Metastatic castration-resistant prostate cancer (mCRPC) is currently a lethal disease. Although several treatment approaches, including second-generation antiandrogens or chemotherapies, have been demonstrated to improve outcomes in patients with mCRPC, each therapy increases median survival by only several months (1, 2). Thus, there is a critical need to develop novel strategies for treating this disease. Protein kinases represent excellent therapeutic targets from both a biological and drug development perspective: The vast majority of signal transduction processes depend on a phosphotransfer cascade, and potent and selective small-molecule kinase inhibitors can be developed with favorable pharmaceutical properties (3). However, to date, there have been no systematic gain-of-function studies exploring kinases that promote metastases in prostate cancer. Coincidentally, there are also no US Food and Drug Administration-approved kinase inhibitors for the treatment of this disease. In PNAS, Faltermeier et al. (4) investigated the landscape of kinases in prostate cancer and performed functional screens to identify candidate kinases that drive metastatic progression in preclinical models of disease.
Although there are over 500 kinases comprising the human kinome, Faltermeier et al. (4) identify 125 kinases of potential relevance in metastatic prostate cancer, from a combination of analyses of phosphoproteomic and genomic/transcriptomic datasets coupled with a literature search. Using an in vivo lung colonization screen based upon overexpression of candidate kinases in prostate tumor-derived, PTEN-null murine Cap8 cells, the authors investigated the ability of their candidate kinases, screening five at a time, to promote metastases in a mouse model. Having identified 20 kinases that promoted metastases in this initial screen, Faltermeier et al. (4) then conducted a second screen in which they overexpressed these 20 kinases in RWPE-1 immortalized normal prostate epithelial cells, and then performed tail vein injections in NOS scid gamma mice. This second screen demonstrated that five kinases, all three rapidly accelerated fibrosarcoma (RAF) family members (ARAF, BRAF, and CRAF), as well as C-Mer proto-oncogene tyrosine kinase (MERTK) and neurotrophic tyrosine kinase receptor, type 2 (NTRK2), drove bone and visceral metastases, as assessed with PET/computed tomography imaging and histology. The authors also confirmed that these five kinases were highly expressed in mCRPC via immunohistochemical evaluation, further supporting the functional relevance of these kinases in this context.
Kinases represent one of the most intensely studied classes of drug targets, and strategies based on kinase inhibition are being explored in numerous disease sites (3). The clinical success achieved with select kinase inhibitors is striking. Dramatic responses are common in the context of targeting kinases that are constitutively activated through gene fusions, such as break point cluster-abelson tyrosine kinase (BCR-ABL) fusions in chronic myelogenous leukemia and EML4-ALK fusions in non–small-cell lung cancer (NSCLC) (5, 6). Clinical gains have also been attained via targeting of kinases modified via activating mutations, such as BRAFV600E in melanoma and EGF receptor mutations in NSCLC (7, 8). However, as noted by several groups, the frequency of genomic alterations resulting in constitutive activation of kinases is rare in prostate cancer (9, 10). In this context, the findings by Faltermeier et al. (4) reinforce previous studies suggesting that overexpression of nonmutated kinases may drive metastatic events in prostate cancer, and that kinase inhibitors should be pursued in the treatment of prostate cancer despite its relatively quiescent genomic landscape of activating kinase alterations.
