Significance
The sympathetic nervous system regulates basic body functions such as heartbeat, blood pressure, and gland activities. Whereas hormone secretion from the adrenal medulla modulates these processes systemically, local and fast responses can be mediated by direct sympathetic innervation. Although many effects of the sympathetic system on skeletal muscle physiology and disease are known, direct sympathetic innervation targets in skeletal muscle have been scarcely studied. We investigated this aspect and found that neuromuscular junctions, the contact points between motor neurons and muscle fibers, are innervated by sympathetic neurons, which is of crucial importance for the integrity and function of nerve–muscle contact. Our findings help to understand and refine treatment of neuromuscular diseases, including myasthenic syndromes.
Keywords: neuromuscular junction, sympathetic neurons, cAMP, beta-agonists, myasthenia
Abstract
The distribution and function of sympathetic innervation in skeletal muscle have largely remained elusive. Here we demonstrate that sympathetic neurons make close contact with neuromuscular junctions and form a network in skeletal muscle that may functionally couple different targets including blood vessels, motor neurons, and muscle fibers. Direct stimulation of sympathetic neurons led to activation of muscle postsynaptic β2-adrenoreceptor (ADRB2), cAMP production, and import of the transcriptional coactivator peroxisome proliferator-activated receptor γ-coactivator 1α (PPARGC1A) into myonuclei. Electrophysiological and morphological deficits of neuromuscular junctions upon sympathectomy and in myasthenic mice were rescued by sympathicomimetic treatment. In conclusion, this study identifies the neuromuscular junction as a target of the sympathetic nervous system and shows that sympathetic input is crucial for synapse maintenance and function.
With the exception of the regulation of blood vessel smooth muscle tonus, functions of sympathetic neurons in skeletal muscle have scarcely been explored (1, 2). Recently, sympathicomimetics (SM) have been introduced successfully as clinical treatment of neuromuscular transmission disorders called congenital myasthenic syndromes (CMSs) (3, 4). Fittingly, muscle weakness is a hallmark of several autonomous nervous system disorders, including chronic fatigue syndrome (5), congenital insensitivity to pain (6), adrenal insufficiency (7, 8), complex regional pain syndromes (9, 10), and Lambert–Eaton myasthenic syndrome (11–13). Because, furthermore, beta-blockers lead to increased susceptibility to muscle fatigue (14) and modulate neuromuscular activity of drugs applied during anesthesia (15), we became interested in addressing sympathetic innervation of skeletal muscle.
Results
A Network of Sympathetic Neurons Contacts Different Targets in Skeletal Muscle.
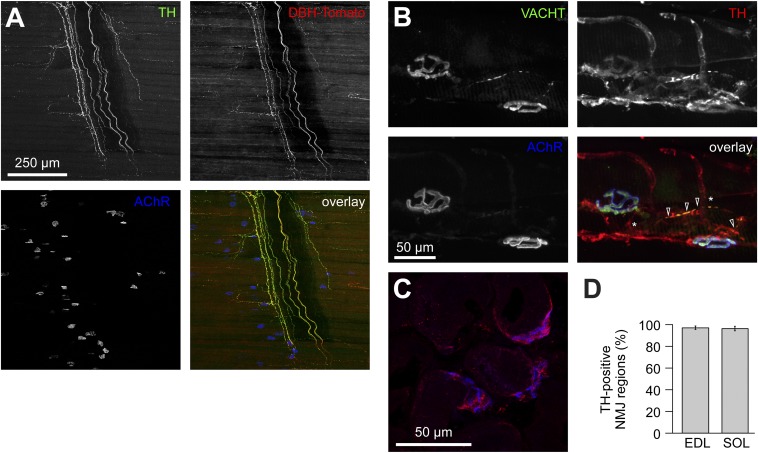
To investigate the distribution of sympathetic neurons in mouse skeletal muscle, we used reporter mice expressing Tomato protein under control of the dopamine β-hydroxylase (DBH) promoter (16) (DBH-Tomato). Tomato colocalized with immunofluorescence (IF) signals of the sympathetic markers tyrosine hydroxylase (TH) (Fig. 1A) and neuropeptide Y (Fig. S1). Sympathetic neurons were present in large amounts throughout the diaphragm muscle (Fig. 1A and Fig. S1A). Axons either innervated or passed in proximity to neuromuscular junctions (NMJs) that were stained with fluorescent alpha-bungarotoxin conjugate (BGT-AF647) (Fig. 1A and Fig. S1). Costaining of extensor digitorum longus (EDL) muscles with wheat germ agglutinin (WGA), TH antibody, and BGT-AF647 showed that TH-positive axons were associated with blood vessels (Fig. S2). However, 69.86 ± 11.22% (mean ± SEM, n = 5) NMJ regions also displayed plaque-like TH immunosignals, which connected to TH-immunopositive axons. The cholinergic presynaptic marker vesicular acetylcholine transporter (VACHT) perfectly colocalized with AChRs but only partially with TH (Fig. 1B); 67.59 ± 8.83% (mean ± SEM, n = 10) of VACHT-positive portions of motor neuron axons (Fig. 1B, open arrowheads) were accompanied by TH-positive axons, which then formed connections to other TH-positive axons (Fig. 1B, asterisks). Muscle transverse sections stained with anti-TH antibody and BGT demonstrated that 95.8 ± 2.6% and 90.6 ± 1.96% (both mean ± SEM, n = 4) of NMJ regions were TH-immunopositive in EDL and soleus muscles, respectively (Fig. 1D).
Fig. 1.
Distribution of sympathetic neurons in skeletal muscle. (A) Diaphragm muscle of a DBH-Tomato mouse expressing Tomato protein in sympathetic neurons was costained with anti-TH antibody and BGT-AF647 (AChR). Signals from TH, Tomato, and BGT are depicted in the overlay in green, red, and blue, respectively. Three-dimensional maximum projection of a confocal z stack of a representative region is shown. All channels were brightness/contrast-enhanced. (B) Longitudinal sections of wild-type EDL muscles were labeled against VACHT, TH, and BGT-AF647. Signals from these markers are depicted in the overlay in green, red, and blue, respectively. Three-dimensional maximum projection of a confocal z stack of a representative region is shown. All channels were brightness/contrast-enhanced. (C and D) EDL and soleus muscles were sectioned transversally, stained with BGT-AF555 (blue in overlay) and anti-TH antibody (red in overlay), and then imaged with confocal microscopy. (C) Representative confocal brightness/contrast-enhanced optical section from EDL. (D) Quantification of TH-positive NMJ regions from EDL and soleus (SOL) muscles. Mean ± SEM (n = 4 muscles each). Negative controls lacking primary antibodies showed 0.7 ± 0.7% (mean ± SEM, n = 4 muscles) in EDL and 0.0% (mean ± SEM, n = 4 muscles) in soleus of TH-positive NMJ regions.
