Significance
Understanding how changes in climate will affect terrestrial ecosystems is particularly important in tropical forest regions, which store large amounts of carbon and exert important feedbacks onto regional and global climates. By combining multiple types of observations with a state-of-the-art terrestrial ecosystem model, we demonstrate that the sensitivity of tropical forests to changes in climate is dependent on the length of the dry season and soil type, but also, importantly, on the dynamics of individual-level competition within plant canopies. These interactions result in ecosystems that are more sensitive to changes in climate than has been predicted by traditional models but that transition from one ecosystem type to another in a continuous, non–tipping-point manner.
Keywords: Amazon forests, biomass, ecological resilience, climate change, ecosystem heterogeneity
Abstract
Amazon forests, which store ∼50% of tropical forest carbon and play a vital role in global water, energy, and carbon cycling, are predicted to experience both longer and more intense dry seasons by the end of the 21st century. However, the climate sensitivity of this ecosystem remains uncertain: several studies have predicted large-scale die-back of the Amazon, whereas several more recent studies predict that the biome will remain largely intact. Combining remote-sensing and ground-based observations with a size- and age-structured terrestrial ecosystem model, we explore the sensitivity and ecological resilience of these forests to changes in climate. We demonstrate that water stress operating at the scale of individual plants, combined with spatial variation in soil texture, explains observed patterns of variation in ecosystem biomass, composition, and dynamics across the region, and strongly influences the ecosystem’s resilience to changes in dry season length. Specifically, our analysis suggests that in contrast to existing predictions of either stability or catastrophic biomass loss, the Amazon forest’s response to a drying regional climate is likely to be an immediate, graded, heterogeneous transition from high-biomass moist forests to transitional dry forests and woody savannah-like states. Fire, logging, and other anthropogenic disturbances may, however, exacerbate these climate change-induced ecosystem transitions.
Amazonia consists of 815 million ha of rainforest, transitional forest, and tropical savannahs; stores approximately half of tropical forest carbon (1); and plays a vital role in global water, energy, and carbon cycling (2). Although uncertainties in climate predictions for the region remain large (3), recent analyses imply that significant portions of the basin will experience both longer and more intense dry seasons by the end of the 21st century (3–6). There is particular concern about southern Amazonian forests that experience longer dry seasons than forests in central and western Amazonia (3) and where a trend of increasing dry season length (DSL) and intensity has already been observed (7). Despite the importance of this region for regional and global climate, the climate sensitivity of the Amazon forests remains uncertain: model predictions range from a large-scale die-back of the Amazon (8, 9) to predictions that the biome will remain largely intact, and may even increase in biomass (10–12). Although some of these differences can be attributed