 100.6 (16.9) |
| Abdominal obesity, >102/88a | 47.7% | 51.8% | 63.6% | 53.8% | 61.7% |
| N | 7,889 | 863 | 4,323 | 1,560 | 1,560 |
| BMI, kg/m2, mean (SD) | 29.0 (8.1) | 29.2 (5.9) | 29.1 (5.1) | 29.3 (6.0) | 29.7 (6.9) |
| Obesity (≥30) | 36.3% | 38.5% | 38.9% | 39.5% | 42.7% |
| N | 7,889 | 863 | 4,323 | 1,571 | 1571 |
For each of the datasets, the Ns refer to the largest analytic sample sizes reported in Table 1 for each of the biomarkers; the statistics for the MIDUS was not weighted because weights are not available for the biomarkers sample.
Sex-specific cut points, 102 for males and 88 for females.
Table S3.
Sample characteristics and weighted descriptive statistics of covariatesa
| Add Health, n = 7,889 | MIDUS, n = 863 | HRS, n = 4,323 | NSHAP, n = 1,571 | |||||
| Variable | Mean (SD) or % | Range | Mean (SD) or % | Range | Mean (SD) or % | Range | Mean (SD) or % | Range |
| Age, years | 28.2 (01.9) | 24–32 | 44.0 (9.9) | 25–64 | 67.3 (10.5) | 50–98 | 67.3 (7.1) | 57–85 |
| Sex, 1 = female | 48.4% | — | 55.5% | — | 54.9% | — | 53.3% | — |
| Race | ||||||||
| White | 69.2% | — | 92.4% | — | 86.5% | — | 85.3% | — |
| Black | 16.3% | — | 2.9% | — | 13.5% | — | 8.0% | — |
| Hispanic | 9.3% | — | 2.1% | — | 6.7% | — | ||
| Others | 5.2% | 2.6% | — | |||||
| Educational attainment | (Parental) | |||||||
| High school or less | 41.4% | — | 26.5% | — | 49.1% | — | 70.8% | — |
| Some college | 30.9% | — | 29.1% | — | 50.9% | — | ||
| Bachelor's degree or higher | 20.8% | — | 44.4% | — | 29.2% | — | ||
| Family income, in thousands | 80.6 (61.4) | 0–300 | 66.5 (128.9) | 0–7,000 | ||||
| Family structure | ||||||||
| Two-parent, biological | 56.4% | — | ||||||
| Two-parent, step | 16.2% | — | ||||||
| Single mother | 19.4% | — | ||||||
| Single father or other | 8.0% | — | ||||||
| Smoking, 1 = current/former smoker | 24.3% | — | 43.3% | — | 56.3% | — | 51.1% | — |
| Physical activity, 1 = <3 times per week | 14.1% | — | 20.9% | — | 46.2% | — | 33.1% | — |
| Alcohol drinks, 1 = excessive drinking | 1.9% | 3.5% | — | |||||
| Depressive symptoms, CES-D | 5 (4.3) | 0–15 | 7.8 (7.7) | 0–49 | 1.4 (1.8) | 0–8 | 4.3 (4.1) | 0–29 |
| Perceived stress, Cohen’s scale | 4.7 (3.1) | 0–16 | 21.6 (6.2) | 10–48 | 5.6 (2.1) | 0–16 | ||
| Diabetes, 1 = ever diagnosed | 0.4% | — | 8.6% | — | 17.9% | — | ||
| Antihypertensive medication use, 1 = yes | 31.9% | — | 46.1% | — | 56.9% | — | ||
| Cholesterol medication use, 1 = yes | 27.1% | — | 13.9% | — | ||||
| Corticosteroid medication use, 1 = yes | 12.7% | — | ||||||
| Antidepressant medication use, 1 = yes | 15.2% | — | ||||||
Most covariates were measured at the baseline. Exceptions include smoking, physical activity, and depressive symptoms (CES-D); perceived stress (Add Health and the MIDUS); and medication uses (MIDUS), which were measured at the same wave as the outcome variables.
In addition to lower risks of poor functioning, more socially integrated individuals also had lower odds of deleterious physical outcomes that are of clinical significance. Table 1 shows that in the basic models adjusting for age, sex, and race, a higher social integration index score was related to a 25% [Odds Ratio (OR) = 0.74, 95% confidence interval (CI) = 0.57–0.97, P = 0.031] and 14% (OR = 0.86, 95% CI = 0.78–0.94, P = 0.001) lower odds of elevated inflammation in adolescence and young adulthood (Add Health) and late adulthood (HRS), respectively. The estimated OR for the NSHAP sample at baseline also shows a negative association resulting in a 40% (OR = 0.59, 95% CI = 0.37–0.95, P = 0.029) lower odds of inflammation. Adjusting for covariates reduced some but not all of these associations, indicating that the physiological effects of social integration are partially mediated by socioeconomic status, health risk behavior (i.e., smoking, physical activity, obesity), and prior chronic disease. To put the magnitude of the effects in perspective, additional analyses revealed that social isolation raised the odds of high inflammation (OR = 1.27, P = 0.05) to a comparable degree with physical inactivity (OR = 1.21, P = 0.05) (Add Health analyses).
Table 1.
Estimated ORs of the linkages between social integration and biomarkers of physiological dysregulation across the life course (95% confidence intervals)
| Adolescence | Young adulthood | Young to midadulthood | Late adulthood | |||
| Biomarker outcomesa | Model | Add Health; age 12–18 | Add Health; age 24–32 | MIDUS; age 25–64 | HRS; age 50–98 | NSHAPb; age 57–91 |
| Inflammation | Basicc | 0.74* | 0.76* | 1.03 | 0.86** | 0.59* |
| (0.57, 0.97) | (0.61, 0.96) | (0.53, 1.98) | (0.78, 0.94) | (0.37, 0.95) | ||
| Fulld | 0.99 | 0.79† | 1.07 | 0.89* | 0.77 | |
| (0.76, 1.29) | (0.62, 1.00) | (0.55, 2.10) | (0.81, 0.98) | (0.48, 1.23) | ||
| N | 6,747 | 6,747 | 863 | 4,323 | 1,153 | |
| Hypertension | Basicc | 0.90 | 1.12 | 1.74 | 0.87* | 0.46** |
| (0.65, 1.24) | (0.80, 1.57) | (0.81, 3.76) | (0.78, 0.89) | (0.25, 0.85) | ||
| Fulld | 1.09 | 1.16 | 1.69 | 0.89* | 0.41** | |
| (0.78, 1.52) | (0.83, 1.63) | (0.73, 3.93) | (0.80, 0.99) | (0.22, 0.76) | ||
| N | 7,561 | 7,561 | 863 | 4,323 | 1,433 | |
| Abdominal | Basicc | 0.52*** | 0.81** | 1.28 | 0.96 | 0.71 |
| Obesity | (0.39, 0.68) | (0.63, 1.03) | (0.62, 2.65) | (0.86, 1.07) | (0.29, 1.79) | |
| Fulld | 0.75 | 0.68† | 0.81 | 1.01 | 0.91 | |
| (0.49, 1.13) | (0.44, 1.04) | (0.26, 2.53) | (0.90, 1.14) | (0.38, 2.20) | ||
| N | 7,889 | 7,889 | 863 | 4,323 | 1,423 | |
| Overall | Basicc | 0.59*** | 0.97 | 1.50 | 1.03 | 0.34* |
| Obesity | (0.44, 0.78) | (0.75, 1.26) | (0.72, 3.12) | (0.93, 1.14) | (0.13, 0.86) | |
| Fulld | 0.66** | 1.07 | 1.70 | 1.09 | 0.44† | |
| (0.49, 0.88) | (0.82, 1.40) | (0.79, 3.65) | (0.97, 1.22) | (0.19, 1.00) | ||
| N | 7,889 | 7,889 | 863 | 4,323 | 1,388 | |
***P < 0.001, **P < 0.01, *P < 0.05, †P < 0.1 (two-tailed test).
Measures and cut points used for each marker in Table S1.
