Abstract
Background:
About 50% of the cerebral ischemia events are induced by intracranial and extracranial atherosclerosis. This study aimed to evaluate the feasibility and accuracy for displaying atherosclerotic plaques in carotid arteries and analyzing their ingredients by using high-resolution new magnetic resonance imaging (MRI) techniques.
Methods:
Totally, 49 patients suspected of extracranial carotid artery stenosis were subjected to cranial MRI scan and magnetic resonance angiography (MRA) examination on carotid arteries, and high-resolution bright-blood and black-blood MRI analysis was carried out within 1 week. Digital subtraction angiography (DSA) examination was carried out for 16 patients within 1 month.
Results:
Totally, 103 plaques were detected in the 49 patients, which were characterized by localized or diffusive thickening of the vessel wall, with the intrusion of crescent-shaped abnormal signal into lumens. Fibrous cap was displayed as isointensity in T1-weighted image (T1WI) and hyperintensities in proton density weighted image (PDWI) and T2-weighted image (T2WI), lipid core was displayed as isointensity or slight hyperintensities in T1WI, isointensity, hyperintensities or hypointensity in PDWI, and hypointensity in T2WI. Calcification in plaques was detected in 11 patients. Eight patients were detected with irregular plaque surface or ulcerative plaques, which were characterized by irregular intravascular space surface in the black-blood sequences, black hypointensity band was not detected in three-dimensional time-of-flight, or the hypointensity band was not continuous, and intrusion of hyperintensities into plaques can be detected. Bright-blood and black-blood techniques were highly correlated with the diagnosis of contrast-enhanced MRA in angiostenosis degree, Rs = 0.97, P < 0.001. In comparison to DSA, the sensitivity, specificity, and accuracy of MRI diagnosis of stenosis for ≥50% were 88.9%, 100%, and 97.9%, respectively.
Conclusions:
High-resolution bright-blood and black-blood sequential MRI analysis can accurately analyze ingredients in atherosclerotic plaques. Determined by DSA, MRI diagnosis of stenosis can correctly evaluate the serious degree of arteriostenosis.
Keywords: Atherosclerosis, Black-blood, Bright-blood, Carotid Arteries, Magnetic Resonance Image
INTRODUCTION
The morbidity and mortality of cerebral ischemic stroke is one of the top three diseases in our country for many years. In contrast, about 50% of the cerebral ischemia events are induced by intracranial and extracranial atherosclerosis. Besides the decrease in cerebral blood flow perfusion induced by arteriostenosis, induction of cerebral ischemia symptoms, instability and abscission of plaques at stenosis sites, thrombopoiesis, and abscission and occlusion of cerebral vessels at distal ends induced by irregular plaque surfaces are also closely related to intracranial and extracranial atherosclerosis.[1,2] Therefore, examinations on tunica media thickness and plaque properties in carotid arteries can predict the incidence of ischemic cerebrovascular diseases.[3] The purpose of the present study was to evaluate the feasibility and accuracy for displaying atherosclerotic plaques in carotid arteries and analyzing their ingredients by using high-resolution new magnetic resonance imaging (MRI) techniques.
METHODS
Patients
Totally, 49 patients (33 male and 16 female, aged 32–77 years) clinically suspected of extracranial carotid artery stenosis were subjected to cranial MRI plain computed tomography (CT) scan and magnetic resonance angiography (MRA) examinations on carotid arteries, and high-resolution bright-blood and black-blood MRI analysis was carried out within 1 week. Among which, seven patients were subjected to enhanced MRI examinations; 32 patients were suffered from symptoms of cerebral ischemia, 29 patients were suffered from hypertension, and 16 patients were suffered from blood fat disturbance, among which 20 patients were suffered from diabetes and hypertension, 19 patients were suffered from diabetes and blood fat disturbance. Digital subtraction angiography (DSA) examinations were carried out for 16 patients within 1 month.