to differences in the predicted future climate forcing of the region (13, 14), accurate predictions of how changes in climate will affect Amazonian forests also rely on an accurate characterization of how the ecosystem is affected by a given change in climate forcing. In this study, we examine the climate sensitivity of the Amazon ecosystem, focusing on the mechanisms underpinning changes in forest dynamics and their implications for the timing and nature of basin-wide shifts in biomass in response to a drying climate.
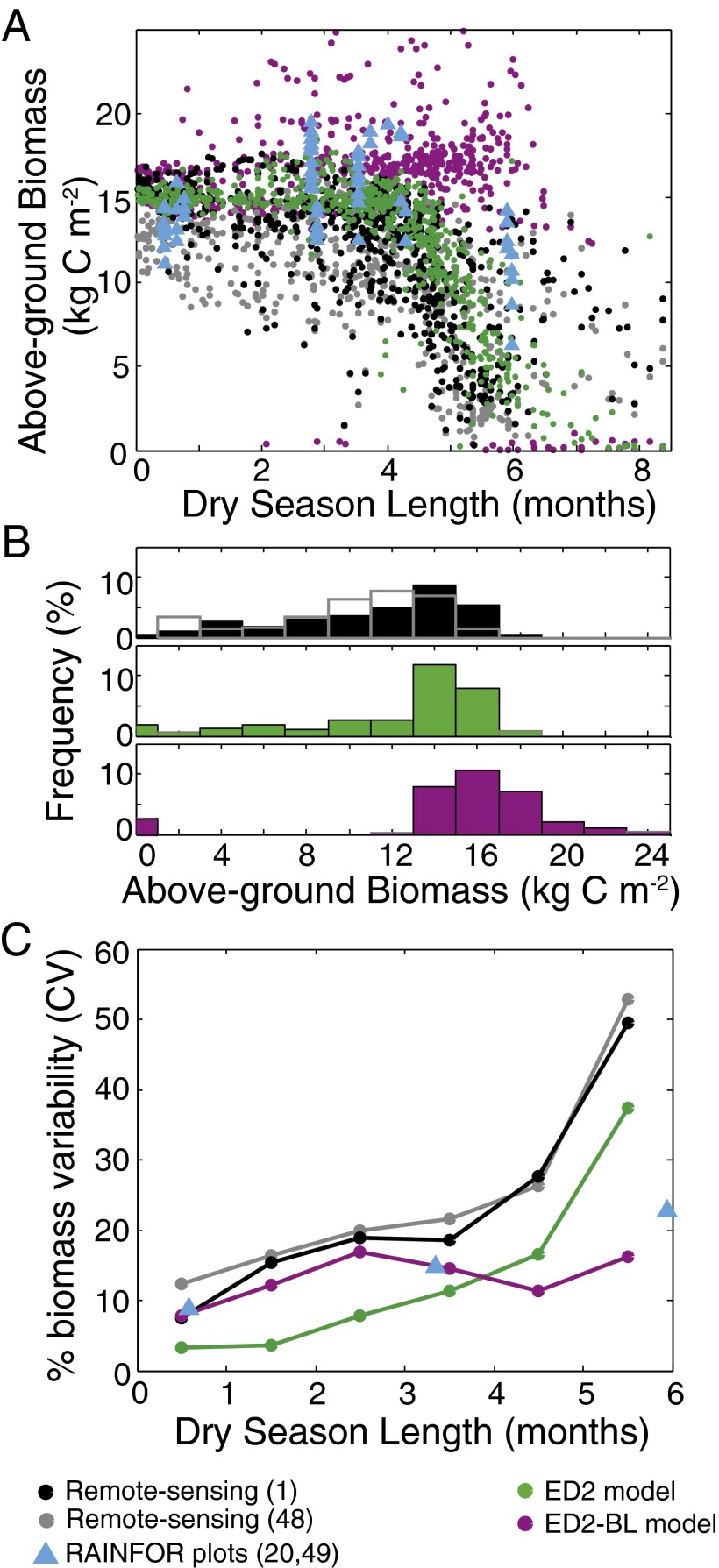
Variation in forest biomass across the Amazon basin (15–17) has been shown to correlate with DSL (16–18) (Fig. 1), soil texture (16), shifts in stem turnover rate (19), and forest composition (20). In general, high-biomass moist tropical forests occur where DSL, defined here as the number of months in which precipitation is <100 mm (6, 9), is short, and low-biomass, savannah-like ecosystems are primarily found when DSLs are long (Fig. 1A). In addition, a significant relationship is observed between regional-scale spatial heterogeneity in above-ground biomass (AGB > 2 kg of carbon per square meter) and DSL, with drier places having greater spatial heterogeneity: This pattern is seen both at the scale of 1° (Fig. 1C; r2 = 0.88, P < 0.01 for remote sensing-based AGB estimates) and at smaller spatial scales (SI Appendix, section S1). In other words, in moist areas, where DSL is short, forests have relatively homogeneous levels of AGB, whereas in drier areas, forests are increasingly heterogeneous. As we show below, this observed heterogeneity in response to increasing DSL has important implications for how the structure, composition, and dynamics of Amazon forests will be affected by changes in climate.
Fig. 1.
(A) Change in AGB with DSL for remote sensing-based estimates (black and gray circles), ground-based plot measurements (blue triangles), ED2 model output (green circles), and ED2-BL model output (purple circles). (B) Distribution of AGB in the observations and the two models. (C) Change in the percentage of biomass variability, with the coefficient of variation (CV) defined as 1σ/mean. Results are for undisturbed primary vegetation forests. Data are from Baccini et al. (1), Saatchi et al. (48), and Baker et al. (20, 49).
The Ecosystem Demography Biosphere (ED2) model, a process-based terrestrial biosphere model that represents individual plant-level dynamics, including competition for light and water (21, 22), was used to investigate the impact of ecosystem heterogeneity on the Amazon forest’s ecological resilience to climate perturbations (SI Appendix, section S3). Here, the term “ecological resilience” is used to describe the ability of a forest to maintain fundamental characteristics, such as carbon pools, composition, and structure, despite changes in climate (23). ED2 model simulations for the Amazon region, forced with a regional climate dataset derived from in situ measurements and remote-sensing observations, correctly reproduce the observed pattern of AGB variability as a function of DSL and soil texture (Fig. 1 and SI Appendix, section S4). In addition, ED2 model simulations for sites with detailed ground-based soil texture, forest structure, turnover, and composition measurements are also consistent with the observed patterns of variation in these quantities (SI Appendix, section S4).