Results for inflammation are based on data at wave 1 only; results for the other three markers are based on data at waves 1 and 2 and longitudinal residual change models.
Adjusted for age, sex, and race.
Adjusted for the full set of covariates in Table S3 for each dataset.
The results for other biomarkers show large age variations in their associations with the social integration index. The protective effects of social integration were particularly large in adolescence (OR = 0.52, 95% CI = 0.39–0.68, P = 0.000) against abdominal obesity and particularly salient in old age against hypertension. In the basic model, the OR estimated for the HRS sample shows a significant 13% lower odds of hypertension (OR = 0.87, 95% CI = 0.78–0.89, P = 0.013) at follow-up for those with a higher mean social integration level earlier in time. The corresponding estimates for the NSHAP sample from the longitudinal residual change model show a 54% reduction in the odds of developing hypertension (OR = 0.46, 95% CI = 0.25–0.85, P = 0.014) over time for those with a higher baseline social integration score.
In both studies, these associations remained robust after adjusting for all of the other covariates in the full models. We note that with an interval of 6 y in the elderly NSHAP sample who already had a high prevalence of hypertension at baseline, a change in risk of this size was substantial. Specifically, the effect of social isolation (OR = 2.42, P = 0.007) on hypertension risk exceeded the effect of diabetes (OR = 1.49, P = 0.059), a well-known risk factor for hypertension at older ages. The associations of social integration with overall obesity are significant in both early and late life. The OR estimates indicate strong protective effects of social connectedness against obesity in adolescence in the basic model and also at old age in the change models estimated for the NSHAP sample.
We did not find any significant results regarding the social integration–biomarker associations in the MIDUS sample, which may be due to empirical and substantive factors. Compared with other studies, the MIDUS sample with biomarkers is more homogeneous, consisting of mostly white respondents with higher levels of educational attainment and household income. However, supplemental analyses restricted to only white respondents with the other three studies did not change their results. [To further examine whether the associations between integration and biomarkers in the MIDUS sample were generalizable, we conducted parallel analyses using a middle-aged subsample (age 34–74) of the National Health and Nutrition Examination Survey (NHANES 1999–2006) (31). Consistent with the MIDUS results, higher social integration among middle-aged NHANES respondents was not protective against physiological risks.] Therefore, the racial and socioeconomic (SES) composition of the MIDUS sample is unlikely to account for its lack of consistency with the other samples. Further scrutiny of the MIDUS data revealed that ∼80% of the sample reported weekly family and friend contact, indicating higher overall reports of integration than the other-aged samples. We therefore used a more stringent cutoff for the frequency of social contact in the MIDUS analysis, but these results continue to show that the degree of social embeddedness has less variation in midlife such that the sheer number of social connections does not differentiate individuals in terms of physical health risks.
We next addressed whether the quality of social relationships matters for health, given the quality of relationships is not informed by a count of social ties and interactions. Social support and strain measures are summarized in Table S2. In general, we found that perceived social support and strain measures are related to physiological indicators differently from social integration in that associations are more modest, nonlinear, and particularly salient for midadulthood. Most respondents in study samples with available information on quality of social relationships reported adequate levels of social support and little strain. Even though variations in the quality of relationships are small, those who perceived more support or strain are different from others in both physical functioning and clinical risk factors. Models including both social integration and social support and strain show similar results from those including them separately, suggesting largely independent associations of each social relationship dimension with biomarkers. The results reported below thus relate to models of social support and social strain without the inclusion of social integration.
Table S2.
Social relationships: Measurements and weighted descriptive statistics
| Variables/items | Coding | Mean (SD) or % |
| 2.1. Add Health; wave I, n = 7,889 | ||
| Social integration index | Sum of 4 items; standardized to range 0–1 | 0.3 (0.3) |
| Parent contact | 1 = average parent activities per week ≥ 5; 0 = otherwise | 27.7% |
| Friend count | 1 = in-degree friend nominations ≥ 7; 0 = otherwise | 24.4% |
| Religious attendance | 1 = attends church ≥ 12 times a year; 0 = otherwise | 59.8% |
| Activities participation | 1 = school activities ≥ 4; 0 = otherwise | 23.8% |
| Social support | Sum of 11 items; 1 = high support for the top quartile | 3.7 (2.6) |
| Parent support | ||
| You feel close to your mother/father | 1 = strongly agree; 0 = disagree, strongly disagree, neither agree or disagree, or agree (average mother and father score if necessary) | 55.0% |
| Your mother/father is warm and loving | 33.3% | |
| You are satisfied with your communication with your mother/father | 28.2% | |
| You are satisfied with your relationship with your mother/father | 38.4% | |
| Family support | ||
| Your parents care about you | 1 = strongly agree; 0 = strongly disagree, disagree, neither agree or disagree, or agree | 85.4% |
| Your family understands you | 19.3% | |
| You have fun with your family | 24.9% | |
| Your family pays attention to you | 29.1% | |
| School cohesion | ||
| You feel close to people at your school | 1 = strongly agree; 0 = strongly disagree, disagree, neither agree or disagree, or agree | 20.4% |
| You feel like a part of your school | 24.0% | |
| You are happy at your school | ||
| 2.2. Add Health; wave IV, n = 7,889 | ||
| Social integration index | Sum of 4 items; standardized to range 0–1 | 0.4 (0.3) |
| Marital status | 1 = married or cohabitating; 0 = otherwise | 59.2% |
| Friend count | 1 = number of close friends ≥6; 0 = otherwise | 31.7% |
| Religious attendance | 1 = attends church ≥12 times a year; 0 = otherwise | 36.5% |
| Volunteering | 1 = volunteered in past year ≥ 1 time; 0 = otherwise | 36.1% |
| 2.3. MIDUS; waves I and II, n = 863a | ||