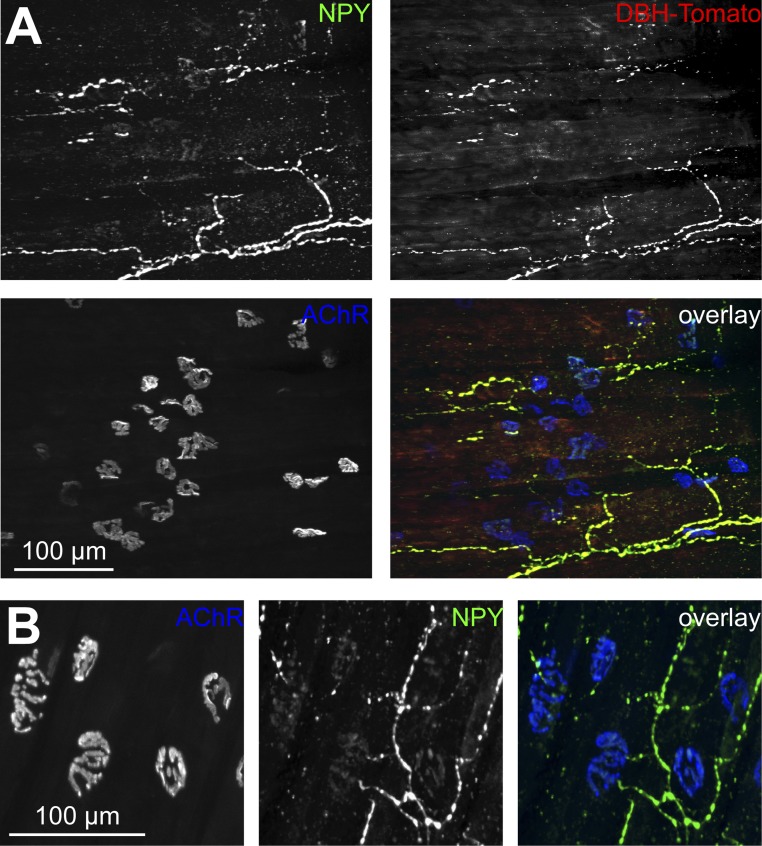
Fig. S1.
Sympathetic neurons innervate neuromuscular junctions. Diaphragm muscles were stained with anti-neuropeptide Y (NPY) antibody and BGT-AF647. For IF of diaphragms and longitudinal TA and EDL muscle sections as shown in Figs. 1 A and B and 3A and Figs. S1 A and B and S2, a modified iDISCO tissue clearing and staining protocol was used (42): Muscles were washed in 1× PBS (three times in 1 h) and preincubated with 0.2% Triton X-100/20% (vol/vol) DMSO/0.3 M glycine in 1× PBS overnight at 37 °C. Samples were then incubated in 0.2% Tween in 1× PBS with 10 µg/mL heparin (PTwH) for 2 d at room temperature and then blocked with 0.2% Triton X-100/10% (vol/vol) DMSO/6% (vol/vol) BSA in 1× PBS (2× PTDB solution) at 37 °C for 24 h. Samples were washed with PTwH three times in 1 h, followed by incubation with primary and secondary antibodies in 1× PTDB solution (diluted with PTwH) for 4 d each. Between primary and secondary antibody staining, muscles were washed with PTwH solution for 2 d and again 2 d before imaging. Antibodies used throughout the study were rabbit anti-TH (AB152, 1:50; Millipore), rabbit anti-ADRB2 (sc-569, 1:200; Santa Cruz Biotechnology), rabbit anti-NPY (11976, 1:200; New England Biolabs), rabbit anti-VACHT (139103, 1:50; Synaptic Systems), Alexa Fluor (AF)488-conjugated wheat germ agglutinin (W11261, 1:200; Invitrogen), BGT-AF555 and BGT-AF647 (1:200; Invitrogen), Alexa Fluor 546-conjugated anti-rabbit (A11010, 1:1,000; Invitrogen), and Alexa Fluor 488-conjugated anti-mouse (A11001, 1:200; Invitrogen). Confocal microscopy used the following parameters: AF488 (excitation 488 nm), AF546 and Tomato (excitation 561 nm), and AF647 (excitation 633 nm) signals were acquired using 500- to 550-nm, 570- to 620-nm, and 650- to 750-nm band-pass settings, respectively, in the SP2 unit. 3D image stacks were taken at 8-bit and 1,024 × 1,024-pixel resolution with maximally 2× zoom. (A) Signals from a reporter mouse expressing Tomato protein in sympathetic neurons (DBH-Tomato). NPY, Tomato, and BGT are depicted in the overlay in green, red, and blue, respectively. Three-dimensional maximum projection of a confocal z stack taken in a representative region is shown. All channels were brightness/contrast-enhanced. (B) Three-dimensional maximum projection of a confocal z stack taken in a representative region is shown from a wild-type mouse diaphragm. NPY and BGT are depicted in the overlay in green and blue, respectively. All channels were brightness/contrast-enhanced. NPY labeling brightness in the end plates was quantified and found to be 20.40 ± 2.54 arbitrary units (AU) (mean ± SEM, n = 8) compared with 118.62 ± 9.74 AU (mean ± SEM, n = 8) in the nerve. This would suggest that the major site of NPY production is in the axonal region.
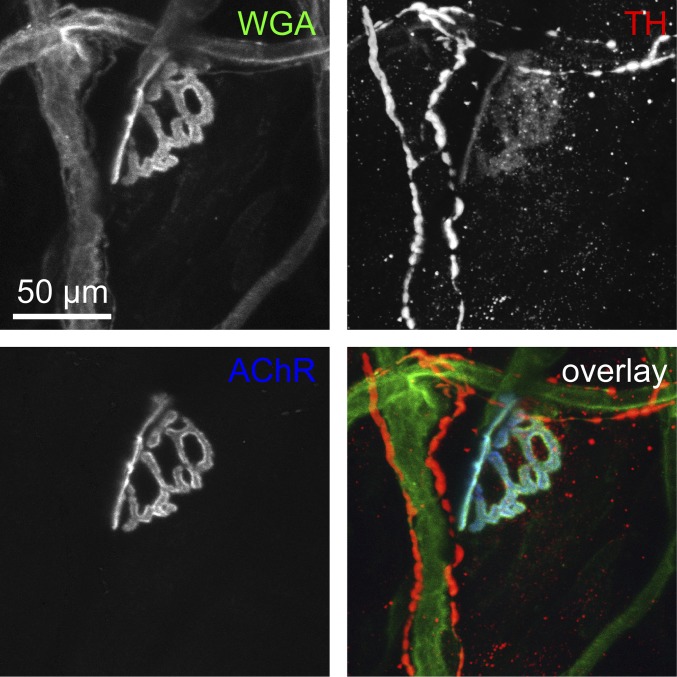
Fig. S2.
Sympathetic neurons contact different targets, including blood vessels and NMJs. Wild-type EDL muscles were sectioned using a Leica VT1000S vibratome into 100-µm-thick sections and then stained with fluorescent wheat germ agglutinin coupled to Alexa Fluor 488 (WGA), anti-TH antibody (TH), and BGT-AF647 (AChR). Signals of WGA, TH, and BGT are depicted in the overlay in green, red, and blue, respectively. Three-dimensional maximum projection of a confocal z stack taken in a representative region is shown. All channels were brightness/contrast-enhanced.
Functional Effects of Sympathetic Stimulation on Muscle and NMJs.
