Significance
This study reveals that atheroprone flow induces integrin α5 translocation into lipid rafts and hence activation to cause endothelial dysfunction in vitro and in vivo. Consequently, this mechanotransduction event leads to the activation of NLRP3 inflammasome. Knockdown of integrin α5 ameliorated endothelial cell (EC) dysfunction in partially ligated carotid arteries of Ldlr−/− mice. Furthermore, haploinsufficiency or functional inhibition of integrin α5 in mice improved EC function in the atheroprone area. These findings reveal a novel mechanism by which atheroprone flow causes endothelial dysfunction that leads to vascular impairments such as atherosclerosis.
Keywords: integrin, lipid rafts, shear stress, proteomics, endothelial dysfunction
Abstract
Local flow patterns determine the uneven distribution of atherosclerotic lesions. Membrane lipid rafts and integrins are crucial for shear stress-regulated endothelial function. In this study, we investigate the role of lipid rafts and integrin α5 in regulating the inflammatory response in endothelial cells (ECs) under atheroprone versus atheroprotective flow. Lipid raft proteins were isolated from ECs exposed to oscillatory shear stress (OS) or pulsatile shear stress, and then analyzed by quantitative proteomics. Among 396 proteins redistributed in lipid rafts, integrin α5 was the most significantly elevated in lipid rafts under OS. In addition, OS increased the level of activated integrin α5 in lipid rafts through the regulation of membrane cholesterol and fluidity. Disruption of F-actin-based cytoskeleton and knockdown of caveolin-1 prevented the OS-induced integrin α5 translocation and activation. In vivo, integrin α5 activation and EC dysfunction were observed in the atheroprone areas of low-density lipoprotein receptor-deficient (Ldlr−/−) mice, and knockdown of integrin α5 markedly attenuated EC dysfunction in partially ligated carotid arteries. Consistent with these findings, mice with haploinsufficency of integrin α5 exhibited a reduction of atherosclerotic lesions in the regions under atheroprone flow. The present study has revealed an integrin- and membrane lipid raft-dependent mechanotransduction mechanism by which atheroprone flow causes endothelial dysfunction.
Shear stress imposed on vascular endothelial cells (ECs) influences vascular phenotype and function. Atherosclerosis preferentially develops at branches and curvatures in the arterial tree where flow is disturbed. In contrast, pulsatile shear stress (PS) in the straight parts of the arteries is atheroprotective (1). At the cellular and molecular levels, disturbed flow pattern increases, while PS inhibits, the inflammatory response in ECs, including the expression of intercellular adhesion molecule 1 (ICAM-1), vascular adhesion molecule 1 (VCAM-1), and interleukin 1β (IL-1β) (2). We found that integrins in ECs are shear stress-sensitive (3). Many subsequent studies demonstrated that integrin activation is essential for transmitting mechanical stimuli to intracellular biochemical pathways (4–6). Additionally, mounting evidence indicates that lipid raft microdomains, membrane receptors, cytoskeletal proteins, and extracellular matrices are linked to integrin activation in the context of mechanotransduction (7–11). However, the key mechanism underlying the differential effects of atheroprone versus atheroprotective flows in activating integrins that in turn induces inflammatory response in ECs remains to be elucidated.
Lipid rafts are membrane microdomains that are enriched in cholesterol, sphingolipids, and a variety of signaling molecules, which function as cellular signaling platforms. These microdomains are more ordered and tightly packed than the surrounding membrane. Removal of membrane cholesterol leads to the dissociation of molecules from lipid rafts and hence deactivation of some of these molecules (12). The F-actin-based cytoskeleton associates with lipid rafts, and many of the structural and functional properties of rafts require an intact F-actin-based cytoskeleton. As many of the lipid raft-associated proteins in ECs are shear stress-sensitive, changes in the cholesterol content of the membrane or disruption of its F-actin-based cytoskeleton abolish mechanosensitivity of the cell.
Both oxidized low-density lipoprotein (OxLDL) and atheroprone flow have been implicated in inducing nuclear factor-κB (NF-κB)-mediated inflammation in ECs. Integrin α5β1 is mainly expressed in ECs (13) and is involved in flow activation of NF-κB (14). With respect to lipid rafts, clustering of ganglioside-abundant lipid rafts has been shown to regulate the activity of integrin β1 (15). Furthermore, a recent study by Yurdagul et al. shows that integrin α5β1 is involved in OxLDL-induced activation of NF-κB in ECs (16). Although it is firmly established that activation of α5β1 mediates the NF-κB inflammatory axis in ECs, it is unknown whether a common molecular basis exists between the ability of atheroprone flow and OxLDL to activate integrin α5β1 and the subsequent molecular mechanisms by which α5β1 increases the inflammatory response, leading to atherosclerosis susceptibility.
The current study was initiated from findings from a quantitative proteomics analysis of lipid raft-associated proteins in ECs regulated by flow. Surprisingly, this high-throughput screening revealed that oscillatory shear stress (OS) drastically increased integrin α5 in lipid rafts of ECs. Because OxLDL treatment of ECs increases their membrane cholesterol content leading to decreased membrane fluidity (17, 18), we hypothesized that alterations in cholesterol composition or lipid packaging in lipid rafts in response to OxLDL or atheroprone flow may alter integrin α5 distribution and/or activity. Furthermore, given that activation of NF-κB in turn activates Nod-like receptor protein 3 (NLRP3) inflammasome (19), integrin α5 would be upstream of the atheroprone flow-induced NLRP3 inflammasome (20).
In this study, we investigate the roles of lipid rafts and integrin α5 in OS-induced EC dysfunction, including NLRP3 inflammasome and their causative effect on atherogenesis. Our in vitro and in vivo experiments demonstrate that the lipid raft translocation and activation of integrin α5, due to changes in membrane fluidity, is a previously unidentified pathway mediating endothelial dysfunction in response to atheroprone flow.
Results
Differential Effects of OS and PS on Lipid Raft Translocation and Activation of Integrin α5.