| Social integration index, n = 863 | Sum of 7 items; standardized to range 0–1 | 0.4 (0.2) |
| Family contact | 1 = once a day or more; 0 = otherwise | 34.6 |
| Friend contact | 1 = once a day or more; 0 = otherwise | 30.4 |
| Marital status | 1 = married; 0 = otherwise | 72.1 |
| Neighbor contact | 1 = almost every day; 0 = otherwise | 43.0 |
| Religious attendance | 1 = once a week or more; 0 = otherwise | 41.7 |
| Activities participation | 1 = top quartile; 0 = lower quartiles; range, 0–37 | 20.9 |
| Volunteer work | 1 = top quartile; 0 = lower quartiles; range, 0–216 | 24.2 |
| Social support, n = 599 | Sum of 14 items; 1 = high support for the top quartile | 9.3 (3.2) |
| Family/friend support—How much: | 1 = a lot; 0 = some, a little, or not at all | Family/friend |
| Do family/friends care about you? | 83.6/54.0 | |
| Do they understand the way you feel? | 44.6/35.8 | |
| Can you rely on them? | 75.0/52.8 | |
| Can you open up to them? | 54.3/41.7 | |
| Spouse support—How much: | ||
| Does your spouse care about you? | 86.6 | |
| Does he/she understand the way you feel? | 46.2 | |
| Can you rely on him/her? | 82.6 | |
| Can you open up to him/her? | 62.0 | |
| Does he/she appreciate you? | 67.4 | |
| Can you be yourself around him/her? | 79.7 | |
| Social strain, n = 599 | Sum of 14 items; 1 = high strain for the top quartile; 1 = a lot or some; 0 = a little or not at all | 3.8 (2.8) |
| Family/friend support—How much: | Family/friend | |
| Do family/friends make too many demands? | 26.5/9.3 | |
| Do they criticize you? | 20.0/6.8 | |
| Do they let you down? | 17.6/11.6 | |
| Do they get on your nerves? | 30.2/16.4 | |
| Spouse support—How much: | ||
| Does your spouse make too many demands? | 33.6 | |
| Does he/she criticize you? | 31.3 | |
| Does he/she let you down? | 15.7 | |
| Does he/she get on your nerves? | 34.7 | |
| Does he/she argue with you? | 39.0 | |
| Does he/she make you feel tense? | 35.7 | |
| 2.4. HRS; waves I–V, n = 4,323a | ||
| Social integration index, n = 4,323 | Sum of 5 items; standardized to range 0–1 | 0.6 (0.2) |
| Marital status | 1 = married or cohabiting; 0 = otherwise | 73.7 |
| Parent contact | 1 = once a week or more; 0 = otherwise | 20.5 |
| Children contact | 86.0 | |
| Neighbor contact | 54.2 | |
| Volunteer | 1 = ever volunteered in the past year; 0 = otherwise | 38.9 |
| Social support, n = 812, waves IV and V | Sum of 12 items; 1 = high support for the top quartile | 4.6 (2.8) |
| Spouse support—How much: | 1 = a lot; 0 = some, a little, or not at all | |
| Does he/she understand the way you feel? | 39.8 | |
| Can you rely on him/her? | 63.7 | |
| Can you open up to him/her? | 44.3 | |
| Children support—How much: | ||
| Do they understand the way you feel? | 35.4 | |
| Can you rely on them? | 55.6 | |
| Can you open up to them? | 35.8 | |
| Family support—How much: | ||
| Do they understand the way you feel? | 24.1 | |
| Can you rely on them? | 39.1 | |
| Can you open up to them? | 28.1 | |
| Friends support—How much: | ||
| Do they understand the way you feel? | 30.1 | |
| Can you rely on them? | 36.2 | |
| Can you open up to them? | ||
| Social strain, n = 812, wave IV and V | Sum of 12 items; 1 = high strain for the top quartile | 1.8 (1.8) |
| Spouse strain—How much: | 1 = a lot or some; 0 = a little or not at all | |
| Do he/she criticize you? | 25.9 | |
| Do he/she let you down? | 13.6 | |
| Do he/she get on your nerves? | 17.3 | |
| Children strain—How much: | ||
| Do they criticize you? | 15.9 | |
| Do they let you down? | 18.4 | |
| Do they get on your nerves? | 16.3 | |
| Family strain—How much: | ||
| Do they criticize you? | 15.6 | |
| Do they let you down? | 17.9 | |
| Do they get on your nerves? | 16.6 | |
| Friends strain—How much: | ||
| Do they criticize you? | 6.7 | |
| Do they let you down? | 10.8 | |
| Do they get on your nerves? | 7.7 | |
| 2.5. NSHAP; wave I, n = 1,571 | ||
| Social integration index, n = 1,433 | Sum of 6 items; standardized to range 0–1 | 0.5 (0.2) |
| Marital status | 1 = married or cohabiting; 0 = otherwise | 73.7 |
| Volunteering | 1 = once a month or more; 0 = otherwise | 32.5 |
| Organized meeting participation | 1 = once a week or more; 0 = otherwise | 28.4 |
| Socializing with neighbors | 1 = several times per month or more; 0 = otherwise | 39.1 |
| Socializing with family or friends | 1 = once a week or more; 0 = otherwise | 53.1 |
| Religious attendance | 1 = every week or more; 0 = otherwise | 50.7 |
| Social support, n = 1,571 | Sum of 6 items; 1 = high support for top quartile | 3.2 (1.5) |
| Spouse support—How often: | 1 = often; 0 = some of the time or never | |
| Can you open up to your spouse? | 61.5 | |
| Can you rely on your spouse? | 67.9 | |
| Family support—How often: | ||
| Can you open up to family? | 46.4 | |
| Can you rely on family? | 69.7 | |
| Friend support—How often: | ||
| Can you open up to friends? | 29.0 | |
| Can you rely on friends? | 47.0 | |
| Social strain, n = 1,527 | Sum of 6 items; 1 = high strain for the top quartile | 1.4 (1.3) |
| Spouse strain—How often: | 1 = often or some of the time; 0 = never | |
| Does your spouse criticize you? | 34.7 | |
| Does your spouse make too many demands? | 29.6 | |
| Family strain—How often: | ||
| Does your family criticize you? | 22.8 | |
| Does your family make too many demands? | 29.3 | |
| Friend strain—How often: | ||
| Do your friends criticize you? | 12.6 | |
| Do your friends make too many demands? | 11.9 |
Longitudinal measures were available. For MIDUS, we averaged the values at waves I and II [no statistically significant difference in measure properties or in association with biomarker outcomes was detected across waves in this and previous studies (23)]. For HRS, we used the values of the social integration index from all five waves to capture the trajectory of change over time (see, e.g., ref. 15) in latent growth curve analyses and averaged the values of social support and social strain variables at waves IV and V in other analyses.
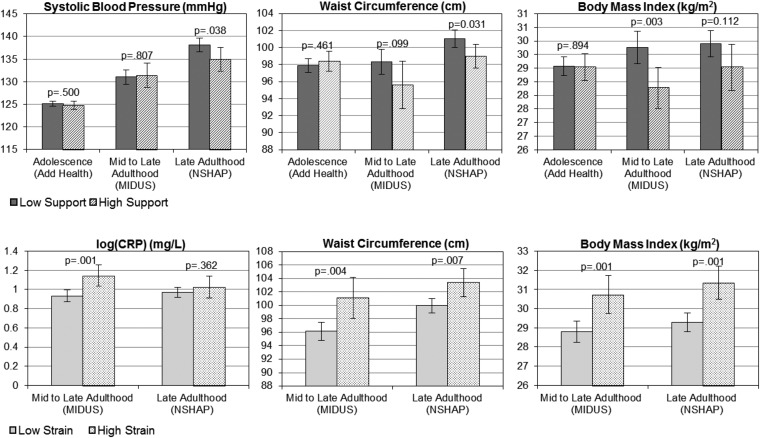
Fig. 3 illustrated that adjusting for age, sex, and race, those with high (top quartile) social support at the baseline show significantly lower mean values of three markers assessed at the follow-ups than those with lower social support, including systolic BP at old age (P = 0.038), WC at young to middle age (P = 0.099) and old age (P = 0.031), and BMI at young to middle age (P = 0.003). Compared with those with low strain, those with high (top quartile) strain at the baseline exhibited significantly higher mean values of CRP at the follow-up in midadulthood and higher mean values of WC and BMI at the follow-up in both mid- and late adulthood. Results from the HRS analyses were similar to the results of the NSHAP analyses and are not shown.
