Abstract
Background:
The influence of blood pressure (BP) lowering on intracerebral hemorrhage (ICH) patients is unclear. To assess the safety and efficacy of aggressive antihypertensive therapies in acute ICH patients, we carried out a systematic review and meta-analysis.
Methods:
PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure, and VIP database up to July 2014 were searched. High-quality randomized controlled trials were included. Low-quality trials were excluded. Serious adverse events were defined as the primary outcome. The secondary outcomes were hematoma enlargement (HE) at 24 h after onset, mortality, and favorable clinical outcome at 90 days.
Results:
Four high-quality trials involving a total of 1427 patients met the inclusion criteria and were analyzed. Odds ratios (ORs) of primary outcome was 0.96 (95% confidence interval [CI ]: 0.82–1.13, P = 0.61). ORs of HE at 24 h after onset, mortality and favorable clinical outcome at 90 days were 0.91 (95% CI: 0.72–1.17, P = 0.47), 0.97 (95% CI: 0.79–1.20, P = 0.81), 1.13 (95% CI: 0.98–1.30, P = 0.09) respectively.
Conclusions:
Aggressive BP management policies are safe and might have a potency of reducing HE and improving clinical outcome.
Keywords: Antihypertensive Therapy, Blood Pressure, Hematoma Enlargement, Hypertension, Intracerebral Hemorrhage
INTRODUCTION
Intracerebral hemorrhage (ICH), characterized by its high morbidity and mortality, is still an tough public health problem throughout the world.[1,2] Despite the progress of ICH diagnostic techniques, knowledge of the pathophysiologic mechanism of ICH remains insufficient, which result in absence of effective therapeutic methods. Volume of hematoma has been generally recognized as a predictor of prognosis and a number of observational studies have revealed that hematoma enlargement (HE) occurs commonly within the first 24 h and independently predicts early neurological deterioration and poor outcome.[3,4,5,6] Thus, it is essentially important to stable the hematoma and prevent HE.
Elevated blood pressure (BP), an independent predictor for poor prognosis for ICH, was deemed to increase the risk of HE.[7,8] In a retrospective analysis of 76 patients with ICH, target systolic BP (SBP) of ≥160 mmHg were significantly associated with HE compared with those of ≤150 mmHg (P = 0.025 < 0.05).[8] Antihypertensive therapy has been widely practiced and conservative target levels were prevalently achieved due to concerning about decreasing cerebral blood flow (CBF) in the past years. The American Heart Association (AHA) guidelines for spontaneous ICH has recommended a target SBP level of <180 mmHg or mean artery pressure (MAP) <110 mmHg.[9] Different from ischemic stroke, in ICH patients, neuroimaging studies have proved that there exists no so-called an ischemic penumbra in the peri-hematoma brain tissues.[10,11] Therefore, this enlightened researchers to intervene high BP more aggressively. Based on these findings, several relevant clinical trials were performed in recent years.
In this study, we attempted to make a systematic review and meta-analysis assessing the safety and efficacy of aggressive BP lowering compared with conservative antihypertensive levels recommended by the AHA guidelines.
METHODS
Searching strategy and selection criteria
Randomized controlled trials (RCTs) of antihypertensive drugs interventions that aimed at lowering BP in patients with acute ICH were identified by searching PubMed, EMBASE, The Cochrane Library, China National Knowledge Infrastructure, and VIP database up to July 2014 with different combinations of the following key words: “intracerebral hemorrhage” or “cerebral hemorrhage” or “antihypertensive” or “blood pressure lowering” or “hematoma enlargement” or “hematoma expansion” or “hematoma growth.” No language limits were imposed.
Inclusion criteria were as follows: (1) Patients with acute spontaneous ICH diagnosed by computed tomography (CT) scan; (2) RCTs on BP management comparing conservative levels and intensive or rapid or aggressive protocols; (3) Studies scoring ≥3 using the Cochrane criteria.
Exclusion criteria were as follows: (1) Low quality studies; (2) Hemorrhage secondary to brain injury, ischemic stroke, tumor, intracranial vascular malformation, and aneurysm; (3) Therapies combining with other interventions such as craniotomy or minimally invasive surgery.
