Significance
The number of species in local ecological assemblages is constrained by competition and other local interactions between populations. However, ecologists have not agreed on the degree to which the unique history and geography of continental regions also influence local species richness. Here, based on forest plots distributed throughout the world, we show that the species richness of forest trees varies among continental regions independently of local climate. In particular, forest plots in Europe and North America contain fewer species than expected from the statistical relationship of diversity to present-day local climate. Thus, the number of species co-occurring locally reflects, to a significant extent, region characteristics, including geographic and geologic history, that influence evolutionary diversification and regional extinction.
Keywords: climate effect, forest dynamics plots, global biodiversity, latitudinal gradient, tree species richness
Abstract
Global patterns of biodiversity reflect both regional and local processes, but the relative importance of local ecological limits to species coexistence, as influenced by the physical environment, in contrast to regional processes including species production, dispersal, and extinction, is poorly understood. Failure to distinguish regional influences from local effects has been due, in part, to sampling limitations at small scales, environmental heterogeneity within local or regional samples, and incomplete geographic sampling of species. Here, we use a global dataset comprising 47 forest plots to demonstrate significant region effects on diversity, beyond the influence of local climate, which together explain more than 92% of the global variation in local forest tree species richness. Significant region effects imply that large-scale processes shaping the regional diversity of forest trees exert influence down to the local scale, where they interact with local processes to determine the number of coexisting species.
Ecologists generally agree that large-scale patterns of diversity reflect a balance between regional processes of species production, extinction, and dispersal, on one hand, and within-region sorting of species based on adaptations to physical conditions of the environment, as well as competition among species for limiting resources, on the other hand (1–4). Nonetheless, the spatial scale down to which region effects extend has not been well resolved (5–7), but has wide-ranging implications for understanding the origins of patterns in local species richness. If the species richness of a local assemblage were strictly limited by competition and other local interactions among populations, new species could not be added without others being forced out, and we would expect to find a common relationship between diversity and local environmental conditions across regions (6). However, if unique historical and biogeographic features of each region influenced within-region diversification and extinction (8–10), these region-specific effects might contribute to the global pattern in local species richness. Efforts to disentangle these effects have met with limited success and have led to a long-standing discussion of the relationship between local and regional diversity (6, 11–21).
Analyses designed to distinguish local and regional influences on diversity have found strong region effects in some cases (8, 22) and weak or nonexistent region effects in others (15, 23–25). However, most studies that failed to find significant region effects either have addressed large, biologically heterogeneous samples, or they have used local samples (e.g., 0.1-ha “Gentry” plots) that are too small to characterize the diversity of local assemblages adequately (26, 27). Additionally, many large-scale samples have been compiled from maps generated from presence-only museum records or from coarse-scale atlases that document the extent of species occupancy, not actual local occurrence. Such data often undercount local species richness. Moreover, many tests of the diversity–environment relationship have analyzed data on local communities that extend over broad ranges of ecological conditions (e.g., tropical rainforests to arctic tundra and hot deserts; ref. 25) with a range of biomes and vegetation types unevenly represented among regions. These sampling issues have confounded the testing of region effects.
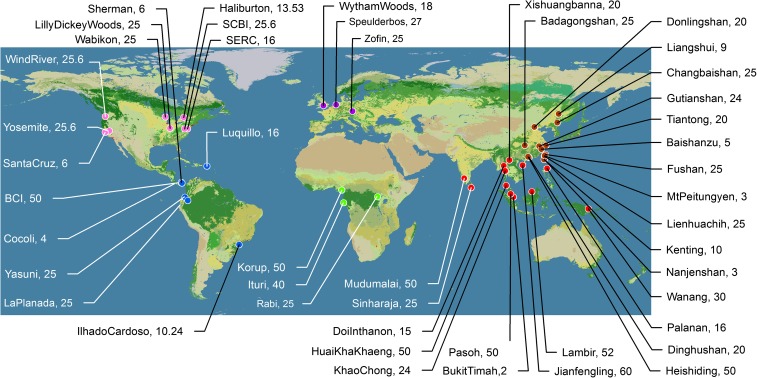
In this study, we analyze a dataset of tree species richness from the Center for Tropical Forest Science—Forest Global Earth Observatories (CTFS—ForestGEO; www.forestgeo.si.edu/; ref. 28) to disentangle the influences of local climate and regional factors, i.e., differences between regions resulting from unique histories and geographic settings, on the global biodiversity pattern. The data represent 47 forest dynamics plots distributed worldwide (Fig. 1) with a median size of 25 ha, within which all individual trees equal to or greater than 1 cm diameter at breast height (DBH) were identified and counted (SI Appendix, Table S1). Plots of this size are large enough to include adequate samples of species richness, but small enough to avoid substantial heterogeneity in climate and vegetation structure within them. The CTFS data are complete censuses, and the species richness in each plot is accurate. Many previous studies have been based on data from herbarium records of coarse-scale species range maps, and species richness is generally underestimated, considerably so in some cases. Moreover, the forest plots represent a single vegetation type surveyed over a range of environmental conditions. We assembled for each plot a set of local plot characteristics and climate data, and used generalized linear models to characterize the relationship between number of tree species and local plot variables and to test the additional statistical effect of region on local species richness. Our analyses were repeated for species richness with tree DBH ≥ 1 cm and DBH ≥ 10 cm.