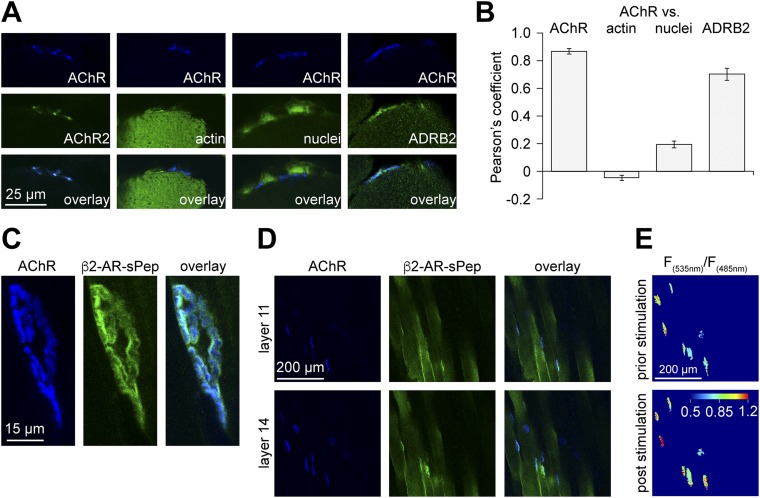
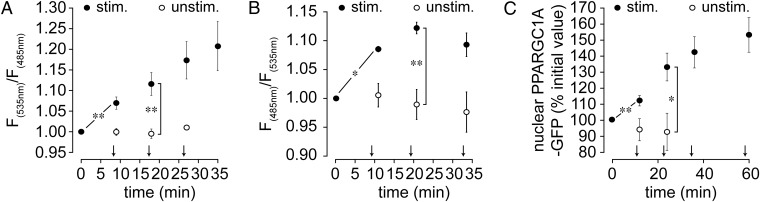
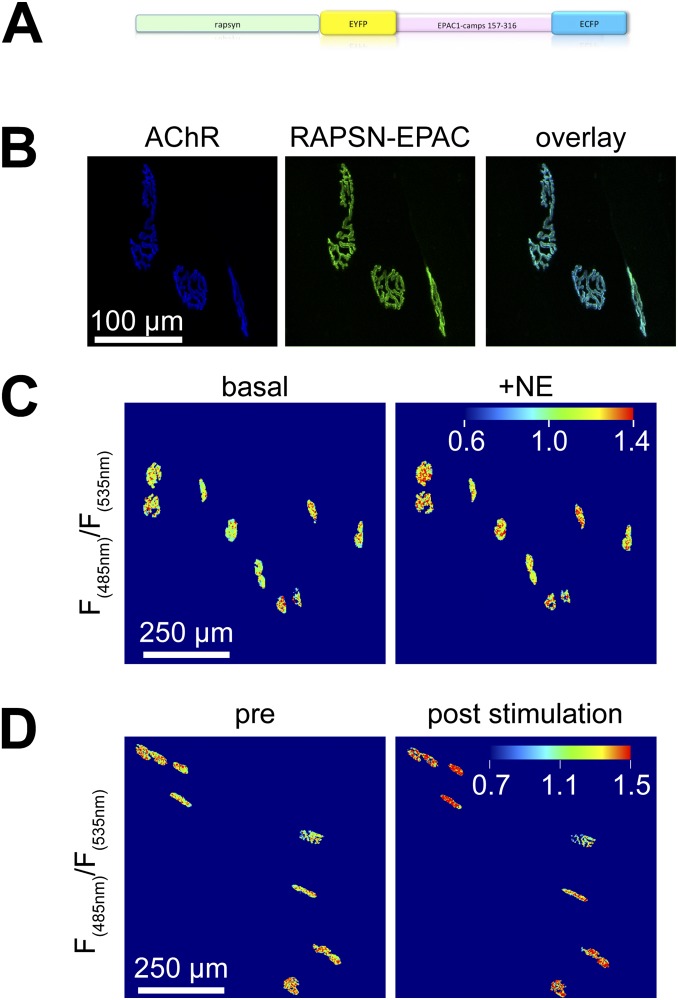
The most abundant targets of norepinephrine in skeletal muscle are β2-adrenoreceptors (ADRB2s) (17, 18). Transverse sections of EDL were stained for ADRB2s and AChRs. Colocalization analysis showed a Pearson’s coefficient of 0.70 ± 0.04 (mean ± SEM, n = 13; Fig. S3 A and B), suggesting enrichment of ADRB2 in the NMJ. Sympathetic neuron activity on postsynaptic ADRB2 signaling was tested using the Förster resonance energy transfer (FRET)-based biosensor β2-AR-s-pep (19), which accumulated at the NMJ (Fig. S3 C and D). In vivo FRET imaging in combination with direct electrical stimulation of the sympathetic chain revealed a fast, sympathetic stimulation-dependent increase in postsynaptic ADRB2 activity (Fig. 2A and Fig. S3E). Next, to monitor postsynaptic cAMP production, a NMJ-specific cAMP sensor, termed “RAPSN-EPAC,” was designed (Fig. S4). RAPSN-EPAC, which is composed of the receptor-associated protein of the synapse (RAPSN) followed by the cAMP-binding domains of the Exchange protein directly activated by cAMP (EPAC), showed specific localization at NMJs (Fig. S4B). In vivo imaging of RAPSN-EPAC demonstrated a consistent increase in postsynaptic cAMP levels upon direct stimulation of the sympathetic chain (Fig. 2B and Fig. S4D).
Fig. S3.
Functional effects of sympathetic stimulation and molecular biosensors. (A) Transverse sections (10-µm-thick) of untransfected EDL muscles were costained using BGT-AF555 (AChR) and either BGT-AF647 (AChR2), phalloidin-TRITC (actin), draq5 (nuclei), or anti-ADRB2 antibody (ADRB2) and then imaged with confocal fluorescence microscopy. Staining of transverse cryosections was as follows. Sections (10-μm-thick) were prepared from snap-frozen EDL muscles, washed with 1× PBS, permeabilized with 0.1% Triton X-100/PBS, blocked with 2% BSA/PBS (2 h), and subsequently stained with antibodies or stains (each for 24 h with extensive washing in-between). Sections were embedded in Mowiol. For quantitative analysis of cross-sections as shown in Fig. 1 C and D, NMJ regions were identified by thresholding on median-filtered BGT fluorescence images. Segments were saved as regions of interest. Mean gray values were measured from corresponding regions in TH IF images (FTH-NMJ). In addition, mean gray values (FTH-fiber) and SD (SDfiber) were measured from the TH IF background fluorescence in fiber cross-sections. NMJ regions were considered TH-positive if the following criterion was met: FTH-NMJ > FTH-fiber + 10 + (2 × SDfiber). (B) Quantitative colocalization analysis for different stainings as indicated. Pearson’s coefficients were derived from NMJ regions using the ImageJ plugin JACoP (NIH). N values for AChR, actin, nuclei, and ADRB2 colocalization were 11, 15, 15, and 13, respectively. (C–E) TA muscles were transfected as described (24, 43, 44) with cDNA encoding for β2-AR-s-pep, a FRET-based ADRB2 activity biosensor (19) (plasmid 47438; Addgene). This sensor consists of full-length ADRB2 with the following C-terminal attachment (in sequence): yellow fluorescent protein mCitrine (FRET acceptor), a linker sequence, cyan fluorescent protein mCerulean (FRET donor), and the G protein-coupled receptor-binding peptide of Gαs, termed s-pep. In β2-AR-s-pep, ligand-induced activation of the ADRB2 portion leads to binding of s-pep to the cytosolic loop of ADRB2. This triggers approximation of mCitrine and mCerulean proteins leading to FRET. Ten days after transfection, a microelectrode was placed at the sympathetic ganglion innervating the hindlimbs. The lumbar sympathetic chain is surrounded by connective tissue ventral to the vertebral column and dorsal to the abdominal aorta and vena cava. Sympathetic ganglia L2 and L3 that innervate hindlimbs are roughly at the height of the renal artery and vein. For stimulation, the chain was exposed under deep anesthesia via a midline laparotomy approach. Animals were kept on a heating pad and received s.c. doses of physiological solution against dehydration. Connective tissue was carefully removed. Stimulation used a microelectrode from Harvard Apparatus that was placed around the chain. An A.M.P.I. Master-8-cp stimulator delivered trains of 10 pulses, 0.5-ms duration, 10-Hz frequency. Ten trains were applied at 4-s intervals. Stimulation occurred at around 2 V. NMJs were marked by injection of 50 μL BGT-AF647 (1:200 in PBS; Invitrogen) into the hindlimb, and muscles were then imaged with in vivo confocal and two-photon microscopy. (C and D) Pictures show high-power 3D projection (C) and low-power single optical layers (D) of confocal fluorescence micrograph stacks revealing enrichment of β2-AR-s-pep (green) at the NMJ (blue). (E) Images show pseudocolored FRET acceptor-to-donor (F535 nm/F485 nm) emission ratios in NMJs upon two-photon microscopy. NMJ regions were segmented, because outside these zones fluorescence intensities were low, leading to arbitrary ratio values obscuring the FRET changes at the level of NMJs. β2-AR-s-pep detects increased ADRB2 activity upon sympathetic ganglion stimulation by increased FRET. 3D image stacks were taken at 12-bit, 200-Hz scan frequency, 2× line average, and 512 × 512-pixel resolution with 1× zoom. For FRET measurements, fluorescence donor mCerulean was excited in two-photon mode at 810 nm. Emission signals from donor (F485 nm) and acceptor (F535 nm) were simultaneously detected by nondescanned detectors using an RSP 505 dichroic mirror and BP485/30 and BP560/50 emission filters (Leica). Photomultiplier settings were kept constant; laser intensity was adjusted according to sample depth. Quantitative analysis of FRET changes used ImageJ 1.49v and the following procedure. Postsynaptic areas enriched in β2-AR-s-pep were segmented in median filtered mCerulean emission image stacks. Masks were made from segmented areas and applied to background-subtracted and mean filtered mCerulean and mCitrine image stacks. Ratio images (mCitrine/mCerulean) were made and average ratios were determined for each postsynaptic area on at least 5 and up to 10 optical slices. All values obtained from one muscle per time point were averaged. Fig. 2A shows mean values of several experiments.