Magnetic resonance imaging protocol
Instruments and scanning sequences: 3.0T MRI device (Achieva, Philips Medical Systems, Best, The Netherlands). Cranial MRI routine scanning sequences included cross-sectional fast spin echo (FSE) sequence T1-weighted (T1W), T2-weighted (T2W), fluid-attenuated inversion recovery, and diffusion weighted imaging. Cervical contrast-enhanced-MRA (CE-MRA) examination was carried out by using 16-channel cranial and cervical united coil, high-pressure injector was used, the contrast agent was gadopentetate dimeglumine (Gd-DTPA, Bayer HealthCare, Germany), the velocity was 2.5 ml/s, breath holding-free scanning was carried out (2D-BOLUSTRAK technique), and the time for the contrast agent reaching the aortic arch was observed. Afterward, a bolus injection of 19 ml contrast agent was carried out, and the mean delay time was 13 s (11–19 s). Coronal high-resolution contrast enhancement volume scan (S2-3D Hi Res) was carried out and the parameters were as followed: Three-dimensional fast field echo sequence, the shortest TR/TE 4.7 ms/1.79 ms, flip angle 270; field of view 320 mm × 320 mm, the pixel after reconstruction was 0.45 mm × 0.45 mm × 0.49 mm, the reconstruction matrix was 704, the number of scanning slices was 150, the slice thickness was 1.0 mm, overlap 50%, SENSE value was 2, and the scanning time was 1 min and 27 s.
Bright-blood and black-blood MRI examination was carried out by using 8-channel cervical surface phased-array coil (Shanghai Chenguan Company, China), the scanning sequence was FSE dual inversion recovery T1W, proton density weighted (PDW), T2W and three-dimensional time-of-flight (3D-TOF) gradient recalled echo, and the parameters were listed in Table 1. The scanning started at distal end of common carotid artery, crotch of common carotid artery, and originated sites of internal carotid artery and external carotid artery, the scanning durations for each slice T1W, T2W, and PDW were 22, 28, and 38 s, respectively, 4–8 slices were scanned, 60 slices were scanned for 3D-TOF for 2 min and 28 s. Fat-suppression technique was used for eliminating the interferences from fats nearby blood vessels for all of the sequences.
Table 1.
Scanning parameters for the MRI sequences
| Sequence | TR (ms) | TE (ms) | BW (KHZ) | FOV (mm) | Slice thickness (mm) | Matrix | NEX | ETL | FA |
|---|---|---|---|---|---|---|---|---|---|
| TSE-DIR T1W | 1R-R | 5.7 | 395 | 160 | 2–3 | 248×264 | 1 | 8 | 90 |
| TSE-DIR PDW | 2R-R | 20 | 180 | 160 | 2–3 | 276×285 | 1 | 8 | 90 |
| TSE-DIR T2W | 2R-R | 40 | 180 | 160 | 2–3 | 248×260 | 1 | 8 | 90 |
| 3D-TOF | 30 | 5.8 | 96 | 160 | 0.6 | 528×257 | 1 | 20 |
MRI: Magnetic resonance imaging; DIR: Dual inversion recovery; T1W: T1-weighted; PDW: Proton density weighted; T2W: T2-weighted; FOV: Field of view; TOF: Time-of-flight; TR: Repetition time; TE: Echo time; BW: Band width; NEX: Number of excitation; ETL: Echo train length; FA: Flip angle.
Image analysis
Thickening in vessel wall for more than 1.5 mm was considered as plaque, the distance between the inner margin of plaque and the exterior margin of vessel wall at the thickest position of plaque was measured, and the mean value was calculated for three measurements. The adjacent sternocleidomastoid signal at the same slice in the same sequence was used as the reference for evaluating the characteristics of plaque signal, which can be divided into hyperintensities, isointensity, hypointensity, and mixed intensity. Ingredients of plaques were analyzed according to previous experiences in references.[4] Types of fibrous caps were observed in 3D-TOF: (1) Thick and intact fibrous caps: Uniform hypointensity band between lumens of hyperintensities and plaques of moderate intensity; (2) Thin and intact fibrous caps: Hypointensity band was not detected, but smooth lumen surface can be displayed in other sequences; (3) Disrupted fibrous caps: Interrupted hypointensity band or no hypointensity band, irregular lumen surface was detected in other sequences. The grading of lumen stenosis induced by plaques was as followed: Lumen stenosis rate (%) = (normal diameter of carotid artery − minimal diameter of incomplete lumen)/normal diameter of carotid artery × 100%.