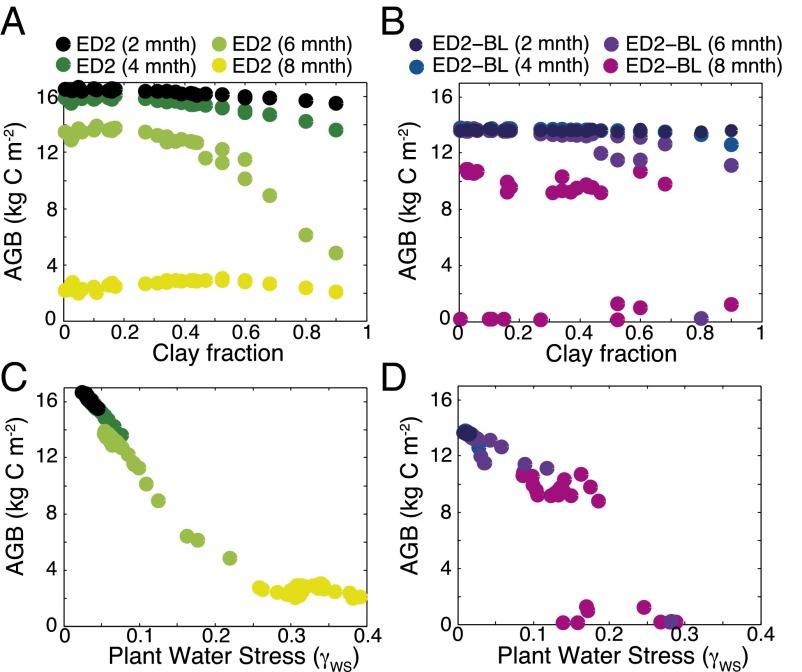
An ensemble of model simulations with varying soil texture was used to investigate the mechanisms that underpin the observed variable response to increasing DSL (SI Appendix, section S3). In the model, individual plant productivity is modified by a measure of plant water stress (γWS) that integrates soil texture, precipitation, and plant transpiration demand such that, as γWS increases, the plants close their stomata to reduce water loss. In the ED2 ensemble simulations, plot biomass is highly correlated with the average γWS for the forested sites (defined here as AGB > 3 kg of carbon per square meter) (Fig. 2C; r2 = 0.96–0.99, P < 0.01; SI Appendix, section S5). Associated with changes in AGB that occur as water stress increases are correlated changes in the productivity and composition of the plant canopy (SI Appendix, section S6).
Fig. 2.
Impact of changes in soil clay fraction (A and B) and plant water stress (C and D) on AGB in the ED2 (A and C) and ED2-BL (B and D) model simulations. Four climatological conditions are shown, a 2-month dry season, a 4-month dry season, a 6-month dry season, and an 8-month dry season.
The important role that water stress operating at the scale of individual plants plays in generating these responses is illustrated by comparing the native ED2 model predictions with output from a horizontally and vertically averaged version of the model (ED2-BL), analogous to a conventional “big leaf” terrestrial biosphere model that represents the canopy in an aggregated manner (SI Appendix, section S3). In the ED2-BL simulations, there is no significant relationship between the spatial heterogeneity of forested sites and DSL (Fig. 1 A and C; r2 = 0.24, P = 0.32). The absence of individual-level plant dynamics in the ED2-BL model results in a markedly different response to variations in soil texture and DSL than the native model formulation: Biomass initially declines as a function of increasing water stress, but a tipping point is then reached, beyond which the high-biomass forest is no longer stable and is replaced by a low-biomass savannah (Fig. 2). The result is a bimodal distribution of AGB across the basin in the ED2-BL model simulations, in contrast to the continuous distribution seen in the native model formulation and the observations (Fig. 1B). This response mirrors the response seen in other big-leaf-type ecosystem models (9). In native ED2 simulations, when water stress is prevented from influencing plant productivity, DSL and soil texture no longer have an impact on AGB (SI Appendix, section S5 and Fig. S5). Taken together, these simulations indicate that the driving mechanism behind the observed heterogeneous response to changes in DSL is the differential performance of individuals within the canopy to declining water availability, and how this response is modulated by soils with different hydrological properties. Specifically, the size and age structure of the ED2 plant canopy results in individuals’ differential access to both light and soil water, influencing the dynamics of individual plant growth and mortality (SI Appendix, section S6). Due to the nonlinear nature of functions governing plant growth, mortality, and recruitment, this heterogeneity results in a more continuous, graded response to changes in water stress than the big leaf (ED2-BL) formulation (Fig. 2). The consequence of this heterogeneity in plant-level responses to changes in soil moisture is that soil texture is likely to become increasingly important for controlling AGB as DSL increases. Soil fertility gradients also influence Amazonian AGB (16–18); however, as we show in SI Appendix, section S2, they do not account for the observed regional-scale pattern of increasing biomass heterogeneity with increasing DSL.