Fig. 2.
Stimulation of the lumbar sympathetic ganglion stimulates muscle adrenergic signal transduction and nuclear import of PPARGC1A. TA muscles were transfected with β2-AR-s-pep (A), RAPSN-EPAC (B), or PPARGC1A-GFP (C). Ten days later, muscles were injected with BGT-AF647 to label NMJs and imaged with in vivo confocal and two-photon microscopy. (A and B) Quantification of FRET measurements. Graphs depict F535 nm/F485 nm (A) or F485 nm/F535 nm (B) emission ratios in all observed biosensor-positive NMJ regions. Ratios were normalized to basal value before stimulation. Arrows indicate time points of lumbar stimulation. Mean ± SEM (n values with stimulation: 5 in A, 3 in B; n values without stimulation: 4 in A, 4 in B; *P < 0.05, **P < 0.01). (C) Quantification of nuclear accumulation of PPARGC1A-GFP upon sympathetic stimulation. Arrows indicate time points of lumbar stimulation. Mean ± SEM (n = 5; *P < 0.05, **P < 0.01).
Fig. S4.
Effect of sympathetic stimulation on subsynaptic cAMP levels. (A) RAPSN-EPAC cAMP sensor was cloned into pcDNA3 using PCR; the complete sequence is available upon request. The EPAC1-camps moiety was as described (45) and consists of the cAMP-binding domains of EPAC protein flanked by EYFP (FRET acceptor) and ECFP (FRET donor). The image shows a schematic of RAPSN-EPAC molecular structure. Upon binding of cAMP to the EPAC1-camps domain, the sensor moiety changes from a closed to an open conformation, mediating a reduction of FRET efficiency (45). The N-terminal addition of RAPSN targets the biosensor to the NMJ. (B) Distribution pattern of RAPSN-EPAC upon transfection as revealed by confocal microscopy. TA muscles were transfected as described (24, 43, 44) with cDNA encoding for RAPSN-EPAC. Ten days later, NMJs were labeled by injection of 50 μL BGT-AF647 (1:200 in PBS; Invitrogen), and muscles were imaged with in vivo confocal microscopy. Note that RAPSN-EPAC efficiently targets NMJs. For the analysis of biosensor distribution or NMJ morphology, EYFP (excitation 488 nm) and BGT-AF647 signals (excitation 633 nm) were acquired using 500- to 550-nm and 650- to 750-nm band-pass settings, respectively, in the Leica SP2 confocal microscope unit. (C and D) TA muscles were transfected with cDNA encoding for RAPSN-EPAC. Ten days later, muscles were imaged with in vivo two-photon microscopy. 3D image stacks were taken at 12-bit, 200-Hz scan frequency, 2× line average, and 512 × 512-pixel resolution with 1× zoom. For FRET measurements, fluorescence donor ECFP was excited in two-photon mode at 810 nm. Emission signals from donor (F485 nm) and acceptor (F535 nm) were simultaneously measured by nondescanned detectors using an RSP 505 dichroic mirror and BP485/30 and BP560/50 emission filters (Leica). Photomultiplier settings were maintained; laser intensity was adjusted according to sample depth. Quantitative analysis of biosensor responses used ImageJ 1.49v and the following procedure. Postsynaptic areas enriched in RAPSN-EPAC were segmented in median filtered ECFP emission image stacks. Masks were made from segmented areas and applied to background-subtracted and mean filtered ECFP and EYFP image stacks. Ratio images (ECFP/EYFP) were made, and average ratios were determined for each postsynaptic area on at least 5 and up to 10 optical slices. (C) RAPSN-EPAC sensor responds to application of norepinephrine (NE) by FRET change. Images show pseudocolored maximum z projections of F(485 nm)/F(535 nm) ratio values before (basal) and after intramuscular injection of 50 µL 10 µM NE (+NE). Note that RAPSN-EPAC detects increased cAMP by decreased FRET, and therefore ratiometric calculations are inverted compared with β2-AR-s-pep. Blue-green and yellow-red cues indicate low and high values of cAMP, respectively. The pseudocolor scale bar shows the color distribution-to-fluorescence ratios. Each colored plaque-like structure represents a single NMJ, which were segmented to eliminate the background signals outside NMJs. Note that most NMJs exhibit a clear red shift upon treatment with NE. (D) RAPSN-EPAC sensor indicates a rise in subsynaptic cAMP levels by FRET change. Before in vivo two-photon microscopy, a microelectrode was placed at the sympathetic ganglion innervating the hindlimbs (for technical details, see also Fig. S3 C–E). Images show 3D maximum z projections of RAPSN-EPAC pseudocolored FRET donor-to-acceptor (F485 nm/F535 nm) emission ratios in NMJs. Each colored plaque-like structure represents a single NMJ, which were segmented to eliminate the background signals outside NMJs. Fig. 2B shows mean values of several experiments, where all values obtained from one muscle per time point were averaged.
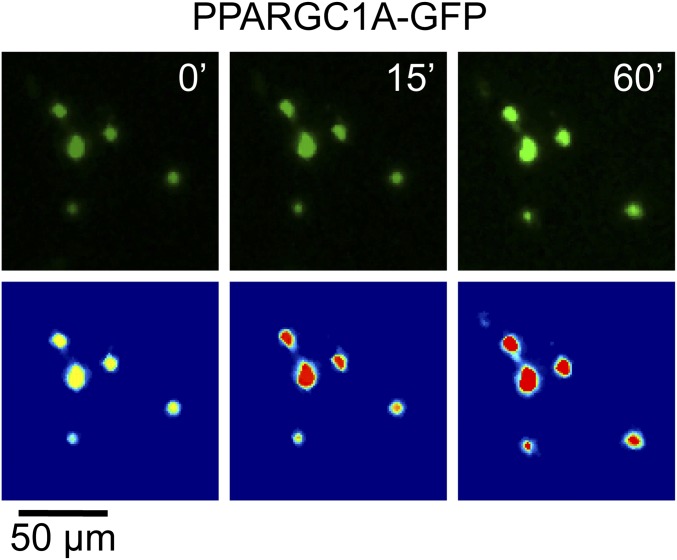
Given the recent success of SM in CMS treatment, we next tested the effect of the SM clenbuterol on AChRα1 subunit gene (CHRNA1) activity. Clenbuterol was applied s.c. daily for 10 d. Then, the amounts of CHRNA1 mRNA were determined from harvested muscles, showing a 10.23 ± 2.0-fold increase (mean ± SEM, n = 4; P = 0.009) in SM-treated muscles compared with saline-injected controls. Given that ADRB2 activation enhances expression of peroxisome proliferator-activated receptor γ-coactivator 1α (PPARGC1A) (20) and that PPARGC1A regulates NMJ-specific genes, including CHRNA1, in an activity-dependent manner (21), we addressed the effect of sympathetic chain stimulation on cytoplasm-to-nucleus shuttling of PPARGC1A. PPARGC1A-GFP was expressed in tibialis anterior (TA) muscles, and its amount in myonuclei upon stimulation of the sympathetic chain was observed by in vivo imaging. This revealed a rapid increase of PPARGC1A-GFP in myonuclei upon sympathetic chain stimulation (Fig. 2C and Fig. S5).