Statistical analysis
Chi-square test was carried out for the comparison in the differences in numeration data; Spearman rank correlation analysis was carried out for the correlation analysis. The results from DSA examinations were used as the criteria for the analysis on sensitivity and specificity for MRI diagnosis on carotid artery stenosis for ≥50% induced by plaques. SPSS statistical software (version 13.0, SPSS Inc., Chicago, IL, USA) was used for the statistical analysis, and P < 0.05 indicated was considered statistically significant.
RESULTS
Analysis on plaque morphology and signal characteristics
Totally, 103 plaques of bigger than 1.5 mm in the vessel wall thickness of vessel wall in carotid arteries were detected in 49 patients, 42 plaques were detected at distal ends and crotch of common carotid arteries, 48 plaques were detected at proximal ends of internal carotid arteries, and 13 plaques were detected at proximal ends of external carotid arteries. The plaques were characterized by localized or diffusive thickening in the vessel wall, with the intrusion of crescent-shaped abnormal signal foci into lumens, and the thickest position was 6.5 mm. Fibrous cap was displayed as isointensity in T1-weighted image (T1WI) and hyperintensities in proton density weighted image (PDWI) and T2-weighted image (T2WI), lipid core was displayed as isointensity or slight hyperintensities in T1WI, isointensity, hyperintensities or hypointensity in PDWI, and hypointensity in T2WI [Figure 1]. Calcification in plaques was detected in 11 patients; it was a strip-like or small patch like, which was manifested by hypointensity in the sequences [Figure 2]. Intact and thin fibrous caps cannot be clearly displayed in TOF. But, smooth lumen surface can be detected in the sequences, the fibrous caps were significantly enhanced and can be clearly displayed after enhancement, the boundary to inner lumen diameter and lipid core was even clear, some lipid cores were enhanced and the signals were not uniform. T1WI inclined sagittal images can display the distribution and longitudinal morphology of plaques, and the surfaces of the plaques were also macroscopically displayed [Figure 3]. Irregular plaque surface or ulcerative plaques were detected in eight patients, which were manifested as irregular vessel lumen surfaces in the black-blood sequences, black hypointensity band was not detected in 3D-TOF, or the hypointensity band was not continuous, and intrusion of hyperintensities into plaques was detected [Figure 2]. Obvious hemorrhagic plaques were not detected in the present study.
Figure 1.
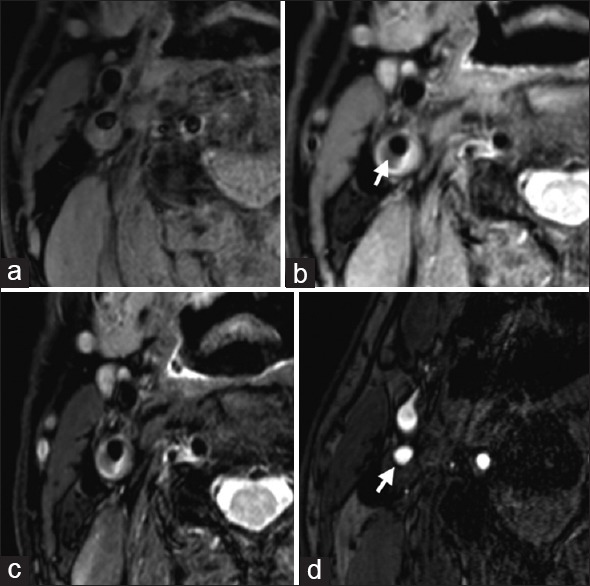
A 72-year-old male, with hypertension and blood fat disturbance. (a): T1-weighted image of right internal carotid artery showed internal carotid artery posterior wall plaque at right side, isointensity and hyperintensities, the surface was smooth and regular. Proton density weighted (b) and T2-weighted image (c) images of right internal carotid artery showed local hypointensity in the plaques, indicating lipid cores (arrow), the hyperintensities nearby indicated large amount of water contents in extracellular matrix of the plaques. (d) Time-of-flight of right internal carotid artery showed hypointensity fibrous cap; the surface was smooth (arrow). Typing of the plaque: Type IV or Va, the lumen stenosis = 50%.
