Significance
Mycobacterium tuberculosis (Mtb) uses type VII secretion systems to secrete cognate protein pairs that alter host interactions. Here, we address the contributions of the ESX-3 secretion system to Mtb growth and pathogenesis through a combination of genetics, proteomics, and growth studies both in vitro and in vivo. ESX-3 is demonstrated to play a critical role in iron acquisition through secretion of a pair pf proteins belonging to the PE–PPE family (PE5–PPE4). In vivo, the importance of PE5–PPE4 secretion was found to depend upon host genotype, likely reflecting a host capacity to restrict iron availability. However, secreted effectors EsxG–EsxH play an iron-independent role in Mtb virulence. Therefore, ESX-3 secretes multiple effectors that target distinct host pathways to influence pathogenesis.
Keywords: Mycobacterium tuberculosis, type VII secretion system, ESX-3, mycobactin, siderophore
Abstract
Mycobacterium tuberculosis (Mtb) encodes five type VII secretion systems (T7SS), designated ESX-1–ESX-5, that are critical for growth and pathogenesis. The best characterized is ESX-1, which profoundly impacts host cell interactions. In contrast, the ESX-3 T7SS is implicated in metal homeostasis, but efforts to define its function have been limited by an inability to recover deletion mutants. We overcame this impediment using medium supplemented with various iron complexes to recover mutants with deletions encompassing select genes within esx-3 or the entire operon. The esx-3 mutants were defective in uptake of siderophore-bound iron and dramatically accumulated cell-associated mycobactin siderophores. Proteomic analyses of culture filtrate revealed that secretion of EsxG and EsxH was codependent and that EsxG–EsxH also facilitated secretion of several members of the proline-glutamic acid (PE) and proline-proline-glutamic acid (PPE) protein families (named for conserved PE and PPE N-terminal motifs). Substrates that depended on EsxG–EsxH for secretion included PE5, encoded within the esx-3 locus, and the evolutionarily related PE15–PPE20 encoded outside the esx-3 locus. In vivo characterization of the mutants unexpectedly showed that the ESX-3 secretion system plays both iron-dependent and -independent roles in Mtb pathogenesis. PE5–PPE4 was found to be critical for the siderophore-mediated iron-acquisition functions of ESX-3. The importance of this iron-acquisition function was dependent upon host genotype, suggesting a role for ESX-3 secretion in counteracting host defense mechanisms that restrict iron availability. Further, we demonstrate that the ESX-3 T7SS secretes certain effectors that are important for iron uptake while additional secreted effectors modulate virulence in an iron-independent fashion.
Bacterial secretion systems play roles in pathogenesis, antigenicity, and virulence and are keys to understanding host–pathogen interactions (1, 2). Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), encodes five type VII secretion systems (T7SS) within genomic loci referred to as “esx-1” through “esx-5” (3). There is considerable interest in understanding these secretion systems, because they appear to be central to Mtb pathogenesis. ESX-1 has been studied most extensively because deletion of a large portion of this coding region is the primary attenuating mutation in Mycobacterium bovis-bacillus Calmette–Guérin (bacillus Calmette–Guérin), the widely used TB vaccine (4–6). In addition to containing the secretion apparatus itself, esx-1 encodes several substrates, including a pair of small helical proteins belonging to the WXG100 family as well as members of the proline-glutamic acid (PE) and proline-proline-glutamic acid (PPE) families (named for conserved N-terminal amino acid signatures) (7). A large body of data points to the importance of ESX-1 in modulating host cell signaling and bacterial trafficking, perhaps in large measure because of a membrane-lytic function (8, 9). However, the precise mechanisms whereby individual substrates contribute to ESX-1 functions are less clear. The less-investigated ESX-3 provides an alternate system by which we can explore how T7SS contribute to growth and virulence. ESX-3 has been thought primarily to play a bacterial intrinsic role in metal homeostasis. This notion is based on the observation that the esx-3 gene cluster (Rv0282–Rv0292) is transcriptionally de-repressed in response to iron and zinc starvation and on genetic data pointing to a role for ESX-3 in using iron bound to the siderophore mycobactin (Mb) in both nonpathogenic mycobacteria and Mtb (10–13). Similar to the esx-1 locus, esx-3 also encodes a pair of WXG100 family members (EsxG and EsxH) and PE–PPE proteins (PE5 and PPE4) (3), but their specific functions remain largely undefined.
Emerging data point to additional roles of ESX-3 in virulence and modulation of immune responses. Introduction of Mtb esx-3 into Mycobacterium smegmatis (Msmeg) strains lacking the endogenous locus generates altered cytokine responses, and, when used as a vaccine, this strain protects against subsequent challenge with Mtb (14). EsxG and EsxH form a heterodimer (15) and generate prominent CD4 and CD8 T-cell responses in mice and humans (16–18). The EsxG–EsxH complex impairs phagosomal maturation by interfering with the host endosomal sorting complex required for transport (ESCRT) (19). Finally, repression of the entire esx-3 locus in bacillus Calmette–Guérin impairs bacterial survival in macrophages (13). Although ESX-3 has been implicated in both metal homeostasis and virulence, it is not known whether these two phenomena are related, and the roles of specific effectors are undefined. Investigation of these questions has been hampered by the essentiality of ESX-3 in standard laboratory medium. Here, we isolated Mtb strains lacking ESX-3 and mutants deficient in the secreted substrates EsxG, EsxH, and PE5–PPE4 by recovering the strains on iron-supplemented media. We evaluated the esx-3 region mutants with regard to their iron and zinc phenotypes, their ability to produce mycobactin, their capacity for siderophore-mediated iron uptake, and their virulence in macrophages and in mice. Further, by comparing culture filtrates (CFs) of WT and knockout strains using label-free quantitative MS, we comprehensively defined ESX-3–secreted factors and determined their codependence on EsxG and EsxH. By comparing the phenotypic data with the secretome analysis, we were able to link secretion of particular substrates to specific effector functions. The phenotypic and proteomic analyses point to a model in which PE5 and PPE4 are the critical ESX-3 substrates involved in metal homeostasis and in counteracting host iron restriction, while EsxG and EsxH also play an essential iron-independent role in virulence.
Results
Hemin and Mb Rescue Growth of Mtb Δesx-3, ΔesxG, ΔesxH, and Δpe5–ppe4.
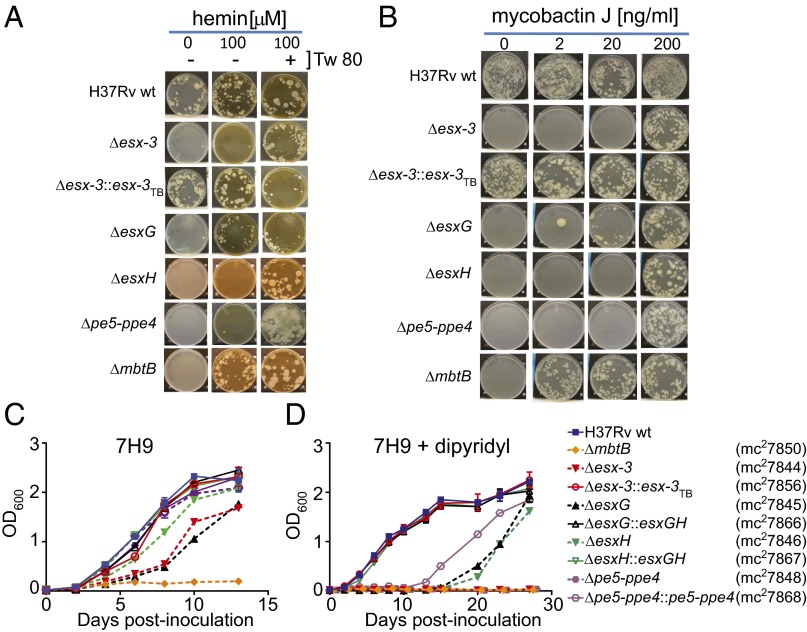
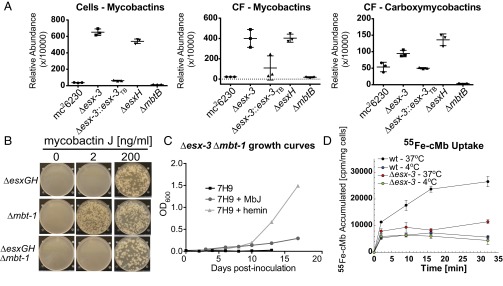
Because ESX-3 has been implicated in using Mb-bound iron (13), we reasoned that we might be able to recover mutants in the esx-3 locus if we used hemin as an alternative iron source (20, 21). Because Esx proteins and PE–PPE family members are implicated as substrates of T7SS (7, 22), we generated strains lacking esxG, esxH, and pe5–ppe4, all of which are encoded within esx-3 (Fig. S1 A and G–I, Table S1, and Dataset S1), as well as a complete esx-3 deletion (i.e., ΔRv0282–Rv0292) (Fig. S1 A–F) and, for comparison of iron phenotypes, a deletion mutant of mbtB (Fig. S1J), which codes for a peptide synthase required to produce Mb (23) (see SI Materials and Methods for details of iron-containing media used to recover mutants). The Δesx-3 mutant, ΔesxG, ΔesxH, the double-knockout Δpe5–ppe4, and ΔmbtB all grew on medium containing 100 μM hemin, failed to grow on unsupplemented medium (Fig. 1 A and B), and were rescued with integrating plasmids expressing the relevant genes under control of the heat-shock protein 60 (hsp60) promoter (Fig. S2A and Table S2) or, for Δesx-3 and ΔmbtB, with a cosmid containing the entire esx-3 region or the entire mbt-1 Mb synthesis locus, respectively (Fig. 1 A and B, Fig. S2A, and Table S2). For unclear reasons, Δesx-3, ΔesxH, and Δpe5–ppe4 also required Tween 80 to grow on hemin-containing medium, whereas ΔesxG and ΔmbtB did not (Fig. 1A).
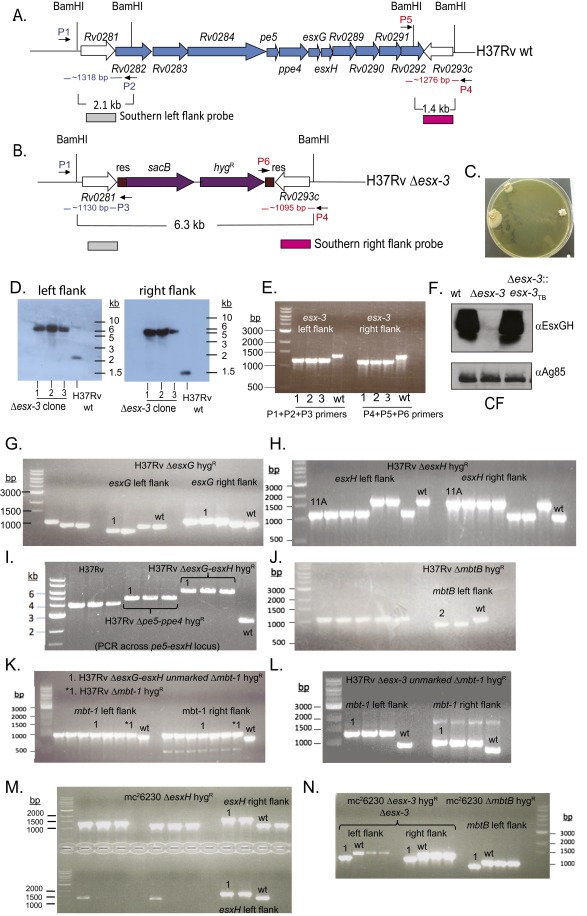
Fig. S1.
Confirmation of mutations in the esx-3 and mbt-1 regions. (A and B) The esx-3 locus (Rv0282–Rv0292) is shown. ORFs removed in the Δesx-3 deletion strain are shown in blue (A). The resulting genomic organization including the sacB-hygR cassette (purple) flanked by γδ-resolvase (res) sites (brown) is shown in B. The location of primers used to verify the esx-3 deletion by PCR in E is indicated in blue (left flank; P1, P2, P3) and red (right flank; P4, P5, P6). (C) Δesx-3 colonies were obtained on plates supplemented with 100 μM hemin and 0.05% Tween 80. Colonies were initially visible after ∼4 wk of incubation and were photographed at ∼7 wk of incubation. (D) Southern blotting was used to confirm deletion of esx-3 from three transductants (1–3), using the probes indicated in A and B. Genomic DNA prepared from H37Rv Δesx-3 transductants (1–3) and H37Rv WT was digested with BamHI. Blots were probed with sequences immediately flanking the deleted esx-3 region at the locations indicated in A and B. Probes were amplified from H37Rv gDNA using the primer pairs ΔRv0282LL+ΔRv0282LR and ΔRv0292RL+ΔRv0292RR, respectively (Dataset S1). Relevant BamHI restriction sites are shown in A and B. The probes for the left flank or the right flank each hybridized to an ∼6.3-kb band in the mutants. In contrast, the WT control yielded the expected band sizes of ∼2.1 and ∼1.4 kb, respectively. (F) Western blotting of CF demonstrated that Δesx-3 failed to secrete EsxG and EsxH. Antigen 85B served as a loading control. (E and G–N) PCR analysis was performed to confirm the mutant genotypes in the H37Rv background for Δesx-3 (mc27788) (E); ΔesxG (mc27789) (G); ΔesxH (mc27790) (H); Δpe5–ppe4 (mc27792) and ΔesxG–esxH (mc27791) (I); ΔmbtB (mc27808) (J); ΔesxG–esxH Δmbt-1 (mc27851) and Δmbt-1 (mc27809) (K); Δesx3 Δmtb-1 (mc27852) (L) and in the mc26230 strain background ΔesxH (mc27819) (M); and Δesx-3 (mc27818) and ΔmbtB (mc27820) (N). Screening primers yielded distinct band sizes for mutant and WT strains and are listed in Dataset S1. The H37Rv WT and mc26230 parental strains are indicated, as are the clones selected for study (numbers above lanes, indicating clone numbers in the laboratory collection). Primers amplify the left and right flanking regions of the targeted genes, except for the Δpe5–ppe4 and ΔesxG–esxH mutants shown in I, where the primers are upstream and downstream of the deleted region. Left- and right-flanking PCRs also were performed subsequently for these mutants and yielded the expected product sizes.
Table S1.