Fig. 3.
Prospective associations of social support and strain with biomarkers of physiological functioning over the life course. OLS models of biomarkers at follow-up regressed on baseline dichotomous measures of social support and social strain, adjusting for age, sex, and race. HRS and NSHAP findings are similar, so we present the larger HRS sample results.
The results for binary outcomes based on clinical thresholds are summarized in Table S4. In these models, continuous measures of social support and social strain yielded superior model fit than the dichotomous measures. Higher social support was associated with lower odds of abdominal and overall obesity in young to midadulthood (MIDUS sample only). Such effects were moderate in size and mostly eliminated or explained away by the adjustment of additional covariates. Higher social strain was predictive of mildly increased odds of inflammatory response and abdominal obesity in young to midadulthood and more substantial increases in the odds of overall obesity in both mid- and late adulthood that persisted after the adjustment of the full set of covariates.
Table S4.
Estimated ORs of the linkages between social support and strain and biomarkers of physiological dysregulation across the life course (95% confidence interval)
| Social support | Social strain | |||||||
| Adolescence | Young to midadulthood | Late adulthood | Young to midadulthood | Late adulthood | ||||
| Biomarker outcomesa | Model | Add Health; age, 12–18 | MIDUS; age, 25–64 | HRS; age, 50–98 | NSHAPb; age, 57–91 | MIDUS; age, 25–64 | HRS; age, 50–98 | NSHAPb; age, 57–91 |
| Inflammation | Basicc | 1.04 | 1.00 | 0.98 | 1.01 | 1.06† | 1.05 | 1.01 |
| (0.90, 1.21) | (0.95, 1.05) | (0.94, 1.03) | (0.92, 1.11) | (1.00, 1.12) | (0.98, 1.13) | (0.93, 1.10) | ||
| Fulld | 1.09 | 1.04 | 1.01 | 0.98 | 1.01 | 1.01 | 0.96 | |
| (0.94, 1.26) | (0.99, 1.10) | (0.96, 1.06) | (0.88, 1.09) | (0.95, 1.08) | (0.94, 1.09) | (0.87, 1.06) | ||
| N | 6,747 | 599 | 801 | 860 | 599 | 801 | 860 | |
| Hypertension | Basic | 0.92 | 0.98 | 0.97 | 0.95 | 1.05 | 0.99 | 0.95 |
| (0.77, 1.11) | (0.93, 1.04) | (0.92, 1.03) | (0.86, 1.05) | (0.99, 1.12) | (0.91, 1.07) | (0.84, 1.08) | ||
| Full | 0.92 | 1.03 | 0.99 | 0.95 | 1.01 | 0.95 | 0.96 | |
| (0.76, 1.11) | (0.97, 1.10) | (0.94, 1.05) | (0.84, 1.06) | (0.94, 1.08) | (0.87, 1.04) | (0.83, 1.11) | ||
| N | 7,561 | 599 | 802 | 1,516 | 599 | 802 | 1,474 | |
| Abdominal obesity | Basic | 1.04 | 0.94* | 0.93* | 0.97 | 1.08* | 1.09* | 1.09 |
| (0.90, 1.22) | (0.89, 0.99) | (0.88, 0.98) | (0.88, 1.06) | (1.01, 1.14) | (1.00, 1.18) | (0.98, 1.22) | ||
| Full | 1.09 | 1.03 | 0.96 | 1.01 | 0.94 | 1.04 | 1.01 | |
| (0.87, 1.36) | (0.95, 1.11) | (0.91, 1.02) | (0.91, 1.11) | (0.84, 1.04) | (0.95, 1.14) | (0.89, 1.15) | ||
| N | 7,889 | 599 | 801 | 1,560 | 599 | 801 | 1,516 | |
| Overall | Basic | 1.03 | 0.95† | 0.96 | 0.93 | 1.12*** | 1.12** | 1.17** |
| Obesity | (0.87, 1.21) | (0.90, 1.00) | (0.91, 1.01) | (0.83, 1.04) | (1.05, 1.19) | (1.03, 1.21) | (1.06, 1.29) | |
| Full | 1.00 | 0.97 | 0.99 | 0.94 | 1.11** | 1.09* | 1.17** | |
| (0.85, 1.18) | (0.91, 1.03) | (0.93, 1.04) | (0.85, 1.05) | (1.03, 1.19) | (1.00, 1.19) | (1.05, 1.30) | ||
| N | 7,889 | 599 | 812 | 1,571 | 599 | 812 | 1,527 | |
***P < 0.001, **P < 0.01, *P < 0.05, †P < 0.1 (two-tailed test).
Measures and cut points used for each marker in Table S1.
Results for inflammation are based on data at wave 1 only; results for the other three markers are based on data at waves 1 and 2 and longitudinal residual change models.
Adjusted for age, sex, and race.
Adjusted for the full set of covariates in Table S3 for each dataset.
Discussion
By combining data from four large nationally representative, population-based, contemporary studies using an innovative longitudinal life course design, this study has provided previously unidentified causal evidence on the mechanisms linking social relationship patterns with health and longevity across the human life span. Although the links represented in Fig. 1 have been found to be strong in past social research that focused on path A, as well as biological research that focused on path B, our study provided evidence that connects paths A and B through path C and, for the first time to our knowledge, documented the life course process in which these paths unfold as depicted in trajectory D.
Our study strengthened support for causal linkages between social relationships and physical functioning embodied in path C by improving both the measurement and analyses of these linkages. First, our study engaged a more comprehensive set of measures of relationship characteristics across multiple social life domains that are specific to each life stage and harmonized across four nationally representative studies. We also examined the associations between these relationship indicators and biomarkers across multiple physiological systems also harmonized across studies. The common patterns that emerged from the study of multiple measures of both social exposures and biological outcomes strongly support the generality of the associations, indicating social integration protects health and promotes longevity. Second, the use of longitudinal data for lagged measures of social exposures in relation to subsequent measures of biomarkers in all study samples ensured the proper temporal order in causal relationships underlying the association. Although the lack of repeated longitudinal measures of biomarkers in three study samples precludes causal inference of the physiological impact of social relationships, the NSHAP study allowed us to model change in physiological function as an outcome using two waves of biomarker measurements and hence provided a more rigorous test of the causal effects of interest. Although we cannot eliminate the possibility that some prior condition affects both social relations and physiology, this limitation relates more to biomarker data measured at one point in time than those that include repeated biomarker measures over time. We cannot rule out the potential effect of mortality selection of older or frailer adults. The results are thus likely conservative regarding the full range of physiological effects of social relations. Furthermore, the results are not only consistent across studies included but also consistent with those obtained in previous research across diverse samples such as laboratory animals (14–16) and clinical populations (5) and study designs such as experiments (14–16) and mortality follow-ups (6–8). They provide robust and uniform support for the existence and causal nature of path C.