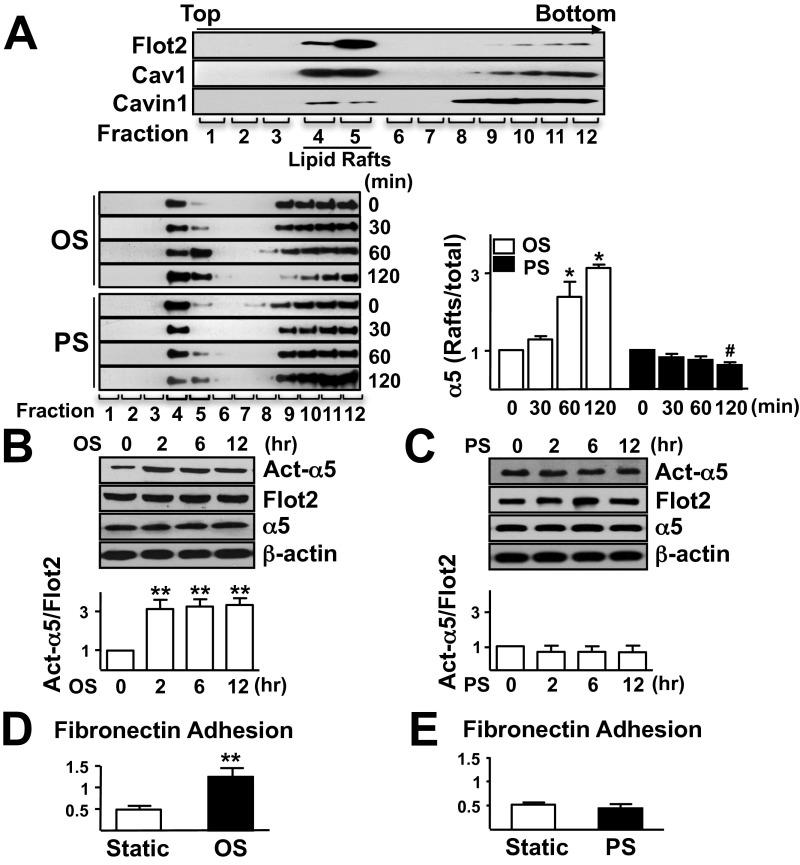
We isolated total proteins from human umbilical vein ECs (HUVECs) that had been exposed to OS (0.5 ± 4 dyn/cm2) or PS (12 ± 4 dyn/cm2) for 2 h. The cell lysates were fractionated using sucrose density gradient ultracentrifugation to separate the lipid raft-associated proteins. Positive blotting of lipid raft markers including flotillin-2 (Flot2), caveolin-1 (Cav1), and cavin1 in fractions 4–5 (Fig. S1A, Top) validated that these fractions contained the lipid raft-associated proteins. Quantitative proteomic analysis was then performed, and 396 proteins were identified in fractions 4–5 (Datasets S1 and S2). We selected proteins that were significantly upregulated or down-regulated in an OS- or PS-dependent manner. Using the Panther Classification System (www.pantherdb.org) (Dataset S3), we categorized these proteins into different functional groups (binding, enzyme regulator, ion channel, motor, receptor, structural molecular, transcription regulator, translation regulator, and transporter). Of the proteins considered mechanosensitive and significantly differentially regulated, the level of integrin α5 was increased by OS, but decreased by PS, in lipid rafts (1.8- vs. 0.6-fold) (Dataset S3).
Fig. S1.
(A) Western blot analysis of the level of Flot2, Cav1, and cavin1 (Top) and distribution of integrin α5 in different fractions under OS or PS (Bottom). Graph shows the relative ratio of raft to total integrin α5. (B and C) ECs were exposed to OS (B) or PS (C) for time durations as indicated, and static cells (0 min) were used as a control. Raft proteins were isolated for Western blot analysis of activated integrin α5 (Act-α5), and whole-cell lysates were isolated to detect total α5. Bar graphs show the relative ratio of Act-α5 to Flot2. (D and E) ECs that had been subjected to OS or PS for 2 h were allowed to adhere to a dish precoated with fibronectin for 20 min, and then adhesive cells were counted. Data are mean ± SEM normalized to static from at least three independent experiments. *P < 0.05, vs. OS control; #P < 0.05, vs. PS control; **P < 0.01, vs. control.
To confirm the results from proteomic analysis, ECs were exposed to OS or PS for increasing time points, and the distribution of integrin α5 in lipid rafts was examined. Under OS, the proportion of integrin α5 in lipid rafts increased in a time-dependent manner, up to threefold at 2 h. In contrast, PS had an opposite effect (Fig. S1A, Bottom). In support of this observation, we used equal amounts of protein purified from lipid rafts and nonlipid rafts under OS vs. PS to detect integrin α5 translocation. As expected, although OS induced integrin α5 translocation into lipid rafts, which became significant at 2 h, PS promoted the exit of integrin α5 from lipid rafts (Fig. 1 A and B).
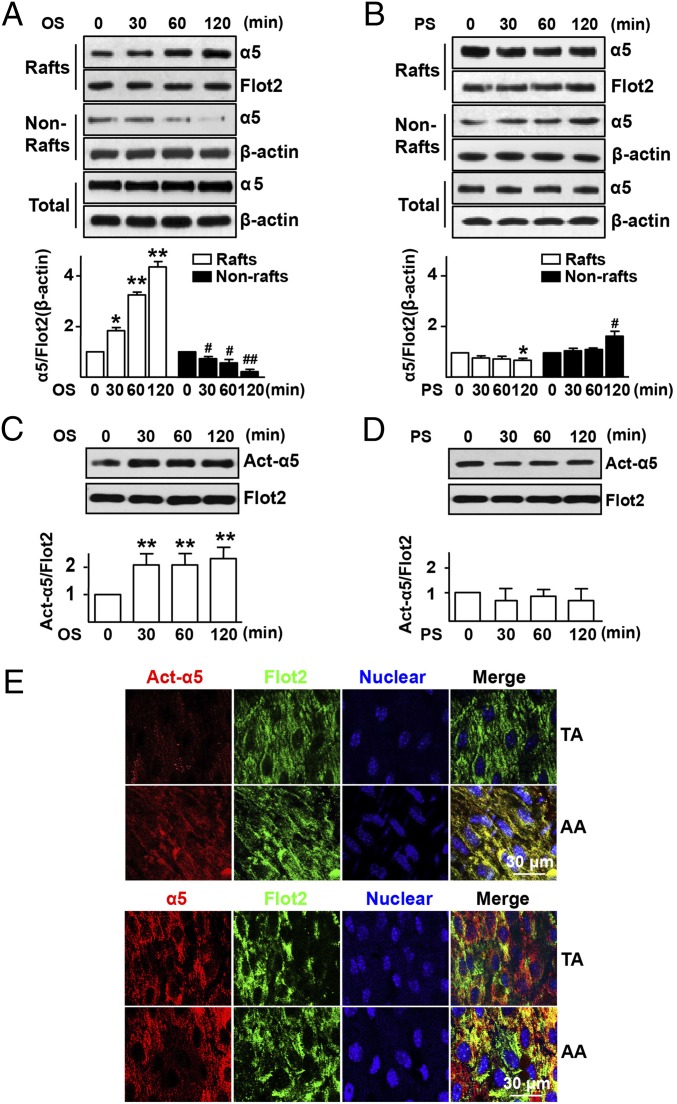
Fig. 1.
OS elevates the level of total and activated integrin α5 in lipid rafts in vitro and in vivo. HUVECs were exposed to OS (0.5 ± 4 dyn/cm2) (A and C) or PS (12 ± 4 dyn/cm2) (B and D) for time durations as indicated. Static cells (0 min) were used as a control. (A and B) Representative Western blots of integrin α5 in lipid rafts (Rafts; fractions 4–5), nonlipid rafts (Nonrafts; fractions 6–12), and whole cellular proteins (Total) in static cells or those exposed to OS or PS. (C and D) Lipid raft proteins were isolated for Western blot analysis of activated integrin α5 (Act-α5) and Flot2, a marker for rafts fractions. Bar graphs show the relative ratio of total or activated α5 to Flot2 or β-actin. ANOVA followed by the Bonferroni post hoc test was used for statistical analysis. Data are mean ± SEM averaged from at least three independent experiments. *P < 0.05, rafts vs. static; #P < 0.05, nonrafts vs. static. (E) The AA and TA were isolated from Ldlr−/− mice (8 wk old; n = 10) for en face immunostaining with Act-α5 (red), Total α5 (α5, red), Flot2 (green), and nuclear (blue).