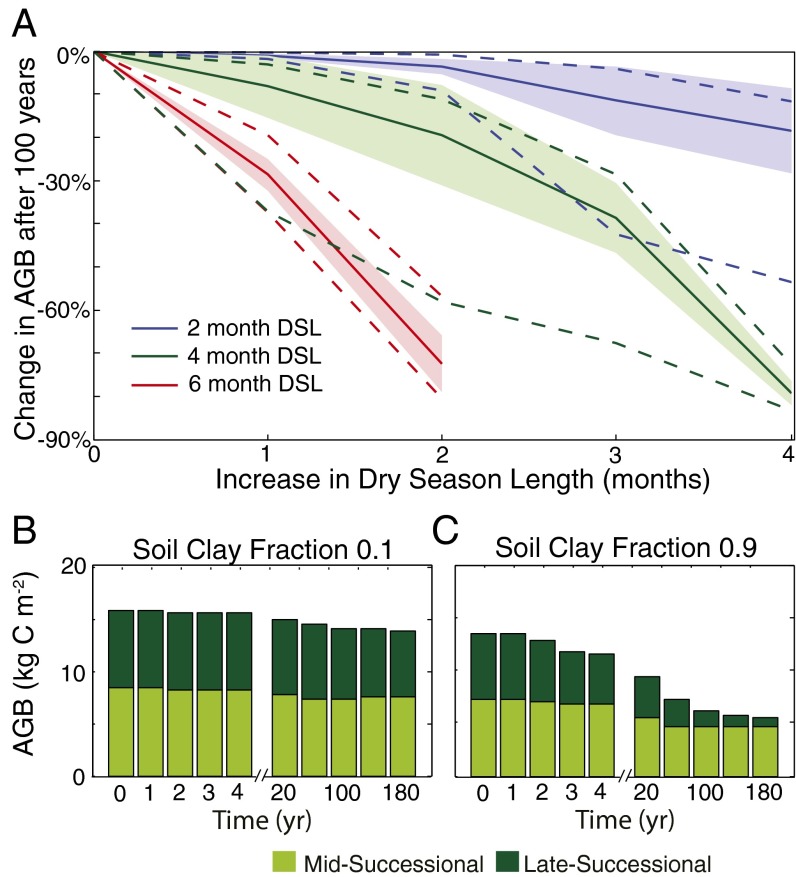
The ED2 biosphere model was used to investigate the expected patterns and time scales of Amazonian ecosystem response to a 1- to 4-month change in DSL over the 21st century (6). Earlier analyses have suggested that by accurately representing the dynamics of individual trees, models such as ED2 that incorporate plant-level dynamics are likely to provide more realistic estimates of forest successional change (21). Forests with a 4-month dry season (24% of the Amazon basin) are projected to lose ∼20% of their biomass with a 2-month increase in DSL (range of 11–58% loss of AGB dependent on clay content), whereas drier forests (6-month DSL) respond more rapidly to changes in climate, losing ∼29% (20–37% loss dependent on clay content) of their biomass with a 1-mo increase in DSL (Fig. 3A and SI Appendix, section S7). As the forests adjust to the new climate regime, the spatial heterogeneity of forest structure, composition, and biomass across the range of soil textures gradually increase. As seen in Fig. 3B, the model predicts that forests in soils with low clay content will be relatively unaffected by the change in climate regime; however, in soils with high clay content, the increase in levels of water stress caused by the onset of a longer dry season will result in marked changes in forest AGB and composition, beginning approximately 3 years after the perturbation (Fig. 3C). The time scale of the predicted initial ecosystem response is consistent with the results from two field-based through-fall exclusion experiments, which showed declining biomass 3–4 years after a drought was introduced (24, 25). Underlying these predicted changes in AGB and canopy composition are reductions in plant growth and increases in mortality rates (SI Appendix, Figs. S14 and S15). Whereas the majority of the change in AGB occurs in the first 100 y, the composition and structure of the forest continue to reorganize for more than 200 years after the perturbation (Fig. 3C). Specifically, the simulations predict a substantial decline in the abundance of late-successional trees in soils with high clay content. This prediction arises as a consequence of the slower rate of growth of late-successional trees that makes them more vulnerable to water stress-induced increases in mortality rates and less competitive against mid-successional species that are favored by drought-induced increases in understory light levels. This prediction of increased vulnerability of late-successional trees to increases in water stress is as yet untested; however, more generally, our analysis highlights how shifts in climate forcing are likely to drive significant shifts in tropical forest composition and structure over decadal and centennial time scales.