Mycobacterial strains used in this study
| Strain name | Genotype | How constructed | Source |
| Parental strains | |||
| H37Rv | WT | N/A | Trudeau Institute |
| mc26230 | H37Rv ΔRD1 ΔpanCD | SpTr and unmarking | (24, 25) |
| H37Rv background | |||
| Hygromycin-marked strains | |||
| mc27788 | Δesx-3 (Rv0282–Rv0292)::res-sacB-hygR-res | SpTr of H37Rv | This work |
| mc27789 | ΔesxG (Rv0287)::res-sacB-hygR-res | SpTr of H37Rv | This work |
| mc27790 | ΔesxH (Rv0288)::res-sacB-hygR-res | SpTr of H37Rv | This work |
| mc27791 | ΔesxG–esxH (Rv0287–Rv0288)::res-sacB-hygR-res | SpTr of H37Rv | This work |
| mc27792 | Δpe5–ppe4 (Rv0285–Rv0286)::res-sacB-hygR-res | SpTr of H37Rv | This work |
| mc27808 | ΔmbtB (Rv2383c)::res-sacB-hygR-res | SpTr of H37Rv | This work |
| mc27809 | Δmbt-1 (Rv2377c–Rv2386c)::res-sacB-hygR-res | SpTr of H37Rv | This work |
| Hygromycin-marked, complemented strains | |||
| mc27827 | Δesx-3 (Rv0282–Rv0292)::res-sacB-hygR-res, attBL5::pYUB1335 | Transformation (pYUB1335) | This work |
| mc27828 | Δesx-3 (Rv0282–Rv0292)::res-sacB-hygR-res, attBL5::pYUB2076 | Transformation (pYUB2076) | This work |
| mc27829 | Δesx-3 (Rv0282–Rv0292)::res-sacB-hygR-res, attBL5::pJP148 | Transformation (pJP148) | This work |
| mc27830 | ΔesxG (Rv0287)::res-sacB-hygR-res, attBL5::pJP148 | Transformation (pJP148) | This work |
| mc27831 | Δesx-3 (Rv0282–Rv0292)::res-sacB-hygR-res, attBL5::pMV361 | Transformation (pMV361) | This work |
| Unmarked strains (sacB-hygR cassette removed) | |||
| mc27844 | Δesx-3 (Rv0282–Rv0292)::res | Phage delivery of γδ-resolvase | This work |
| mc27845 | ΔesxG (Rv0287)::res | Phage delivery of γδ-resolvase | This work |
| mc27846 | ΔesxH (Rv0288)::res | Phage delivery of γδ-resolvase | This work |
| mc27847 | ΔesxG–esxH (Rv0287-Rv0288)::res | Phage delivery of γδ-resolvase | This work |
| mc27848 | Δpe5–ppe4 (Rv0285-Rv0286)::res | Phage delivery of γδ-resolvase | This work |
| mc27850 | ΔmbtB (Rv2383c)::res | Phage delivery of γδ-resolvase | This work |
| Double-deletion strains (second deletion introduced into unmarked strain) | |||
| mc27851 | ΔesxG–esxH (Rv0287–Rv0288)::res; Δmbt-1 (Rv2377c–Rv2386c)::res-sacB-hygR-res | SpTr of mc27847 | |
| mc27852 | Δesx-3 (Rv0282–Rv0292)::res;Δmbt-1 (Rv2377c–Rv2386c)::res-sacB-hygR-res | SpTr of mc27844 | |
| Unmarked complemented strains | |||
| mc27856 | Δesx-3 (Rv0282–Rv0292)::res, attBL5::pYUB1335 | Phage delivery of γδ-resolvase | This work |
| mc27857 | Δesx-3 (Rv0282–Rv0292)::res, attBL5::pYUB2076 | Phage delivery of γδ-resolvase | This work |
| mc27866 | ΔesxG (Rv0287)::res, attBL5::pYUB1944 | Transformation (pYUB1944) | This work |
| mc27867 | ΔesxH (Rv0288)::res, attBL5::pYUB1944 | Transformation (pYUB1944) | This work |
| mc27868 | Δpe5–ppe4 (Rv0285-Rv0286)::res, attBL5::pYUB1945 | Transformation (pYUB1945) | This work |
| mc27874 | ΔmbtB (Rv2383c)::res, attBL5::pYUB1940 | Transformation (pYUB1940) | This work |
| mc26230 background | |||
| Hygromycin-marked strains | |||
| mc27818 | Δesx-3 (Rv0282–Rv0292)::res-sacB-hygR-res | Sptr of mc26230 | This work |
| mc27819 | ΔesxH (Rv0288)::res-sacB-hygR-res | Sptr of mc26230 | This work |
| mc27820 | ΔmbtB (Rv2383c)::res-sacB-hygR-res | Sptr of mc26230 | This work |
| Hygromycin-marked complemented strains | |||
| mc27838 | Δesx-3 (Rv0282–Rv0292)::res-sacB-hygR-res, attBL5::pYUB1335 | Transformation (pYUB1335) | This work |
| Unmarked strains (sacB-hygR cassette removed) | |||
| mc27860 | Δesx-3 (Rv0282–Rv0292)::res | Phage delivery of γδ-resolvase | This work |
| mc27861 | ΔesxH (Rv0288)::res | Phage delivery of γδ-resolvase | This work |
| mc27862 | ΔmbtB (Rv2383c)::res | Phage delivery of γδ-resolvase | This work |
| Unmarked complemented strain | |||
| mc27863 | Δesx-3 (Rv0282–Rv0292)::res, attBL5::pYUB1335 | Phage delivery of γδ-resolvase | This work |
The table lists strain names and relevant genotypes for the mycobacterial strains used in this study; the methods of construction are also indicated as well as the source where appropriate. SpTr, specialized transduction.
Fig. 1.
Defined iron sources rescue the essentiality of esx-3 region mutants. (A and B) The indicated Mtb strains H37Rv WT, Δesx-3 (mc27788), Δesx-3::esx-3TB (mc27827), ΔesxG (mc27789), ΔesxH (mc27790), Δpe5–ppe4 (mc27792), and ΔmbtB (mc27808) (Fig. S1 and Table S1) were plated on 7H10 medium with or without 100 μM hemin and Tween 80 (0.05%) (Tw 80) (A) or with increasing concentrations of MbJ (B). Some late-appearing contamination was present when Δpe5–ppe4 was plated on medium containing hemin and Tween 80 in A. (C and D) The indicated strains, which differ from those used in the plating experiments shown in A and B in that the sacB-hygR cassettes were removed, were grown in 7H9 medium (C) or 7H9 medium supplemented with 100 μM 2,2′-dipyridyl (D). Data points represent the mean ± SEM from duplicate cultures.
Fig. S2.
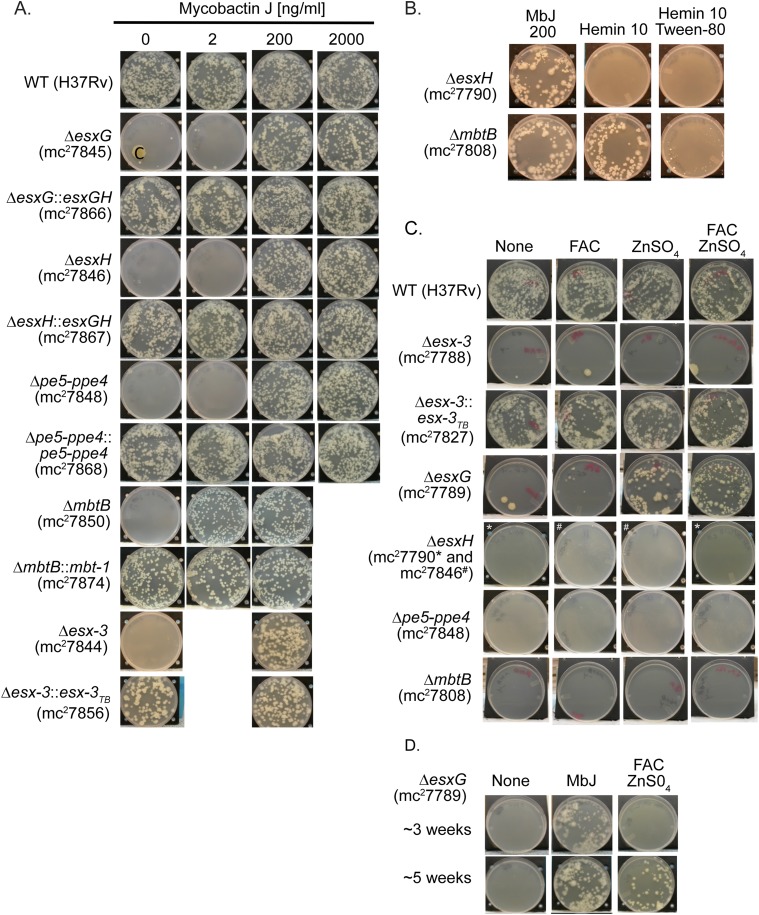
Growth of esx‐3 region mutants with a variety of iron supplements. (A) Indicated strains were grown on 7H10 plates with increasing concentrations of MbJ and were photographed after ∼3–6 wk of incubation at 37 °C. “C” on the ΔesxG plate indicates a region of contamination. (B) ΔesxH and ΔmbtB were grown on 7H10 plates with MbJ (200 ng/mL), hemin (10 μM), or hemin (10 μM) and Tween 80 (0.05%). (C) Strains were grown on 7H10 medium without supplement or with 250 μg/mL FAC, 10 μg/mL ZnSO4, or both FAC and ZnS04 at the indicated concentrations. For ΔesxH, the hygromycin-marked strain (mc27790) was inoculated onto 7H10 plates and onto 7H10 plates with both FAC and ZnSO4 (indicated by an asterisk); the unmarked strain (mc27846) was inoculated onto the other medium types (indicated by a pound sign). (D) ΔesxG was grown on 7H10 plates lacking supplements or with MbJ (200 ng/mL) or FAC (250 μg/mL) and ZnS04 (10 μg/mL) and was photographed at ∼3 wk (Upper) and ∼5 wk (Lower) of incubation at 37 °C.
Table S2.
Plasmids and cosmids used in this study
| Plasmid | Relevant genotype/description | Source |
| pYUB412 | attPL5 λcos int hyg bla ColE1; mycobacterial cosmid, replicates as an episomal vector in Escherichia coli, and integrates into the attBL5 sites of various mycobacterial species. | S. Bardarov and W. R. Jacobs Jr. |
| pYUB1052 | attPL5 λcos int aph bla ColE1; derivative of pYUB412, with hyg cassette replaced with aph gene conferring kanamycin resistance (kanR) | (78) |
| pYUB1335 | pYUB1052::(Rv0278c [portion] to Rv0303 [portion]); cosmid vector containing Mtb esx-3 region | This work |
| pYUB2076 | pYUB1052::(MSMEG_0609 [portion] to MSMEG_0637 [portion]); cosmid vector containing M. smegmatis esx-3 region | (14) |
| pYUB1940 | pYUB412::(Rv2374c [portion]-Rv2387 [portion]); cosmid vector containing Mtb mbt-1 region | This work |
| pMV261 | aph oriM Phsp60 ColE1; episomal mycobacterial shuttle vector, contains hsp60 promoter | (79) |
| pMV361 | aph int attPL5 Phsp60 ColE1; integrating mycobacterial shuttle vector, contains hsp60 promoter | (79) |
| pMV306 | aph int attPL5 ColE1; integrating mycobacterial shuttle vector | (79)* |
| pJP148 | pMV361::esxGMtb-esxHMtb ; esxG (Rv0287)-esxH (Rv0288) of Mtb H37Rv cloned between EcoRI and HindIII sites of pMV361 | This work |
| pYUB1942 | pMV261::esxGMtb-esxHMtb ; esxG (Rv0287)-esxH (Rv0288) of Mtb H37Rv cloned between MscI and HindIII sites of pMV261 | This work |
| pYUB1943 | pMV261::pe5Mtb-ppe4Mtb ; pe5 (Rv0285)-ppe4 (Rv0286) of Mtb H37Rv cloned between MscI and HindIII sites of pMV261 | This work |
| pYUB1944 | pMV306:: Phsp60-esxGMtb-esxHMtb ; XbaI/HindIII fragment of pYUB1942 subcloned into XbaI/HindIII-digested pMV306 vector backbone. | This work |
| pYUB1945 | pMV306:: Phsp60-pe5Mtb-ppe4Mtb ; XbaI/HindIII fragment of pYUB1943 subcloned into XbaI/HindIII-digested pMV306 vector backbone. | This work |
The table lists plasmids and cosmids used in this study, including the relevant genotypes and the source where appropriate.
Derivative of pMV361 lacking Phsp60.
Previous work in Msmeg suggested that ESX-3 participates in the utilization of iron bound to Mb (13). Therefore, we were surprised at the abundant growth of Mtb Δesx-3 when 2 μg/mL mycobactin J (MbJ) (a standard concentration used for culturing Mycobacterium avium subsp. paratuberculosis) was provided. On titrating MbJ, we found that 100-fold more MbJ was needed for growth rescue of the esx-3 mutants than for growth rescue of the ΔmbtB mutant (Fig. 1B). Similarly, 10-fold more hemin was needed to rescue ΔesxH than to rescue ΔmbtB (Fig. 1A and Fig. S2B), and most of the mutants were not effectively rescued by supplementation with an approximately sixfold excess of ferric ammonium citrate (FAC) and a 10-fold excess of zinc sulfate (Fig. S2C), compared with standard 7H10 medium. The only exception was ΔesxG, which grew with zinc supplementation and was further enhanced by additional FAC after prolonged incubation (Fig. S2 C and D). Thus, ΔesxH and Δpe5–ppe4 phenocopy Δesx-3, but ΔesxG exhibits a somewhat distinct phenotype. In contrast to the findings on solid medium, the esx-3 mutants had relatively mild growth defects in 7H9 broth unless the lipophilic iron chelator 2,2′-dipyridyl was included (Fig. 1 C and D), but growth of ΔmbtB was severely restricted even in nonchelated medium (Fig. 1C). In conclusion, the data are consistent with esx-3 playing a role in siderophore-mediated iron acquisition, a role that appears to be more crucial on solid medium than in liquid medium. Furthermore, ESX-3 is nonessential provided that iron is available in an accessible form. This result suggests the presence of a less efficient uptake system(s) that functions in the absence of ESX-3. Finally, the high concentration of hemin required to rescue growth of the esx-3 region mutants suggests potential involvement of ESX-3 in iron uptake from other sources in addition to siderophores.
The esx-3 Mutants Overproduce Mb and Fail to Assimilate Mb-Bound Iron.
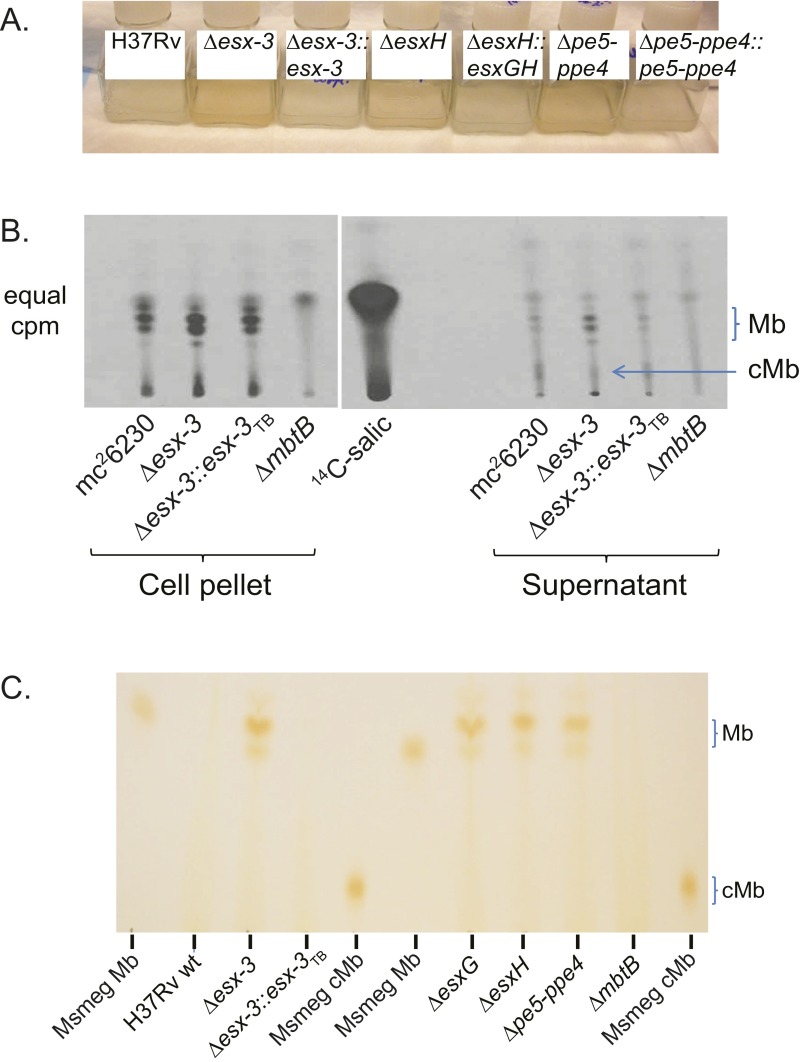
When grown in iron-replete 7H9 broth, the esx-3 region mutants developed a distinct orange pigmentation (Fig. S3A), which we reasoned might represent excess Mb. To address this possibility, bacteria were grown under iron-limited conditions (chelated Sauton’s medium) in the presence of 7-[14C]-salicylic acid, the biosynthetic precursor of Mb. These experiments used esx-3 and mbtB mutants generated in the auxotrophic mc26230 (H37Rv ΔRD1 ΔpanCD) strain background (Table S1), which facilitates work with radiolabeled, infectious samples because it can be used at biosafety level 2 (BSL2) (24, 25). When analyzed by TLC, only Mb was observed in cell pellets, whereas the CF contained both Mb and carboxymycobactin (cMb). ΔmbtB showed the expected absence of Mb and cMb (26, 27). In contrast, Δesx-3 produced Mb slightly in excess of the parental strain, which was restored to WT levels in the complemented strain (Fig. S3B). When cell pellet extracts from Δesx-3, ΔesxG, ΔesxH, and Δpe5–ppe4 grown in iron-replete 7H9 medium were analyzed by TLC, the orange-pigmented material comigrated with Mb; this material was undetectable in H37Rv WT, Δesx-3::esx-3TB, and ΔmbtB (Fig. S3C). These data are consistent with the idea that the esx-3 mutants experience iron deprivation in standard 7H9 medium and respond by up-regulating production of Mb. In iron-limited medium, both WT and esx-3 region mutants experience iron starvation and increase production of Mb, so the differential increase for the mutants is less apparent. To quantify the relative accumulation of Mb in the Δesx-3 and ΔesxH mutant strains, we analyzed them by MS after growth in 7H9 as described in SI Materials and Methods. As expected, Mb and cMb were absent or were present in trace amounts in extracts of the ΔmbtB strain (Fig. 2A). In the Δesx-3 and ΔesxH strains there was >15-fold accumulation of Mb in the cell pellets and CF compared with the parental strain, accounting for >4% of total lipids in both fractions. cMb also was increased in the CF of the mutants, although the changes were more modest (Fig. 2A).