Aggressive or intensive or rapid BP reduction protocols (target SBP ≤140 mmHg or MAP <110 mmHg) were compared with conservative BP treatment strategy (target SBP ≤180 mmHg or MAP <130 mmHg) recommended by the guidelines.
Data collection
Two researchers (Chao Pan and Yang Hu) independently identified the articles following the inclusion and exclusion criteria and assessed the articles’ qualities. Any discrepancies were resolved by discussion or consultation with a third reviewer (Zhou-Ping Tang, an expert in neurology). Serious adverse events (SAEs) were defined as the primary outcome. The secondary outcomes were HE at 24 h after onset, mortality, and favorable clinical outcome at 90 days (a Modified Rankin Scale [mRS] score ≤2 at 90 days).
HE was defined as a substantially increase in volume above 33% or an absolute change in hematoma volume of 12.5–20 ml on repeated CT scan (24 h after ictus).[12]
We used Review Manager version 5.1.2 software (Cochrane Collaboration, Denmark) for data analysis and calculated the I2 to describe the heterogeneity. A fixed-effects model was employed in the absence of significant heterogeneity (I2 <50%); otherwise, a random-effects model was used as an alternative. The pooled results were presented as odds ratio (OR), 95% confidence interval (95% CI) and P value. The funnel plot was drawn to explore the publication bias visually.
RESULTS
Search results
A total of 2534 potentially relevant studies were initially retrieved. Of these, 2474 articles did not meet the inclusion criteria after reading the titles, abstracts, and full-text [Figure 1]. After quality assessment, four high-quality trials were eligible for further pooling analysis [Table 1]. The characteristics of the four included studies have been presented in Table 2.
Figure 1.
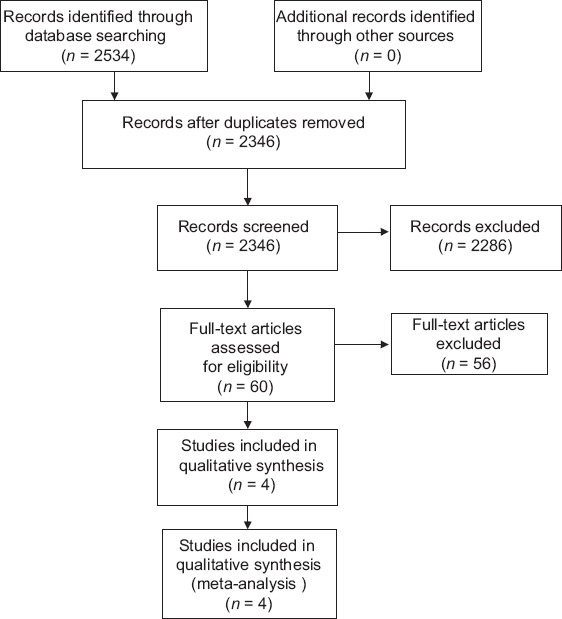
Flow diagram of literature search for this review.
Table 1.
Study quality assessment
| Study | Randomized generation | Outcome blinding | Incomplete data | Allocation concealment | Total score |
|---|---|---|---|---|---|
| INTERACT1 | 2 | 2 | 1 | 2 | 7 |
| INTERACT2 | 2 | 2 | 1 | 2 | 7 |
| ADAPT | 2 | 2 | 1 | 2 | 7 |
| Rapid BP reduction | 1 | 1 | 1 | 2 | 5 |
2: Yes; 1: Unclear; ADAPT: Acutely Decreasing Arterial Pressure Trial; INTERACT: Intensive blood pressure reduction in acute cerebral hemorrhage trial; BP: Blood pressure.
Table 2.