Fig. 3.
Predicted response of forest AGB and composition to an increase in DSL. (A) Change in AGB after 100 y as a result of increasing DSL for forests with historic DSLs of 2, 4, and 6 months for the range of soil textures simulated in the ensemble model simulations (n = 30). The magnitude of the change in AGB is influenced by soil clay fraction: The mean (solid line), 1σ deviation (shaded region), and minimum and maximum values (dashed lines) are shown. (B and C) Bar plots illustrating the impact of a 2-month increase in dry season (from 4 to 6 months) on a forest situated on a low clay content soil and a forest situated on a high clay content soil. The color of the bars indicates the contribution of mid- and late-successional trees, illustrating the shift in composition caused by the increase in DSL.
Recent work has hypothesized that two stable ecosystem states may exist along the boundaries of tropical forests and that a tipping point may occur once a climatological moisture threshold is passed (26, 27). Instead, by combining field observations, remote-sensing estimates, and a terrestrial biosphere model, we find no evidence that an irreversible rapid transition or dieback of Amazon forests will occur in response to a drying climate (8, 9) or that forests will be unresponsive (11, 12). Rather, our results suggest that, at least in the case of Amazonian forests, the ecosystem will exhibit an immediate but heterogeneous response to changes in its climate forcing and that a continuum of transitional forest ecosystem states exists. These conclusions are consistent with experimental observations across Amazonia of short-term drought impacts (28). Furthermore, we find that future climate-induced shifts between a moist tropical forest and a dry forest will be a more graded transition accompanied by increasing spatial heterogeneity in forest AGB, composition, and dynamics across gradients in soil texture. The ability of Amazonian forests to undergo reorganization of their structure and composition in response to climate-induced changes in levels of plant water stress acts as an important buffer against more drastic threshold changes in vegetation state that would otherwise occur; however, it also means that the forests are more sensitive to smaller magnitude changes in their climate forcing than previous studies have suggested.
The analysis conducted here intentionally focused on the direct impacts of changes in climate forcing on vegetation, and did not incorporate the effects of soil nutrients, climate-driven changes in fire frequency, the effects of increasing atmospheric CO2 concentrations, the impacts of land transformation, and biosphere/atmosphere feedbacks. With regard to soil nutrients, at the basin scale, analyses indicate that forest composition, structure, biomass, and dynamics also vary across a gradient in soil fertility (16, 17), with the younger, more fertile soils of western Amazonia supporting forests with lower AGB and higher rates of biomass productivity and stem turnover relative to the forests of the central Amazon and Guianan Shield, which are located on older, more nutrient-poor soils. Meanwhile, landscape-scale studies in central (29) and northwestern (30) Amazonia have found that more fertile clay soils have higher AGB than nutrient-poor sandy soils. Further discussion of the impact of soil nutrients can be found in SI Appendix, section S2).
Plant water availability is affected by both the hydraulic properties of soils and plant hydraulic architecture. Our findings of the importance of individual plant water stress on forest response to changes in climate highlight the need for additional studies into these two important, but relatively understudied, properties of tropical forests. With regard to soil hydraulic properties, recent studies suggest that the relationship between a soil’s texture and its hydraulic properties may differ significantly between tropical and temperate soils (31, 32). However, the impact of these differences on plant water availability remains uncertain. With regard to plant hydraulic architecture, although some measurements exist on rooting properties and vascular architecture of tropical trees (33–36), the above- and below-ground hydraulic attributes of tropical trees remain poorly characterized, especially compared with the hydraulic attributes of their temperate counterparts.
In some areas, particularly those areas with long dry seasons, increasing water stress is likely to be accompanied by increases in fire frequency, which may act to generate more rapid transitions from a higher biomass forested state to a more savannah-like biome (26, 27). Because these two mechanisms have distinct impacts on forest composition, structure, and function, both must be considered when predicting future responses to changes in climate. The potential impacts of fire on patterns of ecosystem change are discussed in SI Appendix, section S1. Recent modeling studies indicate that CO2 fertilization may mitigate the impact of increasing water stress (37); however, experimental studies are needed to quantify the impact of elevated CO2 concentrations better on the physiological functioning of Amazon trees.
Although regional patterns of Amazonian AGB are complex, reflecting the impact of multiple factors, our results suggest that plant-level responses to soil texture heterogeneity and changes in DSL are important in explaining the observed basin-wide pattern of variation in Amazonian AGB, providing a mechanistic explanation for the observed correlations between DSL, AGB, and changes in stand structure and composition (16, 17). These conclusions may also apply to African and Asian tropical forests; however, important differences exist in the future climate predictions for these regions (38) and their soil edaphic and nutrient characteristics and historical fire regimes (39–41).