Fig. S3.
Coloration of esx-3 region mutants in broth culture and production of siderophores by WT and mutant strains. (A) H37Rv WT, Δesx-3 (mc27844), Δesx-3::esx-3TB (mc27856), ΔesxH (mc27846), ΔesxH::esxGH (mc27867), Δpe5–ppe4 (mc27848), and Δpe5–ppe4::pe5–ppe4 (mc27868) were grown in 7H9 medium to log phase and were subcultured in 7H9 medium and grown to stationary phase (13 d). The photograph demonstrates the coloration of esx-3 region mutants. (B) TLC analysis of cell-associated and secreted siderophores extracted from 14C-salicylic acid–labeled cultures of WT (mc26230), Δesx-3 (mc27860), Δesx3::esx-3TB complement (mc27863), and ΔmbtB (mc27862) grown in chelated Sauton’s medium. The location of Mb and cMb is indicated based on the migration of purified standards of ferrated Mb and cMb from Msmeg. (C) Cell-associated siderophores were extracted from H37Rv WT and from Δesx-3 (mc27844), Δesx-3::esx-3TB (mc27856), ΔesxG (mc27845), ΔesxH (mc27846), Δpe5–ppe4 (mc27848), and ΔmbtB (mc27850) grown under iron-replete conditions (7H9 medium) and analyzed by TLC, as described in SI Materials and Methods. Mb and cMb from Msmeg were used as standards.
Fig. 2.
esx-3 region mutants produce Mb but fail to assimilate Mb-bound iron. (A) UPLC-MS analysis of lipids extracted from WT (mc26230) and the indicated mutant strains [Δesx-3 (mc27860), Δesx3::esx-3TB (mc27863), ΔesxH (mc27861), and ΔmbtB (mc27862)] following growth in 7H9 medium without Tween 80 for 3 wk. The amount of Mb and cMb was normalized to total lipids in each sample and reported as a relative abundance out of 10,000 arbitrary units. Data points show the values for individual samples; lines indicate mean ± SD. (B) ΔesxG–esxH (mc27791), Δmbt-1 (mc27809), and ΔesxG–esxH Δmbt-1 (mc27851) were grown on 7H10 medium containing MbJ as indicated. (C) The Δesx3 Δmbt-1 double mutant (mc27852) was inoculated in 7H9 medium or in 7H9 medium supplemented with hemin (100 μM) or MbJ (200 ng/mL). For 7H9 medium, growth curves are in duplicate. Data points represent the mean ± SEM; growth curves for 7H9 + hemin and 7H9 + MbJ were performed in singlicate. (D) The acquisition of 55Fe-loaded cMb was assessed for WT (mc26230) and Δesx-3 (mc27818) at 37 °C and 4 °C.
Because a previously described ΔmmpS4/S5 siderophore export mutant became “intoxicated” by excess intracellular siderophores (28), we asked whether the Mb accumulation in the mutants might contribute to their growth defect. We deleted the entire mbt-1 gene cluster in strains lacking both esxG and esxH or esx-3 (Fig. S1 K and L) and found that the ΔesxG–esxH Δmbt-1 double-deletion strain behaved similarly to ΔesxG–esxH in requiring high concentrations (200 ng/mL) of MbJ for growth on solid medium (Fig. 2B). Similarly, the Δesx-3 Δmbt-1 double mutant was highly impaired for growth in 7H9 medium, even with supplementation (Fig. 2C). Therefore, although esx-3 mutants accumulate Mb, this excess siderophore does not account for the growth defect.
To verify that esx-3 plays a role in siderophore uptake, we examined the accumulation of 55Fe-loaded cMb by WT and Δesx-3. At 37 °C, Δesx-3 accumulated <50% of the radiolabel acquired by the parental strain (Fig. 2D), although the strains exhibited similar levels of nonspecific adsorption at 4 °C. Although uptake of 55Fe-cMb was substantially less for Δesx-3 than for WT, it was not completely absent, supporting the idea that a lower-affinity Mb uptake system functions in the absence of esx-3. Taken together, these results substantiate a role for esx-3 in siderophore-mediated iron acquisition.
Virulence Role of pe5–ppe4 and mbtB Depends on Host Genotype.
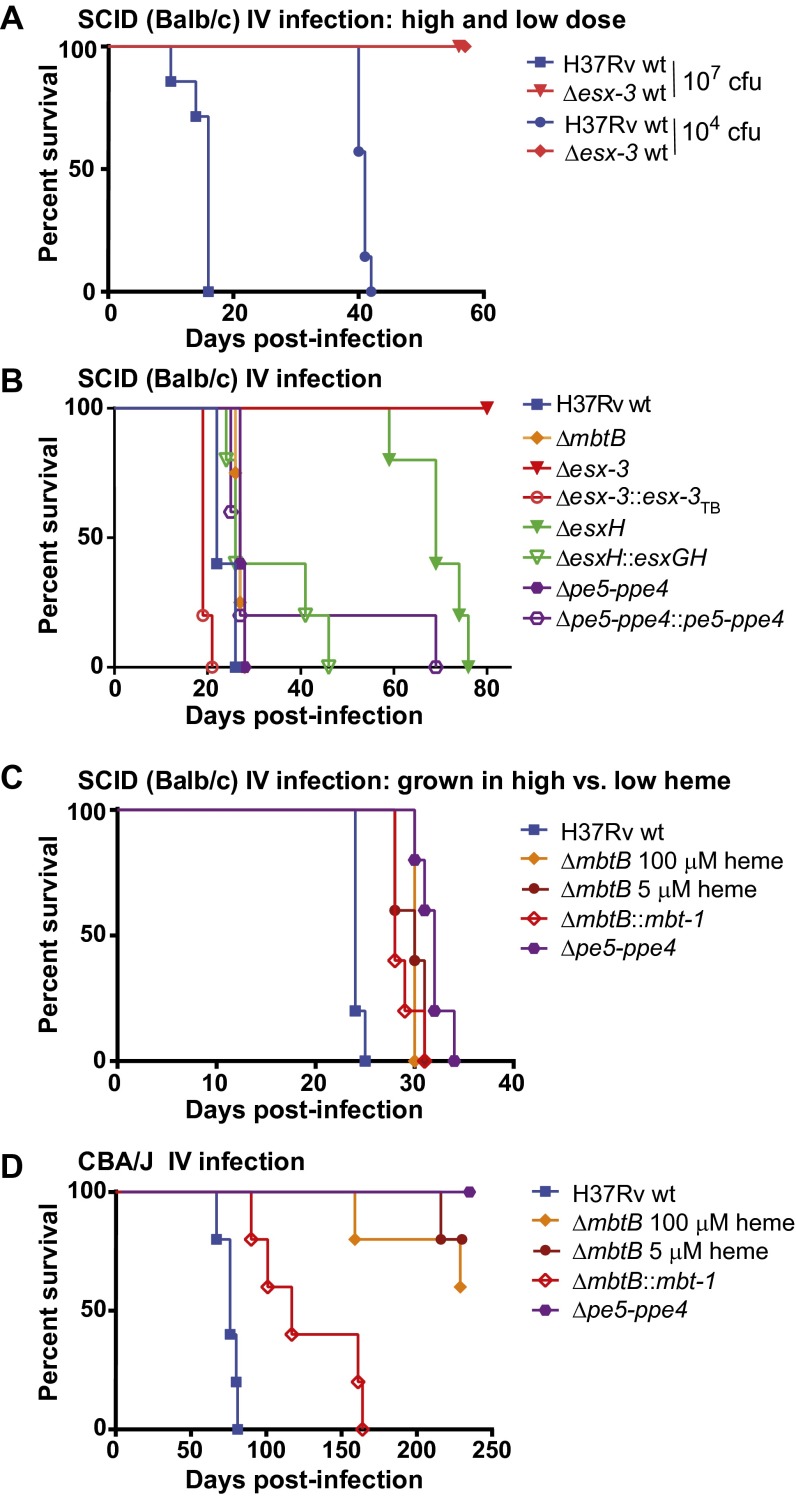
To assess the virulence of numerous mutant strains rapidly, we initially infected BALB/c SCID mice by the i.v. route. A dose of 104 cfu of H37Rv was lethal by ∼40 d postinfection, whereas mice survived ∼107 cfu of Δesx-3 (Fig. 3A). Despite their strikingly similar in vitro iron-related growth defects, mutants harboring individual deletions within the esx-3 region were less severely attenuated. After infection of SCID mice with ∼2 × 106 cfu, the median survival time (MST) was 22 d for mice infected with the WT strain, 27 d for mice infected with ΔmbtB and Δpe5–ppe4, and 65 d for mice infected with ΔesxH; this substantial attenuation of ΔesxH was largely reversed by genetic complementation (Fig. 3B). Most dramatically, the Δesx-3–infected mice survived for 12 mo, whereas complementation of Δesx-3 resulted in slight hypervirulence (MST 19 d). The relatively modest attenuation of Δpe5–ppe4 and ΔmbtB was surprising, given their profound phenotypes in vitro. To address whether hemin supplementation in the growth medium used before i.v. infection provided iron stores that the bacteria could use in vivo, we examined the virulence of the strains following growth in different amounts of hemin. For ΔmbtB, the kinetics of mortality were nearly identical if bacteria were pregrown in 5 or 100 μM hemin (MST 30 d; Fig. 3C). Similarly, when the Δpe5–ppe4 mutant was cultured in the absence of hemin, it was only mildly attenuated (Fig. 3C), similar to when it was pregrown in 100 μM hemin (Fig. 3B). These results show that, despite their profound growth defects in vitro, the Δpe5–ppe4 and ΔmbtB mutants are only mildly attenuated in vivo.
Fig. 3.
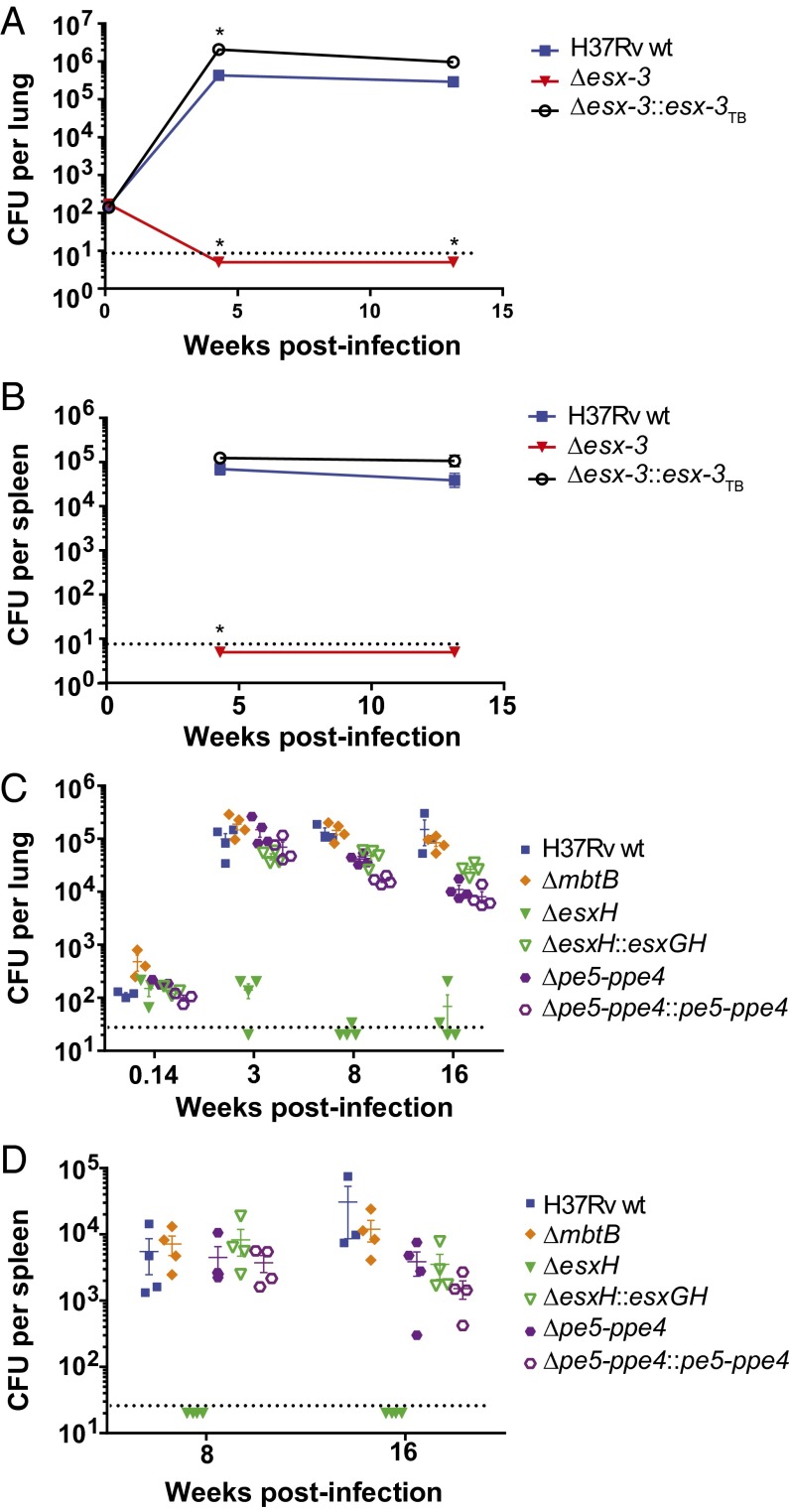
Deletion of the entire esx-3 locus results in dramatic attenuation in vivo, but pe5–ppe4 and mbtB are required for virulence in a mouse strain-dependent manner. (A and B) Survival of SCID mice infected by the i.v. route with H37Rv or Δesx-3 (mc27788) at ∼104 or ∼107 cfu (A) or with H37Rv WT, ΔmbtB (mc27850), Δesx-3 (mc27844), Δesx-3::esx-3TB (mc27856), ΔesxH (mc27846), ΔesxH::esxGH (mc27867), Δpe5–ppe4 (mc27848), or Δpe5–ppe4::pe5–ppe4 (mc27868) at a dose of ∼2 × 106 cfu (B). n = 7 or 8 mice per group in A and 4 or 5 mice per group in B. Before the infections shown in A and B, bacteria were cultured in 7H9 medium with 100 μM hemin. (C) Survival of SCID mice infected by the i.v. route with H37Rv, ΔmbtB (mc27850) pregrown in 100 μM hemin or 5 μM hemin, the ΔmbtB::mbt-1 complemented strain (mc27874), or Δpe5–ppe4 (mc27848). Except for the indicated ΔmbtB strains, the strains were grown without hemin. n = 5 mice per group. (D) Survival of CBA mice infected by the i.v. route with the strains in C. n = 5 mice per group. Survival differences were assessed using the log-rank (Mantel–Cox) test. In B, all groups were significantly different (P < 0.05) from WT except ΔesxH::esxGH and Δpe5–ppe4::pe5–ppe4. In C and D, all groups differed significantly (P < 0.05) from WT. In C, survival of mice infected with ΔmbtB grown in 100 μM hemin did not differ significantly from that of mice infected with ΔmbtB grown in 5 μM hemin or with the ΔmbtB::mbt-1 complemented strain. In D, survival of mice infected with ΔmbtB::mbt-1 differed significantly from that of mice infected with the ΔmbtB strains.