Characteristics of the four included studies
| Study | Design | Number of cases | Intervention | Interclass equilibration | Outcomes |
|---|---|---|---|---|---|
| Rapid BP reduction trial | A prospective, single-center, randomized, single-blinded study | 42 | Standard treatment: MAP 110–130 mmHg; Aggressive BP lowering: MAP <110 mmHg | Good | A clinical decline (NIHSS drop ≥2 points) within 48 h; HE rates at 24 h |
| INTERACT1 | A prospective randomized, parallel assignment, safety, efficacy study, open-label study | 404 | Intensive therapy: BP ≤140 mmHg within 1 h of randomization Control: BP ≤180 mmHg | Good | Intensive BP goals maintained for 24 h safety and tolerability achieved |
| INTERACT2 | A prospective, randomized, open-label, assessor-blinded end-point multicenter, trial | 2839 | Intensive therapy: BP ≤140 mmHg within 1 h of randomization Control: BP ≤180 mmHg | Good | Death and dependency physical function on the mRS; hematoma volume |
| ADAPT | A multi-center randomized open-label, blinded end-point trial | 75 | Target: SBP <150 mmHg or SBP <180 mmHg | Good | Neurological examinations; hematoma and perihematomal edema volumes; the relative CBF within the perihematomal region |
ADAPT: Acutely Decreasing Arterial Pressure Trial; INTERACT: Intensive blood pressure reduction in acute cerebral hemorrhage trial; MAP: Mean artery pressure; SBP: Systolic blood pressure; CBF: Cerebral blood flow; BP: Blood pressure; HE: Hematoma enlargement; NIHSS: National institutes of health stroke scale; mRS: Modified rankin scale.
Koch et al. conducted the rapid BP reduction trial aimed at evaluating the feasibility and safety of rapid BP reduction to lower than previously recommended levels in acute ICH from 2004 to 2006.[13] In this study, subjects satisfying the criteria were randomized into one of two BP lowering groups: A standard BP management group with a target MAP 110–130 mmHg according to the 2007 AHA guidelines or an aggressive BP management group with a target MAP <110 mmHg. There were no significant differences in early neurological deterioration, hematoma and edema growth, and clinical outcome at 90 days.
The first and second intensive blood pressure reduction in acute Intracerebral Hemorrhage Trial (INTERACT1 and 2) study assessed the feasibility, safety and effectiveness of intensive BP lowering protocol (target SBP <140 mmHg) comparing with conservative BP lowering group (target SBP <180 mmHg).[14,15,16,17] The results of INTERACT1 and 2 have showed that intensive and early BP management did decrease hematoma volume modestly but not to the extent of a substantial degree. INTERACT2 failed to show significant improvement in the rate of functionally independence, with early aggressive BP lowering. However, in an ordinal analysis of the distribution of mRS scores, there was a significant shift in favor of those patients who received aggressive BP therapy.
The ICH Acutely Decreasing Arterial Pressure Trial (ICH ADAPT) was conducted to observe the CBF changes secondary to aggressive BP reduction in patients with ICH.[18] Subjects with SBP >150 mmHg were randomized into one of two treatment regimens receiving intravenous antihypertensive therapy: A targeted SBP <150 mmHg or <180 mmHg. Two hours after randomization, all patients underwent a CT perfusion scan. A noncontrast CT was performed at 24 h. The images and statistics of the results dispelled the long-existing concern that lowering BP aggressively may induce cerebral ischemia.
Primary outcome (serious adverse events)
The safety of aggressive BP reduction protocol was assessed. SAEs were defined as ischemic or undifferentiated stroke, acute coronary event, severe hypotension, or others according to the INTERACT trials. A low heterogeneity (I2 = 0%, P = 0.49) was observed, thus we used a fixed-effect model to identify the pooled OR (0.96), 95% CI (0.82–1.13) and P (P = 0.61) [Figure 2]. This result demonstrated no statistically significant difference between these two BP management protocols in SAEs, indicating that aggressive BP protocol is safety enough and would not bring more adverse effects.
Figure 2.
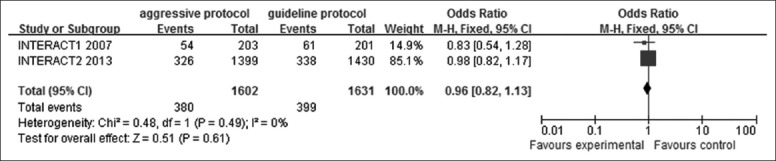
Forest plots depicting safety of aggressive blood pressure reduction (serious adverse effect) in aggressive versus guideline protocol. CI: Confidence interval; INTERACT: Intensive blood pressure reduction in acute cerebral hemorrhage trial.