Fig. S5.
Effect of sympathetic stimulation on translocation of PPARGC1A. TA muscles were transfected with cDNA encoding for PPARGC1A-GFP (46) (plasmid 4; Addgene). Ten days later, muscles were imaged with in vivo confocal microscopy. Then, maximum z projections were made. GFP-positive nuclei were segmented in the first image stack (before stimulation). These segments were shifted in the following image stacks to fit the regions of corresponding nuclei. From each segment, mean gray values were measured. The images show representative confocal images before (Upper) and after segmentation (Lower) illustrating the increase of PPARGC1A-GFP fluorescence in myonuclei with sympathetic stimulation (for technical details, see also Fig. S3 C–E). Numbers in the upper panels indicate time elapsed after start of experiment. (Lower) Brightness values were transformed into clipped pseudocolors [ranging from 500 (blue) to 5,000 (red) arbitrary brightness units] to highlight the increase in fluorescence intensity.
Effects of Sympathectomy and Rescue by Sympathicomimetics on NMJs.
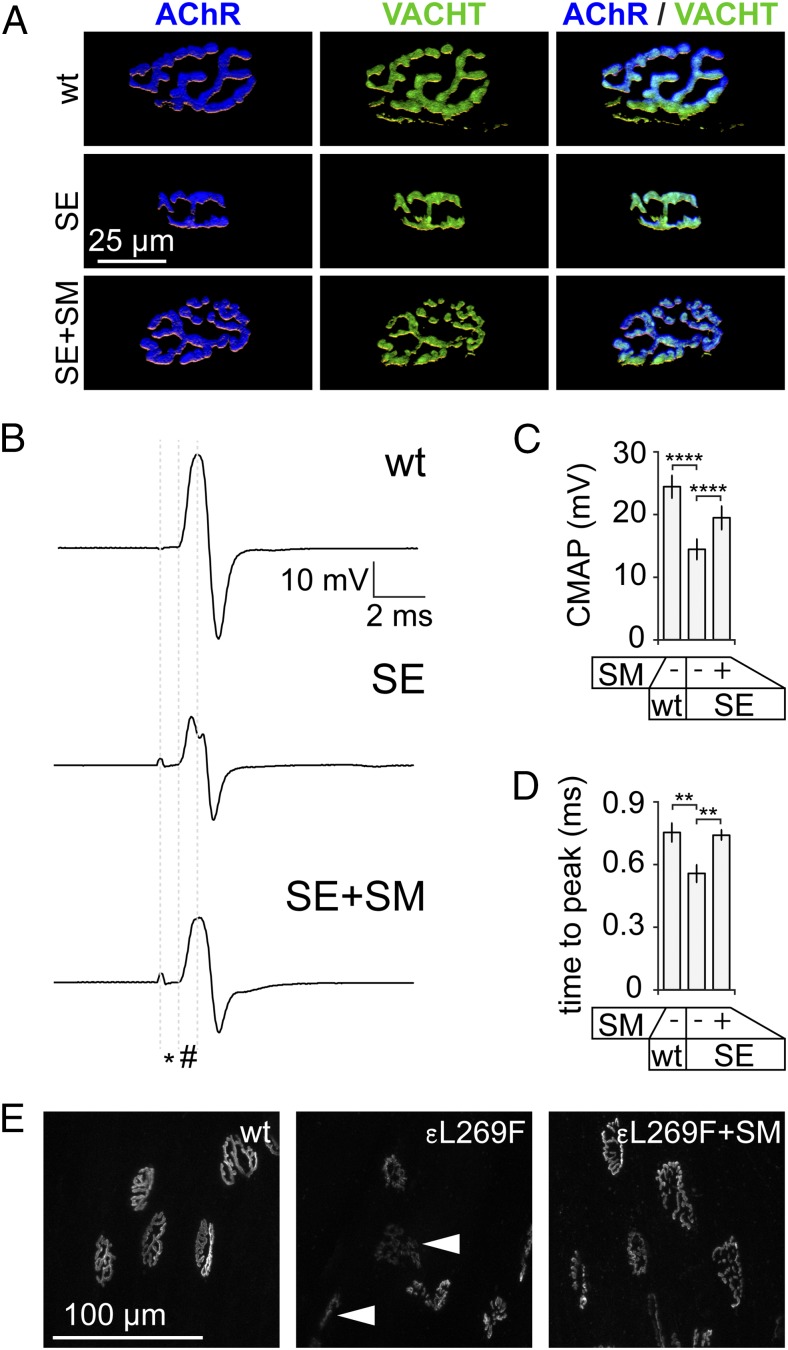
To address a trophic function of sympathetic input for the NMJ, we monitored NMJ morphology in the presence or absence of chemical sympathectomy (SE) by 6-hydroxydopamine (22, 23). As determined by IF, SE led to diminished complexity and size of NMJs (Fig. 3A). Quantitative analysis revealed a size reduction of NMJs upon SE compared with controls by 57 ± 5% (mean ± SEM, n = 10). Colocalization of pre- and postsynaptic structures remained unaffected under this condition and clenbuterol rescued the SE-induced effects (Fig. 3A), suggesting that they were due to lack of sympathetic activity rather than unspecific toxicity of 6-hydroxydopamine. Next, in vivo compound muscle action potentials (CMAPs) were obtained from TA muscles that were pretreated with SE and SM as just described. CMAPs from TA muscles were measured using intramuscular needle electrodes upon repetitive maximal sciatic nerve stimulation. Whereas latency (Fig. 3B, *) was unaltered in all conditions, amplitude and time to peak (Fig. 3B, #) of CMAPs were affected upon SE (Fig. 3 B–D). SM recovered the effects of SE on amplitude partially (Fig. 3C) and on time to peak completely (Fig. 3D).
Fig. 3.