The SCID mice used here were in the BALB/c background, which is defective in natural resistance-associated macrophage protein 1 (Nramp1) (29), a metal transporter localized to the endosomal compartment of macrophages that may restrict phagosomal iron (30). In inbred mouse strains, nramp1 is found in two allelic forms, nramp1R (resistant) and nramp1S (susceptible); the susceptible allele results in degradation of the protein and susceptibility to a variety of intracellular pathogens (31). Nramp1 is functional in CBA mice (29), which we challenged by i.v. infection with ∼2 × 106 cfu of H37Rv, ΔmbtB grown in high or low hemin, or Δpe5–ppe4. Strikingly, in contrast to observations in the nramp1S mice, Δpe5–ppe4 and ΔmbtB were highly attenuated in nramp1R CBA mice (Fig. 3D). These data demonstrate a host-dependent requirement for ESX-3 substrates.
Δesx-3 and ΔesxH Are Attenuated for Growth in Human Macrophages, but Intracellular Growth of Δpe5–ppe4 Depends on Macrophage Polarization.
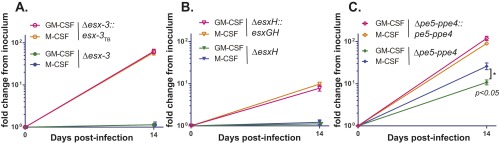
Differences in iron handling have been described for classically (M1) as opposed to alternatively (M2) activated macrophages (32, 33). Similar observations have been made for human macrophages differentiated with macrophage colony-stimulating factor (M-CSF) versus GM-CSF (34), where GM-CSF may promote a more iron-restrictive state. We therefore examined the growth of Δesx-3, ΔesxH, and Δpe5–ppe4 in human macrophages differentiated with M-CSF or GM-CSF (Fig. S4). The Δesx-3 and ΔesxH mutants were highly attenuated for growth in human macrophages, regardless of the cytokine used for differentiation. However, Δpe5–ppe4 showed a modestly improved growth capacity (∼2.4-fold) in the M-CSF–differentiated macrophages, indicating that the requirement for pe5–ppe4 may differ depending upon macrophage polarization, possibly resulting in differences in iron handling of M-CSF– versus GM-CSF–differentiated cells. In contrast, Δesx-3 and ΔesxH were essential for intracellular growth irrespective of macrophage polarization.
Fig. S4.
The esx-3 region is required for growth in human macrophages. Human macrophages from the same donor differentiated with either GM-CSF (pink or green symbols) or M-CSF (gold or blue symbols) were infected with (A) Δesx-3 (closed circles) or Δesx-3::esx-3TB (open circles), (B) ΔesxH (closed triangles) or ΔesxH::esxGH (open triangles), or (C) Δpe5–ppe4 (closed diamonds) or Δpe5–ppe4::pe5–ppe4 (open diamonds), and cfu of triplicate wells were determined 14 d postinfection. Strain numbers are as in Fig. 3B. Error bars indicate SEM.
The in Vitro Iron Phenotype Does Not Correlate with Virulence in SCID or Recombination-Activating Gene Knockout Mice.
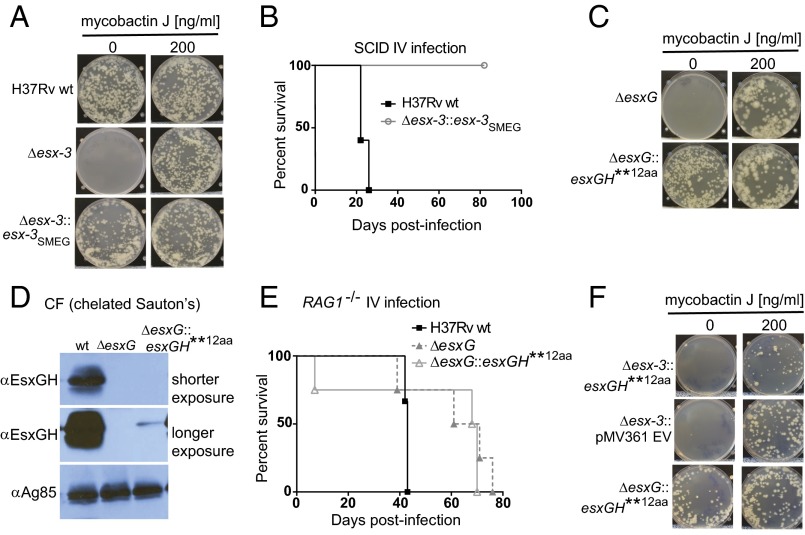
To explore further the relationship between the iron phenotype and virulence, we compared Δesx-3 complemented with the Mtb esx-3 region and a strain complemented with the Msmeg esx-3 region. Although the Msmeg esx-3 region complemented the in vitro iron-related growth defects of Δesx-3 (Fig. 4A), only the Mtb esx-3 region restored growth in vivo. Infection with Δesx-3::esx-3SMEG did not result in any mortality (Fig. 4B), even after 12 mo of infection. Therefore, despite the substantial homology between the Msmeg and Mtb esx-3 regions (14), Msmeg esx-3 lacks functions necessary for virulence. Additional evidence that virulence is separable from the iron requirement comes from analysis of a ΔesxG mutant expressing an aberrant EsxG protein product that has 12 additional N-terminal amino acids (referred to as “esxGH**12aa; pJP148”) (Table S2). Remarkably, the in vitro phenotype of the hygromycin-marked ΔesxG strain (mc27789) was rescued by the EsxG**12aa variant even though EsxG–EsxH secretion was nearly undetectable on Western blotting (Fig. 4 C and D). This finding is consistent with reports in Msmeg, in which <1% of EsxG–EsxH secretion reversed the iron-related growth defects (12). Despite correcting the iron-related growth defect, the strain complemented with EsxG fused to 12 N-terminal amino acids (ΔesxG::esxGH**12aa) behaved almost identically to ΔesxG in recombination-activating gene knockout (RAG1−/−) mice (Fig. 4E). Interestingly, although able to restore growth of ΔesxG on solid medium, the same plasmid (pJP148) was unable to rescue growth of Δesx-3 (Fig. 4F), implying that EsxG–EsxH secretion in Mtb is dependent on the presence of esx-3, consistent with findings in Msmeg (13).
Fig. 4.
esx-3 from Msmeg and the EsxG variant restore in vitro growth but not virulence to esx-3 region mutants. (A) H37Rv WT, Δesx-3 (mc27844), and Δesx-3::esx-3SMEG (mc27857) were plated on 7H10 medium with or without MbJ (200 ng/mL). (B) Survival of BALB/c SCID mice infected i.v. with H37Rv or Δesx-3::esx-3SMEG (mc27857). n = 5 mice per group. (C) ΔesxG (mc27789) and ΔesxG transformed with pJP148 (mc27830) were plated on 7H10 medium and on 7H10 medium with 200 ng/mL MbJ. (D) CF from strains grown in chelated Sauton’s medium was analyzed by Western blotting using an antibody raised against the EsxG–EsxH complex and was compared with H37Rv. Ag85B served as a loading control. (E) Survival analysis of C57BL/6 RAG1−/− mice infected by the i.v. route with ∼5 × 104 cfu of H37Rv, ΔesxG (mc27789), or ΔesxG harboring pJP148 (mc27830). n = 3 mice for H37Rv WT and 4 mice for the other strains. (F) Δesx-3 transformed with pMV361 empty vector (EV) (mc27831) and Δesx-3 or ΔesxG transformed with pJP148 (mc27829 and mc27830, respectively) were plated on 7H10 medium and on 7H10 medium with 200 ng/mL MbJ.
esx-3 and esxH Are Essential for Virulence in Aerosol Infections, but pe5–ppe4 and mbtB Are Dispensable.
We next sought to determine whether the differences in virulence between strains also would be observed in a model that more closely mimics the natural infection. We infected C57BL/6 (nramp1S) mice by aerosol with WT, Δesx-3, and Δesx-3::esx-3TB strains. In contrast to WT, the Δesx-3 mutant could not be recovered from the lungs, and it failed to disseminate to the spleen; Δesx-3::esx-3TB was mildly hypervirulent (Fig. 5 A and B). Similarly, ΔesxH did not proliferate in vivo, failed to disseminate, and was complemented by expression of esxG–esxH (pYUB1944) (Fig. 5 C and D). The marked attenuation of Δesx-3 and ΔesxH was in striking contrast to the Δpe5–ppe4 and ΔmbtB mutants, for which bacterial numbers were similar to WT (Fig. 5 C and D). Overall, these findings support the idea that defects in iron acquisition do not underlie the dramatic in vivo attenuation of Δesx-3. Rather esx-3 must play an additional role in virulence that is dependent upon EsxH and independent of PE5–PPE4.
Fig. 5.
Δesx-3 and ΔesxH are severely attenuated in aerosol infection of C57BL/6 mice, but Δpe5–ppe4 and ΔmbtB remain virulent. (A and B) Bacterial burdens in lungs (A) and spleens (B) were determined at the indicated time points after aerosol infection with H37Rv, Δesx-3 (mc27788), or Δesx-3::esx-3TB (mc27827). n = 4 or 5 mice per group, except n = 3 for H37Rv-infected spleens at 4 wk. Data points indicate mean ± SEM. Data points that are significantly different (P < 0.05) from H37Rv WT using an unpaired Student’s t test are indicated by an asterisk. (C and D) Following aerosol infection with the indicated strains, bacterial burdens were determined at 24 h and at 3, 8, and 16 wk postinfection in lungs (C) and at 8 and 16 wk postinfection in spleens (D). Strains were H37Rv, ΔmbtB (mc27850), ΔesxH (mc27846), ΔesxH::esxGH (mc27867), Δpe5–ppe4 (mc27848), and Δpe5–ppe4::pe5–ppe4 (mc27868); all strains were cultured in 7H9 medium supplemented with 100 μM hemin before use in aerosol infections. Individual data points are shown; lines indicate mean ± SEM. n = 3 or 4 mice per group. Dotted lines indicate approximate limits of detection.
Identification of Rv1386 (PE15)/Rv1387 (PPE20) as ESX-3–Secreted Substrates.
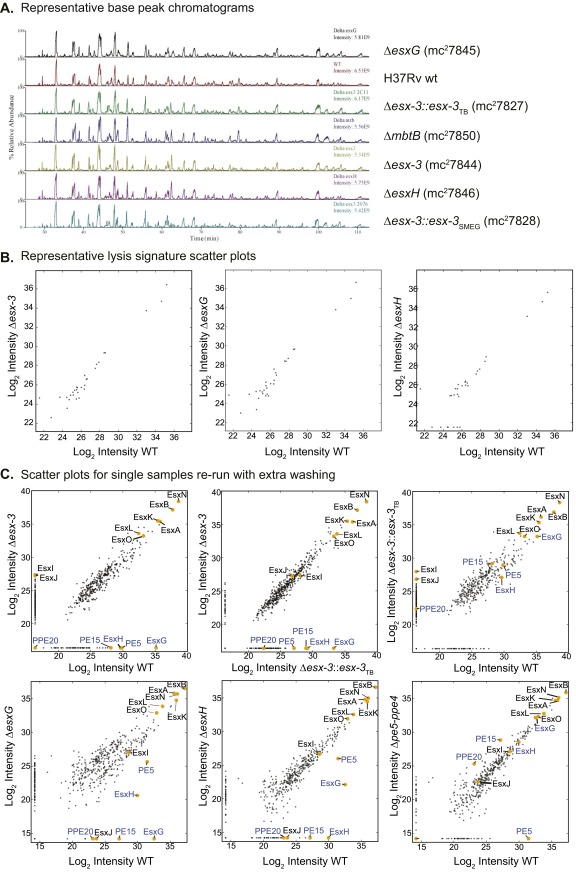
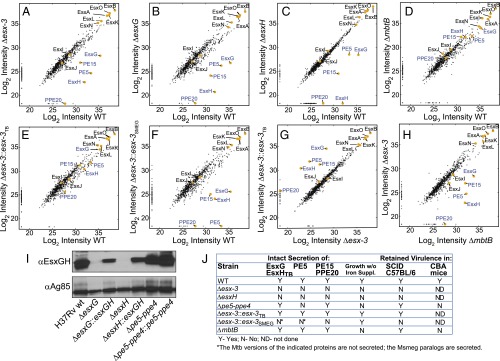
To elucidate further the role(s) played by esx-3 in iron acquisition and virulence, we sought to define the secreted effectors. CF from triplicate samples of WT, Δesx-3, ΔesxG, ΔesxH, Δesx-3::esx-3TB, Δesx-3::esx-3SMEG, and ΔmbtB cultured in chelated Sauton's medium was analyzed by MS using the MaxQuant software suite with the Andromeda search engine for peptide identification (SI Materials and Methods and Fig. S5). Label-free quantitation (LFQ) algorithms were used to determine relative protein amounts (SI Materials and Methods) (35). A panel of ribosomal and chaperonin proteins (Dataset S2) was used to verify similar amounts of lysis in the samples (Fig. S5B). Correcting for multiple testing by controlling for a false discovery rate (FDR) at 5% using the Benjamini–Hochberg method (36), we detected 854 proteins in total (Dataset S2), of which 72 were differentially secreted in the WT strain compared with Δesx-3 (Fig. 6A, and SI Materials and Methods, and Fig. S6A). Esx proteins encoded outside the esx-3 locus that could be unambiguously identified, including EsxA, EsxB, EsxL, EsxN, and EsxO, were present in similar amounts in both samples, demonstrating that the esx-3 deletion does not globally impair Esx protein secretion (Fig. 6A). As expected from analogy to EsxA and EsxB, EsxG and EsxH exhibited codependent secretion; EsxH was present at greatly diminished levels in the CF of the ΔesxG mutant compared with WT, and similarly EsxG was present at greatly reduced levels in the CF of ΔesxH (Fig. 6 B and C and Figs. S5C and S6 B and C). Genetic complementation resulted in partial restoration of both EsxG and EsxH secretion in both mutants (Fig. S6 B and C).
Fig. S5.
Proteomics MS data including representative base–peak chromatograms, lysis signature scatterplots, and scatterplots for single samples rerun with extensive column washing between samples to eliminate carryover. (A) A representative base–peak chromatogram for one biological replicate of all strain types from the triplicate dataset. Results illustrate that the most abundant peptide ions eluting at a given time are similar for each strain. (B) Lysis signature scatterplots for selected comparisons: H37Rv WT vs. Δesx-3 (Left), H37Rv WT vs. ΔesxG (Center), and H37Rv WT vs. ΔesxH (Right). For this analysis, scatterplots show log2-transformed LFQ intensity values of ribosomal and chaperone proteins (Dataset S2). (C) Samples run with extensive washing. (Upper) Single samples from the experiment performed in Fig. 6 were reanalyzed by MS with extensive washing between samples, and scatterplots showing log2-transformed intensity values of proteins identified in the CF were generated from the data after LFQ. Comparisons included H37Rv WT vs. Δesx-3 (Left), Δesx-3::esx-3TB vs. Δesx-3 (Center), and H37Rv WT vs. Δesx-3::esx-3TB (Right). In this case, proteins of interest encoded within the region deleted in the mutant strain (PE5, EsxG, and EsxH) were undetectable in the CF of Δesx-3, as were PE15 and PPE20. (Lower) Additional samples from a second sample set were run in a similar manner. Scatterplot comparisons of log2-transformed LFQ intensity values are shown for H37Rv WT vs. ΔesxG (Left), H37Rv WT vs. ΔesxH (Center), and H37Rv WT vs. Δpe5–ppe4 (Right). Again, proteins of interest encoded within the regions deleted in the various mutant strains were not detected in the CF.
Fig. 6.