This founding would dispel the long-existing doubt that further BP management may induce more unexpected bad results on ICH patients.
Secondary outcomes
Hematoma enlargement at 24 h
The relevant data of these four studies involving 1427 patients (725 in the aggressive BP lowering group, 702 in the guideline recommended group) were presented in Figure 3. The heterogeneity among these trials were quite low (I2 = 41%, P = 0.16). A fixed-effect model was applied. The value of OR was 0.91 (95% CI: 0.72–1.17), indicated that reducing BP intensively or aggressively, maintaining SBP <140 mmHg or MAP <110 mmHg, demonstrated a moderate tendency toward preventing HE compared with guideline group, but was not significant different according to the P value (P = 0.47) and 95% CI.
Figure 3.
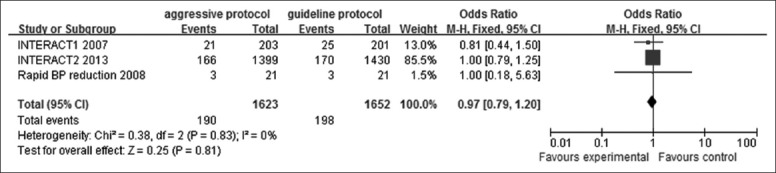
Forest plots depicting hematoma enlargement at 24 h in aggressive versus guideline protocol. CI: Confidence interval; ADAPT: Acutely Decreasing Arterial Pressure Trial; INTERACT: Intensive blood pressure reduction in acute cerebral hemorrhage trial.
Mortality at 90 days
Three studies presented data on 90 days mortality shown in Figure 4. According to our analysis, no heterogeneity (P = 0.83, I2 = 0%) was found among the trials, thus a fixed-effect model was chosen for the pooling analysis. The mortality rate was slightly greater in the guideline group than in the intensive group but did not reach significant values with a pooled OR of 0.97 (95% CI: 0.79–1.20). These results demonstrated that intensive BP lowering brought small effects on improving mortality rates at 90 days.
Figure 4.
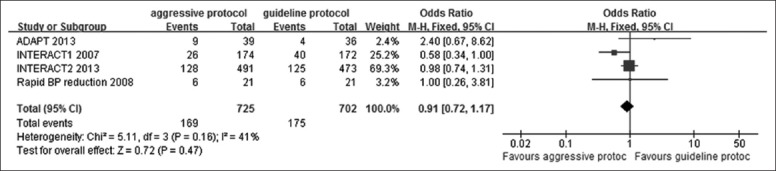
Forest plots depicting mortality at 90 days in aggressive versus guideline protocol. CI: Confidence interval; ADAPT: Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial; INTERACT: Intensive blood pressure reduction in acute cerebral hemorrhage trial.
Favorable clinical outcome at 90 days (a Modified Rankin Scale score ≤2 at 90 days)
Data on favorable clinical outcome (mRS ≤2) were available in 3 trials [Figure 5]. There existed no heterogeneity (I2 = 0%, P = 0.60) and the pooled OR were identified using a fixed-effect model (OR: 1.13; 95% CI: 0.98–1.30). Although no significant difference were achieved between the aggressive BP group and the guideline group (95% CI: 0.98–1.30, P = 0.09), a tendency that lowering BP aggressively may benefit the ICH patients through promoting their operational abilities in everyday life was still observed.
Figure 5.
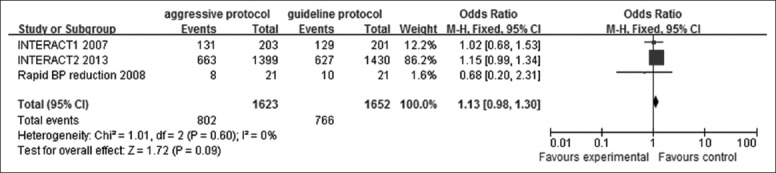
Forest plots depicting favorable clinical outcome at 90 days (a Modified Rankin Scale score ≤2 at 90 days) in aggressive versus guideline protocol. CI: Confidence interval; INTERACT: Intensive blood pressure reduction in acute cerebral hemorrhage trial
DISCUSSION
Treatment of high BP was a crucial question, which directly affects early outcome and long-time prognosis of patients, whereas there was too much disagreement, especially with regard to targeted BP levels. Consensuses that hypertensive response should be handled deliberately, based on relatively deficient and inferior evidences, were temporarily reached in consideration of preserving cerebral blood supply.