Fig. 1.
Global distribution of the 47 CTFS plots. The number associated with each plot is its size in hectares. The base vegetation map is the 2012 MODIS global land cover map (www.landcover.org/data/lc/) with IGBP Land Cover Type Classification.
Results
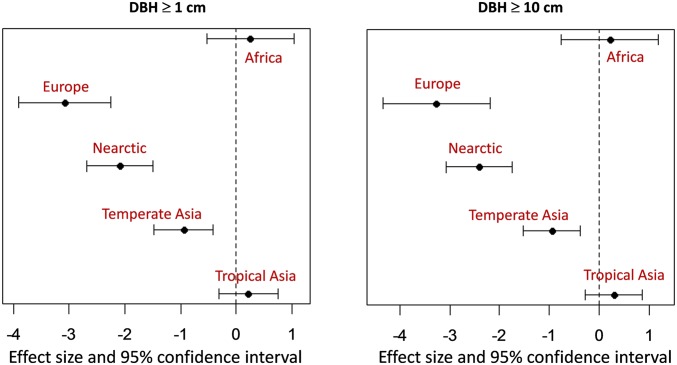
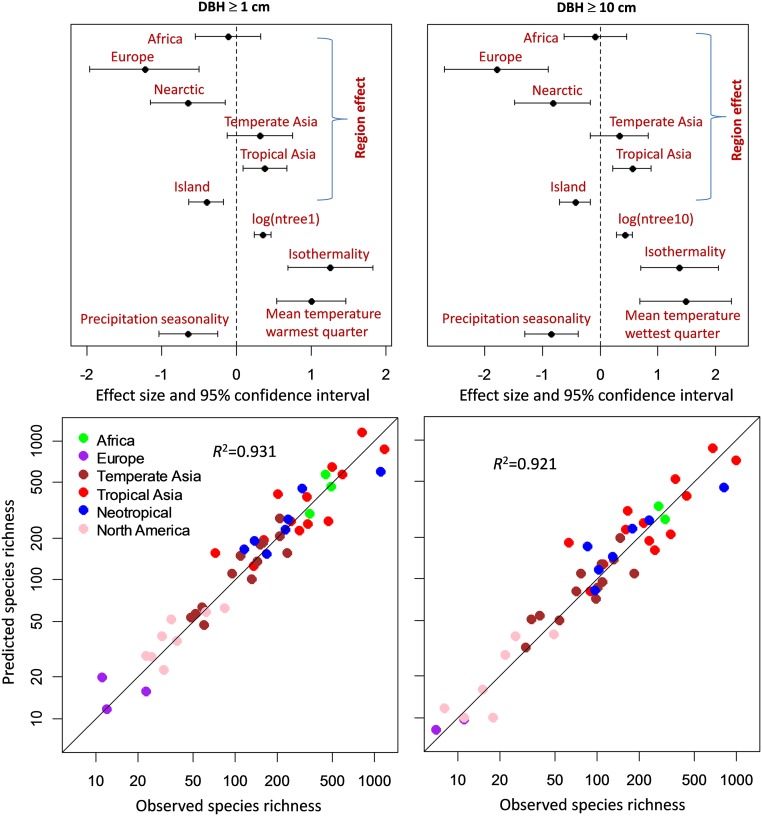
Differences between continental-scale regions (“region effects”) alone explained 73% of the variance in species richness among plots for both DBH ≥ 1 and DBH ≥ 10 cm (Fig. 2 and Table 1). Because region effects are confounded with local climate and habitat attributes, we further included climate and additional local plot variables listed in SI Appendix, Table S1 and selected among these full models by a stepwise procedure. The “best” model selected by the Akaike Information Criterion (AIC) for each DBH class is presented in Fig. 3 and Table 2. Although the influence of individual climate variables can be complex, the overall results are consistent and clear: The models for DBH ≥ 1 and DBH ≥ 10 cm retain a reduced set of local climate variables, in addition to sample size per plot (number of stems) and region, to explain >92% of the global variation in local tree diversity (Fig. 3 and Table 2). Thus, three major sources of variation are statistically associated with local tree species richness: (i) biogeographic region, (ii) sample size, and (iii) local climate.
Fig. 2.