Figure 2.
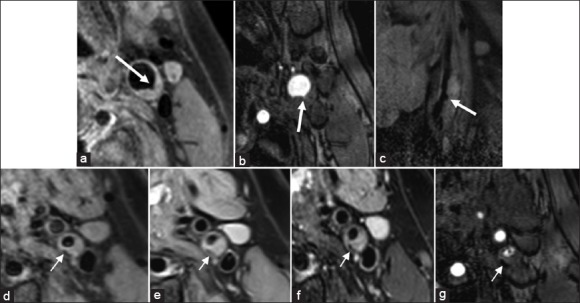
A 52-year-old male, with diabetes, hyperlipemia, and ischemic cerebral infarction in left anterior circulation. (a-c) T1-weighted image (a), time-of-flight (b), and sag T1-weighted image (c) showed plaques in posterior wall at the crotch of left catastrophic coverage act, irregular ulcers were detected on the surface (thick white arrows), type VI. (d-g) T1-weighted image (d), proton density weighted image (e), T2-weighted image (f), and time-of-flight (g) indicated plaques in posterior wall at the initial segment of left internal carotid artery, hypointensity patch-like calcification can be detected in the inner wall (thin white arrows).
Figure 3.
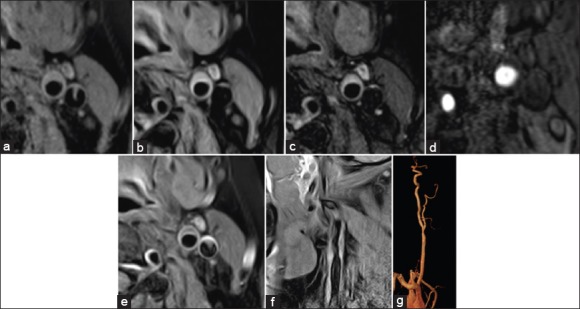
A 59-year-old female, with diabetes, hypertension, and blood fat disturbance. (a-d) T1-weighted image (a), proton density weighted image (b), T2-weighted image (c), and time-of-flight (d) indicated plaques in posterior wall at the crotch of left catastrophic coverage act, T1-weighted image isointensity, proton density weighted, T2-weighted image hyperintensities, the surface was smooth and regular, and no obvious fibrous cap was detected in time-of-flight. (e) T1-weighted with contrast of left catastrophic coverage act showed a significant enhancement in fibrous caps in T1-weighted after enhancement, the surface was regular and smooth, and the boundary to the relative hypointensity lipid cores was clear. (f and g) sag T1-weighted image (f) and contrast-enhanced magnetic resonance angiography (g) of left catastrophic coverage act, the morphologies were coincident (type V).
Accuracy for diagnosing arteriostenosis by using black-blood and bright-blood magnetic resonance imaging analysis
Totally, among the 294 vessel segments of 49 patients’ stenosis, 181 segments were negative. Stenosis for 50–99% in common carotid artery induced by plaques was detected in two patients; lumen stenosis for 50–99% in internal carotid artery induced by plaques was detected in 15 patients, while lumen stenosis for 50–99% in external carotid artery induced by plaques was detected in four patients. Three cases of internal carotid artery occlusion and one case of external carotid artery occlusion were detected. Five patients of stenosis for <50% in internal carotid artery and two patients of stenosis for <50% in external carotid artery were evaluated as more than 50% in the cervical CE-MRA, and one patient of external carotid artery stenosis for more than 90% was displayed as occlusion in the CE-MRA. No obvious plaques or vessel wall thickening for <1.5 mm was detected in five patients suspected of mild stenosis in CE-MRA, and no obvious plaque was detected in the four patients showing no stenosis in CE-MRA. “Black-blood” and “bright-blood” techniques were highly correlated with CE-MRA in the diagnosis on angiostenosis degree, Rs = 0.97, P < 0.001 [Table 2].