SM treatment rescues NMJ phenotypes of sympathectomized muscle. (A–D) TA muscles of wild-type mice received injections of saline (wt) or 6-hydroxydopamine (SE) on alternate days for 2 wk. In the last 10 d, one SE group was also treated with s.c. daily doses of the SM clenbuterol (SE+SM). Then, muscles were harvested for IF (A) or CMAP recordings were made (B–D). (A) Muscles were taken and longitudinal sections were made and stained with BGT-AF555 and anti-VACHT antibody. Confocal microscopy was performed, signals were segmented, and 3D projections were calculated. Images show projections of representative NMJs. (B–D) In vivo CMAPs were recorded from TA muscles. Maximal stimuli of 0.25-ms duration were applied by a shielded microelectrode to the sciatic nerve. CMAP recordings used intramuscular needle electrodes. (B) Curves depict representative CMAPs from muscles treated as indicated. Latency (*), time to peak (#), and amplitude of CMAPs were determined. (C) Quantitative analysis revealed a significant reduction of CMAP amplitudes upon SE and partial rescue by SM. Mean ± SEM (n = 8, 5, and 5 for wt, SE, and SE+SM, respectively; ****P < 0.0001). (D) Quantitative analysis revealed a significant reduction of time to peak upon SE and full rescue by SM. Mean ± SEM (n = 6, 5, and 5 for wt, SE, and SE+SM, respectively; **P < 0.01). (E) TA muscles of wild-type and myasthenic slow-channel CHRNE L269F mice (εL269F) received s.c. daily doses of saline or the SM clenbuterol for 10 d. At the start of s.c. treatments, NMJs were labeled with BGT-AF647. At the end of the treatment period, NMJs were imaged with in vivo confocal microscopy. Maximum z projections showing representative NMJs under the conditions indicated in the images: wt, wild type treated with saline; εL269F, εL269F treated with saline; εL269F+SM, εL269F treated with clenbuterol.
The efficacy of SM for many CMS patients has been proven (3, 4). Here we used a myasthenic CHRNE L269F mouse model to test the effects of SM on NMJ morphology. AChRs were labeled with BGT. Then, mice received either saline or clenbuterol for 10 d. In vivo imaging of TA muscles was performed to reveal NMJ morphology. Similar to SE-treated wild-type mice (Fig. 3A), saline-treated myasthenic animals had aberrant NMJs with reduced size and complexity and weak BGT staining, indicating a diminished presence of AChRs. Due to scarce amounts of AChRs, many NMJs were hardly visible (Fig. 3E, Center, arrowheads). Conversely, NMJs of SM-treated CHRNE L269F mice looked largely normal (Fig. 3E). Compared with wild-type synapses, myasthenic NMJs were 44 ± 3% (mean ± SEM, n = 6; P = 0.003) smaller upon saline treatment but only 6 ± 14% (mean ± SEM, n = 6; P = 0.36) smaller upon clenbuterol application.
Discussion
This study addresses the distribution and functions of sympathetic neurons in skeletal muscle with several approaches. It reveals that sympathetic neurons innervate most NMJs in skeletal muscles, and that this innervation is crucial for synaptic integrity and function. At this moment it cannot be concluded whether the stimulation of sympathetic neurons acts directly on skeletal muscle or indirectly via nearby blood vessels, motor neurons, or other cells. However, the sympathetic activation effect on cAMP signaling is consistent with a trophic function of sympathetic input for NMJs. The data corroborate the antagonistic activity of SE and SM with respect to expression of AChR. Previous studies have shown that a cAMP/PKA microdomain at the NMJ is important for synapse maintenance (24–28). Sympathetic input at NMJs is a candidate for maintaining this microdomain. The observed import of PPARGC1A into myonuclei upon sympathetic nerve stimulation is consistent with the transcriptional changes by SM postexercise (29). Because PPARGC1A is crucial in mitochondrial biogenesis (30), reduced mitochondrial protein synthesis postexercise after treatment with beta-blockers (31) could be explained as well. Because sympathetic input to skeletal muscles is via the sciatic nerve, various effects using sciatic denervation as an atrophy model might also have a sympathetic contribution. Fittingly, ganglionic sympathectomy increases proteolysis in rat muscles and norepinephrine treatment of denervated rat muscles reduces mRNA levels of the atrogenes MAFbx (syn. atrogin-1) and TRIM63 (syn. MuRF1) (2).
Future studies need to investigate whether sympathetic neurons form synaptic contacts with the NMJs or abut their neurotransmitters more distantly. Both options might be equally functional. As known from autonomous innervation of the central nervous system, monoamines, such as norepinephrine, can be distributed by volume transmission to reach several targets (32, 33). A general modulatory role of muscle function by sympathetic tone would benefit from an organization as a nerve net that elicits effects on several tissue components. This could harmonize muscle function and local circulation similar to respiratory–cardiovascular coupling (34).
Our findings provide a possible link to the success of SM in treating CMSs, and may help to explain phenomena of muscle weakness in many autonomous nervous system disorders. It remains to be studied whether SM primarily improves sarcomeric function, neuromuscular transmission, or a mixture of both. Although SM is effective in some forms of myasthenic syndromes (NMJ pathology) (3, 35–37) and spinal muscular atrophy (motor neuron defect involving the NMJ) (38), it was not effective in a trial for facioscapulohumeral muscular dystrophy (primary muscle pathology) (39). In clinics, sympathectomy is used to treat hyperhidrosis. Although sympathectomy is not reported to induce severe muscle weakness in patients, changes in muscle metabolism have been documented (40). Also, sympathectomy in rabbits evoked muscle atrophy and fiber splitting (40). Our experiments involving sympathectomy and SM treatment led to atrophy and its recovery, respectively (Fig. S6). Given the intimate relationship between synaptic function, muscle activity, and muscle trophicity, it is unlikely that these factors can be fully separated. However, the effects of beta-blockers on muscle fatigue (14) and anesthesia (15) suggest that an important component of sympathetic activity affects synaptic integrity and function.
Fig. S6.
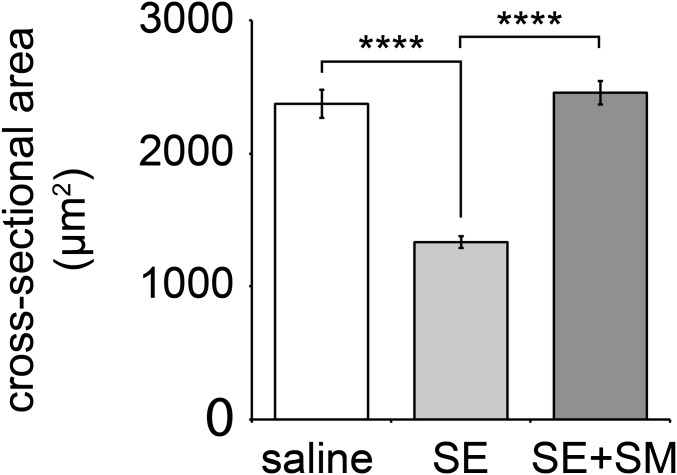
Sympathectomy reduces fiber cross-sectional area, and SM treatment recovers it. TA muscles were treated with either saline or 6-hydroxydopamine (SE) for 2 wk on alternate days. In addition, one group of SE-treated mice received clenbuterol during the last 10 d (SE+SM). Cross-sectional areas (CSA) were determined as previously described (24). Mean CSA ± SEM (n = 6, 12, and 11 muscles for saline, SE, and SE+SM, respectively; ****P < 0.0001).
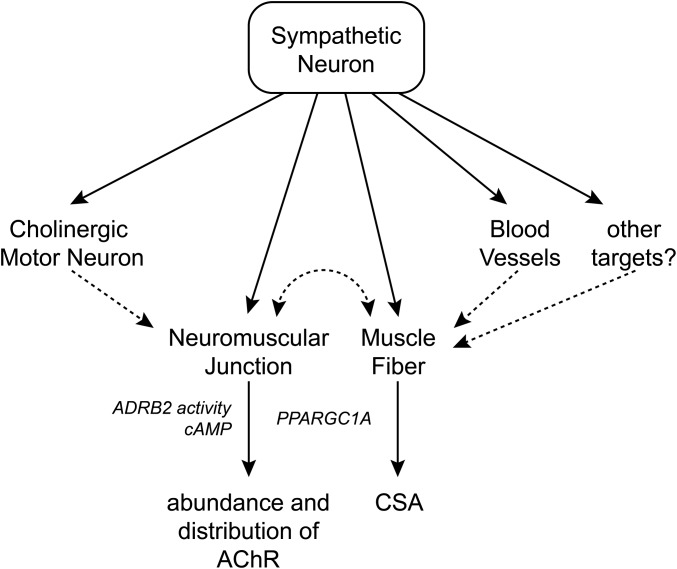
The data presented here are consistent with a model where sympathetic neurons coinnervate several targets in muscle, including blood vessels, motor neurons, muscle fibers, and NMJs (Fig. S7). Complex cross-talk between these cell types might occur using feedback mechanisms that need to be addressed further. Sympathetic innervation controls muscle metabolism as well as maintenance and function of NMJs. The success of SM treatment of several forms of myasthenic syndromes should spur further elucidation of the involved signaling pathways to develop more selective and potent therapies.