Interestingly, the systematic analysis by Faltermeier et al. (4) identifies the RAF family of kinases as drivers of metastasis. RAF kinases have previously been implicated as drivers of prostate cancer, through gene fusions involving RAF family members that activate the RAF-MEK-ERK pathway (11). However, these fusions occur in less than 1% of patients with mCRPC (9, 10). The RAS/RAF pathway has been shown to be potentially activated by other mechanisms, such as copy number alterations and transcriptional up-regulation, in a large majority of prostate cancer metastases (12). The kinase inhibitor sorafenib, which targets BRAF and CRAF in addition to the vascular endothelial growth factor (VEGF) receptors, mast/stem cell growth factor receptor (c-Kit), and other kinases, has been assessed in phase II clinical trials for patients with mCRPC with mixed results, with minimal to moderate responses in the context of monotherapy or in combination with other systemic therapies (13, 14). Given the subtle, yet important, differences in the activity of ARAF, BRAF and CRAF, a more detailed understanding of RAF kinases in prostate cancer may be required to define the mechanism by which RAF family members promote metastases and to define the role of RAF inhibitors in prostate cancer. As pointed out by Faltermeier et al. (4), given that RAF inhibitors may paradoxically activate WT BRAF in certain contexts (15), inhibition of downstream RAF effectors, such as mitogen-activated protein kinase kinase 1 (MEK), may ultimately represent a more efficacious strategy.
Of note, further studies are required to validate the role of the nominated kinases fully in prostate cancer progression. Ultimately, the translational significance of any preclinical study is dependent upon the used model systems. The strength, and novelty, of the study from Faltermeier et al. (4) is the use of a gain-of-function overexpression system to investigate the functional role of kinases in metastases systematically. However, although tail vein injections allow one to assess tumor cell extravasation and growth at a secondary site, this approach does not allow for evaluation of the impact of kinases on earlier steps necessary for metastases, such as local tumor invasion and intravasation. Additionally, the primary screen focused on lung metastases, which are relatively uncommon, compared with bone metastases, in prostate cancer. Thus, the identification of the RAF family, MERTK, and NTRK2 as mediators of mCRPC represents just the tip of the iceberg, and additional studies using complementary model systems will be necessary to define the metastases-promoting kinome more comprehensively.
A final critical point to reemphasize is that at this point in time, conventional treatment for mCRPC does not include any kinase inhibitors, because no kinase inhibitors have yielded improvements in overall survival in a large phase III randomized trial. Although numerous kinase inhibitors, such as sorafenib, sunitinib, gefitinib, erlotinib, lapatinib, imatinib, cabozantinib, and dasatinib, among others, have been assessed in early phase I/II clinical trials for patients with metastatic or aggressive prostate cancer, the majority have not demonstrated sufficient activity to be advanced to phase III trials (reviewed in 16). Of these inhibitors, cabozantinib (which targets MET, VEGF receptor 2, and RET) and dasatinib (which targets Src family kinases, BCR-ABL, c-KIT, and other kinases) progressed furthest in clinical development. Unfortunately, despite extremely promising results from phase II trials, neither cabozantinib nor dasatinib significantly improved overall
Faltermeier et al. investigated the landscape of kinases in prostate cancer and performed functional screens to identify candidate kinases that drive metastatic progression in preclinical models of disease.
survival compared with the standard-of-care therapies on phase III trials (17, 18).
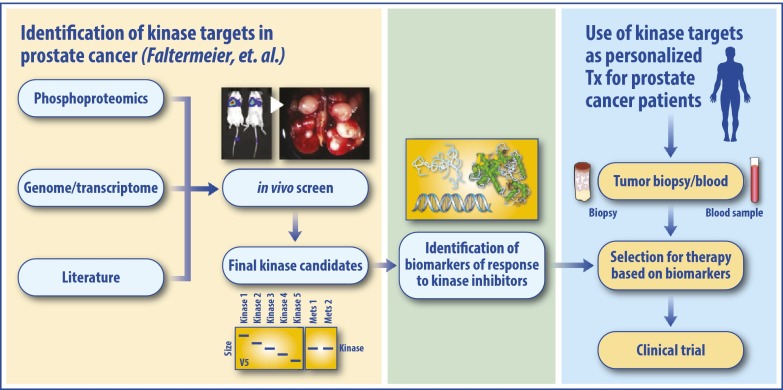
These findings underscore an important lesson: In addition to identifying kinases that drive mCRPC, the field needs to define biomarkers of response to determine which patients are most likely to respond to select kinase inhibitors in the appropriate clinical context (Fig. 1). It is unlikely that any particular kinase inhibitor will be effective in the majority of patients and improve survival in an unselected population of mCRPC patients. Although the RAF family, MERTK, and NTRK2 were nominated using in vivo tail injection metastases screens using Cap8 and RWPE-1 cells, these particular kinases may not consistently drive metastases across all prostate cancer model systems, and across all human mCRPC cases. To identify subpopulations of patients most likely to respond to a particular therapy, tissue and blood samples should be interrogated for molecular determinants of treatment response or resistance. Potentially, an integrated proteomic and/or transcriptomic analysis of patient samples may eventually allow for more judicious use of existing kinase inhibitors while concurrently uncovering unexplored target kinases for drug development. Thus, although Faltermeier et al. (4) should be commended for their innovative study, their findings represent an initial step in exploring the metastases-promoting kinome in prostate cancer, and one that needs to be investigated further.