Table 2.
Correlation analysis between CE-MRA and black-blood and bright-blood techniques on 49 cases (294 vessel segments) of carotid artery stenosis
| Black-blood and bright-blood MRI | CE-MRA | |||
|---|---|---|---|---|
| No stenosis | <50% stenosis | 50–99% stenosis | Occlusion | |
| No stenosis | 181 | 10 | ||
| <50% stenosis | 71 | 7 | ||
| 50–99% stenosis | 20 | 1 | ||
| Occlusion | 4 | |||
The results of correlation analysis between MRA and MRI black-blood and bright-blood techniques in angiostenosis degree were: Rs=0.97, P<0.001. MRI: Magnetic resonance imaging; MRA: Magnetic resonance angiography; CE-MRA: Contrast-enhanced MRA.
Sixteen patients were subjected to DSA examinations, 14 vessels among the total 96 vessels at distal ends of common carotid arteries at both ends and crotch, as well as originate site of internal and external carotid arteries showed angiostenosis for ≥50%, four vessels were occluded, except that one case of stenosis <50% induced by plaques in internal carotid artery and one case of stenosis <50% induced by plaques in external carotid artery by MRI corresponded to ≥50% in DSA, other results were all coincident. The sensitivity, specificity, and accuracy of MRI diagnosis of stenosis for ≥50% were 88.9%, 100%, and 97.9%, respectively [Table 3].
Table 3.
Correlation analysis between DSA and black-blood and bright-blood techniques on 16 cases (96 vessel segments) of carotid artery stenosis (serious)
| No. of case | MRI | DSA |
|---|---|---|
| 50–99% stenosis | 12 | 14 |
| Occlusion | 4 | 4 |
The sensitivity, specificity, and accuracy of MRI diagnosis of stenosis for ≥50% were 88.9%, 100%, and 97.9%, respectively. MRI: Magnetic resonance imaging; DSA: Digital subtraction angiography.
DISCUSSION
Correlation between pathological typing of atherosclerotic plaques and apoplexy
Atherosclerosis is a kind of systemic disease frequently seen in great vessels, and the major pathological changes are fatty streaks and fibrous plaques in tunica intima of arteries. American Heart Association divided the histomorphological changes in atheromatosis into six types according to its severity in 1995: Type I corresponded to early lesions, lipid deposition was detected in tunica intima; type II corresponded to fatty streaks; type III corresponded to early stage of plaques and extracellular lipid droplet accumulation can be detected; type IV corresponded to plaque stage and lipid cores were detected, but thick fibrous caps were not detected; type V was characterized by the appearance of fibrous caps on the basis of relatively big lipid cores and protrusion into lumens; when secondary pathological changes were detected in type IV and type V, it corresponded to type VI. Type VII characterized by calcification was increased on the basis in 2000, as well as type VIII characterized by fibrous tissues.[5,6] The lesions became irreversible from type IV and were termed as “vulnerable plaques.” Thin fibrous caps, big necrotic lipid cores, internal hemorrhage in plaques, large amount of macrophage infiltration in fibrous caps or plaques are also vulnerable plaques, which were closely related to cerebral apoplexy.
In a previous study, in the comparison between Chinese patients and white people from USA, in the symptoms induced by carotid artery lesions by stenosis for more than 50%, the lipid/necrotic cores in the plaques in the patients from our country were big, the percentage of calcified slices in the type VII lesions was low, while the percentage of slices for early type III was high.[7] The lipid cores in the plaques showing stenosis of ≥50% in the present study were relatively big and calcified into small patches, which were coincident with previous reports and also indicated the instability of the plaques.