We systematically evaluated the safety and effectiveness of aggressive BP treatment protocols compared with conservative BP lowering levels and made a review on antihypertensive studies on ICH. On the basis of low heterogeneity, we pooled these trials and expanded the sample size for a meta-analysis. Our results suggested that aggressive BP treatment policies are quite safe and tend to show a potential to reduce HE in acute ICH patients with elevated BP, though the difference was not significant. Results of mortality and favorable clinical outcome, in accordance with that of HE, indicated that for ICH patients with rising BP, to reduce BP aggressively and early might benefit patients through restrict HE. The 95% CI was narrowed compared with that of INTERACT2, the largest RCTs among these trials, containing 2839 ICH subjects.
MAP was known to be associated with cerebral perfusion pressure. It was deemed that low MAP would induce poor cerebral perfusion. Interestingly, in the rapid BP reduction trial, patients assigned into the aggressive BP management group with a target MAP <110 mmHg demonstrated no more SAEs than patients assigned into conservative level group, indicating that this MAP level may be safe. Similarly, the ICH ADAPT trial used CT perfusion to observe the CBF and the results dispelled the concern that lowering BP aggressively may induce cerebral ischemia. This may explain the safety of early aggressive BP lowing therapy.
Most of the observational studies found a tight association between BP and HE, while the results did not reach statistically significances strictly. The inconformity may be explained as follow: HE is a complicated process with multiple factors involved in apart from BP, such as liver diseases, hyperglycemia, history of stroke, alcohol consumption, hematoma volume, locations of hematoma, and irritability.[12,19,20,21] Different antihypertensive agents and other therapies besides BP lowering, for example, hemostasis medication, therapeutic hypothermia, abuse of mannitol may influence the results. Moreover, however, there is a noteworthy fact that opinions differ on whether BP plays an important role in HE.[20,22,23] In an analysis of recombinant activated factor VII ICT (FAST), HE was found not associated with baseline BP.[20] Theoretically speaking, even a modest decrease of hematoma would ameliorate recovery and in this regard, an aggressive BP management was worth implementing. Indeed, in INTERACT2, the ordinal analysis of mRS demonstrated a significantly lower score in intensive treatment group (OR: 0.87; 95% CI: 0.77–1.00; P = 0.04).[14]
There were several limitations in this meta-analysis. Only four randomized studies were eligible for analyzing. Some studies were not included since a lack of controls or randomization. For example, the antihypertensive treatment of acute cerebral hemorrhage trial was excluded for nonrandomization. Three levels of SBP reduction in patients suffering ICH were achieved using intravenous nicardipine within 6 h after bleeding: 170–200, 140–170, or 110–140 mmHg.[24]
What's more, most cases were provided by INTERACT2, leading to a larger weight of INTERACT2. In ICH ADAPT study, the relatively aggressive SBP reduction level was 150 mmHg, instead of 140 mmHg in other trials. Therefore an uncertainty may arise from an approximate 10 mmHg interval of SBP. BP management was supposed to be beneficial through reducing cerebral edema, but the impact of antihypertensive therapy on peri-hematomal edema was not discussed for a lack of materials. The absolute increase of hematoma volume was not within the scope of analysis because of limited original data. Since the number of included studies was < 10, it was improper to draw a funnel plot to explore the publication bias.
In conclusion, we hold that aggressive BP management was safe enough and show its promising potential compared with a more conservative BP policy previously recommended for acute ICH patients. It might be worth implementing aggressive BP lowing therapy for ICH patients presenting with SBP between 150 and 220 mmHg. Our exploratory results are for reference. More RCTs were desperately needed to further investigate optimal BP reduction levels, drug selection and the most likely candidates that will benefit from aggressive BP management.