We next investigated whether OS regulates the activity of integrin α5. Using an antibody recognizing the activated integrin α5, we found that OS, but not PS, activated integrin α5 (Fig. 1 C and D). This differential effect between OS and PS in activating integrin α5 persisted up to 12 h following flow application (Fig. S1 B and C). In line with integrin α5 activation, OS enhanced EC adhesion on fibronectin, a specific ligand for integrin α5. By contrast, PS decreased such adhesion (Fig. S1 D and E). To validate the in vitro observations, we investigated integrin α5 activation in different regions of the aorta of low-density lipoprotein receptor-deficient (Ldlr−/−) mice on normal diet. En face immunostaining with the use of an antibody recognizing the activated integrin α5 showed enhanced staining in atheroprone areas [i.e., aortic arch (AA)] in comparison with the atheroprotective area [i.e., thoracic aorta (TA)] (Fig. 1E, Top). Noticeably, there was no difference of total integrin α5 expression between TA and AA (Fig. 1E, Bottom).
Lipid Raft Translocation of Integrin α5 Is Associated with Its Activation.
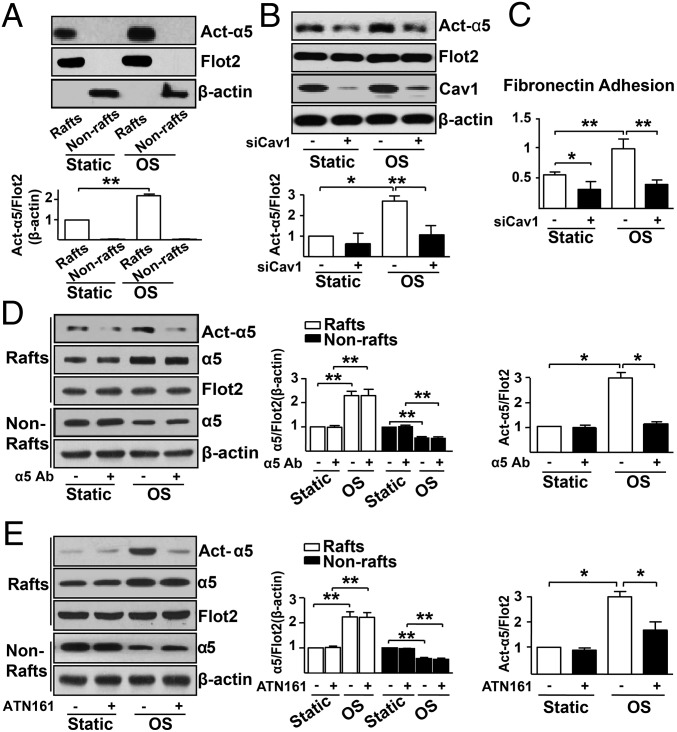
Western blot analysis shown in Fig. 2A indeed verified that OS activated integrin α5 and that the activated integrin α5 was mainly located in the lipid raft fractions. We then examined the causative effect of integrin α5 translocation and activation, namely, whether α5 translocation is necessary for its activation or vice versa. When we knocked down Cav1 to disrupt the structure of lipid rafts to prevent α5 translocation, OS could not activate α5 nor could it enhance EC adhesion to fibronectin (Fig. 2 B and C), which indicates that α5 translocation instigates its activation by OS. However, when ECs were treated with an α5 neutralizing antibody (α5 Ab) or ATN161 (an α5-specific inhibitory peptide) to prevent α5 activation, OS was still able to induce the α5 translocation into lipid rafts (Fig. 2 D and E). Collectively, results in Fig. 2 suggest that the Cav1-dependent integrin α5 translocation into lipid rafts is necessary for its activation by OS but not vice versa.
Fig. 2.
The OS-activated integrin α5 is associated with lipid rafts translocation. (A) HUVECs were exposed to OS or kept static for 2 h, and proteins were isolated for Western blot analysis of activated α5 (Act-α5) in Rafts and Nonrafts. (B) HUVECs pretreated with scramble siRNA (siCav1, −) or Cav1 siRNA (siCav1, +) for 48 h. Lipid raft and EC proteins were isolated for Western blot analysis of Act-α5, Flot2, Cav1, and β-actin. Bar graphs show the relative ratio of Act-α5 to Flot2 or β-actin. (C) HUVECs pretreated with or without siCav1 were subjected to OS for 2 h. ECs adhesion to fibronectin was measured. (D and E) HUVECs were pretreated with integrin α5 neutralizing antibody (10 μg/mL) for 1 h (D) or with ATN161 or control peptide (10 μmol/L) for 48 h (E), and then exposed to OS for 2 h. Western blot was performed with antibodies as indicated. Bar graphs show the relative ratio of Raft α5 to Flot2, or Nonraft α5 to β-actin. ANOVA followed by the Bonferroni post hoc test was used for statistical analysis. Data are mean ± SEM averaged from at least three independent experiments. *P < 0.05, vs. static.
Integrin α5 Activity Is Regulated by Membrane Fluidity and Cholesterol Content.
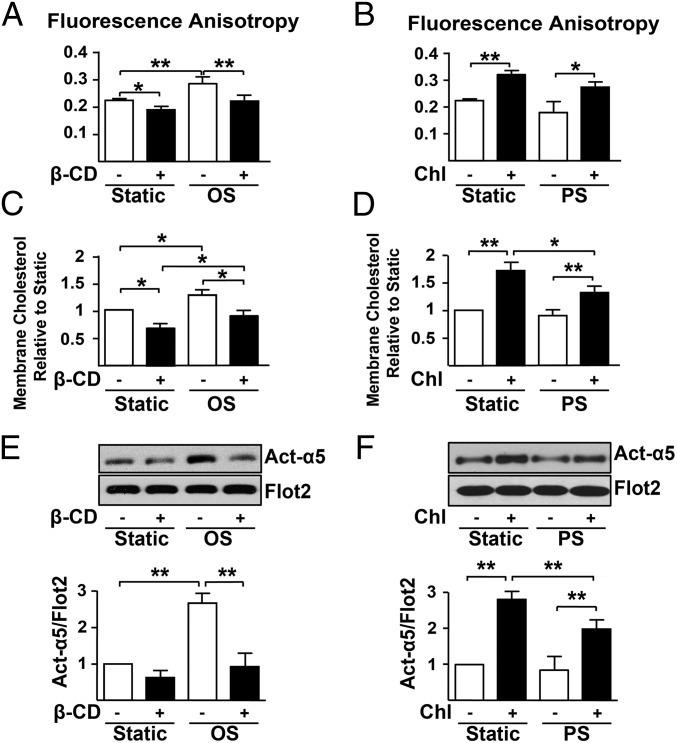
As membrane fluidity affects the mobility of membrane proteins, and because we previously reported that shear stress regulates cholesterol content of the EC membrane, in turn altering membrane fluidity (21), we next investigated the involvement of membrane fluidity in flow regulation of integrin α5 activity. We found that OS increased 1,6-diphenyl-1,3,5-hexatriene (DPH) anisotropy, which is indicative of a decrease in membrane fluidity (Fig. 3A). Such change was consistent with the increase in cholesterol content in the membrane (Fig. 3C). These OS-imposed effects were not seen in ECs pretreated with methyl-β-cyclodextrin (β-CD) to deplete cholesterol (Fig. 3 A and C). In contrast, PS increased membrane fluidity and attenuated cholesterol content, which were abolished if EC cultures were supplemented with cholesterol (Fig. 3 B and D). Importantly, cholesterol depletion with β-CD prevented α5 activation by OS (Fig. 3E), whereas cholesterol supplementation increased the level of activated α5 under PS (Fig. 3F). These findings suggest that membrane fluidity affected by cholesterol content is crucial for OS activation of integrin α5.