Magnitude of the regional effects on species richness. Neotropical sites define the baseline for the region effects. Coefficients of the negative binomial generalized linear models (Table 1) and their 95% confidence intervals for DBH ≥ 1 cm (Left) and 10 cm (Right), respectively. The regional effects models explain 73.3% of variation in richness with DBH ≥ 1 cm (i.e., R2 = 0.733; Left) and 72.6% of variation in richness with DBH ≥ 10 cm (Right).
Table 1.
The negative binomial generalized linear regression for modeling region effects on species richness with DBH ≥ 1 cm and DBH ≥ 10 cm
| Explanatory variables | SE | z | Pr(>|z|) | |
| DBH ≥ 1 cm | ||||
| Intercept | 5.801 | 0.217 | 26.6 | <2e-16 |
| Africa | 0.256 | 0.397 | 0.7 | 0.52 |
| Europe | −3.071 | 0.422 | −7.3 | 3.48e-13 |
| Nearctic | −2.087 | 0.302 | −6.9 | 4.85e-12 |
| Temperate Asia | −0.946 | 0.270 | −3.5 | 4.66e-04 |
| Tropical Asia | 0.217 | 0.269 | 0.8 | 0.42 |
| DBH ≥ 10 cm | ||||
| Intercept | 5.465 | 0.235 | 23.3 | <2e-16 |
| Africa | 0.213 | 0.497 | 0.4 | 0.67 |
| Europe | −3.268 | 0.549 | −6.0 | 2.59e-09 |
| Nearctic | −2.407 | 0.341 | −7.1 | 1.64e-12 |
| Temperate Asia | −0.951 | 0.292 | −3.3 | 0.0011 |
| Tropical Asia | 0.298 | 0.291 | 1.0 | 0.31 |
The log link function is , where β is the vector of regression coefficients (including the intercept) and x is the character region variable (Africa, Europe, Nearctic, Neotropical, Temperate Asia, Tropical Asia). Because the variables have been standardized to a range of 0–1, the β coefficients are standardized effect sizes. The region effects were coded in reference to the Neotropical forests (the intercept). Compared with the baseline forests, European, North American, and Temperate Asian forests have reduced tree diversity.
Fig. 3.
Magnitude of the effects of region, number of plot stems, and significant local climate and plot variables predicting species richness. (Upper) Coefficients of the negative binomial generalized linear models (Table 2) and their 95% confidence intervals for DBH ≥ 1 cm (Left) and 10 cm (Right), respectively. Neotropical sites define the baseline for the region effects. (Lower) Relationships between the observed species richness and the richness predicted from the corresponding generalized linear models for the two DBH classes. If the models predicted species richness perfectly, the points would fall on the diagonal lines.
Table 2.
The output of the negative binomial generalized linear model for species richness with DBH ≥ 1 cm and ≥10 cm
| Explanatory variables | SE | z | Pr(>|z|) | |
| DBH ≥ 1 cm) | ||||
| Intercept | 0.335 | 0.729 | 0.460 | 0.65 |
| Region effect | ||||
| Africa | −0.113 | 0.223 | −0.505 | 0.61 |
| Europe | −1.232 | 0.372 | −3.310 | 9.31e-04 |
| Nearctic | −0.653 | 0.253 | −2.580 | 9.89e-03 |
| Temperate Asia | 0.307 | 0.222 | 1.385 | 0.17 |
| Tropical Asia | 0.377 | 0.152 | 2.483 | 0.013 |
| Island | −0.407 | 0.117 | −3.472 | 5.17e-04 |
| log(ntree1) | 0.347 | 0.055 | 6.355 | 2.08e-10 |
| Isothermality (BIO3) | 1.253 | 0.289 | 4.337 | 1.44e-05 |
| Mean temp of warmest quarter (BIO10) | 0.993 | 0.236 | 4.207 | 2.59e-05 |
| Precipitation seasonality (BIO15) | −0.647 | 0.201 | −3.215 | 1.30e-03 |
| DBH ≥ 10 cm | ||||
| Intercept | −0.599 | 0.873 | −0.685 | 0.49 |
| Region effect | ||||
| Africa | −0.085 | 0.276 | −0.307 | 0.76 |
| Europe | −1.786 | 0.456 | −3.913 | 9.11e-05 |
| Nearctic | −0.823 | 0.337 | −2.443 | 0.015 |
| Temperate Asia | 0.333 | 0.256 | 1.303 | 0.19 |
| Tropical Asia | 0.551 | 0.169 | 3.263 | 1.10e-03 |
| Island | −0.431 | 0.134 | −3.209 | 1.33e-03 |
| log(ntree10) | 0.424 | 0.069 | 6.163 | 7.14e-10 |
| Isothermality (BIO3) | 1.370 | 0.345 | 3.976 | 7.01e-05 |
| Mean temp of wettest quarter (BIO8) | 1.472 | 0.402 | 3.658 | 2.54e-04 |
| Precipitation seasonality (BIO15) | −0.842 | 0.237 | −3.546 | 3.91e-04 |
The log link function is , where β is the vector of regression coefficients (including the intercept) and x is the vector of explanatory variables. The region effects were coded in reference to the Neotropical forests (the intercept). The effect of island (i.e., isolation effect) is in reference to the mainland plots; −0.407 for the island plots (for DBH ≥ 1 cm) indicates that species richness on islands is significantly (P = 0.0005) lower than that in the mainland plots (given that the effects of other variables were accounted for). The model was selected by stepwise procedure using the criterion of AIC.