In addition, our study identified the multifaceted links of different dimensions of social relations to physical health. A measurement approach including more than one type of relationship measure may better represent the multiple pathways by which social relations affect health (2). By assessing both the structural and functional dimensions of social relationships, we found that particular network and support characteristics may have unique influences on health. Extending previous research, we found that the links between social embeddedness and better physical functioning, as well as lower clinically significant disease risks, are exceptionally strong across most biological markers examined. Greater perceived quality of network ties was also beneficial for some markers with more variation across the life course. We further assessed the quality of social relationships by distinguishing positive and negative functions of network connections. We found evidence for social strain as a more significant predictor of (worsening) physiological outcomes than commonly examined social support measures hypothesized to improve health. This suggests the importance of considering psychosocial distress that may arise from negative social exchanges in future investigations of social relationships and health links.
The longitudinal life course design that we used in trajectory D elucidates when and how social integration matters for health as the aging process unfolds. We found evidence for the early emergence and persistence of the path C linkage between social relationships and physical health over the life course. We did not include the childhood life stage in this study because we focus more on meaningful social connections beyond familial contexts. Embeddedness in social networks seems to be especially critical for health during the formative years of building social relationships in adolescence and in the later adult years when the maintenance of social connections are relevant. The robust findings that social integration during adolescence (and not during young adulthood) matters for young adult metabolic and cardiovascular healthy functioning suggest that these early relationship contexts and connections play a role in predisease pathways into adulthood. The young adult years are considered to be the healthy years of the life span, yet adolescent social connections differentiate health risks in young adults, long before symptoms or overt signs of disease emerge.
After the busy years of midlife, maintaining social connections in older adulthood plays a vital role in protecting health. Chronic conditions naturally increase during late adulthood as part of the aging process. However, socially embedded older adults experience fewer disease risks, and our results from the NSHAP analyses suggest a causal role of social connections in reducing hypertension and obesity in old age. The deleterious effect of social isolation, in particular, was estimated to exceed that of diabetes, a well-known clinical risk factor for many chronic diseases including hypertension. Our findings therefore point to two life stages when the development and maintenance of social relationships can be especially critical for reducing future health risks and, in turn, reducing the high cost and consequences of chronic disease for individuals, families, and society as a whole.
The age variations we found for these associations also depend on relationship measures. Whereas the size of social networks was consistently important to physical health in both early and late adulthood, network size was not found to be significantly related to any biomarkers in midadulthood. Our additional analyses suggest that this is unlikely to be a methodological artifact. Several substantive explanations are possible. On one hand, adults in midlife are naturally embedded in multiple social networks associated with this life stage, including those at work, in the community, with children and other parents, and aging parents. Social integration is not a discriminating factor in midlife for most adults. On the other hand, these multiple social connections in midlife are potentially stressful in nature. Prior research shows that greater role conflict across multiple social domains and across generations is especially prevalent during this life stage (32). Therefore, the perceived quality of social relationships rather than the density of one’s social network may better capture the link between social ties and health in midlife.
In fact, this conclusion is supported by our finding that social support and strain, which measured qualitative characteristics of social connections that are distinct from relationship quantity, mattered more for physical health in midadulthood and continued to have impacts in late adulthood. In adolescence, however, social integration was more pertinent to physical health than social support as perceived by individuals. This life course pattern of variation in the relative importance of social integration and support to physiological functioning was previously unknown and can have new practical import for policy intervention.
Our study sheds light on the biological mechanisms through which social relationships impact health across the human life span. Our findings suggest the early emergence and continuity of the physiological impacts of social relationships across the life course. They also suggest physiological vulnerabilities to social stress that may be specific to life course stages and relationship stressors. Disrupting various social relation deficits and physiological risk connections could directly arrest early progression toward chronic diseases and delay disease onset or lessen the disease burden in late life. The findings, therefore, provide a strong scientific basis for effective prevention and intervention that will lead to further improvement in life expectancy.
Materials and Methods
We used longitudinal data from four nationally representative studies to test path C and collectively cover all stages of the life course depicted in developmental trajectory D (Fig. 1). Refer to Data Description for details on data description.
The physical health biomarkers that we examine are continuous measures of CRP, systolic and diastolic BP, WC, and BMI, which represent important biological pathways underlying stress response that strongly predict future disease and mortality (12). We also used categorical measures based on clinical cut points that indicate the corresponding disease outcomes including inflammation, hypertension, abdominal obesity, and obesity. Weighted descriptive statistics for each biomarker sample and dataset were reported in Table S1. For social relationship variables, we measured (i) social integration to capture the structural and quantitative components of relationships and (ii) social support and social strain to assess the quality of social connections in terms of the functions these connections served. Details on scale construction and descriptive statistics for relationship variables are included in Table S2.
We conducted multivariate regression analyses to examine the prospective associations between social relationship characteristics and biomarkers using the longitudinal design unique to each dataset. For three studies where biomarkers were assessed at the follow-up surveys only (Add Health, MIDUS, and HRS), we estimated models of biomarker outcomes in relation to social relationship measures assessed at prior points in time. Because the HRS included five waves of data on social integration (1998–2006), we estimated the latent growth curve models to most effectively summarize individual trajectories (19) (see Fig. S1 for HRS model specification). The NSHAP provided repeated measures of three other biomarkers at both baseline and the follow-up, including BP, WC, and BMI. Therefore, we used longitudinal residual change models to examine the effects of social relationships at wave I on changes in these biomarkers over time from wave I to wave II. This design offers a more rigorous test of potential causality, as it minimizes the risks of both reverse causality and spuriousness (e.g., that some other variable is affecting both biomarkers and social relationships). For each outcome variable, we estimated models in a stepwise fashion: (i) basic models of individual social relationship variables adjusting for age, sex, and race; (ii) models of all social relationship variables (when available for the samples) adjusting for age, sex, and race; and (iii) full models adjusting for all covariates that are strong risk factors for inflammation, metabolic, and cardiovascular dysregulation and may contribute to their associations with social relationships. See Data, Measures, and Methods for a detailed description of analytic approaches for each sample and Table S3 for covariates included in fully adjusted models.
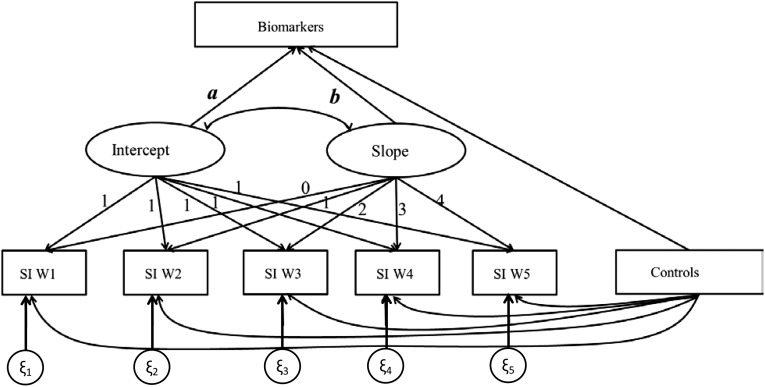
Fig. S1.