It was reported by North American Symptomatic Carotid Endarterectomy Trial in 1995 that cerebral infarction was not detected in a considerable percentage of patients showing stenosis higher than 70%, while manifestations of cerebral infarction were detected in some patients showing stenosis of <70% or even 50%. Therefore, it was supposed that the degree of lumen stenosis was not the best method for judging prognosis and the key point was whether the carotid artery plaque was stable. Prabhakaran et al. carried out ultrasound analysis that the 5-year accumulative risks for cerebral ischemic stroke without calibration in the patients showing no plaque in carotid arteries, those showing regular plaque surface and those showing irregular plaque surface were 1.3%, 3.0%, and 8.5%, respectively. They pointed that the plaques with irregular surfaces were still independently correlated with ischemic apoplexy.[8] Totally, 8 cases of ulcerative plaque were detected in the present study, all of these patients showed cerebrovascular symptoms, among which four patients showed stenosis of ≥50%, and thus even low degree of stenosis can also lead to cerebral apoplexy if vulnerable plaques were detected. The fibrous caps in the seven patients in the present study after contrast agent enhancement were significantly enhanced, and enhancement in lipid cores in different degrees was also detected, indicating the possibilities of abundant neovascularization and unstable plaques. Enhancement was also detected in the plaques in the patients of obsolete cerebral apoplexy, indicating the possibility of recurrence and further studies are required.
Advantages for magnetic resonance imaging analysis on atherosclerotic plaques in carotid arteries
Carotid arteries are elastic arteries and predilection sites for atherosclerosis, thickening in tunica media complex, and formation of atheromatous plaques in carotid arteries are indicators for atherosclerosis, these pathological changes are always earlier than those in coronary artery and cerebral arteries, which are windows for evaluating atherosclerosis.[9,10]
The expenses on ultrasound analysis are low, the operations are simple and convenient, and thus it is a kind of noninvasive imaging method, and the sensitivity and specificity for serious stenosis cases of higher than 70% can be as high as 91–95% and 86–97%, respectively.[11] The shortage is that the accuracy of the diagnosis is dependent on operating techniques and practiced degree of operators, peak systolic velocity analysis on vascular tortuosity is difficult, stenosis in internal carotid artery at distal end cannot be examined, and serious calcified plaques may also interfere with the detection of blood flow.
Multirow spiral CT angiography can display the images for cross-sections of blood vessels, detect lumen stenosis, and accurately detect all of the calcified plaques. However, the detection of morphology on the surface of plaques and the internal ingredients is still unsatisfactory, and the predictive values for plaque ulcers are not high.[12,13]
DSA is the golden criterion for morphological examinations on atherosclerosis, but its values for judging lesions in vessel wall such as structural ingredients and properties of artery plaques are limited, the capability for displaying surface ulcers is relatively low in comparison to histological examinations on specimens by carcinoembryonic antigen, its sensitivity was only 46% and the specificity was 74%;[14] if atherosclerosis is widespread, the stenosis degree may be underestimated; moreover, DSA is a kind of invasive examination and it may lead to ischemic cerebrovascular disease and other complications.
High-resolution magnetic resonance analysis can accurately judge the stability of plaques. Due to the complexity of plaque ingredients, the program for examinations on multiple comparison sequences is frequently used now, among which bright-blood and black-blood technique is one of the major techniques. With the applications of 3.0T high electric field intensity magnetic resonance (MR) machine and surface phased-array coil, the signal-to-noise ratio, and the spatial resolution are further improved.[15,16] 3D-TOF bright-blood technique can well display calcification of plaques and fibrous caps containing compact collagen, and identify unstable fibrous caps of plaques.[17] Black-blood technique refers to MR technique for inhibiting flow blood signal, which makes the imaging of vessel wall adjacent to the intravascular space of hypointensity clearer, the optimal plaque ingredients can be displayed by alternating echo and repetition time, and the frequently used sequences are T1WI, PDWI, and T2WI.