Significant region effects show that species richness varies among continents after accounting for the influence of local climate and plot characteristics. The unique contribution of the region effects to variation in species richness can be quantified by comparing the difference in explained variance between the full model with region effects, number of stems, and climate (R2 = 0.931 for DBH ≥ 1 cm and 0.921 for DBH ≥ 10 cm) and the reduced model that excludes the region effects (R2 = 0.863 and 0.821, respectively). Although the unique contribution of region amounts to only 7% and 10% of the total variance, the unique contributions of local plot variables are also small—20% and 19%, respectively. The strong association of variation in climate with region (SI Appendix, Fig. S2) makes it difficult to partition the effects of each unambiguously, except to say that both make significant contributions to variation in diversity. Several of the forest plots are located on islands (Taiwan, Hainan Island, the Philippines, Borneo, New Guinea, Sri Lanka, and Puerto Rico), and we tested for an isolation effect differentiating continental and island localities. We did not detect an additional effect of isolation when region effects were considered alone (Fig. 2 and Table 1). However, in the full model including climate variables, isolation (i.e., island locations) was significantly associated with lower species richness (P ≤ 1.33e-03) for both DBH ≥ 1 and DBH ≥ 10 cm size classes (Fig. 3 and Table 2).
The sampling effect, as measured by the number of stems in a plot, is another strong predictor of local diversity. Because plot size (area) is strongly correlated with the number of stems (R2 = 0.57 for DBH ≥ 1 and 0.66 for DBH ≥ 10 cm), plot size itself was not retained as a significant effect by the stepwise model selection.
The climate variables represent two types of factors (Fig. 3 and Table 2): (i) energy factors, including mean temperature of warmest quarter (BIO10) (standardized effect size, = 0.99, P = 2.6e-05) for DBH ≥ 1 cm, and Mean temperature of wettest quarter (BIO8) ( = 1.47, P = 2.5e-04) for DBH ≥ 10 cm; and (ii) climate seasonality factors (variability in temperature and water availability), including isothermality (BIO3: reduced temperature seasonality) ( = 1.25, P = 1.4e-05) and precipitation seasonality (BIO15) ( = −0.65, P = 1.3e-03) for DBH ≥ 1 cm, and these same two factors with = 1.37 (P = 7.0e-05) and = −0.84 (P = 3.9e-04), respectively, for DBH ≥ 10 cm. Although the effect of specific climatic variables on species richness can differ, both higher temperature (BIO8 and BIO10) and isothermality (BIO3) are associated with higher local plot diversity. Precipitation seasonality (BIO15), for which high values are associated with prolonged dry seasons, is associated with reduced tree species richness.
Actual evapotranspiration (AET, a measure of water flux) and potential evapotranspiration (PET, a measure of thermal input) were not retained as significant independent variables in the multiple regressions in our study although they are positively correlated with species richness in some analyses (23, 25). However, because variation in evapotranspiration across the CTFS plots is strongly correlated with mean temperature of the warmest quarter (BIO10) (R2 = 0.59 for AET; R2 = 0.55 for PET), mean temperature of wettest quarter (BIO8) (R2 = 0.59, 0.41), and isothermality (BIO3) (R2 = 0.50, 0.50), our results support these previous studies. Similarly, although the number of tree species decreased with increasing absolute latitude north or south of the equator (SI Appendix, Fig. S3; R2 = 0.77 for DBH ≥ 1 and R2 = 0.74 for DBH ≥ 10 cm), latitude per se was not retained under stepwise model selection because of its close correlation with climate variables: isothermality (R2 = 0.75), mean temperature of wettest quarter (R2 = 0.47), and mean temperature of warmest quarter (R2 = 0.58), respectively. Finally, we note that topographic variables (i.e., elevation and elevation range within a plot) were not significant effects for either the 1-cm DBH or the 10-cm DBH size class. Thus, within-plot habitat variation probably does not strongly influence the species richness of local assemblages, at least among these forest plots in the context of global variation.