The response of forests to changes in their climate forcing is an emergent ecosystem-level response that is ultimately driven by individual trees responding to changes in their local environments. Nonlinearities in the performance of individual plants, such as their rates of photosynthetic assimilation and mortality, as environmental conditions change imply that terrestrial biosphere models need to represent these differential responses of individuals to capture emergent ecosystem properties accurately (42). This analysis demonstrates that the conventional approach of modeling average plants in average environments within climatological grid cells underestimates the direct, near-term response of tropical forests to climatological change but overestimates the direct impacts of larger scale changes in forcing. Consequently, accurate predictions for the timing and nature of forest responses to changes in climate require consideration of how climate and soils affect the performance of individuals within plant canopies. As we have shown here, models that incorporate plant-level dynamics are able to characterize observed extant patterns of variation in the structure, composition, and dynamics of Amazonian ecosystems more accurately, and accounting for these patterns has important implications for the sensitivity and ecological resilience of Amazon forests to different levels of climatological perturbation.
Methods
The ED2 model is an integrated terrestrial biosphere model that incorporates land-surface biophysics, vegetation dynamics, hydrology, and soil carbon biogeochemistry, and it uses a size- and age-structured system of partial differential equations to approximate the individual-level dynamics of plant canopies (21, 22, 43). The horizontally and vertically averaged ED2-BL model represents exactly the same biophysical and biogeochemical processes as the ED2 model, but the size- and age-structured canopy is replaced with a horizontally and vertically averaged canopy akin to those canopies used by conventional terrestrial biosphere models. Additional information on the model formulation is provided in SI Appendix, section S3.
The ED2 and ED2-BL models were run for the entire Amazon basin forced with a rescaled National Centers for Environmental Prediction (NCEP) reanalysis product (44) and observation-based soil maps (45, 46) at 1° resolution, and with increasing atmospheric CO2 (47) (SI Appendix, section S3). The model results were compared against remote-sensing estimates (1, 48) aggregated to the same resolution as the model simulations. Plot-based observations were made on the scale of 0.4–10 ha (20), and were compared against model simulations forced with site-specific inputs (SI Appendix, Table S4).
The water stress factor (γWS) was used in both the ED2 and ED2-BL models to scale photosynthesis in response to water stress. The γWS was calculated for each individual (i) as:
| [1] |
where γWS ranges from 0 (unstressed) to 1 (stressed). Timax is the maximum transpiration (kg of water per year) for individual i, is the root biomass (kg of carbon) for individual i, θ(z) is the soil moisture (kg of water per cubic meter) at soil depth z, K is the root conductance (m2⋅kg of carbon per year), and θWP is the soil wilting point (kg of water per cubic meter). The available soil water (kg of water per square meter), θ(z) − θWP, is integrated over the rooting depth (RD) of the individual.
Spatial heterogeneity () was calculated over 1-month DSL intervals for model simulations and remote-sensing based estimates. These calculations were done at 1-ha resolution for plot-based observations and model simulations for these locations, at 500-m and 1-km resolution for remote-sensing based estimates, and at 1° resolution for the regional model simulations and remote-sensing based estimates. Due to the relatively low number of plots, the spatial heterogeneity of the plot-based observations was calculated for three DSL categories: 0–2 months, 2–5 months, and 5–8 months.
Supplementary Material
Acknowledgments
We thank Amazon Forest Inventory Network (RAINFOR) partners for generously providing plot data, particularly T. Baker; G. López-Gonzalez; and the late S. Almeida, A. Gentry, and S. Patiño. Funding was provided by the Gordon and Betty Moore Foundation Andes-Amazon Initiative and a National Oceanic and Atmospheric Administration Climate and Global Change fellowship (to N.M.L.). RAINFOR inventories have been funded by the Natural Environment Research Council and the Gordon and Betty Moore Foundation. O.L.P. is supported by a European Research Council Advanced Grant and is a Royal Society-Wolfson Research Merit Award holder.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511344112/-/DCSupplemental.