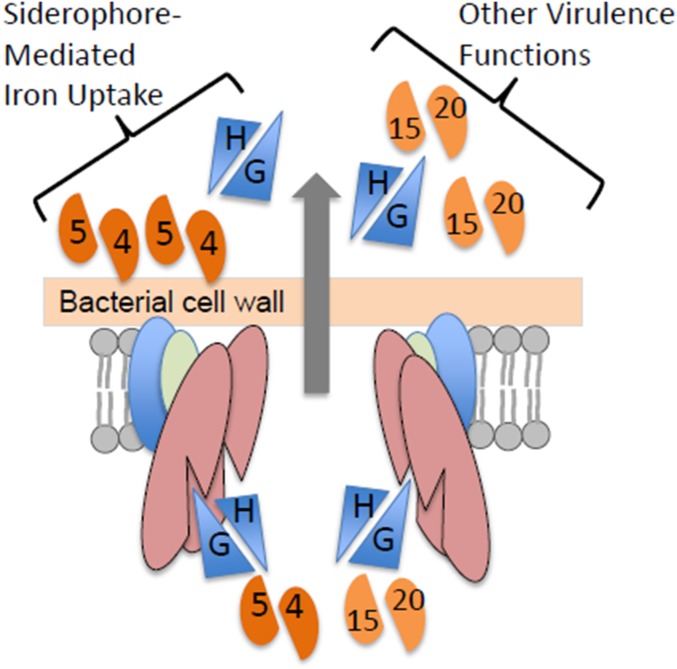
Proteomics MS analysis of CF reveals codependent secretion of PE5 with EsxG–EsxH and identifies PE15/PPE20 as ESX-3 substrates. (A–H) Scatterplots show log2-transformed LFQ intensity values of proteins identified in the CF with the following strain comparisons: H37Rv vs. Δesx-3 (mc27844) (A); H37Rv vs. ΔesxG (mc27845) (B); H37Rv vs. ΔesxH (mc27846) (C); H37Rv vs. ΔmbtB (mc27850) (D); H37Rv vs. Δesx-3::esx-3TB (mc27827) (E); H37Rv vs. Δesx-3::esx-3SMEG (mc27828) (F); Δesx-3 vs. Δesx-3::esx-3TB (G); and ΔmbtB vs. Δesx-3 (H). Values for proteins of interest (Esx proteins and select PE–PPE proteins) are in yellow and are labeled; proteins implicated as ESX-3 substrates are indicated by blue text. Trace amounts of PE5, EsxG, and EsxH were detected in the Δesx-3 samples because of carryover from other samples; when the columns were washed extensively between runs, PE5, EsxG, and EsxH were absent from Δesx-3, as anticipated (Fig. S5C). See Fig. S6 for lists of proteins that are present at significantly different levels for the various comparisons and Dataset S2 for lists of all proteins detected in the secretome analysis. (I) Western blot analysis of CF from strains grown in chelated Sauton’s medium using the EsxG–EsxH antibody. Ag85B served as a loading control. (J) Summary of secretome findings, in vitro growth requirements, and in vivo growth characteristics for the various strains.
Fig. S6.
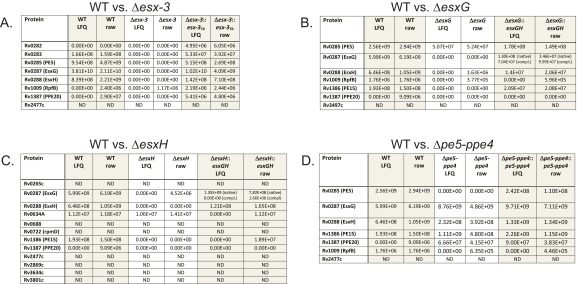
Proteins present at significantly reduced levels in the CF of Δesx‐3, ΔesxG, and ΔesxH compared with WT. The tables list candidate substrates of the ESX-3 secretion system. The lists in the left column include only proteins that differed significantly in abundance between WT and mutant (analysis as described in SI Materials and Methods) based on the triplicate dataset (Dataset S2), only proteins present in three of three WT samples, and only proteins present at a lower level in mutants than in WT. Each table also includes both the LFQ and the raw (from Thermo Fisher .RAW files) intensity values for the indicated proteins in WT, mutant, and complemented strains, in individual (singlicate) samples run with extensive column washing between samples to eliminate carryover. (A) For WT vs. Δesx‐3, the individual samples rerun with extra column washing were taken from the triplicate set (i.e., were one of the three samples). (B–D) The individual samples were from a separate single analysis for WT vs. ΔesxG (B), WT vs. ΔesxH (C), and WT vs. Δpe5‐ppe4 (D) (Dataset S2). Because there was only a single analysis of the Δpe5–ppe4 mutant, statistical analysis is not available for this strain. Therefore, the WT vs. Δpe5–ppe4 comparison in D includes the suggested substrates identified in the other analyses. For strains complemented with the pYUB1944 plasmid, two peptide signals are indicated for EsxG. One is an internal peptide, which is the same for the endogenous protein and the complementing construct (native); the other is the unique N terminus of the complementation construct (compl.), with methionine and alanine added at the N terminus of EsxG as a result of cloning sites. ND, the protein was not detected in any of the samples from the sample set, according to the inclusion criteria described in SI Materials and Methods.
To identify ESX-3 substrates, we focused on eight proteins that were present in all three WT samples and were diminished in Δesx-3 (Fig. 6 A, E, and G and Fig. S6). Of these, secretion of Rv1009/RpfB was not restored by complementation (Dataset S2), suggesting that it may not be a substrate for secretion. Of the remaining proteins, five (Rv0282, Rv0283, Rv0285/PE5, EsxG, and EsxH) were encoded within the region deleted in the mutant strain. We detected trace levels of these five proteins in the Δesx-3 samples (below 3% of WT levels), consistent with a typical level of carryover. To verify this result, we reran one sample from each strain on an extensively washed column; as expected, these proteins were not detected (Fig. S5C). Rv1387/PPE20 and Rv2477c were the only other proteins that satisfied our criteria for being potential substrates. However, Rv2477c also was undetectable in two of three ΔmbtB samples (Dataset S2), suggesting that its absence might reflect an adaptation to iron deprivation as opposed to its being a direct ESX-3 substrate. Thus, only Rv1387/PPE20 fulfilled criteria as a potential ESX-3 substrate. Although it did not meet our strict criteria for significance, Rv1386 (PE15) also was diminished in the esx-3 mutant (P value 0.007, which corresponds to an FDR of 13%) (Fig. 6A) and was undetectable when the Δesx-3 sample was reanalyzed after carryover removal (Fig. S5C). CF samples prepared from ΔesxG and ΔesxH also had greatly reduced or undetectable PE15 and PPE20 (Fig. 6 B and C and Figs. S5C and S6 B and C), demonstrating that secretion of these proteins is dependent on EsxG and EsxH. Combined, these data strongly suggest that PE15 and PPE20 are ESX-3 substrates.
Secretion of PE5–PPE4 Is Required for Iron Acquisition, Whereas Secretion of EsxG and EsxH Is Important for Virulence.
We next examined whether secretion of EsxG–EsxH and PE5–PPE4 are codependent. Although still detectable, secretion of PE5 was substantially reduced in ΔesxG and ΔesxH (Fig. 6 B and C and Figs. S5C and S6B). This diminution reached significance for ΔesxG but not for ΔesxH, using our strict criteria [P values for PE5 of 0.0003 (FDR = 6%) in WT vs. ΔesxG and 0.001 (FDR 26%) in WT vs. ΔesxH]. We did not detect PPE4 in the CF of any sample, and this protein likely remains membrane localized because it contains several hydrophobic, potentially membrane-spanning domains, analogous to the situation in ESX-1, where PE35 is found in the CF and PPE68 remains membrane associated (37, 38). In contrast, the Δpe5–ppe4 mutant secreted nearly WT levels of EsxG and EsxH (Figs. S5C and S6D), a finding confirmed on Western blotting using a polyclonal antibody recognizing the EsxG–EsxH complex (Fig. 6I). Thus, the Δpe5–ppe4 mutant exhibits a severe iron phenotype but secretes EsxG and EsxH normally and is not attenuated in vivo in nramp1S hosts. This observation shows that the iron-acquisition function of ESX-3 is not simply related to the secretion of EsxG–EsxH. Taken together with the phenotypic studies, the data suggest that PE5–PPE4 is important for the iron-acquisition function of ESX-3, whereas virulence correlates with secretion of EsxG, EsxH, PE15, and PPE20 (Fig. 6J).
SI Materials and Methods
Bacterial Strain Construction.
A detailed list of strains is provided in Table S1.
Allelic exchange via specialized transduction was used to generate a deletion of the entire esx-3 locus in Mtb H37Rv (68, 69). DNA fragments containing ∼900 bp of sequence upstream of Rv0282 and 867 bp downstream of Rv0292 were PCR-amplified from H37Rv genomic DNA using the primers listed in Dataset S1, digested with Van91I, and cloned into the Van91I-digested p0004S (pYUB1471) vector (69) flanking an sacB-hygR cassette. The allelic exchange substrate (AES) then was digested with PacI and ligated into PacI-digested temperature-sensitive phAE159, a derivative of TM4. Phage was packaged, propagated in Msmeg mc2155, and high-titer lysates were prepared as described (69). The resulting specialized transducing phage was used to delete the esx-3 locus in Mtb H37Rv, replacing the coding sequence with a γδres-sacB–hygR-γδres cassette. Transductions were plated onto 7H10 agar supplemented with 10% OADC enrichment, 0.5% glycerol, 100 μM hemin, 0.05% Tween 80, and 50 μg/mL hygromycin B. Genomic DNA prepared from hygromycin-resistant colonies was analyzed by Southern blotting to confirm the deletion (Fig. S1). The deletion also was confirmed by PCR of flanking regions (Fig. S1) using the primers listed in Dataset S1, and by whole-genome sequencing using Illumina MiSeq technology (available upon request).
Deletions of esxG, esxH, esxG–esxH combined, pe5–ppe4 combined, mbtB, and the complete mbt-1 Mb synthesis cluster (Rv2377c–Rv2386c) in Mtb H37Rv were generated using the methods described for the esx-3 deletion. Phasmids for deletion of esxG, esxH, and mbtB were constructed in collaboration with the Genomics Institute of the Novartis Research Foundation to generate a set of gene-deletion constructs for Mtb (76). PCR primers used to amplify the flanking regions for the construction of AESs are listed in Dataset S1. Transductants were recovered on 7H10 agar with 10% OADC enrichment, 0.5% glycerol, and 50 μg/mL hygromycin B plus 200 μg/mL FAC with 10 μg/mL zinc sulfate for ΔesxG. For ΔesxH, ΔesxG–esxH, Δpe5–ppe4, and Δmbt-1 the iron source was 200 ng/mL MbJ. For ΔmbtB, 100 μM hemin with 0.05% Tween 80 was used. Genomic DNA was prepared from the isolates using a cetyltrimethylammonium bromide/chloroform-based method (77), and deletions were verified by PCR, in which distinct band sizes were diagnostic for mutant and WT (Fig. S1; primers are listed in Dataset S1), as well as by whole-genome sequencing showing an absence of reads at the relevant loci (available upon request).
In addition, a select subset of esx-3 region mutants was generated in mc26230, an H37Rv-derived auxotrophic strain with deletions at the RD1 and panCD loci (24, 25) that has been approved by the Albert Einstein College of Medicine Biosafety committee for use under BSL2 containment, facilitating the processing of radioactively labeled, infectious samples. Deletions of esx-3, mbtB (both recovered with 100 μM hemin/0.05% Tween 80 supplementation) and esxH (recovered with 200 ng/mL MbJ supplementation) were introduced into mc26230 using specialized transduction and the phasmid constructs described above for the deletions in H37Rv. Deletions were confirmed by PCR (Fig. S1; primers are listed in Dataset S1 and were obtained from Fisher Scientific) and whole-genome sequencing (available upon request). The Δesx-3 and ΔmbtB mutants in the mc26230 background displayed iron-related growth phenotypes similar to their counterparts in H37Rv.
We also removed the sacB-hygR cassette, which is flanked by γδ-resolvase sites, from the deletion mutants to minimize the likelihood of polar effects on neighboring genes in the operons; the resulting strains are referred to as “unmarked.” The TM4-derived phage phAE280 was used to deliver a construct expressing the γδ-resolvase enzyme (69); transductants were plated onto 7H10 medium without hygromycin containing 10% (wt/vol) sucrose and 200 ng/mL MbJ (Allied Monitor) or 100 μM hemin. PCR across the residual resolvase site followed by sequence confirmation of PCR products was used to verify the unmarking.
Following excision of the sacB-hygR cassette, additional deletions were introduced into selected strains (Δmbt-1 deletion into ΔesxG–esxH and Δmbt-1 deletion into Δesx-3) using the method of specialized transduction described above. Complemented strains were prepared by introducing constructs into the deletion mutants by electroporation using standard protocols (77) and the presence of the constructs was verified by PCR. The ΔesxG, ΔesxH, Δpe5–ppe4, and ΔmbtB strains were complemented after unmarking, whereas Δesx-3::esx-3TB (pYUB1335) and Δesx-3::esx-3SMEG (pYUB2076) were unmarked after complementation. Hygromycin-marked versions of Δesx-3 and ΔesxG were also complemented with plasmid pJP148 (see Plasmid Construction below).
Plasmid Construction.
pYUB1336 and pYUB2076, cosmids containing the entire Mtb and Msmeg esx-3 regions, respectively, have been described previously (14). pYUB1335 differs from pYUB1336 in the antibiotic resistance marker, as detailed in Table S2. The esxG–esxH and pe5–ppe4 loci were amplified by PCR from H37Rv genomic DNA using the primers listed in Dataset S1 and were cloned into the pMV261 episomal plasmid under control of the hsp60 promoter using the MscI and HindIII restriction sites; this cloning strategy resulted in the addition of two amino acids—methionine and alanine—to the N termini of EsxG and PE5. Following sequence verification of the constructs, the hsp60 promoter–gene pair cassette was liberated by digestion with XbaI and HindIII and was subcloned into the pMV306 integrating plasmid to generate pYUB1944 and pYUB1945 (Table S2). Alternatively, for esxG–esxH complementation, a construct was generated by cloning the PCR-amplified esxG–esxH locus from Mtb between the EcoRI and HindIII sites of pMV361, resulting in the addition of 12 amino acids to the N terminus of EsxG (pJP148; Table S2). The ΔmbtB deletion was complemented with an integrating cosmid (pYUB1940) (Table S2) containing the entire mbt-1 Mb synthesis locus, so that the mbtB gene would be expressed from its native promoter. Escherichia coli used for cloning experiments was grown in LB medium at 37 °C, using the following antibiotics when required: ampicillin or carbenicillin (100 μg/mL), kanamycin (25–40 μg/mL), and hygromycin (150 μg/mL).
Radiolabeling of Siderophores from Bacteria Grown in Iron-Deficient Medium.
Starter cultures of mc26230, mc27860 (Δesx-3), mc27863 (Δesx-3::esx-3TB), and mc27862 (ΔmbtB) were inoculated from frozen stocks into 7H9 medium supplemented with 24 μg/mL d-pantothenic acid (pantothenate; Sigma or Fisher); medium also contained 100 μM hemin for mc27860, 10 μM hemin for mc27862, and 25 μg/mL kanamycin for mc27863. Once grown, bacteria from starter cultures were washed in PBS-T and transferred to 7H9 medium with 24 μg/mL pantothenate; this medium had no further supplements, except for the inclusion of 10 μM hemin for mc27862 and 25 μg/mL kanamycin for mc27863. Following subculture in 7H9 medium, the bacteria were washed in chelated Sauton’s medium and then were transferred to chelated Sauton’s medium, which had no additional supplement and no detergent. To label Mb siderophores, the washed bacteria were inoculated at a starting OD600 of 0.2 into 5 mL of medium in the presence of 1 µCi/mL [7-14C]-salicylic acid (PerkinElmer) and were allowed to grow with shaking for 20 d at 37 °C. Approximately 100 μL of zirconia/silica beads (Biospec products) were added to promote dispersed growth in the absence of detergent. At the end of the growth period, siderophores were extracted as described previously (46). Briefly, bacteria were pelleted by centrifugation (2,000 × g for 7 min); then ferric chloride hexahydrate (20 mg/mL; 0.04 mL) was added to the supernatant (5 mL). After 1 h incubation at room temperature, supernatants were extracted three times with chloroform; the organic fractions were pooled and dried under nitrogen. The cell pellets were resuspended in 2.5 mL ethanol and incubated overnight at 37 °C with shaking. Following centrifugation (2,000 × g for 7 min), the supernatant was collected and diluted 1:1 with water; then ferric chloride hexahydrate was added to a final concentration of 2.2 mM. Following 1-h incubation at room temperature, the material was extracted three times with chloroform, and organic fractions were pooled and dried under nitrogen. The extracts (both from the initial supernatant and from the cell pellet) were resuspended in 0.2 mL of chloroform and spotted on TLC silica gel 60F254 plates. The samples were normalized based on equivalent cpms. In various experiments, MbJ as well as cMb (EMC Microcollections) and Mb (a kind gift of Colin Ratledge, Department of Biological Sciences, University of Hull, Hull, UK) from Msmeg were included as controls, as was the radiolabeled salicylic acid substrate. The TLC plate was developed in ethanol/hexanes/water/ethyl acetate/acetic acid (5:25:2.5:35:0.5) and subjected to autoradiography by exposure for 72 h at −80 °C.