Fig. 1.
Kinase inhibitors as a therapeutic strategy for mCRPC. Using preclinical models of prostate cancer, kinases that potentially drive metastatic progression may be identified through in vivo screening assays for metastases, as described by Faltermeier et al. (4). Upon identification of biomarkers of response, patients could be selected for treatment with a specific kinase inhibitor to optimize the potential for disease response.
Despite these challenges, the identification and therapeutic targeting of kinases that drive metastases hold great promise in prostate cancer. Given the success of kinase inhibitors across multiple other cancer types, it is likely that this drug class will eventually improve outcomes in prostate cancer. However, similar to other disease sites, where the utilization of kinase inhibitors is usually guided by molecular biomarkers, it will be paramount to develop companion biomarker assays to personalize therapy.
Acknowledgments
F.Y.F. is supported by the A. Alfred Taubman Medical Research Institute, the Prostate Cancer Foundation, the Evans Foundation, and the University of Michigan Prostate Cancer Specialized Programs of Research Excellence (S.P.O.R.E.) (Grant P50 CA186786). V.K. is supported by the Prostate Cancer Foundation.
Footnotes
Conflict of interest statement: F.Y.F. has previously served on advisory boards for Celgene and Medivation/Astellas, and has received a research grant from Celgene for investigation of the use of DNA-dependent protein kinase (DNAPK) inhibitors in prostate cancer.
See companion article on page E172 in issue 2 of volume 113.
References
- 1.Beer TM, et al. PREVAIL Investigators Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bono JS, et al. TROPIC Investigators Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 4.Faltermeier CM, et al. Functional screen identifies kinases driving prostate cancer visceral and bone metastasis. Proc Natl Acad Sci USA. 2015;113:E172–E181. doi: 10.1073/pnas.1521674112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Druker BJ, et al. IRIS Investigators Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17(8):2081–2086. doi: 10.1158/1078-0432.CCR-10-1591. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty KT, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 9.Grasso CS, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson D, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palanisamy N, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16(7):793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aragon-Ching JB, et al. Final analysis of a phase II trial using sorafenib for metastatic castration-resistant prostate cancer. BJU Int. 2009;103(12):1636–1640. doi: 10.1111/j.1464-410X.2008.08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beardsley EK, et al. A phase II study of sorafenib in combination with bicalutamide in patients with chemotherapy-naive castration resistant prostate cancer. Invest New Drugs. 2012;30(4):1652–1659. doi: 10.1007/s10637-011-9722-5. [DOI] [PubMed] [Google Scholar]
- 15.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limvorasak S, Posadas EM. Kinase inhibitors in prostate cancer. Anticancer Agents Med Chem. 2009;9(10):1089–1104. doi: 10.2174/187152009789735080. [DOI] [PubMed] [Google Scholar]
- 17.Araujo JC, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): A randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14(13):1307–1316. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MR, et al. Final analysis of COMET-1: Cabozantinib (Cabo) versus prednisone (Pred) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) previously treated with docetaxel (D) and abiraterone (A) and/or enzalutamide (E) J Clin Oncol. 2015;33(Suppl 7):abstr 139. [Google Scholar]