Representation of the latent growth curve model and hypothesized relationships between social integration and biomarkers from HRS.
Data, Measures, and Methods
We used longitudinal data from four nationally representative NIH-funded studies that include high-quality measures of both social relationships and physiological markers key to test path C and collectively cover all stages of the life course depicted in developmental trajectory D. Data for adolescence and young adulthood come from 7,889 participants in Add Health aged 12–19 at wave I (1994–95) and followed up at ages 24–32 in wave IV (2008–09). Data for young to midadulthood come from 863 respondents aged 25–64 in MIDUS surveyed at wave I (1995–96) and followed up at wave II (2004–09). Data for late adulthood come from two studies, including 4,323 participants aged 50 and older in the HRS at the baseline year of 1998 and followed up every 2 y to 2006, as well as from 1,571 participants aged 57–85 in theNSHAP at wave I (2005–06) and followed up at wave II (2010–11). Please refer to Data Description for details on data description.
The biomarkers of interest to this study represent important biological pathways underlying stress response that strongly predict future disease and mortality (12). The collection of these biomarkers varied across studies. We focused on four biomarker measurements that were available in all studies at the follow-up surveys and also at the baseline for the NSHAP (Data Description). Innate immune function was assessed by CRP levels, an acute phase protein whose elevation in circulating level indicates systemic inflammation (12). Cardiovascular function was assessed by diastolic and systolic BP, the latter of which is strongly related to vascular and other chronic disease events across the life course (33). Metabolic function was assessed by WC and BMI based on measured weight and height. WC complements BMI by accounting for body composition that has shown to be more directly linked to metabolic disorders and obesity-related health risks than BMI alone (34). Inflammatory and metabolic disorders represent risk factors for cardiovascular disease, so these markers do not correspond to mutually exclusive categories of physical functions. We used both the continuous measures that directly indicate physiological functioning and categorical measures based on clinical cut points that indicate the corresponding disease outcomes including inflammation, hypertension, abdominal obesity, and obesity. Weighted descriptive statistics for each biomarker sample and dataset were reported in Table S1.
We used social relationship measures at baseline that were collected before the biomarker outcomes in all studies and also at the follow-ups for MIDUS and HRS (Data Description). We measured social integration, the structural and quantitative components of relationships, by a summary index that indicates the number and nature of social ties across relationship domains including romantic relationships (e.g., marital/cohabitation status), family and friends (e.g., frequency of interaction with family, friends, and relatives), religious institutions (e.g., church attendance), and the community (e.g., membership in social organizations, volunteering) (8). The details on scale construction and descriptive statistics for this and other relationship variables are included in Table S2. Items used in the social integration index for each study sample are largely similar and measure the same relationship domains specific to each life course stage. The one exception is for the adolescent life stage measured in Add Health, which focuses on the more salient parental relationships than more temporary and immature romantic relationships during adolescence. To facilitate comparison of results across datasets, we constructed standardized scores of the social integration index.
In addition to the social integration index, we assessed the quality of social connections in terms of the functions these connections served. Whereas the vast majority of research on social relationships and health has focused on positive relationship functions such as social support, few studies have investigated negative functions or poor relationship quality. We included measurements of both. Social support was assessed by a summary index of the level of support as perceived by respondents from each relationship domain, including family, friends, school (for Add Health only), and spouse (except for Add Health). Social strain was similarly assessed by a scale indicating the level of strain from the corresponding relationships. This scale was not available for the Add Health sample. Like the social integration index, we used the average values across two waves for both social support and social strain variables in the MIDUS and HRS analyses. For all three social relationship measures, we assessed both continuous and categorical variables in regression analyses to test for linear and threshold effects. The choice of definitions of each in the final analyses was based on tests of statistical significance of regression coefficients and model fit using the Bayes Information Criterion.
We conducted multivariate regression analyses to examine the prospective associations between measured social relationship characteristics and biomarkers using the longitudinal design unique to each dataset. For three studies where biomarkers were assessed at the follow-up surveys only (Add Health, MIDUS, and HRS), we estimated models of biomarker outcomes in relation to social relationship measures assessed at prior points in time. Because the HRS included five waves of data on social integration (1998–2006), we estimated the latent growth curve models to most effectively summarize individual trajectories defined by both initial status or mean baseline level and the growth rate or average change with time (35) (see Fig. S1 for HRS model specification). The results presented from the HRS include the mean estimates only because the slope estimates were not statistically significant at α < 0.05 for any markers, suggesting no effect of change in social integration index over time on biomarkers. This result may reflect the fact that individual trajectories of social integration index were relatively stable and constant over time, which is consistent with the findings of previous studies using MIDUS (28) and HRS (19).
NSHAP provided repeated measures of three biomarkers at both baseline and the follow-up, including BP, WC, and BMI. Therefore, we used longitudinal residual change models to examine the effects of social relationships at wave I on changes in these biomarkers over time from wave I to wave II. This design offers a rigorous test of potential causality, as it minimizes the risks of both reverse causality (e.g., that biomarkers affect social relationships) and spuriousness (e.g., that some other variable is affecting both biomarkers and social relationships) (36). NSHAP data on CRP were not available for the follow-up at the time of this study, so the association estimated is cross-sectional at baseline.
We estimated the OLS models for continuous measures of biomarkers (CRP was log-transformed to account for skewness) and generalized linear models for categorical outcomes of biomarkers, including ordinal logit models for inflammation and logistic models for the others. For each outcome variable, we estimated models in a stepwise fashion: (i) basic models of individual social relationship variables adjusting for age, sex, and race; (ii) models of all social relationship variables (when available for the samples) adjusting for age, sex, and race; and (iii) full models adjusting for all covariates that are strong risk factors for inflammation, metabolic, and cardiovascular dysregulation and may contribute to their associations with social relationships. These covariates include socioeconomic status, health behaviors, psychosocial factors, chronic conditions, and medications. Their coding and descriptive statistics across samples are included in Table S3. All analyses adjusted for survey design effects and nonresponse using sampling weights (Data Description).
Data Description
Add Health.
Add Health is a nationally representative study of over 20,000 adolescents in grades 7–12 in the US in 1994–95 who have been followed into adulthood. The scientific purpose of Add Health is to understand the social, behavioral, and biological linkages in health across the life course (37). Add Health used a stratified school-based design and selected a nationally representative sample of all high schools and a feeder school in the United States. An in-school questionnaire was administered to all students who attended the selected schools during 1994–95 (wave I). An in-home sample was then selected from the school rosters for more in-depth interviews in the home setting with adolescents and a parent at wave I. The Add Health cohort was followed up in 1996 (wave II), 2001–2002 (wave III), and finally in 2008–2009 (wave IV) when individuals were 24–32 y old. The independent variables and covariates for the present study are drawn from the in-school questionnaire at wave I and the in-home interviews at waves I and IV. Biomarker data are drawn from the wave IV interview. High-sensitive C-reactive protein (hsCRP) data come from assays of dried blood spots. Field interviewers measured systolic and diastolic BP, height, weight, (which were used to calculate the BMI) and WC for the respondents during the in-home interview. For more information about Add Health biomarker collection, see the protocol in ref. 38.