Discussion
Global patterns of biodiversity continue to attract attention (2, 25, 29–31), but the relative influence of historical and regional factors versus local habitat and climate on global variation in taxonomic diversity remains controversial. The problem derives to some degree from incomplete and heterogeneous diversity data, uncertainties about the regional distributions of species, and inconsistencies in sampling scale. We have tried to circumvent these issues by relating local diversity in plots of similar size, whose tree diversity has been assessed by identical census methods, to a widely used set of climate variables in addition to region and plot-specific traits. The local tree taxonomic diversity of the 47 plots included in this study showed a clear global pattern, exhibiting the typical latitudinal gradient of declining values away from the equator (SI Appendix, Fig. S3), which parallels gradients in several climate variables. In addition, our analyses show that differences in tree species richness between regions, presumably related to their unique history and physiography (32–34), contribute substantially to global variation in local tree species richness in forested habitats.
The most consistent effects of Region were the low climate- and stem density-adjusted species richness for both DBH ≥1 and ≥10 cm trees in the three European plots and the North American forests compared with forests in temperate Asia (Fig. 3). The relatively low diversity in North America and, especially, in Europe compared with the forests of eastern Asia is well known. Explanations include more moderate effects of late Cenozoic climate cooling and glaciation in eastern Asia (see ref. 10 for effects in Europe), lower time-integrated regional area in Europe (32), extinction related to mountain building and increasing dryness in Western North America (35), and the geographic complexity of temperate eastern Asia, providing more opportunity for species formation (8). It is striking that the effect on species richness of regional processes appears to extend down to the local scale, emphasizing the spatial and temporal continuity of pattern and process (36). The effect of isolation on the local diversity of forests on several islands around the globe is also evident in our analyses.
African forests are considered to be less diverse than tropical Asian and tropical American forests (37–40); however, the African forests represented in the CTFS forest plots have comparable species richness to those elsewhere. Possibly the few African plots were selected for their high diversity compared with drier forests elsewhere on the continent. The climate variables at the African CTFS sites group closely with those at the tropical Asian and Neotropical sites (SI Appendix, Fig. S2). Given the matching climates at the tropical sites, and acknowledging the small number of selected African plots, the CTFS plots provide no evidence of region effects influencing the diversity of trees across the tropics.
Variation in AET, which is associated with habitat productivity, and PET, which measures the heat load (water evaporation potential) of the environment, are often associated with variation in regional species richness (e.g., refs. 23 and 25). However, in the presence of other related climate variables, AET and PET were not retained by our model selection. Our results suggest that tree species richness is influenced by energy and water flux through the environment (and their synergetic combination), as reflected by the variables our models selected (e.g., BIO8, mean temperature of wettest quarter; and BIO10, mean temperature of warmest quarter). However, some climate variables, such as BIO3 (isothermality = daily temperature range divided by the annual temperature range) and BIO15 (precipitation seasonality), which are not correlated with AET and PET across the forest locations, are retained in our models for forest tree species richness. This result suggests that energy and water alone are probably not sufficient predictors; absence of seasonal variation in energy (BIO3) and water (BIO15) also plays an important role in determining the global richness pattern, consistent with the analysis of Kreft and Jetz (25).
Finally, although it is clear that regional processes, along with variation in climate and sample size, jointly explain variation in local diversity of forest trees, it is important to note the difficulty of separating these effects statistically. Climate variables show strong latitudinal gradients, which are associated with the pervasive pattern of decreasing diversity with increasing latitude (SI Appendix, Fig. S3), and they also differ consistently between regions at the same latitude, particularly within temperate latitudes (SI Appendix, Fig. S2). Because the forest plots in each region are distributed across a unique range of climate variables, the qualitative variable region is redundant, to some degree, on the quantitative climate variables, and region effects per se tend to be obscured in multiple regression models. Previous studies failing to find region effects at the local scale may have confounded region and climate in their analyses.
Differences in diversity between regions, independent of present climate, are broadly appreciated and are generally attributed to region size and unique history and geography (8, 32, 33, 40), including the recent influence of Pleistocene glaciations (10, 41–43) at temperate latitudes. Regional diversity is built up through an excess of species formation over extinction. The rates of both these processes presumably vary among regions owing to differences in their size and geographic complexity, leading to different regional levels of diversity (32). How species are sorted within regions, which establishes patterns of local (alpha) diversity and turnover of species between locations (beta diversity), should depend on how populations interact, primarily through competition for resources, which can also promote evolutionary diversification over environmental gradients. The strong statistical influence of region on local species richness suggests that membership in local communities is not limited locally, but rather responds to regional pressures of species production and the spread of populations within regions to occupy extended geographic areas and ranges of ecological conditions.
Local diversity is the outcome of complex interactions of local and regional processes, as emphasized by the present analysis. The ambiguity of the local–regional relationship in the ecological literature arises, in large part, from the difficulty of separating processes acting on local and regional scales. This problem is exacerbated by the uneven sampling effort associated with much empirical data, which typically have been gathered for other purposes, and by the unique climate variables associated with each biogeographic region. Nonetheless, understanding the nature of the local–regional diversity relationship is essential for ecologists seeking to integrate theory addressing the maintenance of diversity at the local community scale, including species–habitat relationships and species interactions, and at the regional level, including continental-scale attributes that influence species formation and extinction.