Fig. 3.
OS promotes integrin α5 translocation to lipid rafts through alteration of membrane fluidity. HUVECs were treated with β-CD (5 mmol/L) for 2 h (A, C, and E) or Chl (30 μg/mL) for 2 h (B, D, and F) before being exposed to OS or PS or kept under static condition for 2 h. Cell membrane was then isolated for fluorescence anisotropy (A and B) or cholesterol content (C and D) measurements. (E and F) Lipid raft proteins were isolated for Western blot analysis of Act-α5 and Flot2. ANOVA followed by the Bonferroni post hoc test was used for statistical analysis. Data are mean ± SEM averaged from at least three independent experiments. *P < 0.05, vs. static controls.
Integrin α5 Translocation Is Mediated by F-Actin-Based Cytoskeleton Rearrangements.
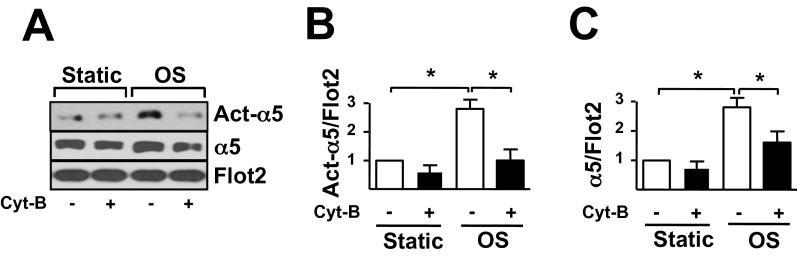
Given the important role of the F-actin-based cytoskeleton rearrangements in protein translocation from the cytoplasm to lipid rafts (i.e., caveolae) (21, 22), we further investigated whether the OS-induced integrin α5 translocation is dependent on F-actin-based cytoskeleton. Disruption of the F-actin-based cytoskeleton by cytochalasin B (Cyt-B) markedly attenuated the OS-induced integrin α5 translocation and activation (Fig. S2).
Fig. S2.
(A) ECs pretreated with or without 2.5 μmol/L Cyt-B for 2 h were subjected to OS for 2 h. Act-α5 and total-α5 (α5) in lipid rafts (Rafts) were measured. (B and C) Bar graphs show the relative ratio of act-α5 (B) and α5 (C) to Flot2 in A. Data are mean ± SEM from at least three independent experiments normalized to the static control. *P < 0.05, vs. static control.
OS-Induced EC Dysfunction Is Integrin α5-Dependent.
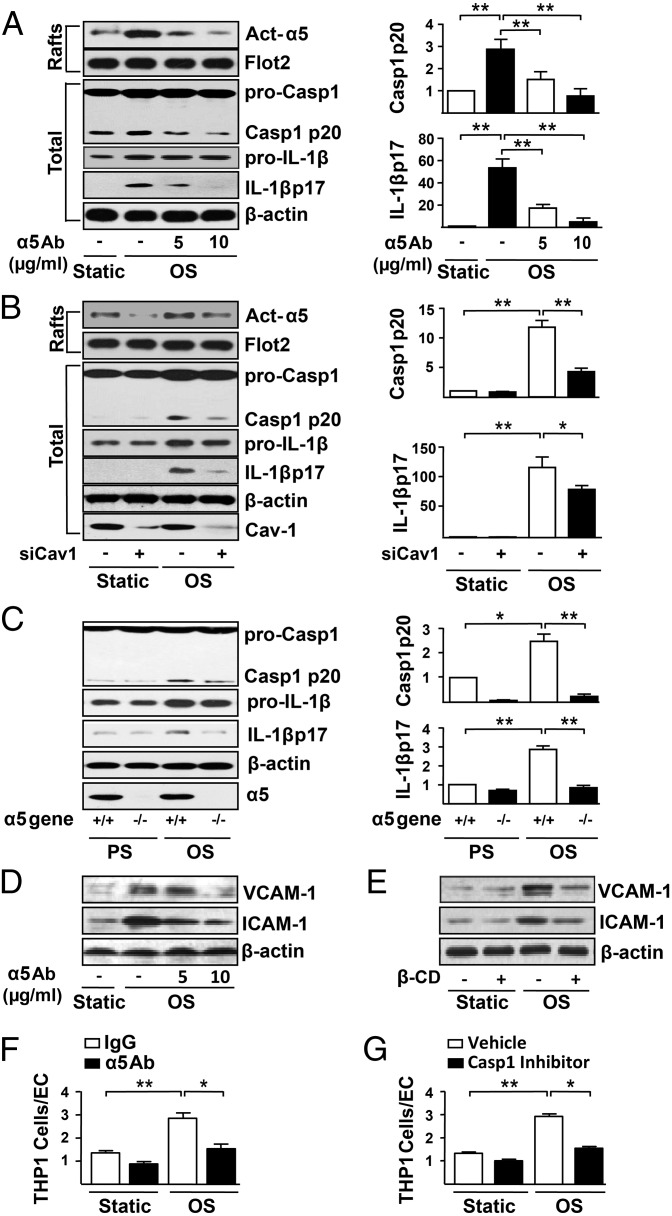
Integrin α5 activates the NLRP3 inflammasome in macrophages (23), and OS has been shown to induce NLRP3 inflammasome in ECs (20). Because OxLDL-induced NF-κB is α5-dependent (16) and NF-κB activation primes the induction of NLRP3 inflammasome (19), we reasoned that α5 activation is necessary for OS-induced inflammasome activation. Indeed, the OS-induced cleavage of procaspase1 and pro-IL-1β, hallmarks of NLRP3 inflammasome induction, was reduced by α5 neutralizing antibody in a dose-dependent manner (Fig. 4A). A similar inhibitory effect was seen in ECs in which Cav1 was knocked down (Fig. 4B). Moreover, there was a decrease in OS activation of NLRP3 inflammasome in mouse embryonic fibroblasts (MEFs) isolated from α5-null embryos (Fig. 4C). Downstream of the OS-induced NLRP3 inflammasome is the enhanced expression of the chemoattractant and adhesion molecules such as VCAM-1 and ICAM-1. The OS-augmented VCAM-1 and ICAM-1 expression was attenuated by either α5 neutralizing antibody or β-CD treatment (Fig. 4 D and E). Additionally, the enhanced binding of monocytes to EC under OS was blocked by both the α5 neutralizing antibody (Fig. 4F) and Z-YVAD-FMK, a caspase-1 inhibitor (Fig. 4G). Thus, OS activation of integrin α5 is functionally linked to endothelial dysfunction.
Fig. 4.