References
- 1.Baccini A, et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat Clim Chang. 2012;2(3):182–185. [Google Scholar]
- 2.Werth D, Avissar R. The local and global effects of Amazon deforestation. J Geophys Res Atmos. 2002;107(D20):LBA 55-1–LBA 55-8. [Google Scholar]
- 3.Boisier JP, Ciais P, Ducharne A, Guimberteau M. Projected strengthening of Amazonian dry season by constrained climate model simulations. Nat Clim Chang. 2015;5(7):656–660. [Google Scholar]
- 4.Malhi Y, et al. Climate change, deforestation, and the fate of the Amazon. Science. 2008;319(5860):169–172. doi: 10.1126/science.1146961. [DOI] [PubMed] [Google Scholar]
- 5.Chou C, Neelin JD. Mechanisms of global warming impacts on regional tropical precipitation. J Clim. 2004;17(13):2688–2701. [Google Scholar]
- 6.Malhi Y, et al. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc Natl Acad Sci USA. 2009;106(49):20610–20615. doi: 10.1073/pnas.0804619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu R, et al. Increased dry-season length over southern Amazonia in recent decades and its implication for future climate projection. Proc Natl Acad Sci USA. 2013;110(45):18110–18115. doi: 10.1073/pnas.1302584110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox PM, et al. Amazonian forest dieback under climate-carbon cycle projections for the 21st century. Theor Appl Climatol. 2004;78(1-3):137–156. [Google Scholar]
- 9.Good P, et al. Quantifying environmental drivers of future tropical forest extent. J Clim. 2011;24(5):1337–1349. [Google Scholar]
- 10.Thompson SL, et al. Quantifying the effects of CO2-fertilized vegetation on future global climate and carbon dynamics. Geophys Res Lett. 2004;31(23):L23211. [Google Scholar]
- 11.Cox PM, et al. Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature. 2013;494(7437):341–344. doi: 10.1038/nature11882. [DOI] [PubMed] [Google Scholar]
- 12.Huntingford C, et al. Simulated resilience of tropical rainforests to CO2-induced climate change. Nat Geosci. 2013;6(4):268–273. [Google Scholar]
- 13.Zhang K, et al. The fate of Amazonian ecosystems over the coming century arising from changes in climate, atmospheric CO2, and land use. Glob Change Biol. 2015;21(7):2569–2587. doi: 10.1111/gcb.12903. [DOI] [PubMed] [Google Scholar]
- 14.Galbraith D, et al. Multiple mechanisms of Amazonian forest biomass losses in three dynamic global vegetation models under climate change. New Phytol. 2010;187(3):647–665. doi: 10.1111/j.1469-8137.2010.03350.x. [DOI] [PubMed] [Google Scholar]
- 15.Malhi Y, et al. The above-ground coarse wood productivity of 104 Neotropical forest plots. Glob Change Biol. 2004;10(5):563–591. [Google Scholar]
- 16.Quesada CA, et al. Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences. 2012;9(6):2203–2246. [Google Scholar]
- 17.ter Steege H, et al. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature. 2006;443(7110):444–447. doi: 10.1038/nature05134. [DOI] [PubMed] [Google Scholar]
- 18.Baraloto C, et al. Disentangling stand and environmental correlates of aboveground biomass in Amazonian forests. Glob Change Biol. 2011;17(8):2677–2688. [Google Scholar]
- 19.Phillips OL, et al. Pattern and process in Amazon tree turnover, 1976-2001. Philos Trans R Soc Lond B Biol Sci. 2004;359(1443):381–407. doi: 10.1098/rstb.2003.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker TR, et al. Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob Change Biol. 2004;10(5):545–562. [Google Scholar]
- 21.Moorcroft PR, Hurtt GC, Pacala SW. A method for scaling vegetation dynamics: The ecosystem demography model (ED) Ecol Monogr. 2001;71(4):557–585. [Google Scholar]
- 22.Medvigy D, Wofsy SC, Munger JW, Hollinger DY, Moorcroft PR. Mechanistic scaling of ecosystem function and dynamics in space and time: Ecosystem Demography model version 2. J Geophys Res Biogeosci. 2009;114:G01002. [Google Scholar]
- 23.Walker B, Holling CS, Carpenter SR, Kinzig A. Resilience, adaptability and transformability in social–ecological systems. Ecol Soc. 2004;9(2):5. [Google Scholar]
- 24.Nepstad DC, Tohver IM, Ray D, Moutinho P, Cardinot G. Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology. 2007;88(9):2259–2269. doi: 10.1890/06-1046.1. [DOI] [PubMed] [Google Scholar]
- 25.da Costa ACL, et al. Effect of 7 yr of experimental drought on vegetation dynamics and biomass storage of an eastern Amazonian rainforest. New Phytol. 2010;187(3):579–591. doi: 10.1111/j.1469-8137.2010.03309.x. [DOI] [PubMed] [Google Scholar]