Materials and Methods
Since the early 1980s, when Steven Hubbell established the first 50-ha plot on Barro Colorado Island, Panama (44, 45), the CTFS of the Smithsonian Institution has developed a global network of forest plots across Africa, Europe, Asia, and the Americas. The network comprises of more than 60 plots within which it monitors approximately 6 million free-standing trees/shrubs with DBH ≥ 1 cm, belonging to nearly 10,000 species. We compiled species richness data for 47 of these plots (Fig. 1 and SI Appendix, Table S1). Most of the plots are 5–50 ha in area and contain an average of 1.1 × 105 individuals (≥1 cm DBH) and 1.4 × 104 individuals (≥10 cm DBH). We analyzed the data for the two size classes of individuals separately; the ≥1 cm DBH data include the ≥10 cm DBH data and, thus, are not completely independent.
Most of the plot data were accompanied by local measurements of the physical environment: elevation, annual precipitation, mean annual temperature, and number of dry months (SI Appendix, Table S1) (28). For each of the 47 plots, we also downloaded climate variables from www.worldclim.org/bioclim, a dataset widely used for ecological niche modeling (46, 47). The 19 bioclimatic variables, BIO1–BIO19, describe average temperature and precipitation, and seasonal variation in temperature and precipitation (48). A canonical correlation analysis between 14 nonredundant bioclimatic variables (SI Appendix, Table S1) and the locally measured climate variables yielded three significant canonical correlations with adjusted R2 values ranging from 0.989 (with local mean annual temperature) to 0.682 (local number of dry months) (SI Appendix, Table S2). Thus, the bioclimatic variables, which were available for all of the plots, provide an adequate description of the local climate, particularly temperature. Accordingly, the explanatory variables included in this analysis were as follows:
(i) Local plot characteristics: latitude; plot area; number of stems; elevation; elevation range within each plot, which is related to local heterogeneity (49), although additional heterogeneity related to variation in soils (50) is not accounted for in this analysis.
(ii) Local climate variables: the 19 bioclimatic variables (48) plus AET and PET (compiled from the Global Evapotranspiration and Water Balance Data Sets of Ahn and Tateishi; ref. 51). Because some of the bioclimatic variables, AET, and PET are highly intercorrelated, we excluded variables having pairwise Pearson correlation coefficients > 0.95 to avoid collinearity. Twelve bioclimatic variables plus AET and PET were finally included in our analyses (SI Appendix, Table S1).
(iii) Region: a character variable indicating tropical Africa, temperate Europe, tropical Asia, temperate Asia, tropical America (Neotropics), and Nearctic (North America). We conducted additional analyses with the Nearctic separated into eastern and western temperate North America, but obtained almost identical results. The models without separation were slightly favored by AIC and are therefore reported in this study.
(iv) Isolation: plots on islands (Taiwan, Hainan Island, the Philippines, Borneo, Puerto Rico, Sri Lanka, and New Guinea) were coded as island (isolation) in contrast to the continental plots (nonisolation).
Plot area and number of stems were log-transformed to account for power relationships with species richness. All other numeric variables (x) were standardized to 0–1 range by (x − xmin)/(xmax − xmin).
Because species richness values for the 47 plots exhibit a negative binomial distribution (SI Appendix, Fig. S1), we used negative binomial generalized linear models with a log link function to model richness as a function of the region effects, isolation, stem density, areal effect, and local climatic and environmental variables. We initially modeled species richness as a function of region (Fig. 2 and Table 1). Because the six regions can be distinguished by different climate conditions (SI Appendix, Fig. S2), region effects can be confounded with local climate and habitat conditions. Accordingly, we extended the region effects model to include all of the local climate and plot variables listed in SI Appendix, Table S1.
A stepwise selection procedure was used for model selection based on AIC. The importance of a variable can be evaluated by comparing a full model that includes all related variables and a reduced model excluding the variable, or variables, of interest. The deviance difference between the two models follows a χ2 distribution.
Statistical analyses in this study were conducted by using R (www.r-project.org/). The function “glm.nb” in the package MASS was used to model the negative binomial generalized linear models.
Supplementary Material
Acknowledgments
We thank many people for generously providing us with their plot data for this work, including Norm Bourg, Sarayudh Bunyavejchewin, Deliang Chen, Rick Condit, Stuart Davies, Gregory Gilbert, Dong He, Robert Howe, Patrick Jansen, Minxi Jiang, Guangze Jin, Kamil Kral, Yiching Lin, Yanyan Liu, Zhengrong Luo, Jim Lutz, William J. McShea, Vojtech Novotny, Alexandre A. de Oliveira, Jan den Ouden, I-Fang Sun, Sean Thomas, Xihua Wang, Yi-Hui Wang, George Weiblen, Amy Wolf, Han Xu, Yan Zhu, and many others who have contributed to establishing the the Center for Tropical Forest Science (CTFS) global plots. Xinghua Sui prepared Fig. 1. This work was supported by Sun Yat-sen University, the Natural Sciences and Engineering Research Council (Canada), and the Curators of the University of Missouri.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523683113/-/DCSupplemental.