Fig. S7.
Model of the sympathetic innervation network in skeletal muscle and its role in NMJs. Sympathetic neurons coinnervate several targets in muscle, including blood vessels, motor neurons, muscle fibers, NMJs, and potentially other cell types. This innervation modulates muscle ADRB2 activity, postsynaptic cAMP levels, and the import of PPARGC1A into myonuclei, and controls muscle CSA as well as the abundance and distribution of AChRs in NMJs. It is an open question as to whether all observed effects on muscle and NMJs are due to direct sympathetic innervation (solid arrows) or indirect signaling (dashed arrows), for example, from sympathetic neurons over blood vessels to skeletal muscle fibers. Furthermore, it remains to be clarified whether the effects on NMJs and muscle trophicity are linked or independent. Because SM works efficiently in several forms of CMS, sympathetic innervation might have pleiotropic functions for the synapse.
Materials and Methods
Animals.
All animals were kept and treated according to guidelines of the Brazilian College of Animal Experimentation and EU Directive 2010/63/EU. Experimental protocols were approved by the commission of ethics in animal research of the School of Medicine of Ribeirão Preto and national authorities in France, Germany, and the United Kingdom. Adult male and female C57BL/10J, CHRNE L269F, B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J x B6-Tg(DBH-iCre) (called DBH-Tomato) were used for experiments. Anesthesia and preparation of mice for microscopy and electrophysiology were performed as described (25).
Immunofluorescence and Molecular Biosensors.
IF stainings of diaphragm, muscle cross-sections, and EDL longitudinal sections are described in Figs. S1–S3. EDL and soleus were used as paradigms for fast and slow muscles, respectively, diaphragm for its band-like array of NMJs, and TA for electroporation because of its in vivo accessibility. Molecular biosensors and their transfection are described in Figs. S3–S5.
Microscopy and Image Analysis.
All microscopy used a DMRE TCS SP2 confocal and two-photon microscope equipped with Leica Confocal Software 2.61, a KrAr laser (488 nm, 514 nm), a diode-pumped laser (561 nm), an HeNe laser (633 nm), a tunable Mai Tai pulsed two-photon laser, and a 63×/1.2 NA HCX PL APO CS water immersion objective for fixed samples as well as 20×/0.7 N.A. HC PL APO CS IMM/CORR UV and 63×/1.2 N.A. HCX PL APO CS W CORR objectives (all Leica Microsystems) for in vivo imaging (immersion medium, Visc-Ophtal gel; Dr. Winzer Pharma). For further details regarding microscopy of fixed samples, in vivo imaging, and image analysis, see Figs. S1 and S3. Image composition used Photoshop and Illustrator (Adobe Systems Software).
Sciatic Denervation, Stimulation of Lumbar Sympathetic Ganglionic Chain, and Pharmacological Treatments.
Sciatic denervation was as described (41). For details regarding the stimulation of the sympathetic ganglionic chain, see Fig. S3. Clenbuterol solution in PBS was always freshly prepared and then injected s.c. (3 mg/kg) for 10 d before microscopy. Chemical sympathectomy used 6-hydroxydopamine (22) (in 0.3% ascorbic acid oxygen-free water), which was injected into hindlimbs (100 mg/kg) on alternate days for 2 wk before imaging.
Compound Muscle Action Potential Measurement.
Mice were kept under isoflurane anesthesia. CMAP measurements were performed after saline, SE, or SE+SM treatment (Fig. 3 B–D) with maximal electrical stimulations delivered by a microelectrode (Harvard Apparatus) to the sciatic nerve. An A.M.P.I. Master-8-cp stimulator provided pulses at 0.25-ms duration and 5-Hz frequency. CMAPs were recorded by needle electrodes connected to a Bio Amp and a PowerLab 8/35 running LabChart 8 software (ADInstruments). Data analysis used LabChart 8 and Microsoft Excel:mac2011.
Statistical Analysis.
Graphic representation of data used Microsoft Excel:mac2011 and Adobe Illustrator. Significance was tested with Student t test or Welch test, where applicable. Kolmogorov–Smirnov test for normal distribution and F test for homo/heteroscedasticity were performed. Sample sizes were calculated to reach 80% power and β <0.2.
Acknowledgments
We thank Dr. Steve Laval for critical discussions, and acknowledge the help of animal facilities at the host institutes. T.P. was funded by the National Research Council (CNR) Special Project “Bioimaging.” S.L. and R.R. were supported by European Commission International Research Staff Exchange Scheme (IRSES) Grant SarcoSi, Deutsche Forschungsgemeinschaft (DFG) Grants La668/15-1, RU923/7-1, and RU923/8-1, and a grant from Hector Stiftung. H.L. is supported by Medical Research Council (MRC) UK (Reference G1002274, Grant 98482) and by the European Commission (FP7/2007-2013) under Grant Agreements 305444 (RD-Connect) and 305121 (Neuromics). D. Lustrino, W.A.S., I.C.K., and L.C.C.N. were funded by Sao Paolo Research Foundation (FAPESP) (2012/51456-1; 2012/24524-6; 2012/05697-7; 2010/11083-6).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524272113/-/DCSupplemental.