TLC Analysis of Unlabeled Siderophores from Mtb in Iron-Replete Medium.
The indicated strains were inoculated from frozen stocks into 7H9 medium without additional iron supplements except that 5 μM hemin was added to facilitate the growth of mc27850 (ΔmbtB), and 25 μg/mL kanamycin was included for mc27856 (Δesx-3::esx-3TB). Starter cultures were expanded to ∼50 mL and were allowed to grow to stationary phase. Bacteria were harvested by centrifugation; cell pellets were extracted with ethanol overnight at 37 °C with shaking. After centrifugation, the supernatants were diluted 1:1 with water and were filtered twice with 0.22-μm filters before removal from the BSL3 laboratory. This filtering was followed by the addition of ferric chloride hexahydrate and extraction with chloroform. The combined organic phases were dried, resuspended in 0.2 mL of chloroform, and analyzed by TLC as described above. Following photography of the plate, the TLC plate was sprayed with a solution of 10% sulfuric acid in ethanol and heated at 150 °C for 15 min in an oven to visualize the lipids. A similar amount of extracted material was observed for all strains, indicating that differences in the amount of starting material or incapacity to extract lipids from the strains did not underlie the observed differences in Mb content.
MS Analysis of Unlabeled Siderophores from Mtb Grown in Iron-Replete Medium.
Indicated strains were grown from freezer stocks in 10-mL aliquots of Middlebrook 7H9 liquid medium containing 0.2% glycerol, 0.05% Tween 80, 10% OADC, and 24 μg/mL d-calcium pantothenate (Fisher Scientific). Cultures were grown for 8 d until midlog phase, were pelleted, washed once with Dulbecco’s PBS (Corning), and concentrated to an OD600-equivalent of 10 absorbance units in PBS. Ten microliters of concentrated cells were then used to inoculate subcultures of each strain in triplicate in the same medium as above. These cultures were grown for 21 d and then were extracted for UPLC-MS analysis.
Lipid extraction for UPLC-MS analysis.
Cultures were pelleted by centrifugation. Supernatants were decanted and filtered twice with 0.22-μm syringe filters. Lipid extraction was performed essentially as described by Madigan et al. (70). Cell pellets were washed once with ice-cold PBS, quenched with 1 mL ice-cold methanol, and transferred into glass vials. Chloroform (2 mL) was added to each methanol suspension, and the vials were mixed overnight at room temperature by inversion on a rotating mixer. Chloroform–methanol suspensions were centrifuged to pellet cell debris, and liquid fractions were transferred to new glass vials and dried under nitrogen. Filtered supernatants were acidified with 60 μL of 3-M hydrochloric acid, after which 2.5 mL of each replicate was transferred to a glass vial and mixed with 1.4 volumes (3.5 mL) of ethyl acetate overnight at room temperature by inversion on a rotating mixer. Supernatant extractions were centrifuged at 1,100 × g for 10 min, and ethyl acetate fractions were transferred to new glass vials and dried under nitrogen. Dried extracts from both cell pellets and supernatants were dissolved in 0.75 mL and 0.20 mL isopropanol/acetonitrile/water (2:1:1, vol/vol/vol), respectively.
UPLC-MS analysis.
Separation and analysis of lipid extracts was performed as described (71) using a Waters ACQUITY UPLC system and a Waters Synapt G2 quadrupole TOF hybrid mass spectrometer. Total ion chromatograms of the three biological replicates of each sample type were aligned in Waters MassLynx software. Markers of exact mass–retention time pairs were obtained with intensities normalized to the total ion current, and mycobactins were identified using a modified MycoMass database: www.brighamandwomens.org/research/depts/medicine/rheumatology/labs/moody/default.aspx. Markers used for analysis were restricted to those that matched the retention times of known standards of MbJ and mycobactin S and bacillus Calmette–Guérin cMb (mycobactins courtesy of Colin Ratledge).
cMb Uptake Experiment.
Uptake of cMb was quantified as described previously (46). cMb prepared from Msmeg was deferrated followed by labeling with 55Fe as described. The strains mc26230 and mc27818 (Δesx-3) were grown in 7H9 medium supplemented with 20 μM hemin to midlogarithmic phase (OD600 1–2). Cells were washed on ice with a low-iron medium consisting of 500 µM MgCl2⋅6H2O, 7 µM CaCl2⋅2H2O, 1 µM NaMoO4⋅2H2O, 2 µM CoCl2⋅6H2O, 6 µM MnCl2⋅4H2O, 7 µM ZnSO4⋅7H2O, 1 µM CuSO4⋅5H2O, 15 mM (NH4)2SO4, 12 mM KH2PO4, pH = 6.8, 1% (wt/vol) glucose, which was supplemented with 10% OADC and 0.2% casamino acids, and were resuspended in the same medium to an OD600 of ∼3.0 on ice [mean cell concentrations (dry weight cell per milliliter of medium): mc26230 = 0.717 ± 0.08 mg/mL, mc27818 = 0.725 ± 0.05 mg/mL, P = 0.886, two-tailed Student’s t test). For uptake experiments, 2 mL of cell suspensions were equilibrated at 37 °C for 15 min and shaken at ∼400 rpm. 55Fe-labeled cMb was added to the cells at a final concentration of ∼0.1 µM cMb, 0.367 µCi 55Fe. Samples (200 μL) were removed at 2, 9, 16, and 32 min and were added to 400 µL of a killing buffer consisting of 100 mM LiCl and 50 mM EDTA in 4% formaldehyde in Spin-X filter microcentrifuge tubes. Cells were centrifuged immediately and were washed twice in killing buffer. The radioactivity of the cells was quantified using liquid scintillation counting (Beckman Coulter LS6500). All experiments were done in triplicate.
Intracellular Bacterial Growth Assays.
Preparation of primary human macrophages.
Human macrophages were prepared as described (72) following the method of Vogt and Nathan (73). Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare) from de-identified human peripheral blood obtained from the New York Blood Center and were selected with CD14 magnetic beads (Miltenyi Biotec) per the manufacturer’s protocol. The CD14+ PBMCs were resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco-Life Technologies) supplemented with 10% non–heat-inactivated human AB serum (Gemini Bio-Products), 20 mM Hepes buffer, and 2 ng/mL recombinant human GM-CSF or 10 ng/mL recombinant human M-CSF (R&D Systems) and were seeded into 48-well tissue-culture plates (Costar) at a density of 2 × 105 cells per well. To allow differentiation into macrophages, PBMCs were incubated for 14 d at 37 °C, 5% CO2 and 10% O2 (achieved by flushing the chamber with N2); tissue culture medium was replenished at days ∼7 and 11.
Macrophage infections.
Mtb strains were cultured in 7H9 medium, supplemented with 25 μg/mL kanamycin for the complemented strains. Initial cultures of the mutants contained residual hemin from the frozen stocks. Following growth of the initial cultures, the strains were passaged again in the medium noted above before use in infections. Log-phase cultures were prepared for infection by washing in PBS and sonication, then were diluted in RPMI medium with 10% non–heat-inactivated human AB serum and 20 mM Hepes buffer (no cytokine), and were added to macrophages at an MOI of 0.02 based on the OD600 estimate; the inocula were plated on 7H10 medium with 10% (vol/vol) OADC, 0.5% glycerol, and 200 ng/mL MbJ to determine bacterial counts. Mycobacterial growth in macrophages was enumerated at 14 d postinfection by lysis of both supernatant and cell monolayers in 0.025% SDS in H2O, followed by plating serial dilutions on agar (7H10 with 10% OADC, 0.5% glycerol, 200 ng/mL MbJ). Colonies were counted after ∼2–3 wk of incubation.
Virulence Studies in Mice.
Intravenous and aerosol infections of mice were carried out as described in Materials and Methods. Bacterial culture conditions for the individual experiments as well as inoculum amounts for the i.v. infections are indicated below. Mycobacterial strains were grown in 7H9 medium with antibiotics as appropriate for strains containing hygromycin or kanamycin resistance cassettes and additional supplements as indicated.
Intravenous Infections.
SCID infections.
For the SCID infections, strains were initially passaged in 7H9 medium with 100 μM hemin. Inocula were plated onto 7H10 medium (H37Rv WT), 7H10 medium with 100 μM hemin, 0.05% Tween 80 (Δesx-3) (for the experiment shown in Fig. 3A), or 7H10 medium containing 200 ng/mL MbJ (for the experiment shown in Figs. 3B and 4B).
Doses (cfu per 200 μL PBS-T) determined by plating inocula for the SCID experiment in Fig. 3A were 8.4 × 106 for H37Rv WT and 4.2 × 106 for Δesx-3 (mc27788) when the target dose was 1 × 107 and were 8.6 × 103 for H37Rv WT and 7.2 × 103 for Δesx-3 (mc27788) when the target dose was 1 × 104.
Doses (cfu per 200 μL PBS-T) determined by plating inocula for the SCID experiment in Figs. 3B and 4B (2 × 106 target dose) were 3.60 × 106 for H37Rv WT; 2.04 × 106 for ΔmbtB (mc27850); 1.85 × 106 for Δesx-3 (mc27844); 1.61 × 106 for Δesx-3::esx-3TB (mc27856); 1.79 × 106 for ΔesxH (mc27846); 2.45 × 106 for ΔesxH::esxGH (mc27867); 1.37 × 106 for Δpe5–ppe4 (mc27848); 2.09 × 106 for Δpe5–ppe4::pe5–ppe4 (mc27868); and 1.52 × 106 for Δesx-3::esx-3SMEG (mc27857).
Experiments comparing virulence of bacteria grown under different culture conditions in SCID and CBA mice.
For experiments comparing the virulence of bacteria grown under different culture conditions in SCID and CBA mice (Fig. 3 C and D), strains were passed twice in the desired medium [7H9 with 5 μM vs. 100 μM hemin for ΔmbtB and 7H9 with no hemin for H37Rv WT, ΔmbtB::mbt-1 (pYUB1940) complemented, and Δpe5–ppe4] before use in infections. Inocula were plated onto 7H10 medium containing 200 ng/mL MbJ.
Doses (cfu per 200 μL PBS-T) determined by plating inocula for the SCID and CBA experiments in Fig. 3 C and D (2 × 106 target dose) were 3.1 × 106 for H37Rv WT; 3.4 × 106 for ΔmbtB (mc27850) grown in 7H9 + 100 μM hemin; 3.4 × 106 for ΔmbtB (mc27850) grown in 7H9 + 5 μM hemin; 2.5 × 106 for ΔmbtB::mbt-1 (mc27874); and 2.5 × 106 for Δpe5–ppe4 (mc27848).
RAG1−/− experiment.
For the RAG1−/− experiment (Fig. 4E) comparing H37Rv with H37Rv ΔesxG (mc27789) and H37Rv ΔesxG complemented with the variant of pMV361-esxGH including 12 additional amino acids at the N terminus of EsxG (mc27830), strains were cultured in 7H9 medium, which also contained 100 μM hemin for the marked ΔesxG mutant. Inocula were plated onto 7H10 medium, which was supplemented with MbJ 2000 ng/mL for the ΔesxG mutant.
Doses (cfu per 200 μL PBS-T) determined by plating inocula for the RAG1−/− experiment in Fig. 4E (5 × 104 target dose) were 2.50 × 104 for H37Rv WT; 2.16 × 104 for ΔesxG (mc27789); and 2.10 × 104 for ΔesxG::esxGH**12aa (mc27830).
Aerosol Infections.
For the initial C57BL/6 aerosol experiment (Fig. 5 A and B) comparing H37Rv to H37Rv Δesx-3 (mc27788) and to H37Rv Δesx-3 complemented with Mtb esx-3 cosmid (mc27827), bacteria were cultured for two passages in 7H9 medium that also contained 100 μM hemin for Δesx-3. Initial inocula and organ homogenates were diluted accordingly at the indicated time points and were plated onto 7H10 medium, which also was supplemented with 100 μM hemin and 0.05% Tween 80 for the Δesx-3 mutant.
For the C57BL/6 aerosol experiments in which the panel of deletion mutants was compared (Fig. 5 C and D), bacteria were passaged twice in 7H9 medium containing 100 μM hemin before infection. Initial inocula and organ homogenates were diluted accordingly at the indicated time points and were plated onto 7H10 medium containing 200 ng/mL MbJ.
CF Preparation.
Starter cultures were grown in 7H9 medium with 100 μM hemin and antibiotics as appropriate for the complemented strains. Strains for which samples were prepared in triplicate were H37Rv WT and the following mutants in the H37Rv background: Δesx-3 (mc27844), ΔesxG (mc27845), ΔesxH (mc27846) Δesx-3::esx-3TB (mc27827), Δesx-3::esx-3SMEG (mc27828), and ΔmbtB (mc27850). For each strain, two samples were prepared in one experiment, and the third was prepared in a separate experiment. Bacteria from starter cultures were pelleted by centrifugation, washed in PBS-T, and expanded into 7H9 (with 10 μM hemin for ΔmbtB and without hemin for the other strains) at an initial OD600 of ∼0.04 (0.02 for H37Rv WT, 0.08 for ΔmbtB). Cultures were grown to the target OD600 of ∼1.0, were washed twice in chelated Sauton’s medium, and then were inoculated into chelated Sauton’s medium (no detergent) at OD600 0.50. After incubation in roller bottles at 37 °C for ∼48 h, cultures were centrifuged to pellet the bacteria, and supernatants were filtered twice through 0.22-μm filters. TCA was added to a final concentration of 10–12% (vol/vol), and CFs were incubated overnight at 4 °C. The material then was centrifuged at 15,000 × g for 15 min at 4 °C, and the pellets were washed in cold acetone and were centrifuged again at 15,000 × g for 15 min at 4 °C. Pellets were dried and stored at −80 °C until further processing for MS. The same protocol was used to prepare a second set of samples in singlicate: H37Rv WT, ΔesxG (mc27845), ΔesxG::esxGH (mc27866), ΔesxH (mc27846), ΔesxH::esxGH (mc27867), Δpe5–ppe4 (mc27848), and Δpe5–ppe4::pe5–ppe4 (mc27868).
Proteomics MS.
Trypsin digestion.
Samples were reconstituted in 200 μL of a 2-M urea solution, reduced with DTT at 57 °C for 1 h (4 μL of 0.2 M), and subsequently alkylated with iodoacetamide at room temperature in the dark for 45 min (4 μL of 0.5 M). Digestion with 400 ng of sequencing-grade modified trypsin (Promega) was allowed to proceed overnight on a shaker at room temperature.
Peptide extraction/sample desalting.
A slurry of R2 20-μm POROS beads (Life Technologies Corporation) in 5% formic acid and 0.2% trifluoroacetic acid (TFA) was added to each sample. Samples were incubated at 4 °C for 2 h with shaking, and the beads were loaded onto 0.1% TFA-equilibrated C18 Zip Tip pipette tips (Millipore) by microcentrifugation. The tubes were rinsed three times with 0.1% TFA, and each rinse was added to its corresponding Zip Tip followed by microcentrifugation. The extracted POROS beads were washed again with 0.5% acetic acid. Peptides were eluted with 40% acetonitrile in 0.5% acetic acid followed by 80% acetonitrile in 0.5% acetic acid. The organic solvent was removed using a SpeedVac concentrator.