References
- 1.Lortie CJ, et al. Rethinking plant community theory. Oikos. 2004;107(2):433–438. [Google Scholar]
- 2.Ricklefs RE. A comprehensive framework for global patterns in biodiversity. Ecol Lett. 2004;7(1):1–15. [Google Scholar]
- 3.Weiher E, Keddy PA. Assembly rules, null models, and trait dispersion: New questions from old patterns. Oikos. 1995;74(1):159–164. [Google Scholar]
- 4.Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW. The merging of community ecology and phylogenetic biology. Ecol Lett. 2009;12(7):693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 5.Harrison S, Cornell H. Toward a better understanding of the regional causes of local community richness. Ecol Lett. 2008;11(9):969–979. doi: 10.1111/j.1461-0248.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- 6.Cornell HV, Harrison SP. Regional effects as important determinants of local diversity in both marine and terrestrial systems. Oikos. 2012;122(2):288–297. [Google Scholar]
- 7.White EP, Hurlbert AH. The combined influence of the local environment and regional enrichment on bird species richness. Am Nat. 2010;175(2):E35–E43. doi: 10.1086/649578. [DOI] [PubMed] [Google Scholar]
- 8.Qian H, Ricklefs RE. Large-scale processes and the Asian bias in species diversity of temperate plants. Nature. 2000;407(6801):180–182. doi: 10.1038/35025052. [DOI] [PubMed] [Google Scholar]
- 9.Ricklefs RE, Schwarzbach AE, Renner SS. Rate of lineage origin explains the diversity anomaly in the world’s mangrove vegetation. Am Nat. 2006;168(6):805–810. doi: 10.1086/508711. [DOI] [PubMed] [Google Scholar]
- 10.Svenning JC. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecol Lett. 2003;6(7):646–653. [Google Scholar]
- 11.Srivastava D. Using local-regional richness plots to test for species saturation: Pitfalls and potentials. J Anim Ecol. 1999;68(1):1–16. [Google Scholar]
- 12.Loreau M. Are communities saturated? On the relationship between α, β, and γ diversity. Ecol Lett. 2000;3:73–76. [Google Scholar]
- 13.Cornell HV. Unsaturation and regional influences on species richness in ecological communities: A review of the evidence. Ecoscience. 1999;6(3):303–315. [Google Scholar]
- 14.Cornell HV, Lawton JH. Species interactions, local and regional processes, and limits to the richness of ecological communities: A theoretical perspective. J Anim Ecol. 1992;61:1–12. [Google Scholar]
- 15.Francis AP, Currie DJ. A globally consistent richness-climate relationship for angiosperms. Am Nat. 2003;161(4):523–536. doi: 10.1086/368223. [DOI] [PubMed] [Google Scholar]
- 16.Huston MA. Local processes and regional patterns: Appropriate scales for understanding variation in the diversity of plants and animals. Oikos. 1999;86(3):393–401. [Google Scholar]
- 17.Karlson RH, Cornell HV. Species richness of coral assemblages: Detecting regional influences at local spatial scales. Ecology. 2002;83(2):452–463. [Google Scholar]
- 18.Belmaker J, Jetz W. Relative roles of ecological and energetic constraints, diversification rates and region history on global species richness gradients. Ecol Lett. 2015;18(6):563–571. doi: 10.1111/ele.12438. [DOI] [PubMed] [Google Scholar]
- 19.Eiserhardt WL, Borchsenius F, Plum CM, Ordonez A, Svenning JC. Climate-driven extinctions shape the phylogenetic structure of temperate tree floras. Ecol Lett. 2015;18(3):263–272. doi: 10.1111/ele.12409. [DOI] [PubMed] [Google Scholar]
- 20.Kerkhoff AJ, Moriarty PE, Weiser MD. The latitudinal species richness gradient in New World woody angiosperms is consistent with the tropical conservatism hypothesis. Proc Natl Acad Sci USA. 2014;111(22):8125–8130. doi: 10.1073/pnas.1308932111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lessard JP, et al. Strong influence of regional species pools on continent-wide structuring of local communities. Proc Biol Sci. 2012;279(1727):266–274. doi: 10.1098/rspb.2011.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latham RE, Ricklefs RE. Global patterns of tree species richness in moist forests: energy-diversity theory does not account for variation in species richness. Oikos. 1993;67(2):325–333. [Google Scholar]
- 23.Currie DJ, Paquin V. Large-scale biogeographical patterns of species richness of trees. Nature. 1987;329:326–327. [Google Scholar]
- 24.Francis AP, Currie DJ. Global patterns of tree species richness in moist forest: Another look. Oikos. 1998;81:598–602. [Google Scholar]