OS-activated integrin α5 leads to endothelial dysfunction. ECs were pretreated with integrin α5 neutralizing antibody (5 μg/mL or 10 μg/mL) for 1 h (A, D, and F), scramble siRNA or Cav1 siRNA (40 nmol/L) for 48 h (B), β-CD (5 mmol/L) for 2 h (E), or caspase-1 inhibitor Z-YVAD-FMK (2 μmol/L) for 24 h (G); α5−/− MEFs or littermate wild-type MEFs (α5+/+) were used in C. All cell groups were kept under static condition or exposed to PS or OS for 12 h. In A−E, Western blots were performed for activated integrin α5 (Act-α5), procaspase1 (pro-Casp1), caspase1 p20 (Casp1 p20), pro-IL-1β, IL-1β p17, ICAM-1, and VCAM-1. Lipid raft fractions (Rafts) were used to detect Act-α5, whereas total cell lysates were used for all other proteins. Bar graphs showed the relative ratio of casp1 p20 and IL-1β p17 to β-actin. Data are mean ± SEM normalized to the static control from at least three independent experiments. ANOVA followed by the Bonferroni post hoc test was used. *P < 0.05, vs. static or PS. (F and G). The BCECF-AM-labeled THP1 cells were added to EC monolayer and incubated for 30 min. Fluorescence microscopy was used to assess the attached THP1 cells. The ratio of adherent THP1 cells to ECs was measured. ANOVA followed by the Bonferroni post hoc test was used for statistical analysis. Data are mean ± SEM averaged from at least three independent experiments. *P < 0.05, vs. static controls.
EC Dysfunction in Atheroprone Regions in Vivo Is Integrin α5-Dependent.
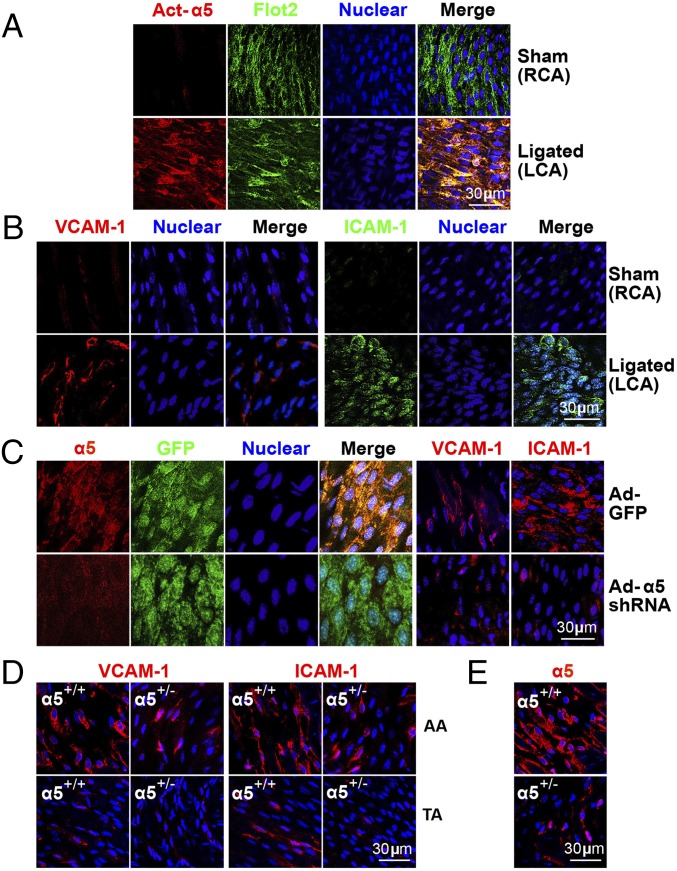
To demonstrate the relevance of OS-activated integrin α5 in proinflammatory response in ECs in vivo, we used Ldlr−/− mice subjected to partial ligation of the carotid artery, which induces an acute disturbed flow pattern (24). Compared with the sham-ligated contralateral artery in the same animal, inflammation was aggravated in the partially ligated artery, as evidenced by the activation of integrin α5 (Fig. 5A) and the upregulation of VCAM-1 and ICAM-1 (Fig. 5B). We used adenovirus (Ad)-mediated integrin α5 shRNA to infect partially ligated carotid arteries in Ldlr−/− mice and confirmed the knockdown of integrin α5 (Fig. 5C, first four panels). Compared with control experiments (using Ad-GFP), Ad-integrin α5 shRNA decreased the OS-induced expressions of VCAM-1 and ICAM-1 (Fig. 5C, fifth and sixth panels). Because homozygous deletion of α5 is embryonic lethal, we created α5+/− mice and their α5+/+ wild-type littermates to further validate the deleterious role of integrin α5 in inducing EC dysfunction in relation to atherosclerosis. The reduced expression of integrin α5 in α5+/− mice was verified by en face immunostaining (Fig. 5E). Haploinsufficiency of integrin α5 markedly blocked the OS-induced expression of VCAM-1 and ICAM-1 in the inner curvature of AA (atheroprone area) of integrin α5+/− mice, compared with the corresponding area in α5+/+ littermates (Fig. 5D).
Fig. 5.
EC dysfunction in atheroprone region of mouse aorta is integrin α5-dependent. (A and B) Male Ldlr−/− mice (8 wk-old, n = 10) that had undergone partial ligation of the carotid artery were fed a WD for 1 wk. The left (partially ligated) and right (sham-operated) carotid arteries (LCA and RCA, respectively) were isolated for en face immunostaining. Whereas A shows representative images of Act-α5 (red), Flot2 (green), and nuclear (blue) from RCA and LCA, images in B are VCAM-1 (red), ICAM-1 (green), and nuclear (blue). In C, the experimental conditions and number of animals were the same as those in A and B except that carotid arteries were infused with Ad-α5 shRNA or Ad-GFP during vessel ligation. The first four panels show en face immunostaining of integrin α5 (α5, red), GFP (green), nuclear (blue), and merged images, and fifth and sixth panels show VCAM-1 (red, fifth panel) and ICAM-1 (red, sixth panel). (D) En face immunostaining of VCAM-1 (red), ICAM-1 (red), α5 (red), and nuclear (blue) in AA and TA from integrin α5+/− mice and the wild-type littermates (α5+/+) (8 wk-old, n = 10 in each group). (E) The expressions of integrin α5 in α5+/− mice and the wild-type littermates were verified by en face immunostaining.
Atherogenesis in Ldlr−/− Mice Is Integrin α5-Dependent.