We used a separate analytical sample for each biomarker outcome that included respondents who had complete data on social relationship measures and all covariates used in the analysis and those with valid sampling weights, resulting in three subsamples: one for CRP (n = 6,747), one for BP (n = 7,561), and a third for BMI and waist analysis (n = 7,889). The largest source of missing data are due to those without a wave I social integration score, most due to respondents missing in-school survey data from wave I (3,474). The next largest group of missing data comes from the biomarker data, with 1,553 missing CRP assays, 371 missing BP measures, and 96 missing anthropometric measures. In addition, we excluded those missing covariates, including those missing measures of smoking (89) and wave IV social integration measures (190). Those excluded from the sample were slightly more likely to be older (P = 0.023) and have higher Center for Epidemiological Studies Depression (CESD) scores (P = 0.000).
All analyses use survey weights to account for the unequal chances of selection, and error variances are adjusted for the clustered sampling design.
MIDUS.
MIDUS is a longitudinal and national longitudinal study of behavioral, psychological, and social factors that contribute to age-related differences in overall health and well-being. A total of 7,108 participants aged 25–74 were recruited for the original study in 1995–96 (wave I) by random digit dialing. Phone interviews and self-administered questionnaires were completed by MIDUS participants. The wave II data were collected 9–10 y later (2004–2006), with a mortality-adjusted retention rate of 75% (39). The independent variables for this study were drawn from self-administered questionnaires from both waves 1 and 2 and were averaged across waves. All covariates were drawn from wave 2. Biological data come from the MIDUS II biomarkers study (2004–2009), which assessed key biological parameters indicative of physical health. Eligible participants lived in the continental United States and completed the MIDUS II core phone interview and self-administered questionnaire. Fasting whole-blood samples were collected and serum aliquots were assayed for hsCRP. BP, WC, and BMI were measured by MIDUS staff members during the physical examination. More detailed information about the MIDUS biospecimen collection and protocol can be found in ref. 40.
Of the 1,255 participants in the biomarkers study, 1,054 participated in both survey waves. Our analytic sample was limited to participants who were aged 25–64 at wave 1, leaving 965 of the initial sample. For the social integration analysis, we limited our sample to 863 participants who were within the age range of interest and had complete data for all independent variables, covariates, and biomarker measures. The greatest source of missing data were social integration (82 missing), followed by income (22 missing) and biomarker measurements (15 missing; mostly CRP). Compared with the analytic sample for social integration, participants excluded due to age or missing data were significantly more likely to be older (P < 0.001; due to the age restriction), more likely to be diagnosed with diabetes (P < 0.001), more likely to report more depressive symptoms (P = 0.014), and were slightly less likely to be current cigarette smokers (P = 0.051). The excluded sample did not differ significantly on sex, educational, physical activity, perceived stress, and BMI indicators. The analytic sample for social relationship quality was further limited to 599 married individuals who reported on all social relationship indicators; therefore, the only additional exclusion criteria for the social relationship analytic sample was marital status (301 unmarried) and missing social relationship items (370 missing, primarily due to missing spouse support and strain items). Compared with the analytic sample for social relationships, excluded participants were more likely to be older (P < 0.001) and female (P = 0.001), were less physically active (P = 0.005), and reported more depressive symptoms (P < 0.001).
Survey weights are not available for the MIDUS II biomarkers study. To account for sampling of family members, we clustered the sample by family membership and reported robust SEs.
HRS.
The HRS is a nationally representative longitudinal survey of the US population aged 50 y and older conducted every 2 y from1992 to 2008, using a multistage sampling of households design with an oversample of black or Hispanics and overall response rate of about 87% (41). We used information from noninstitutionalized respondents included in the random one-half of the 2006 sample who consented to the biomarker collection and completed the blood test. Respondents’ BP, height, weight, and WC were measured by trained interviewers, and the CRP data were collected by blood spots. The physical and blood test consent procedure, laboratory measures, equipment, and protocols are described in ref. 42.
Of the 4,435 participants in the biomarker sample in 2006 who had complete biomarker and physical measures included in this study, 112 had missing data on covariates and were excluded. The final sample for the social integration analyses consists of 4,323 respondents who had social integration assessments beginning in 1998, the first wave of the HRS with adequate social integration measures, and also four subsequent waves until 2006. The social support/strain variables were only collected from a subset of respondents in a leave-behind survey in 2004 and 2006. The final sample for the social relationship quality analyses included 812 who had completed measures on social support/strain in both 2004 and 2006 and biomarkers in 2006.
The 2006 biomarker sample weights were implemented in the analyses and were supplemented to the core sample weights of the 2006 survey. The 2006 core sample weights were the product of the baseline sample weights, which adjust for age, gender, race/ethnicity, and geography and a poststratification correction based on the American Community Survey at year 2006 to account for the wave-specific nonresponse.
NSHAP.
The NSHAP is a nationally representative longitudinal study of older adults for the years 2005–2006 (wave 1) and followed up in 2010–2011 (wave 2). NSHAP wave I used a national area probability sample of community-residing adults ages 57–85 at the time of the interview and oversampled African-American and Hispanic areas. The original wave 1 sample included 3,005 respondents, and 2,261 of these wave 1 respondents were reinterviewed at wave 2. The overall response rate for wave 1 was 75.5%, and the response rate for respondents who were reinterviewed at wave 2 was 87.8%. More information about the survey design can be found in ref. 43. NSHAP data collection consisted of an in-person questionnaire, biomeasure collection, and a paper leave-behind questionnaire. The in-person questionnaire and biomeasures were administered by a National Opinion Research Center (NORC) field interviewer in the respondent’s home. The independent variables and covariates for the study are drawn from the in-person and leave-behind questionnaires from wave I. Biomarker data are drawn from waves 1 and 2 of the study. To assess levels of hsCRP, NSHAP collected dried blood spots at both waves 1 and 2; however, only wave 1 CRP data were used for this study because the wave 2 CRP data had not yet been released at the time of analysis. Detailed information on the collection of dried blood spots by NSHAP can be found in ref. 43. BP was measured at both waves 1 and 2. Respondents completed two or three seated BP measures on their left arms. We used the mean of each respondent’s BP readings. Finally, at both waves 1 and 2, respondents were weighed and their height and WC were also measured by the field interviewers.
Each set of analyses used a different sample size, which included respondents with complete data on the variables used in the analysis: CRP analyses (n = 1,153 for integration; n = 860 for support; and n = 860 strain), longitudinal hypertension analyses (n = 1,433 for integration; n = 1,516 for support; and n = 1,474 for strain), longitudinal WC analyses (n = 1,423 for integration; n = 1,560 for support; and n = 1,516 for strain), longitudinal BMI analyses (n = 1,388 for integration; n = 1,571 for support; and n = 1,527 for strain). A total of 788 respondents had missing data on CRP, 222 respondents had missing BMI data, 106 respondents had missing WC data, and 44 respondents had missing BP data. In addition to missing biomarker data, the greatest sources of missing data were on the smoking (318 missing), social relationship (471 missing), and psychosocial (160 missing) measures. In general, compared with those in the analytic samples, those excluded from the analysis because of missing data were older (P < 0.001) and more likely to be male (P = 0.072). In addition, those excluded from the analyses reported less education (P < 0.001), less physical activity (P < 0.001), more depression (P < 0.001) and perceived stress (P < 0.001), and higher rates of diabetes (P = 0.016). To the extent that the analytic sample had higher SES and was healthier, the results of our analyses may be conservative given positive associations between high SES and social relationships.