MS analysis.
Aliquots of each sample were loaded onto an EASY spray 50-cm C18 analytical HPLC column with <2-μm bead size using the auto sampler of an EASY-nLC 1000 HPLC (Thermo Fisher) and solvent A (2% acetonitrile, 0.5% acetic acid). The peptides were gradient eluted into a Q Exactive (Thermo Scientific) mass spectrometer using a 2-h linear gradient from 2–40% solvent B (95% acetonitrile, 0.5% acetic acid), followed by 10 min from 40–100% solvent B. Solvent B was held at 100% for another 10 min for column washing. High-resolution full MS spectra were acquired with a resolution of 70,000, an automatic gain control (AGC) target of 1e6, with a maximum ion time of 120 ms and scan range of 300–1500 m/z. Following each full MS scan, 20 data-dependent high-resolution higher-energy collisional dissociation MS/MS spectra were acquired. All MS/MS spectra were collected using the following instrument parameters: resolution of 17,500, AGC target of 2e5, maximum ion time of 250 ms, one microscan, 2 m/z isolation window, fixed first mass of 150 m/z, and normalized collision energy of 27.
LFQ and peptide and protein identification was performed using the MaxQuant software suite (35). The dataset was filtered to remove proteins with only a single unique peptide and to remove proteins that were not detected in all three replicates of at least one group (WT, Δesx-3, and so forth). LFQ intensity values were log2 transformed. Scatterplots were generated to compare the log2 LFQ intensity values between each group. A t test was used to determine which proteins differ significantly between two groups. This determination encompasses those proteins present in only one of the two strains as well as proteins differing in abundance to a statistically significant degree based upon a two-sided t test with correcting for multiple testing by controlling for a FDR at 5% using the Benjamini–Hochberg method (36). To estimate the relative amount of lysis in the samples, a panel of ribosomal and chaperonin proteins was selected (Dataset S2), and scatterplots of log2 LFQ intensity values were created as described above (representative lysis signature plots are shown in Fig. S5B).
Discussion
Mtb encodes five evolutionarily related T7SS, ESX-1–ESX-5 (7, 39–41). These systems are believed to influence the outcome of infection by directing the secretion of specific effector proteins. However, apart from the intensively investigated ESX-1, there are limited data characterizing the secretomes of individual ESX systems or correlating these findings with in vivo data to define the contributions of individual effectors to pathogenesis. This undertaking is complex, because ESX systems have been shown to secrete a variety of substrates lacking classical N-terminal signal sequences, including Esx family members, members of the diverse PE and PPE protein families, and, in some cases, additional substrates (such as Esp proteins secreted via ESX-1) (41). The studies of ESX-3 described here demonstrate how a single ESX system can secrete multiple effectors to modulate varied host defenses to influence pathogenesis.
Previous findings have suggested that ESX-3 functions in the acquisition of iron (11, 13); in the context of host infection, this iron-uptake capacity is of critical importance to nearly all bacterial pathogens (42, 43). Here, using a panel of Mtb deletion mutants, we demonstrate that not only the entire esx-3 locus but specifically the secreted substrates EsxG–EsxH and PE5–PPE4 are required for iron acquisition. We further demonstrate that the contribution of iron acquisition to Mtb virulence in human macrophages and mouse models depends on the host phenotype. Remarkably, analysis of the esx-3 region mutants showed that ESX-3 plays a critical role in virulence, which correlates with the secretion of effectors distinct from those required for iron acquisition (Fig. 6J). Although all mutants required iron supplementation to grow on solid medium, we found striking differences in their impact on virulence in mice. The Δpe5–ppe4 and ΔmbtB strains, which retained the ability to secrete EsxG–EsxH and PE15–PPE20, were virulent in i.v. infections of SCID mice and aerosol infections of C57BL/6 mice; this virulence may reflect the ability of Mtb to exploit in vivo sources of iron such as heme (20, 21). Still, this result was surprising, given the reported phenotype of other mutants defective in Mb synthesis or trafficking. Some of the data are not directly comparable to ours, because the other studies used bacillus Calmette–Guérin rather than Mtb or guinea pigs rather than a murine model (27, 44). However, both mbtK, which transfers fatty acids to the Mb peptide core, and mmpS4/S5, which are required for siderophore export, are essential for virulence in mice (45, 46). Notably, however, both of these mutants also have additional phenotypes (altered lipid profiles and toxic accumulation of siderophores, respectively) that could contribute to their in vivo attenuation (28, 45). In contrast to Δpe5–ppe4 and ΔmbtB, the ΔesxH mutant behaved similarly to the strain bearing a deletion of the entire esx-3 region in aerosol infections of C57BL/6 mice. Both ΔesxH and Δesx-3 were substantially attenuated, failing to proliferate in the lungs and to disseminate to the spleen. This finding suggests that the virulence defect of Δesx-3 is not caused solely by defective iron acquisition and further suggests that the attenuation is primarily attributable to absence of EsxH, either directly or through the loss of other substrates that rely on EsxH for secretion.
We considered the possibility that the requirement for iron-acquisition systems in Mtb might depend on the capacity of the host to restrict iron by comparing infection in mouse lines that differ in the nramp1 gene (29, 47). Nramp1 is thought to export divalent metals out of the phagosome, and that export is postulated to starve bacteria of essential metals and/or to heighten oxidative stress (30). Remarkably, both the ΔmbtB and Δpe5–ppe4 mutants were highly attenuated in nramp1R CBA mice, in contrast to their relatively preserved virulence in the nramp1S mice (C57BL/6 and BALB/c). Interestingly, an extensive body of literature suggests that the nramp1R locus does not confer resistance to infection with fully virulent Mtb in mice (48, 49), although in humans polymorphisms in NRAMP1 (termed “SLC11A1”) are associated with the development of pulmonary tuberculosis in West African and Asian populations (50, 51). The pe5–ppe4 and mbtB mutants, with defects related to iron utilization, may reveal a role of Nramp1 in host defense against Mtb and point to siderophore-mediated iron uptake as an immune evasion mechanism. Of course, additional differences between the inbred strains also could underlie the distinct phenotypes (52, 53), and further studies using congenic strains will clarify the contribution of Nramp1. Our macrophage data also indicate that the importance of pe5–ppe4 for intracellular growth of Mtb may depend on macrophage gene expression and polarization. Overall, our studies reveal a relationship between host genotype and bacterial iron handling in determining the outcome of infection with Mtb.
Our proteomic analysis points to a limited set of ESX-3–secreted substrates: EsxG, EsxH, the PE–PPE pair encoded within the locus (PE5–PPE4), an evolutionarily related pair of proteins encoded outside the locus (PE15–PPE20), and perhaps a few others such as Rv2477c. However, Rv2477c lacks the characteristic YxxxD/E T7SS motif and also is reduced in CF from ΔmbtB, making its assignment as a direct ESX-3 substrate less likely. The limited number of substrates assigned to the Mtb ESX-3 secretion system differs somewhat from ESX-1, which secretes a number of Esp substrates, and from ESX-5, which appears to secrete multiple PE–PPE family members (41). However, additional ESX-3 effectors may remain membrane associated and undetectable in CF or may be secreted under culture conditions different from those tested here. Nonetheless, several features of ESX-1 and ESX-5 also appear to be shared by ESX-3. EsxG and EsxH, which form a heterodimer (15, 54), exhibited codependent secretion, as did EsxA and EsxB (55, 56). In addition, elimination of EsxG or EsxH impaired the secretion of PE5 and PE15–PPE20. This suggests that EsxG may behave like its counterpart EsxB, which recently has been shown to promote the ATPase activity of the secretion apparatus (57).
Our data also clearly support the observation that specific PE–PPE pairs are targeted to specific ESX systems (58, 59). To our knowledge, PE15–PPE20 is the first PE–PPE pair encoded outside an esx locus for which the data support secretion by an ESX system other than ESX-5. It is not surprising that ESX-3 secretes this pair, because there is a strong connection between PE5 and PE15: They are evolutionarily closely related (60) and are highly conserved in members of the Mtb complex, share 67% identity, and are suggested to play role(s) in intracellular survival in macrophages (61). Moreover, their relationship is underscored by transcriptional data, because both pe5–ppe4 and pe15–ppe20 appear to belong to the ZuR regulon (62, 63) and are down-regulated by nutrient starvation (64). Interestingly, however, two lines of evidence suggest that PE15 and PPE20 are not responsible for the iron-acquisition function of ESX-3. First, complementation of Δesx-3 with esx-3SMEG corrected the in vitro growth defect (Fig. 4A) but failed to restore secretion of PE15/PPE20 (Fig. 6F). Second, Δpe5–ppe4 exhibited iron-related growth defects very similar to Δesx-3 but secreted PE15/PPE20 in excess of WT (Fig. S5C). Our finding that the Δpe5–ppe4 mutant has preserved secretion of EsxG/EsxH is also consistent with findings from genetic analysis of the ESX-1 locus, where deletion of ppe68 resulted in a modest increase in EsxA secretion (55, 65). However, the data are more complex for ESX-5, in which deletion of the ppe25–pe19 gene cluster in H37Rv led to loss of EsxN and PPE41 secretion (66), although differing results were obtained for a ppe27 mutant in the CDC1551 background (67).
In summary, our findings implicate the PE5–PPE4 proteins as the critical ESX-3 effectors involved in iron acquisition. EsxG–EsxH may be required for iron utilization simply by virtue of promoting PE5–PPE4 secretion, or both complexes may act in concert to assimilate Mb-bound iron (see the model in Fig. 7). Our data also indicate that ESX-3 regulates Mtb growth and virulence in vivo in a manner independent of the iron-acquisition functions that are so critical in vitro. This regulation may be related to recent observations that the ESX-3 region can modulate host immune responses; removal of the Msmeg esx-3 region attenuated the organism as the result of clearance by an MyD88-dependent innate immune response (14). Further, EsxH has been demonstrated to interact with and inhibit the mammalian cell ESCRT machinery that plays critical roles in membrane trafficking (19). Additional studies will be required to elucidate the in vivo functions of EsxG–EsxH and to clarify how ESX-3 facilitates iron acquisition and under what circumstances different ESX-3 effector proteins are required for virulence. Finally, this work reinforces the significance of T7SS for mycobacterial–host interactions and highlights the need for continued investigation into their role in pathogenesis.
Fig. 7.
The schematic illustrates ESX-3 substrates and their distinct functional roles. EsxG and EsxH are cosecreted and facilitate secretion of PE5–PPE4 (the latter remaining membrane-localized). PE5–PPE4, perhaps in concert with EsxG–EsxH, plays a role in siderophore-mediated iron uptake. EsxG and EsxH also facilitate secretion of PE15–PPE20, and, separately or together, these proteins play additional, iron-independent roles in virulence.
Materials and Methods
Bacterial Strains.
Mtb H37Rv and mc26230 (H37Rv ΔRD1 ΔpanCD) were used to generate mutants as listed in Table S1. Mutants were constructed using specialized transduction (68, 69). The antibiotic cassette was removed from selected strains using phage-delivered γδ-resolvase, with sacB counterselection facilitating the isolation of unmarked strains (69). The L5 phage integration system was used for complementation. Details of strain and plasmid construction are provided in SI Materials and Methods.
Liquid Growth Conditions.
Mycobacterial strains were cultured at 37 °C in Middlebrook 7H9 medium (Difco) with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC) enrichment (BBL; Becton Dickinson), 0.2% glycerol (Fisher), and 0.05% Tween 80 or 0.05% tyloxapol (referred to as “7H9”), with additional supplements as indicated. Strains bearing antibiotic cassettes were cultured with 50–75 μg/mL hygromycin or 25 μg/mL kanamycin as appropriate. Chelated Sauton’s medium consisted of 0.5 g KH2PO4, 4 g l-asparagine⋅H2O, 2 g citric acid, 60 g glycerol/L, adjusted to pH ∼7.0, and treated overnight with chelex-100 (10 g/L) (Bio-Rad) to remove trace iron contaminants. After filtration, 0.5 g/L of MgSO4·7H2O was added.
Solid Medium Growth Experiments.
Bacterial cultures were washed in PBS containing 0.05% Tween 80 or 0.05% tyloxapol (PBS-T), were resuspended in PBS-T, and were adjusted to equivalent OD600 values. Serial dilutions were plated onto 7H10 base medium containing 10% OADC and 0.5% glycerol with additional additives as indicated. Supplements included bovine hemin (Sigma), 0.05% Tween 80, FAC (Sigma), zinc sulfate (Sigma or Fisher), and MbJ (Allied Monitor). Plates were incubated for ∼3–6 wk at 37 °C.
Liquid Growth Curves.
Bacteria were cultured in 7H9 medium with ∼5 μM residual hemin from the frozen stock. In some experiments 10 μM hemin was added to promote growth of ΔmbtB; 100 μM hemin was added for the Δesx-3 Δmbt-1 double mutant. Bacteria were pelleted by centrifugation, washed in PBS-T, and inoculated into growth medium at a starting OD600 of ∼0.02. Medium was unsupplemented 7H9 or included 100 μM 2,2′-dipyridyl (Sigma), 100 μM hemin, or 200 ng/mL MbJ. MbJ was prepared as described (27) by solubilizing 100 μg in a solution containing 250 μg tyloxapol in 1.25 mL ethanol (20% wt/vol). The ethanol was evaporated under vacuum, and the tyloxapol and MbJ were resuspended in 7H9 medium and added to 500 mL 7H9.
Siderophore Uptake and Synthesis.
Uptake of cMb was quantified as described previously (46), using Msmeg cMb, which was deferrated and then labeled with 55Fe (see SI Materials and Methods for details). Analysis of radiolabeled siderophores from Mtb grown in iron-deficient medium and extraction of unlabeled siderophores from iron-replete cultures was performed essentially as described previously (46); additional details are provided in SI Materials and Methods. For MS analysis of siderophores, strains were cultured in 7H9 medium with 24 μg/mL d-pantothenate. Cell pellets were washed with PBS and were extracted with CHCl3/MeOH (2:1). Two and a half milliliters of sterile filtered supernatants were acidified with 15 μL 3M HCl and extracted with 3.5 mL ethyl acetate (70). The organic phases of pellet and supernatant extractions were dried, resuspended in isopropanol/acetonitrile/water (2:1:1; vol/vol/vol), and subjected to ultra high-performance liquid chromatography (UPLC)-MS analysis as described in SI Materials and Methods and ref. 71.
Intracellular Bacterial Growth Assays.
Human macrophages were prepared as described (72) following the method of Vogt and Nathan (73) and differentiation with either 2 ng/mL recombinant human GM-CSF or 10 ng/mL recombinant human M-CSF (R&D Systems), as detailed in SI Materials and Methods. Following infection at a multiplicity of infection (MOI) of 0.02, mycobacterial growth was enumerated at 14 d postinfection by plating on 7H10 plates containing 200 ng/mL MbJ (Allied Monitor). Colonies were counted after ∼2–3 wk of incubation.
CF Preparation.
CFs were prepared as described (19). Following growth to log phase in 7H9 medium (with 10 μM hemin for the ΔmbtB strain), Mtb were subcultured for 2 d in chelated Sauton’s medium, pelleted by centrifugation, and the supernatants were filtered through 0.22-μM filters followed by precipitation with 12% trichloroacetic acid (TCA) (also see SI Materials and Methods).
Proteomics MS.
Protein pellets were reconstituted in 2-M urea buffer, pH 7.8, before reduction, alkylation, and digestion with trypsin. After desalting the peptide mixtures were gradient eluted directly into a Q Exactive mass spectrometer (Thermo Scientific). The MaxQuant software suite was used for protein identifications and LFQ (35). Protein intensities were compared using a two-sided t test and correcting for multiple testing by controlling for FDR at 5% using the Benjamini–Hochberg method (36). Details are provided in SI Materials and Methods.
EsxG–EsxH Antibody Production and Western Blotting.