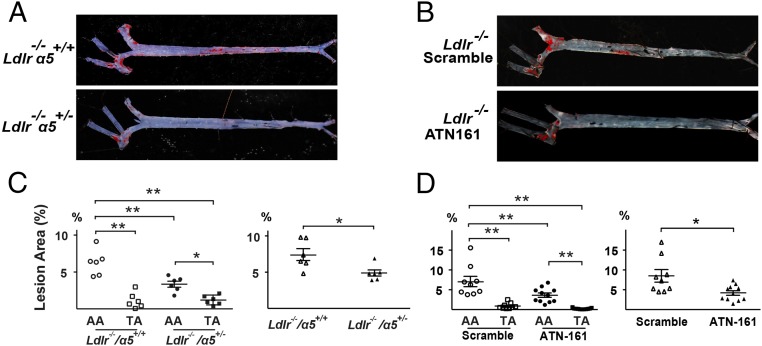
We used two different animal models to demonstrate that the OS-regulated α5 is relevant to atherogenesis in vivo. First, 8 wk-old Ldlr−/− and Ldlr−/−/α5+/− mice were fed a western diet (WD) for 4 wk. The extent of atherosclerosis in the aorta was determined accordingly. There was a ∼50% decrease in total lesion area as well as that in the AA in the Ldlr−/−/α5+/− mice (Fig. 6 A and C). In a second model, Ldlr−/− mice were fed with WD and injected with ATN-161, which is an inhibitory peptide of integrin α5. Compared with Ldlr−/− mice injected with a scrambled peptide, inhibition of integrin α5 by ATN-161 significantly decreased the total atherosclerosis area. The lesion areas in both AA and TA were decreased by ATN-161 treatment (Fig. 6 B and D). These data demonstrate the importance of integrin α5 in atherogenesis in the Ldlr−/− mice, similar to that in Apolipoprotein E-deficient (Apoe−/−) mice, reported by Yurdagul et al. (16).
Fig. 6.
Atherosclerosis in atheroprone region of mouse aorta is integrin α5-dependent. (A) The 8-wk-old Ldlr−/− α5+/+ and Ldlr−/− α5+/− mice were fed a WD for 4 wk. (B) Ldlr−/− mice received i.p. injection of ATN-161 or scramble peptide 1 wk before first receiving WD, which was given every third day until the termination of the experiment. (A and B) Representative of Oil Red O staining of aortas from the various groups of animals. (C and D) Quantification of percent lesion areas in the AA, TA, and total aorta from groups A and B. ANOVA followed by the Bonferroni post hoc test was used for statistical analysis. Data are mean ± SEM normalized to control. *P < 0.05, vs. control.
Discussion
The present study has revealed a mechanotransduction mechanism by which atheroprone flow causes endothelial dysfunction through integrin α5 translocation into lipid rafts and subsequent activation. Consequently, NLRP3 inflammasome is induced by the activated integrin α5. The results of our proteomics analysis of proteins suggested that OS leads to α5 translocation into lipid rafts. In ensuing studies, we confirmed that OS increased integrin α5 translocation into lipid rafts, and that this was a prerequisite for integrin α5 activation. In contrast, PS caused integrin α5 to reside largely outside lipid rafts, and therefore the activity of α5 showed little changes. We also demonstrated a link between integrin α5 activation and NLRP3 inflammasome induction in ECs under OS. Thus, the novelties of our study are twofold. First, OS activates integrin α5 exclusively in the lipid raft. Second, integrin α5 activation in turn mediates NLRP3 inflammasome induction by OS. Thus, the OS-induced activation of integrin α5, in the context of atheroprone flow and hypercholesterolemia, appears to be an essential step in the atherogenic process.
Shear stress may modulate EC functions in a rapid manner through its action on the plasma membrane. We have previously reported that shear stress changes membrane fluidity temporally and spatially (25). Specifically, OS increases the cholesterol content in EC membranes, which results in decreased membrane fluidity (21). Such alterations of biophysical properties would affect the structure and function of membrane-associated proteins, especially those found in lipid rafts, such as integrin α5. Significantly, OS, but not PS, induced the translocation of integrin α5 into lipid rafts (Fig. 1). Because OS increases the cholesterol content of EC membranes, the cholesterol-enriched lipid rafts may thus sequester integrin α5 to allow for its sustained activation. This notion is supported by our previous observation that Cav1 and the F-actin-based cytoskeleton are involved in protein translocation into lipid rafts (21, 22). Indeed, results in Fig. 2 and Fig. S2 show that Cav1 and F-actin-based cytoskeleton are required for OS-induced α5 translocation.
Atheroprone flow activates NF-κB and increases the expression of chemoattractants (e.g., monocyte chemotactic protein-1) and adhesion molecules (e.g., ICAM-1, VCAM-1) in vitro and in vivo (26–30). Recent studies suggest that atheroprone flow regulates gene expression at the genome level via epigenetic regulation, in particular, DNA methylation (31–33). At the signaling level, atheroprone flow can induce NLRP3 inflammasome in ECs, which contributes to atherosclerosis susceptibility (20). The results in Fig. 4 show that functional blocking or genetic ablation of α5 inhibited the OS-induced NLRP3 inflammasome. Because inflammasome induction leads to the increase in the level of IL-1β and IL-18, the OS-induced inflammation in ECs is at least in part mediated by integrin α5 activation of NLRP3 inflammasome. The mechanosensitive integrin α5-inflammation axis in vivo was validated by the loss-of-function experiments with the use of integrin α5 shRNA, and haploinsufficiency of integrin α5 in mice. All these experiments showed decreased EC inflammation under atheroprone flow (Fig. 5 C and D). Importantly, we demonstrated that the in vivo relevance of integrin α5 activation to the atherogenic process using two different murine models that have either haploinsufficiency of α5 or inhibition of α5 activation by an inhibitory peptide led to marked protection against atherosclerosis even in the presence of sustained hypercholesterolemia. In our studies, we used the WD-fed Ldlr−/− model to test the role of integrin α5 in atherogenesis. The study by Yurdagul et al. (16) also demonstrated the important role of integrin α5 activation for the atherogenic process using the Apoe−/− model; they found that α5 activation could also occur via a different mechanism that involved OxLDL (16). The fact that integrin α5 was demonstrated to play an obligatory role in atherogenesis in two different murine models (i.e., Ldlr−/− and Apoe−/−), and can be activated by two different mechanisms (i.e., atheroprone flow and OxLDL), adds to the confidence that this pathway is highly relevant in vivo.
It is intriguing that both OS and OxLDL can activate integrin α5 in vitro. Given the reduced atherosclerosis found in the atheroprone areas in the Ldlr−/−/α5+/− mice, we propose that OS and OxLDL could share similar mechanism in activating integrin α5. Integrin α5 activation would occur at regions of profound atheroprone flow, where atherosclerosis is most prevalent. Seemingly, OxLDL is not present in the circulation, but it is believed that OxLDL is generated in the intima at sites of lesion formation. How this in turn would lead to EC α5 activation is less clear. Whatever the mechanism is, one could envision the regulation of α5 activation by both mechanical and biochemical actions to play a key role in EC dysfunction and atherosclerosis. This thesis is in line with that proposed by Davies and colleagues that the synergism between flow characteristics and hypercholesterolemia deserves “further evaluation of the epigenetic and epigenomic regulation of endothelial phenotype adaptation during early pathological change” (31, 34).
In summary, our study reveals a novel mechanism by which atheroprone flow increases endothelial inflammation that involves an integrated response of lipid rafts, F-actin-based cytoskeleton, and integrin α5. Our results provide a novel experimental basis for the use of integrin α5-specific antagonists for preventing dysfunctional endothelium and alleviating atherosclerosis.
Materials and Methods
All animal protocols were approved by Peking University Health Science Center Animal Care and Use Committee. The sources of antibodies and reagents, and detailed methods for cell culture, shear stress, purification of membrane and lipid raft protein, fluorescence anisotropy measurements, iTRAQ labeling and proteomics analysis, en face immunostaining, RNA interference, monocyte adhesion, fibronectin adhesion, carotid partial ligation, generation of α5+/− mice, adenovirus construction, lesion area assessment, and statistical analysis are described in SI Materials and Methods.