References
- 1.Barker D, Saito M. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc R Soc Lond B Biol Sci. 1981;212(1188):317–332. doi: 10.1098/rspb.1981.0042. [DOI] [PubMed] [Google Scholar]
- 2.Silveira WA, et al. Activating cAMP/PKA signaling in skeletal muscle suppresses the ubiquitin-proteasome-dependent proteolysis: Implications for sympathetic regulation. J Appl Physiol. 2014;117(1):11–19. doi: 10.1152/japplphysiol.01055.2013. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez Cruz PM, Palace J, Beeson D. Congenital myasthenic syndromes and the neuromuscular junction. Curr Opin Neurol. 2014;27(5):566–575. doi: 10.1097/WCO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 4.Engel AG, Shen X-M, Selcen D, Sine SM. Congenital myasthenic syndromes: Pathogenesis, diagnosis, and treatment. Lancet Neurol. 2015;14(4):420–434. doi: 10.1016/S1474-4422(14)70201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto LE, Raj SR, Biaggioni I. Chronic fatigue syndrome and the autonomic nervous system. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, editors. Primer on the Autonomic Nervous System. 3rd Ed. Academic; Amsterdam: 2012. pp. 531–534. [Google Scholar]
- 6.Tachi N, Ohya K, Chiba S, Nihira H, Minagawa K. Muscle involvement in congenital insensitivity to pain with anhidrosis. Pediatr Neurol. 1995;12(3):264–266. doi: 10.1016/0887-8994(95)00043-f. [DOI] [PubMed] [Google Scholar]
- 7.Munver R, Volfson IA. Adrenal insufficiency: Diagnosis and management. Curr Urol Rep. 2006;7(1):80–85. doi: 10.1007/s11934-006-0046-5. [DOI] [PubMed] [Google Scholar]
- 8.Trikudanathan S, Williams GH. Altered adrenal function and the autonomic nervous system. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, editors. Primer on the Autonomic Nervous System. 3rd Ed. Academic; Amsterdam: 2012. pp. 571–574. [Google Scholar]
- 9.Jänig W. Complex regional pain syndrome. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, editors. Primer on the Autonomic Nervous System. 3rd Ed. Academic; Amsterdam: 2012. pp. 583–587. [Google Scholar]
- 10.Jänig W. The fascination of complex regional pain syndrome. Exp Neurol. 2010;221(1):1–4. doi: 10.1016/j.expneurol.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: From clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10(12):1098–1107. doi: 10.1016/S1474-4422(11)70245-9. [DOI] [PubMed] [Google Scholar]
- 12.Morgan-Followell B, de Los Reyes E. Child neurology: Diagnosis of Lambert-Eaton myasthenic syndrome in children. Neurology. 2013;80(21):e220–e222. doi: 10.1212/WNL.0b013e318293e14e. [DOI] [PubMed] [Google Scholar]
- 13.Khurana RK. Paraneoplastic autonomic dysfunction. In: Robertson D, Biaggioni I, Burnstock G, Low RA, Paton JFR, editors. Primer on the Autonomic Nervous System. 3rd Ed. Academic; Amsterdam: 2012. pp. 593–596. [Google Scholar]
- 14.Hunter AM, et al. The effect of selective beta1-blockade on EMG signal characteristics during progressive endurance exercise. Eur J Appl Physiol. 2002;88(3):275–281. doi: 10.1007/s00421-002-0710-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim KS, Kim KH, Shin WJ, Yoo HK. Neuromuscular interactions between mivacurium and esmolol in rabbits. Anaesthesia. 1998;53(2):140–145. doi: 10.1046/j.1365-2044.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- 16.Stanke M, et al. Target-dependent specification of the neurotransmitter phenotype: Cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development. 2006;133(1):141–150. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Sainz RD, Molenaar P, Summers RJ. Characterization of beta 1- and beta 2-adrenoceptors in rat skeletal muscles. Biochem Pharmacol. 1991;42(9):1783–1789. doi: 10.1016/0006-2952(91)90516-8. [DOI] [PubMed] [Google Scholar]
- 18.Williams RS, Caron MG, Daniel K. Skeletal muscle beta-adrenergic receptors: Variations due to fiber type and training. Am J Physiol. 1984;246(2 Pt 1):E160–E167. doi: 10.1152/ajpendo.1984.246.2.E160. [DOI] [PubMed] [Google Scholar]
- 19.Malik RU, et al. Detection of G protein-selective G protein-coupled receptor (GPCR) conformations in live cells. J Biol Chem. 2013;288(24):17167–17178. doi: 10.1074/jbc.M113.464065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura S, et al. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology. 2007;148(7):3441–3448. doi: 10.1210/en.2006-1646. [DOI] [PubMed] [Google Scholar]
- 21.Handschin C, et al. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21(7):770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thoenen H, Tranzer JP. Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-hydroxydopamine. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;261(3):271–288. doi: 10.1007/BF00536990. [DOI] [PubMed] [Google Scholar]
- 23.Picklo MJ. Methods of sympathetic degeneration and alteration. J Auton Nerv Syst. 1997;62(3):111–125. doi: 10.1016/s0165-1838(96)00121-x. [DOI] [PubMed] [Google Scholar]
- 24.Röder IV, et al. Myosin Va cooperates with PKA RIalpha to mediate maintenance of the endplate in vivo. Proc Natl Acad Sci USA. 2010;107(5):2031–2036. doi: 10.1073/pnas.0914087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi K-R, et al. Rapsyn mediates subsynaptic anchoring of PKA type I and stabilisation of acetylcholine receptor in vivo. J Cell Sci. 2012;125(Pt 3):714–723. doi: 10.1242/jcs.092361. [DOI] [PubMed] [Google Scholar]
- 26.Röder IV, et al. Participation of myosin Va and Pka type I in the regeneration of neuromuscular junctions. PLoS One. 2012;7(7):e40860. doi: 10.1371/journal.pone.0040860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson PG, Lanuza MA, Jia M, Li MX, Tomas J. Phosphorylation reactions in activity-dependent synapse modification at the neuromuscular junction during development. J Neurocytol. 2003;32(5-8):803–816. doi: 10.1023/B:NEUR.0000020625.70284.a6. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Pena y Valenzuela I, Pires-Oliveira M, Akaaboune M, Akaaboune M. PKC and PKA regulate AChR dynamics at the neuromuscular junction of living mice. PLoS One. 2013;8(11):e81311. doi: 10.1371/journal.pone.0081311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruno NE, et al. Creb coactivators direct anabolic responses and enhance performance of skeletal muscle. EMBO J. 2014;33(9):1027–1043. doi: 10.1002/embj.201386145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng T, et al. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. 2010;298(3):C572–C579. doi: 10.1152/ajpcell.00481.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson MM, Bell C, Peelor FF, III, Miller BF. Beta-adrenergic receptor blockade blunts postexercise skeletal muscle mitochondrial protein synthesis rates in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R327–R334. doi: 10.1152/ajpregu.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agnati LF, et al. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: The volume transmission and the wiring transmission. Acta Physiol Scand. 1986;128(2):201–207. doi: 10.1111/j.1748-1716.1986.tb07967.x. [DOI] [PubMed] [Google Scholar]
- 33.Fuxe K, et al. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal-glial networks. Front Physiol. 2012;3:136. doi: 10.3389/fphys.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anrep GV, Pascual W, Rössler R. Respiratory variations of the heart rate. II. The central mechanism of the sinus arrhythmia and the inter-relationship between central and reflex mechanism. Proc R Soc Lond B Biol Sci. 1936;119:218–230. [Google Scholar]
- 35.Liewluck T, Selcen D, Engel AG. Beneficial effects of albuterol in congenital endplate acetylcholinesterase deficiency and Dok-7 myasthenia. Muscle Nerve. 2011;44(5):789–794. doi: 10.1002/mus.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke G, et al. Salbutamol benefits children with congenital myasthenic syndrome due to DOK7 mutations. Neuromuscul Disord. 2013;23(2):170–175. doi: 10.1016/j.nmd.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Schara U, et al. Ephedrine therapy in eight patients with congenital myasthenic syndrome due to DOK7 mutations. Neuromuscul Disord. 2009;19(12):828–832. doi: 10.1016/j.nmd.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Pane M, et al. Daily salbutamol in young patients with SMA type II. Neuromuscul Disord. 2008;18(7):536–540. doi: 10.1016/j.nmd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Kissel JT, et al. FSH-DY Group Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 2001;57(8):1434–1440. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- 40.Hashmonai M, Kopelman D. The pathophysiology of cervical and upper thoracic sympathetic surgery. Clin Auton Res. 2003;13(Suppl 1):I40–I44. doi: 10.1007/s10286-003-1105-3. [DOI] [PubMed] [Google Scholar]
- 41.Strack S, et al. A novel labeling approach identifies three stability levels of acetylcholine receptors in the mouse neuromuscular junction in vivo. PLoS One. 2011;6(6):e20524. doi: 10.1371/journal.pone.0020524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renier N, et al. iDISCO: A simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159(4):896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Röder IV, et al. Role of myosin Va in the plasticity of the vertebrate neuromuscular junction in vivo. PLoS One. 2008;3(12):e3871. doi: 10.1371/journal.pone.0003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudolf R, Hafner M, Mongillo M. Investigating second messenger signaling in vivo. Methods Enzymol. 2012;505:363–382. doi: 10.1016/B978-0-12-388448-0.00027-9. [DOI] [PubMed] [Google Scholar]
- 45.Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279(36):37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 46.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]