- 26.Staver AC, Archibald S, Levin SA. The global extent and determinants of savanna and forest as alternative biome states. Science. 2011;334(6053):230–232. doi: 10.1126/science.1210465. [DOI] [PubMed] [Google Scholar]
- 27.Hirota M, Holmgren M, Van Nes EH, Scheffer M. Global resilience of tropical forest and savanna to critical transitions. Science. 2011;334(6053):232–235. doi: 10.1126/science.1210657. [DOI] [PubMed] [Google Scholar]
- 28.Phillips OL, et al. Drought sensitivity of the Amazon rainforest. Science. 2009;323(5919):1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- 29.Laurance WF, et al. Relationship between soils and Amazon forest biomass: A landscape-scale study. For Ecol Manage. 1999;118(1–3):127–138. [Google Scholar]
- 30.Jimenez EM, et al. Edaphic controls on ecosystem-level carbon allocation in two contrasting Amazon forests. J Geophys Res Biogeosci. 2014;119(9):1820–1830. [Google Scholar]
- 31.Hodnett MG, Tomasella J. Marked differences between van Genuchten soil water-retention parameters for temperate and tropical soils: A new water-retention pedo-transfer functions developed for tropical soils. Geoderma. 2002;108(3–4):155–180. [Google Scholar]
- 32.Marthews TR, et al. High-resolution hydraulic parameter maps for surface soils in tropical South America. Geosci Model Development. 2014;7(3):711–723. [Google Scholar]
- 33.Nepstad DC, et al. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature. 1994;372:666–669. [Google Scholar]
- 34.Nepstad DC, et al. The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. J Geophys Res Atmoss. 2002;107(D20):LBA 53-1–LBA 53-18. [Google Scholar]
- 35.Schuldt B, Leuschner C, Brock N, Horna V. Changes in wood density, wood anatomy and hydraulic properties of the xylem along the root-to-shoot flow path in tropical rainforest trees. Tree Physiol. 2013;33(2):161–174. doi: 10.1093/treephys/tps122. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira RS, Dawson TE, Burgess SS, Nepstad DC. Hydraulic redistribution in three Amazonian trees. Oecologia. 2005;145(3):354–363. doi: 10.1007/s00442-005-0108-2. [DOI] [PubMed] [Google Scholar]
- 37.Rammig A, et al. Estimating the risk of Amazonian forest dieback. New Phytol. 2010;187(3):694–706. doi: 10.1111/j.1469-8137.2010.03318.x. [DOI] [PubMed] [Google Scholar]
- 38.Chadwick R, Boutle I, Martin G. Spatial patterns of precipitation change in CMIP5: Why the rich do not get richer in the tropics. J Clim. 2012;26(11):3803–3822. [Google Scholar]
- 39.Dwyer E, Pereira JMC, Gregoire JM, DaCamara CC. Characterization of the spatio-temporal patterns of global fire activity using satellite imagery for the period April 1992 to March 1993. J Biogeogr. 2000;27(1):57–69. [Google Scholar]
- 40.Baillie IC. Soils of the humid tropics. In: Richards PW, editor. The Tropical Rainforest. 2nd Ed. Cambridge Univ Press; Cambridge, UK: 1996. pp. 256–286. [Google Scholar]
- 41.Vitousek PM, Sanford RL. Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst. 1986;17:137–167. [Google Scholar]
- 42.Levin SA, Grenfell B, Hastings A, Perelson AS. Mathematical and computational challenges in population biology and ecosystems science. Science. 1997;275(5298):334–343. doi: 10.1126/science.275.5298.334. [DOI] [PubMed] [Google Scholar]
- 43.Hurtt GC, Moorcroft PR, Pacala SW, Levin SA. Terrestrial models and global change: Challenges for the future. Glob Change Biol. 1998;4(5):581–590. [Google Scholar]
- 44.Sheffield J, Goteti G, Wood EF. Development of a 50-year high-resolution global dataset of meteorological forcings for land surface modeling. J Clim. 2006;19(13):3088–3111. [Google Scholar]
- 45.Quesada CA, et al. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences. 2010;7(5):1515–1541. [Google Scholar]
- 46.Global Soil Task 2000. Global Soil Data Products CD_ROM, ed IGBP-DIS, 10.3334/ORNLDAAC/565.
- 47.Nakicenovic N, et al. IPCC Special Report on Emissions Scenarios. Cambridge Univ Press; Cambridge, UK: 2000. [Google Scholar]
- 48.Saatchi SS, et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc Natl Acad Sci USA. 2011;108(24):9899–9904. doi: 10.1073/pnas.1019576108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker TR, et al. Increasing biomass in Amazonian forest plots. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2004;359(1443):353–365. doi: 10.1098/rstb.2003.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.