SI Materials and Methods
Antibodies and Reagents.
Anti-activated-integrin α5, anti-Cav1, and anti-cavin1 antibodies were from Abcam; anti-total-integrin α5 and horseradish peroxide-conjugated anti-rabbit and anti-mouse antibodies were from Cell Signaling Technology; anti-Flot2, anti-β-actin, anti-VCAM-1, and anti-integrin α5 neutralizing antibodies were from Santa Cruz Biotechnology; anti-ICAM-1 antibody was from BD Biosciences Pharmingen; anti-rabbit and anti-mouse dylight 488-conjugated and anti-rabbit and anti-mouse cy3-conjugated secondary antibodies were from EarthOx; and β-CD, Cyt-B, fibronectin, cholesterol-methyl-β-cyclodextrin (Chl), and DPH were from Sigma. The integrin α5 inhibitor ATN161 (Ac-PHSCN-NH2) and scrambled peptide (Ac-HSPNC-NH2) were synthesized with 98% peptide purity by ChinaPeptides Co., Ltd. The cholesterol determination kit was from BIOSINO Inc.
Cell Culture and Shear Stress Experiments.
HUVECs were isolated and cultured as described (35). All HUVECs were used before passage 5. For flow experiments, confluent monolayers of HUVECs were seeded on glass slides, and a parallel plate flow system was used to impose OS (0.5 ± 4 dyn/cm2) or PS (12 ± 4 dyn/cm2). The flow system was enclosed in a chamber held at 37 °C and ventilated with 95% humidified air plus 5% CO2.
Purification of Membrane and Lipid Raft Protein.
Membrane proteins were isolated from ECs by a multiple-centrifugation procedure (36). Lipid raft fractions were purified from ECs by a modified sucrose density gradient ultracentrifugation procedure (37).
Fluorescence Anisotropy Measurements.
Fluorescence anisotropy was determined by the use of the fluorescent probe DPH as reported (38). Membranes were suspended at a final concentration of 10 mg/mL and labeled with a 1 μmol/L DPH at 25 °C for 20 min. Steady-state fluorescent was measured in a Flex station 3 spectrofluorometer. Excitation and emission wavelengths were set at 350 nm and 452 nm. Anisotropy(r) values were calculated from the equation
where Iv and Ih are the intensities when the excitation and emission polarizers are parallel and perpendicular to each other.
iTRAQ Labeling and Proteomics Analysis.
The labeling of lipid raft proteins with isobaric tags and nano-LC/MS/MS was performed as described (39). The iTRAQ experiments were repeated twice, and data were analyzed by a paired t test based on the fold changes of all peptide fragments resulting from a specific protein. An increase by a minimum of 1.5-fold (40) was considered as upregulation, whereas those less than 0.7-fold (41) were considered down-regulation. Proteins inversely upregulated or down-regulated by PS and OS, with P ≤ 0.05 under both flow conditions, were considered to be significant.
En Face Immunostaining.
Mouse aortas were fixed with 4% paraformaldehyde for 20 min, and then stained with anti-activated integrin α5, anti-integrin α5, anti-Flot2, anti-VCAM-1, and anti-ICAM-1 antibodies overnight. The fluorescence signal was visualized by a Leica confocal laser scanning microscopy.
RNA Interference.
Subconfluent HUVECs were transfected with siRNA for Cav1 or control siRNA at 40 nmol/L with use of Lipofectamine RNAi Max (Invitrogen) for 48 h. All siRNA molecules were purchased from Invitrogen. Sequences corresponding to the siRNAs were as follows: Cav1 sense, 5′-CCCUAAACACCUCAACGAU-3′, and antisense, 5′-AUCGUUGAGGUGUUUAGGG-3′.
Monocyte Adhesion.
ECs were cultured for 24 h on coverslips in six-well plates until confluent, and then pretreated with shear stress or kept as static control. THP1 cells were labeled with BCECF-AM (Invitrogen) and then plated onto the EC plates at 2 × 106 cells/well. After incubation for 30 min at 37 °C, nonadherent cells were removed by washing three times with PBS. The numbers of stained adhering cells in five random fields were counted for each group under a fluorescence microscope.
Fibronectin Adhesion.
Plates with 24 wells were coated with fibronectin (5 μg/mL in PBS) overnight at 4 °C, blocked with 1% BSA for 1 h at 37 °C, and washed three times with PBS. ECs were plated at 1 × 105 cells/well for 20 min at 37 °C, and then nonadherent cells were removed by washing three times with PBS. The remaining cells were stained with a solution containing 0.5% crystal violet. To quantify the number of adhesion cells, absorbance of the remaining cells was measured by use of Thermo Multiskan Ascent at 595 nm.
Animal Experiments and Carotid Artery Partial Ligation Model.
The 8-wk-old Ldlr−/− male mice were obtained from Peking University Health Science Center. The mice were fed a WD (Research Diets, D12109) containing 40 kcal% fat, 1.25% cholesterol, and 0.5% cholic acid or a chow diet for 1 wk before determining the indicated protein levels in the aortic intima by en face immunostaining. In some experiments, partial ligation of the left carotid artery was performed as described (24). ATN-161 and scramble peptide injections were conducted as reported (42).
Generation of Integrin α5+/− Knockout Mice.
The integrin α5 knockout vector pronuclear injection was performed on fertilized C57BL/6J mouse oocytes. To determine the mutations generated in individual founders, we analyzed the sequence of integrin α5 by PCR analysis using the following primers: 5′-CAAAGGTCTGGGTGCATCTT-3′ and 5′-TTCCAGCCTACCTTTGCTGT-3′. PCR products were TA cloned and sequenced. The size of the deletion on a single chain was 11 bp in the exon 2. En face immunostaining were performed to confirm integrin α5 expression in the integrin α5 knockout mice. Heterozygous knockout mice and their wild-type littermates were used for all experiments (n = 10, each group).
Adenovirus Construction and Partial Ligation-Based Intima Infection.
The recombinant adenovirus expressing mouse integrin α5 shRNA was obtained commercially from Yingrun Biotechnologies Inc. Partial ligation of the LCA was carried out as previously described (24) with left external carotid artery left intact, through which 5 × 108 plaque-forming units of adenovirus was introduced into the lumen of LCA (about a 1.5-cm segment) and instilled for 40 min. Following infection, the adenovirus was released, and blood flow to the common carotid artery was restored. Ad-GFP was used as an infection control. Sham surgery was conducted on the right carotid artery as flow pattern control. Some of the infected arteries were then detected for infection efficiency; other mice (n = 10, each group) were kept for en face immunostaining for ICAM-1 and VCAM-1 until 7 d of WD.
Assessment of Atherosclerosis.
The 8-wk-old Ldlr−/− and Ldlr−/− α5+/− mice and their littermates were fed a WD diet for 4 wk. Aortas were isolated to assess lesion formation and distribution by Oil Red O staining.
Statistical Analysis.