Financial support and sponsorship
This research was supported by grants from the National Natural Science Foundation of China (No. 81171089; 81471201) and the Key Clinical Program of the Ministry of Health of China (2010).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: Epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–64. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Luna D, Rubiera M, Ribo M, Coscojuela P, Piñeiro S, Pagola J, et al. Ultraearly hematoma growth predicts poor outcome after acute intracerebral hemorrhage. Neurology. 2011;77:1599–604. doi: 10.1212/WNL.0b013e3182343387. [DOI] [PubMed] [Google Scholar]
- 5.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 6.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–81. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 7.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–5. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 8.Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage: Relationship between elevated blood pressure and hematoma enlargement. Stroke. 2004;35:1364–7. doi: 10.1161/01.STR.0000128795.38283.4b. [DOI] [PubMed] [Google Scholar]
- 9.Morgenstern LB, Hemphill JC 3 r rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–29. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herweh C, Jüttler E, Schellinger PD, Klotz E, Jenetzky E, Orakcioglu B, et al. Evidence against a perihemorrhagic penumbra provided by perfusion computed tomography. Stroke. 2007;38:2941–7. doi: 10.1161/STROKEAHA.107.486977. [DOI] [PubMed] [Google Scholar]
- 11.Schellinger PD, Fiebach JB, Hoffmann K, Becker K, Orakcioglu B, Kollmar R, et al. Stroke MRI in intracerebral hemorrhage: Is there a perihemorrhagic penumbra? Stroke. 2003;34:1674–9. doi: 10.1161/01.STR.0000076010.10696.55. [DOI] [PubMed] [Google Scholar]
- 12.Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11:101–18. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- 13.Koch S, Romano JG, Forteza AM, Otero CM, Rabinstein AA. Rapid blood pressure reduction in acute intracerebral hemorrhage: Feasibility and safety. Neurocrit Care. 2008;8:316–21. doi: 10.1007/s12028-008-9085-8. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–65. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 15.Delcourt C, Huang Y, Arima H, Chalmers J, Davis SM, Heeley EL, et al. Hematoma growth and outcomes in intracerebral hemorrhage: The INTERACT1 study. Neurology. 2012;79:314–9. doi: 10.1212/WNL.0b013e318260cbba. [DOI] [PubMed] [Google Scholar]
- 16.Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): A randomised pilot trial. Lancet Neurol. 2008;7:391–9. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 17.Manning L, Hirakawa Y, Arima H, Wang X, Chalmers J, Wang J, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: A post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014;13:364–73. doi: 10.1016/S1474-4422(14)70018-3. [DOI] [PubMed] [Google Scholar]
- 18.Butcher KS, Jeerakathil T, Hill M, Demchuk AM, Dowlatshahi D, Coutts SB, et al. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44:620–6. doi: 10.1161/STROKEAHA.111.000188. [DOI] [PubMed] [Google Scholar]
- 19.Wartenberg KE, Mayer SA. Reducing the risk of ICH enlargement. J Neurol Sci. 2007;261:99–107. doi: 10.1016/j.jns.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Broderick JP, Diringer MN, Hill MD, Brun NC, Mayer SA, Steiner T, et al. Determinants of intracerebral hemorrhage growth: An exploratory analysis. Stroke. 2007;38:1072–5. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 21.Kimura K, Iguchi Y, Inoue T, Shibazaki K, Matsumoto N, Kobayashi K, et al. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. J Neurol Sci. 2007;255:90–4. doi: 10.1016/j.jns.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Martí-Fàbregas J, Martínez-Ramírez S, Martínez-Corral M, Díaz-Manera J, Querol L, Suárez-Calvet M, et al. Blood pressure is not associated with haematoma enlargement in acute intracerebral haemorrhage. Eur J Neurol. 2008;15:1085–90. doi: 10.1111/j.1468-1331.2008.02254.x. [DOI] [PubMed] [Google Scholar]
- 23.Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–6. doi: 10.1161/01.str.29.6.1160. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi AI, Palesch YY, Martin R, Novitzke J, Cruz-Flores S, Ehtisham A, et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: Results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol. 2010;67:570–6. doi: 10.1001/archneurol.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