Analyses use survey weights to adjust for survey design and account for the probability of selection, with poststratification adjustments for nonresponse based on age and urbanicity.
Acknowledgments
We are grateful to our colleagues Jenny Tung, Jim House, Jason Lieb, Martha McClintock, Linda Waite, Philip Morgan, and Michael Shanahan for helpful suggestions and comments. This research is supported by NIH Grants P01 HD31921 (to K.M.H.) and K01 AG036745 (toY.C.Y.), and training and research support was provided by Carolina Population Center Grants T32 HD007168 and R24 HD050924, respectively. This research uses data from Add Health, a program project directed by K.M.H. and designed by J. Richard Udry, Peter S. Bearman, and K.M.H. at the University of North Carolina at Chapel Hill and funded by National Institute of Child Health and Human Development Grant P01 HD31921, with cooperative funding from 23 other federal agencies and foundations.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511085112/-/DCSupplemental.
References
- 1.Marmot MG. Status syndrome: A challenge to medicine. JAMA. 2006;295(11):1304–1307. doi: 10.1001/jama.295.11.1304. [DOI] [PubMed] [Google Scholar]
- 2.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 4.Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 2000;51(6):843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 5.Penwell LM, Larkin KT. Social support and risk for cardiovascular disease and cancer: A qualitative review examining the role of inflammatory processes. Health Psychol Rev. 2010;4(1):42–55. [Google Scholar]
- 6.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110(15):5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stringhini S, et al. Socioeconomic status, structural and functional measures of social support, and mortality: The British Whitehall II Cohort Study, 1985-2009. Am J Epidemiol. 2012;175(12):1275–1283. doi: 10.1093/aje/kwr461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YC, McClintock MK, Kozloski M, Li T. Social isolation and adult mortality: The role of chronic inflammation and sex differences. J Health Soc Behav. 2013;54(2):183–203. doi: 10.1177/0022146513485244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KP, Christakis NA. Social networks and health. Annu Rev Sociol. 2008;34(1):405–429. [Google Scholar]
- 10.Thoits PA. Mechanisms linking social ties and support to physical and mental health. J Health Soc Behav. 2011;52(2):145–161. doi: 10.1177/0022146510395592. [DOI] [PubMed] [Google Scholar]
- 11.Umberson D, Crosnoe R, Reczek C. Social relationships and health behavior across life course. Annu Rev Sociol. 2010;36:139–157. doi: 10.1146/annurev-soc-070308-120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finch CE. The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Academic, Burlington, MA; 2010. [Google Scholar]
- 13.Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA. 2006;103(38):14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClintock MK, Conzen SD, Gehlert S, Masi C, Olopade F. Mammary cancer and social interactions: Identifying multiple environments that regulate gene expression throughout the life span. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec No 1):32–41. doi: 10.1093/geronb/60.special_issue_1.32. [DOI] [PubMed] [Google Scholar]
- 15.Hermes GL, et al. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci USA. 2009;106(52):22393–22398. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermes GL, Rosenthal L, Montag A, McClintock MK. Social isolation and the inflammatory response: Sex differences in the enduring effects of a prior stressor. Am J Physiol Regul Integr Comp Physiol. 2006;290(2):R273–R282. doi: 10.1152/ajpregu.00368.2005. [DOI] [PubMed] [Google Scholar]
- 17.Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46(3) Suppl:S39–S52. [PubMed] [Google Scholar]
- 18.Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: Inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci. 2011;66(5):493–500. doi: 10.1093/gerona/glr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YC, Li T, Ji Y. Impact of social integration on metabolic functions: Evidence from a nationally representative longitudinal study of US older adults. BMC Public Health. 2013;13:1210. doi: 10.1186/1471-2458-13-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeman TE, Singer BH, Ryff CD, Dienberg Love G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 2002;64(3):395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. Am J Public Health. 2008;98(7):1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeman TE, Kaplan GA, Knudsen L, Cohen R, Guralnik J. Social network ties and mortality among the elderly in the Alameda County Study. Am J Epidemiol. 1987;126(4):714–723. doi: 10.1093/oxfordjournals.aje.a114711. [DOI] [PubMed] [Google Scholar]
- 23.Kittleson MM, et al. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Arch Intern Med. 2006;166(21):2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- 24.Miller GE, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll JE, et al. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci USA. 2013;110(42):17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanahan MJ. Social genomics and the life course: Opportunities and challenges for multilevel population research. In: Waite L, editor. The Future of the Sociology of Aging: An Agenda for Action. National Academies Press; Washington, DC: 2013. pp. 255–276. [Google Scholar]
- 28.Yang YC, Schorpp K, Harris KM. Social support, social strain and inflammation: Evidence from a national longitudinal study of U.S. adults. Soc Sci Med. 2014;107:124–135. doi: 10.1016/j.socscimed.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 30.Franklin SS, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention The National Health and Nutrition Examination Survey: Sample design, 1999-2006. 2012 Available at www.cdc.gov/nchs/data/series/sr_02/sr02_155.pdf.
- 32.Coverman S. Role overload, role conflict, and stress: Addressing consequences of multiple role demands. Soc Forces. 1989;67(4):965–982. [Google Scholar]
- 33.Pardue M-L, Wizemann TM. Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academies, Washington, DC; 2001. [PubMed] [Google Scholar]
- 34.Curran PJ, Hussong AM. Modeling Intraindividual Variability with Repeated Measures Data: Methods and Applications. Psychology Press; Mahwah, NJ: 2001. [Google Scholar]
- 35.Allison PD. Change scores as dependent variables in regression analysis. Sociol Methodol. 1990;20(1):93–114. [Google Scholar]
- 36.Harris KM. An integrative approach to health. Demography. 2010;47(1):1–22. doi: 10.1353/dem.0.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Entzel P, et al. Add Health Wave IV documentation: Cardiovascular and anthropomorphic measures. 2009 Available at www.cpc.unc.edu/projects/addhealth/data/guides/Wave%20IV%20cardiovascular%20and%20anthropometric%20documentation%20110209.pdf.
- 38.Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. J Aging Health. 2010;22(3):307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. J Aging Health. 2010;22(8):1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heeringa SG, Connor JH. Technical Description of the Health and Retirement Study Sample Design. 1995 Available at hrsonline.isr.umich.edu/sitedocs/userg/HRSSAMP.pdf.
- 41.Crimmins E, et al. HRS documentation report: Documentation of biomarkers in the 2006 and 2008 Health and Retirement Study. 2013 Available at hrsonline.isr.umich.edu/sitedocs/userg/Biomarker2006and2008.pdf.
- 42.O’Muircheartaigh C, Eckman S, Smith S. Statistical design and estimation for the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i12–i19. doi: 10.1093/geronb/gbp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams SR, McDade TW. The use of dried blood spot sampling in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i131–i136. doi: 10.1093/geronb/gbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]