Data are expressed as mean ± SEM from at least three independent experiments. Two groups were compared by the Student t test. Differences among multiple groups were evaluated by ANOVA followed by the Bonferroni post hoc test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors thank Dr. Joseph Witztum for his valuable comments. This work was supported by the National Natural Science Foundation of China (81130002 and 30821001 to Y.Z.; 81270349 to J.Y.-J.S.), the Major National Basic Research Grant of China (2010CB517504 to Y.Z.), and National Institutes of Health Grant HL108735 (to J.Y.-J.S. and S.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524523113/-/DCSupplemental.
References
- 1.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6(1):16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20(17):4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goncalves I, Nesbitt WS, Yuan Y, Jackson SP. Importance of temporal flow gradients and integrin αIIbβ3 mechanotransduction for shear activation of platelets. J Biol Chem. 2005;280(15):15430–15437. doi: 10.1074/jbc.M410235200. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Mohan S, Baylink DJ, Lau KH. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J Biol Chem. 2005;280(20):20163–20170. doi: 10.1074/jbc.M501460200. [DOI] [PubMed] [Google Scholar]
- 6.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17(11):4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radel C, Carlile-Klusacek M, Rizzo V. Participation of caveolae in β1 integrin-mediated mechanotransduction. Biochem Biophys Res Commun. 2007;358(2):626–631. doi: 10.1016/j.bbrc.2007.04.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, et al. Interplay between integrins and FLK-1 in shear stress-induced signaling. Am J Physiol Cell Physiol. 2002;283(5):C1540–C1547. doi: 10.1152/ajpcell.00222.2002. [DOI] [PubMed] [Google Scholar]
- 10.Brown WS, Khalili JS, Rodriguez-Cruz TG, Lizee G, McIntyre BW. B-Raf regulation of integrin α4β1-mediated resistance to shear stress through changes in cell spreading and cytoskeletal association in T cells. J Biol Chem. 2014;289(33):23141–23153. doi: 10.1074/jbc.M114.562918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jalali S, et al. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA. 2001;98(3):1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110(5):597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai WJ, et al. Activation of the integrins αβ51 and ανβ3 and focal adhesion kinase (FAK) during arteriogenesis. Mol Cell Biochem. 2009;322(1-2):161–169. doi: 10.1007/s11010-008-9953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk SD, et al. Matrix-specific protein kinase A signaling regulates p21-activated kinase activation by flow in endothelial cells. Circ Res. 2010;106(8):1394–1403. doi: 10.1161/CIRCRESAHA.109.210286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell JS, Brown WS, Woodside DG, Vanderslice P, McIntyre BW. Clustering T-cell GM1 lipid rafts increases cellular resistance to shear on fibronectin through changes in integrin affinity and cytoskeletal dynamics. Immunol Cell Biol. 2009;87(4):324–336. doi: 10.1038/icb.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yurdagul A, Jr, et al. α5β1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(7):1362–1373. doi: 10.1161/ATVBAHA.114.303863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorin E, Hamilton C, Dominiczak AF, Dominiczak MH, Reid JL. Oxidized-LDL induced changes in membrane physico-chemical properties and [Ca2+]i of bovine aortic endothelial cells. Influence of vitamin E. Atherosclerosis. 1995;114(2):185–195. doi: 10.1016/0021-9150(94)05482-x. [DOI] [PubMed] [Google Scholar]
- 18.Lénárt N, et al. Cultured cells of the blood-brain barrier from apolipoprotein B-100 transgenic mice: Effects of oxidized low-density lipoprotein treatment. Fluids Barriers CNS. 2015;12(1):17. doi: 10.1186/s12987-015-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13(4):325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao H, et al. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128(6):632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu Y, et al. A novel mechanism of γ/δ T-lymphocyte and endothelial activation by shear stress: The role of ecto-ATP synthase β chain. Circ Res. 2011;108(4):410–417. doi: 10.1161/CIRCRESAHA.110.230151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Chen Z, Wang X, Shyy JY, Zhu Y. Cholesterol loading increases the translocation of ATP synthase beta chain into membrane caveolae in vascular endothelial cells. Biochim Biophys Acta. 2006;1761(10):1182–1190. doi: 10.1016/j.bbalip.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Jun HK, Lee SH, Lee HR, Choi BK. Integrin α5β1 activates the NLRP3 inflammasome by direct interaction with a bacterial surface protein. Immunity. 2012;36(5):755–768. doi: 10.1016/j.immuni.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Nam D, et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297(4):H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol. 2001;280(4):C962–C969. doi: 10.1152/ajpcell.2001.280.4.C962. [DOI] [PubMed] [Google Scholar]
- 26.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99(2):315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng C, et al. Shear stress affects the intracellular distribution of eNOS: Direct demonstration by a novel in vivo technique. Blood. 2005;106(12):3691–3698. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 28.Hajra L, et al. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97(16):9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagel T, Resnick N, Dewey CF, Jr, Gimbrone MA., Jr Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler Thromb Vasc Biol. 1999;19(8):1825–1834. doi: 10.1161/01.atv.19.8.1825. [DOI] [PubMed] [Google Scholar]
- 30.Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995;15(1):2–10. doi: 10.1161/01.atv.15.1.2. [DOI] [PubMed] [Google Scholar]
- 31.Jiang YZ, et al. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circ Res. 2014;115(1):32–43. doi: 10.1161/CIRCRESAHA.115.303883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn J, et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 2014;124(7):3187–3199. doi: 10.1172/JCI74792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Li YS, Wang KC, Chien S. Epigenetic mechanism in regulation of endothelial function by disturbed flow: Induction of DNA hypermethylation by DNMT1. Cell Mol Bioeng. 2014;7(2):218–224. doi: 10.1007/s12195-014-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang YZ, Manduchi E, Jiménez JM, Davies PF. Endothelial epigenetics in biomechanical stress: Disturbed flow-mediated epigenomic plasticity in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2015;35(6):1317–1326. doi: 10.1161/ATVBAHA.115.303427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, et al. LDL induces transcription factor activator protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18(3):473–480. doi: 10.1161/01.atv.18.3.473. [DOI] [PubMed] [Google Scholar]
- 36.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273(37):24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, et al. Lipoprotein promotes caveolin-1 and Ras translocation to caveolae: Role of cholesterol in endothelial signaling. Arterioscler Thromb Vasc Biol. 2000;20(11):2465–2470. doi: 10.1161/01.atv.20.11.2465. [DOI] [PubMed] [Google Scholar]
- 38.Wojciak-Stothard B, et al. Aberrant chloride intracellular channel 4 expression contributes to endothelial dysfunction in pulmonary arterial hypertension. Circulation. 2014;129(17):1770–1780. doi: 10.1161/CIRCULATIONAHA.113.006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu MX, et al. Proteomic analysis of endothelial lipid rafts reveals a novel role of statins in antioxidation. J Proteome Res. 2012;11(4):2365–2373. doi: 10.1021/pr300098f. [DOI] [PubMed] [Google Scholar]
- 40.Grønborg M, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5(1):157–171. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 41.Boukli NM, et al. Unique and differential protein signatures within the mononuclear cells of HIV-1 and HCV mono-infected and co-infected patients. Clin Proteomics. 2012;9(1):11. doi: 10.1186/1559-0275-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoeltzing O, et al. Inhibition of integrin αβ51 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer. 2003;104(4):496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.