A His-tagged EsxG–EsxH fusion construct (74) was produced in Escherichia coli as described (19) and was used to immunize rabbits. Rabbit polyclonal serum was used in Western blotting.
Mice and Infections.
Mouse studies were performed in accordance with National Institutes of Health guidelines (75), and all work was approved by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee (Protocol #20120114). Female C57BL/6 and SCID mice were from the National Cancer Institute, and female CBA/J mice and B6.129S7-Rag1tm1Mom/J (RAG1−/−) mice were from Jackson Laboratories. Mycobacterial strains were grown in 7H9 medium with antibiotics as appropriate. For all infections, bacteria were washed in PBS-T and were sonicated. For i.v. infections, mice were injected via the lateral tail vein with the indicated doses of bacteria in 200 μL PBS-T. The actual dose delivered was determined by serial dilution and plating. For aerosol infections, the bacterial suspension also contained 0.04% antifoam Y-30 emulsion (Sigma) and was targeted to deliver a dose of ∼50–150 cfu per lung. Mice from each infection group were killed 24 h after aerosol exposure, and lung homogenates were plated to determine initial bacterial numbers delivered per strain. Bacterial burdens in lungs and spleen were determined by plating organ homogenates, prepared and diluted in PBS-T, from three or four mice per infection group onto appropriate medium. Details are provided in SI Materials and Methods.
Statistical Analysis.
Differences between groups were analyzed by Student’s t test. A P value ≤0.05 was considered statistically significant. Survival curves were compared with the log-rank (Mantel–Cox) test using GraphPad Prism for Windows (GraphPad Software). Statistical comparisons of protein intensities in CF samples were performed as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Bing Chen, Mei Chen, and John Kim for assistance with mouse infections; Annie Dai for phage preparation; Colin Ratledge for providing mycobactins; Brian Weinrick for mutant verification by whole genome sequencing; Michelle Larsen and Anthony Orth for sharing constructs from the Genomics Institute of the Novartis Research Foundation phasmid collection; Katherine Moccia and Eva Yang for assistance with strain generation and verification; and members of the W.R.J. and J.A.P. laboratories for numerous helpful discussions. This work was supported by NIH Grants AI26170 and AI098925 (to W.R.J.) and T32GM007288 for support of C.A.K. at the Albert Einstein College of Medicine; and NIH Grant AI087682 and funds from the New York University School of Medicine (to J.A.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523321113/-/DCSupplemental.
References
- 1.Stoop EJ, Bitter W, van der Sar AM. Tubercle bacilli rely on a type VII army for pathogenicity. Trends Microbiol. 2012;20(10):477–484. doi: 10.1016/j.tim.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 2.van der Woude AD, Luirink J, Bitter W. Getting across the cell envelope: Mycobacterial protein secretion. Curr Top Microbiol Immunol. 2013;374:109–134. doi: 10.1007/82_2012_298. [DOI] [PubMed] [Google Scholar]
- 3.Bitter W, et al. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 2009;5(10):e1000507. doi: 10.1371/journal.ppat.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu T, et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA. 2003;100(21):12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis KN, et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J Infect Dis. 2003;187(1):117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46(3):709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdallah AM, et al. Type VII secretion--mycobacteria show the way. Nat Rev Microbiol. 2007;5(11):883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- 8.Champion PA, Cox JS. Protein secretion systems in Mycobacteria. Cell Microbiol. 2007;9(6):1376–1384. doi: 10.1111/j.1462-5822.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 9.Stanley SA, Cox JS. Host-pathogen interactions during Mycobacterium tuberculosis infections. Curr Top Microbiol Immunol. 2013;374:211–241. doi: 10.1007/82_2013_332. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez GM, Voskuil MI, Gold B, Schoolnik GK, Smith I. ideR, An essential gene in mycobacterium tuberculosis: Role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect Immun. 2002;70(7):3371–3381. doi: 10.1128/IAI.70.7.3371-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serafini A, Boldrin F, Palù G, Manganelli R. Characterization of a Mycobacterium tuberculosis ESX-3 conditional mutant: Essentiality and rescue by iron and zinc. J Bacteriol. 2009;191(20):6340–6344. doi: 10.1128/JB.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegrist MS, et al. Mycobacterial Esx-3 requires multiple components for iron acquisition. MBio. 2014;5(3):e01073. doi: 10.1128/mBio.01073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegrist MS, et al. Mycobacterial Esx-3 is required for mycobactin-mediated iron acquisition. Proc Natl Acad Sci USA. 2009;106(44):18792–18797. doi: 10.1073/pnas.0900589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweeney KA, et al. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat Med. 2011;17(10):1261–1268. doi: 10.1038/nm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilghari D, et al. Solution structure of the Mycobacterium tuberculosis EsxG·EsxH complex: Functional implications and comparisons with other M. tuberculosis Esx family complexes. J Biol Chem. 2011;286(34):29993–30002. doi: 10.1074/jbc.M111.248732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J. Induction of CD8 T cells against a novel epitope in TB10.4: Correlation with mycobacterial virulence and the presence of a functional region of difference-1. J Immunol. 2007;179(6):3973–3981. doi: 10.4049/jimmunol.179.6.3973. [DOI] [PubMed] [Google Scholar]
- 17.Hervas-Stubbs S, et al. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infect Immun. 2006;74(6):3396–3407. doi: 10.1128/IAI.02086-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skjot RL, et al. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect Immun. 2002;70(10):5446–5453. doi: 10.1128/IAI.70.10.5446-5453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehra A, et al. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog. 2013;9(10):e1003734. doi: 10.1371/journal.ppat.1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CM, Niederweis M. Mycobacterium tuberculosis can utilize heme as an iron source. J Bacteriol. 2011;193(7):1767–1770. doi: 10.1128/JB.01312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tullius MV, et al. Discovery and characterization of a unique mycobacterial heme acquisition system. Proc Natl Acad Sci USA. 2011;108(12):5051–5056. doi: 10.1073/pnas.1009516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdallah AM, et al. PPE and PE_PGRS proteins of Mycobacterium marinum are transported via the type VII secretion system ESX-5. Mol Microbiol. 2009;73(3):329–340. doi: 10.1111/j.1365-2958.2009.06783.x. [DOI] [PubMed] [Google Scholar]
- 23.McMahon MD, Rush JS, Thomas MG. Analyses of MbtB, MbtE, and MbtF suggest revisions to the mycobactin biosynthesis pathway in Mycobacterium tuberculosis. J Bacteriol. 2012;194(11):2809–2818. doi: 10.1128/JB.00088-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain P, et al. φ(2)GFP10, a high-intensity fluorophage, enables detection and rapid drug susceptibility testing of Mycobacterium tuberculosis directly from sputum samples. J Clin Microbiol. 2012;50(4):1362–1369. doi: 10.1128/JCM.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambandamurthy VK, et al. Mycobacterium tuberculosis DeltaRD1 DeltapanCD: A safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine. 2006;24(37-39):6309–6320. doi: 10.1016/j.vaccine.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 26.De Voss JJ, et al. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc Natl Acad Sci USA. 2000;97(3):1252–1257. doi: 10.1073/pnas.97.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tullius MV, Harth G, Maslesa-Galic S, Dillon BJ, Horwitz MA. A Replication-Limited Recombinant Mycobacterium bovis BCG vaccine against tuberculosis designed for human immunodeficiency virus-positive persons is safer and more efficacious than BCG. Infect Immun. 2008;76(11):5200–5214. doi: 10.1128/IAI.00434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones CM, et al. Self-poisoning of Mycobacterium tuberculosis by interrupting siderophore recycling. Proc Natl Acad Sci USA. 2014;111(5):1945–1950. doi: 10.1073/pnas.1311402111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malo D, et al. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics. 1994;23(1):51–61. doi: 10.1006/geno.1994.1458. [DOI] [PubMed] [Google Scholar]
- 30.Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9(8):397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- 31.Bellamy R. The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1999;1(1):23–27. doi: 10.1016/s1286-4579(99)80010-0. [DOI] [PubMed] [Google Scholar]
- 32.Corna G, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95(11):1814–1822. doi: 10.3324/haematol.2010.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recalcati S, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40(3):824–835. doi: 10.1002/eji.200939889. [DOI] [PubMed] [Google Scholar]
- 34.Sierra-Filardi E, Vega MA, Sánchez-Mateos P, Corbí AL, Puig-Kröger A. Heme Oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010;215(9-10):788–795. doi: 10.1016/j.imbio.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Cox J, et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13(9):2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc, B. 1995;57(1):289–300. [Google Scholar]
- 37.Fortune SM, et al. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci USA. 2005;102(30):10676–10681. doi: 10.1073/pnas.0504922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Målen H, Berven FS, Fladmark KE, Wiker HG. Comprehensive analysis of exported proteins from Mycobacterium tuberculosis H37Rv. Proteomics. 2007;7(10):1702–1718. doi: 10.1002/pmic.200600853. [DOI] [PubMed] [Google Scholar]
- 39.Houben EN, Korotkov KV, Bitter W. Take five - Type VII secretion systems of Mycobacteria. Biochim Biophys Acta. 2014;1843(8):1707–1716. doi: 10.1016/j.bbamcr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Simeone R, Bottai D, Brosch R. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr Opin Microbiol. 2009;12(1):4–10. doi: 10.1016/j.mib.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Simeone R, Bottai D, Frigui W, Majlessi L, Brosch R. 2015. ESX/type VII secretion systems of mycobacteria: Insights into evolution, pathogenicity and protection. Tuberculosis (Edinb) 95:5150–5154. [DOI] [PubMed]
- 42.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neyrolles O, Wolschendorf F, Mitra A, Niederweis M. Mycobacteria, metals, and the macrophage. Immunol Rev. 2015;264(1):249–263. doi: 10.1111/imr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy PV, et al. Disruption of mycobactin biosynthesis leads to attenuation of Mycobacterium tuberculosis for growth and virulence. J Infect Dis. 2013;208(8):1255–1265. doi: 10.1093/infdis/jit250. [DOI] [PubMed] [Google Scholar]
- 45.Madigan CA, et al. Lipidomic analysis links mycobactin synthase K to iron uptake and virulence in M. tuberculosis. PLoS Pathog. 2015;11(3):e1004792. doi: 10.1371/journal.ppat.1004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells RM, et al. Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis. PLoS Pathog. 2013;9(1):e1003120. doi: 10.1371/journal.ppat.1003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vidal S, et al. The Ity/Lsh/Bcg locus: Natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182(3):655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medina E, North RJ. Evidence inconsistent with a role for the Bcg gene (Nramp1) in resistance of mice to infection with virulent Mycobacterium tuberculosis. J Exp Med. 1996;183(3):1045–1051. doi: 10.1084/jem.183.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.North RJ, LaCourse R, Ryan L, Gros P. Consequence of Nramp1 deletion to Mycobacterium tuberculosis infection in mice. Infect Immun. 1999;67(11):5811–5814. doi: 10.1128/iai.67.11.5811-5814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellamy R, et al. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998;338(10):640–644. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- 51.Li HT, Zhang TT, Zhou YQ, Huang QH, Huang J. SLC11A1 (formerly NRAMP1) gene polymorphisms and tuberculosis susceptibility: A meta-analysis. Int J Tuberc Lung Dis. 2006;(1):3–12. [PubMed] [Google Scholar]
- 52.Beamer GL, Cyktor J, Carruthers B, Turner J. H-2 alleles contribute to antigen 85-specific interferon-gamma responses during Mycobacterium tuberculosis infection. Cell Immunol. 2011;271(1):53–61. doi: 10.1016/j.cellimm.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beamer GL, et al. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J Immunol. 2008;181(8):5545–5550. doi: 10.4049/jimmunol.181.8.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arbing MA, et al. Heterologous expression of mycobacterial Esx complexes in Escherichia coli for structural studies is facilitated by the use of maltose binding protein fusions. PLoS One. 2013;8(11):e81753. doi: 10.1371/journal.pone.0081753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brodin P, et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun. 2006;74(1):88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanley SA, Raghavan S, Hwang WW, Cox JS. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci USA. 2003;100(22):13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg OS, et al. Substrates Control Multimerization and Activation of the Multi-Domain ATPase Motor of Type VII Secretion. Cell. 2015;161(3):501–512. doi: 10.1016/j.cell.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daleke MH, et al. General secretion signal for the mycobacterial type VII secretion pathway. Proc Natl Acad Sci USA. 2012;109(28):11342–11347. doi: 10.1073/pnas.1119453109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ekiert DC, Cox JS. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci USA. 2014;111(41):14758–14763. doi: 10.1073/pnas.1409345111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gey van Pittius NC, et al. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol Biol. 2006;6:95. doi: 10.1186/1471-2148-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiwari BM, Kannan N, Vemu L, Raghunand TR. The Mycobacterium tuberculosis PE proteins Rv0285 and Rv1386 modulate innate immunity and mediate bacillary survival in macrophages. PLoS One. 2012;7(12):e51686. doi: 10.1371/journal.pone.0051686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maciag A, et al. Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J Bacteriol. 2007;189(3):730–740. doi: 10.1128/JB.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dam JC, Schaap PJ, Martins dos Santos VA, Suárez-Diez M. Integration of heterogeneous molecular networks to unravel gene-regulation in Mycobacterium tuberculosis. BMC Syst Biol. 2014;8:111. doi: 10.1186/s12918-014-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43(3):717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 65.Demangel C, et al. Cell envelope protein PPE68 contributes to Mycobacterium tuberculosis RD1 immunogenicity independently of a 10-kilodalton culture filtrate protein and ESAT-6. Infect Immun. 2004;72(4):2170–2176. doi: 10.1128/IAI.72.4.2170-2176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bottai D, et al. Disruption of the ESX-5 system of Mycobacterium tuberculosis causes loss of PPE protein secretion, reduction of cell wall integrity and strong attenuation. Mol Microbiol. 2012;83(6):1195–1209. doi: 10.1111/j.1365-2958.2012.08001.x. [DOI] [PubMed] [Google Scholar]
- 67.Houben EN, et al. Composition of the type VII secretion system membrane complex. Mol Microbiol. 2012;86(2):472–484. doi: 10.1111/j.1365-2958.2012.08206.x. [DOI] [PubMed] [Google Scholar]
- 68.Bardarov S, et al. Specialized transduction: An efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148(Pt 10):3007–3017. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- 69.Jain P, et al. Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. MBio. 2014;5(3):e01245–14. doi: 10.1128/mBio.01245-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madigan CA, et al. Lipidomic discovery of deoxysiderophores reveals a revised mycobactin biosynthesis pathway in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2012;109(4):1257–1262. doi: 10.1073/pnas.1109958109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prados-Rosales R, et al. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J Bacteriol. 2014;196(6):1250–1256. doi: 10.1128/JB.01090-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong KW, Jacobs WR., Jr Mycobacterium tuberculosis exploits human interferon γ to stimulate macrophage extracellular trap formation and necrosis. J Infect Dis. 2013;208(1):109–119. doi: 10.1093/infdis/jit097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogt G, Nathan C. In vitro differentiation of human macrophages with enhanced antimycobacterial activity. J Clin Invest. 2011;121(10):3889–3901. doi: 10.1172/JCI57235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Callahan B, et al. Conservation of structure and protein-protein interactions mediated by the secreted mycobacterial proteins EsxA, EsxB, and EspA. J Bacteriol. 2010;192(1):326–335. doi: 10.1128/JB.01032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 76.Tufariello JM, et al. Enhanced specialized transduction using recombineering in Mycobacterium tuberculosis. MBio. 2014;5(3):e01179–14. doi: 10.1128/mBio.01179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larsen MH, Biermann K, Tandberg S, Hsu T, Jacobs WR. 2007. Genetic manipulation of Mycobacterium tuberculosis. Curr Protoc Microbiol 6:(A):10A.2.1–10A.2.21. [DOI] [PubMed]
- 78.Braunstein M, et al. Identification of genes encoding exported Mycobacterium tuberculosis proteins using a Tn552'phoA in vitro transposition system. J Bacteriol. 2000;182(10):2732–2740. doi: 10.1128/jb.182.10.2732-2740.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stover CK, et al. New use of BCG for recombinant vaccines. Nature. 1991;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.