- 25.Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. Proc Natl Acad Sci USA. 2007;104(14):5925–5930. doi: 10.1073/pnas.0608361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simová I, et al. Global species-energy relationship in forest plots: Role of abundance, temperature and species climatic tolerances. Glob Ecol Biogeogr. 2011;20(6):842–856. [Google Scholar]
- 27.Baraloto C, et al. Rapid simultaneous estimation of aboveground biomass and tree diversity across Neotropical forests: A comparison of field inventory methods. Biotropica. 2013;45(3):288–298. [Google Scholar]
- 28.Anderson-Teixeira KJ, et al. CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Glob Change Biol. 2015;21(2):528–549. doi: 10.1111/gcb.12712. [DOI] [PubMed] [Google Scholar]
- 29.Allen AP, Gillooly JF. Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecol Lett. 2006;9(8):947–954. doi: 10.1111/j.1461-0248.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 30.Currie DJ, et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol Lett. 2004;7(12):1121–1134. [Google Scholar]
- 31.Gaston KJ. Global patterns in biodiversity. Nature. 2000;405(6783):220–227. doi: 10.1038/35012228. [DOI] [PubMed] [Google Scholar]
- 32.Fine PVA, Ree RH. Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am Nat. 2006;168(6):796–804. doi: 10.1086/508635. [DOI] [PubMed] [Google Scholar]
- 33.Jetz W, Fine PV. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 2012;10(3):e1001292. doi: 10.1371/journal.pbio.1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreft H, Jetz W. A framework for delineating biogeographical regions based on species distributions. J Biogeogr. 2010;37(11):2029–2053. [Google Scholar]
- 35.Wolfe JA. Tertiary climatic changes at middle latitudes of western North America. Palaeogeogr Palaeoclimatol Palaeoecol. 1994;108(3–4):195–205. [Google Scholar]
- 36.Ricklefs RE. Disintegration of the ecological community. Am Nat. 2008;172(6):741–750. doi: 10.1086/593002. [DOI] [PubMed] [Google Scholar]
- 37.Gentry AH. Patterns of plant community diversity and floristic composition on environmental and geographical gradients. Ann Mo Bot Gard. 1988;75:1–34. [Google Scholar]
- 38.Whitmore TC. Tropical Rain Forests of the Far East. Clarendon; Oxford: 1984. [Google Scholar]
- 39.Parmentier I, et al. The odd man out? Might climate explain the lower tree α-diversity of African rain forests relative to Amazonian rain forests? J Ecol. 2007;95(5):1058–1071. [Google Scholar]
- 40.Slik JWF, et al. An estimate of the number of tropical tree species. Proc Natl Acad Sci USA. 2015;112(24):7472–7477. doi: 10.1073/pnas.1423147112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latham RE, Ricklefs RE. Continental comparisons of temperate-zone tree species diversity. In: Ricklefs RE, Schluter D, editors. Species Diversity: Historical and Geographical Perspectives. Univ of Chicago Press; Chicago: 1993. pp. 294–314. [Google Scholar]
- 42.Sauer JD. Plant Migration. The Dynamics of Geographic Patterning in Seed Plant Species. Univ of California Press; Berkeley, CA: 1988. [Google Scholar]
- 43.Svenning J-C, Skov F. Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol Lett. 2007;10(6):453–460. doi: 10.1111/j.1461-0248.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- 44.Hubbell SP, Foster RB. Short-term dynamics of a neotropical forest: Why ecological research matters to tropical conservation and management. Oikos. 1992;63:48–61. [Google Scholar]
- 45.Condit R. Research in large, long-term tropical forest plots. Trends Ecol Evol. 1995;10(1):18–22. doi: 10.1016/s0169-5347(00)88955-7. [DOI] [PubMed] [Google Scholar]
- 46.Hijmans RJ, Graham CH. The ability of climate envelope models to predict the effect of climate change on species distributions. Glob Change Biol. 2006;12(12):1–10. [Google Scholar]
- 47.Elith J, Leathwick JR. Species distribution models: Ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst. 2009;40:677–697. [Google Scholar]
- 48.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25(15):1965–1978. [Google Scholar]
- 49.Brown C, et al. Multispecies coexistence of trees in tropical forests: Spatial signals of topographic niche differentiation increase with environmental heterogeneity. Proc Biol Sci. 2013;282(1800):20130502. doi: 10.1098/rspb.2013.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldeck CA, et al. Soil resources and topography shape local tree community structure in tropical forests. Proc Biol Sci. 2012;280(1753):20122532. doi: 10.1098/rspb.2012.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn C-H, Tateishi R. Development of a global 30-minute grid potential evapotranspiration data set. Photogrammetry and Remote Sensing. 1994;33(2):12–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.