The present study utilized two inversion recovery saturated pulse and rapid acquisition interleaved spin echo to deprive the proton flowing into the slices of transverse magnetization, no signal was produced and it was black, and thus effectively inhibited blood flow signal. Fat-suppression technique was used to eliminate the interferences from hyperintense fats nearby blood vessels, while the fats in plaques mainly existed in the forms of cholesterol esters and free cholesterol, and fat-suppression may not affect the comparison in various kinds of ingredients in plaques. Cappendijk et al. supposed that a single TFE T1W sequence can be used for rapid and accurate quantitative analysis on lipid necrotic cores in plaques.[18] It was found in the present study that the time for T1W scanning was short, the resolution was relatively high, but combination with other sequences was still required to improve the correct analysis on ingredients in the plaques. Bright-blood and black-blood MRI and CE-MRA in the present study are highly correlated in the diagnosis on angiostegnosis degree, and the accuracy for diagnosing angiostegnosis degree by bright-blood and black-blood MRI was very high by using the results of DSA as the criteria. The angiostegnosis degrees were underestimated in two cases by MRI, which was attributed to less scanning slices and relatively big normal vessel diameter as the reference.
The shortage of multi-sequence magnetic resonance plaque imaging is that the duration for imaging is relatively long, respiratory movements, blood vessel beats, swallow and involuntary movements can all lead to motion artifacts. Electrocardiogram-gating technique can improve the quality of images for vessel wall, but it is required to prolong the time for imaging. The present study utilized finger pulse in substitution for electrocardiogram-gating technique, and relatively satisfactory results were obtained. Delayed scanning after injection of the contrast agent can also reduce artifacts in lumens.
In conclusion, high-resolution bright-blood and black-blood sequential MRI can accurately analyze ingredients of atherosclerotic plaques, and enhanced MRI is helpful for identifying vulnerable plaques. Determined by DSA, the sensitivity, specificity, and accuracy of MRI diagnosis of stenosis for ≥50% were 88.9%, 100%, and 97.9%, respectively, and it can accurately evaluate seriousness degree of arteriostenosis. The shortage is that the duration for imaging is long, which may be significantly affected by respiration and motion artifact.
Financial support and sponsorship
This study was supported by a grant of Shanghai Municipal Health and Family Planning Commission (No. 201440420).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Saam T, Cai J, Ma L, Cai YQ, Ferguson MS, Polissar NL, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology. 2006;240:464–72. doi: 10.1148/radiol.2402050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan C, Mitsumori LM, Beach KW, Maravilla KR. Carotid atherosclerotic plaque: Noninvasive MR characterization and identification of vulnerable lesions. Radiology. 2001;221:285–99. doi: 10.1148/radiol.2212001612. [DOI] [PubMed] [Google Scholar]
- 3.Degnan AJ, Young VE, Gillard JH. Advances in noninvasive imaging for evaluating clinical risk and guiding therapy in carotid atherosclerosis. Expert Rev Cardiovasc Ther. 2012;10:37–53. doi: 10.1586/erc.11.168. [DOI] [PubMed] [Google Scholar]
- 4.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–73. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- 5.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A Report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–31. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 6.Stary HC. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler Thromb Vasc Biol. 2000;20:1177–8. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 7.Saam T, Cai JM, Cai YQ, An NY, Kampschulte A, Xu D, et al. Carotid plaque composition differs between ethno-racial groups: An MRI pilot study comparing mainland Chinese and American Caucasian patients. Arterioscler Thromb Vasc Biol. 2005;25:611–6. doi: 10.1161/01.ATV.0000155965.54679.79. [DOI] [PubMed] [Google Scholar]
- 8.Prabhakaran S, Rundek T, Ramas R, Elkind MS, Paik MC, Boden-Albala B, et al. Carotid plaque surface irregularity predicts ischemic stroke: The Northern Manhattan study. Stroke. 2006;37:2696–701. doi: 10.1161/01.STR.0000244780.82190.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomura M, Kasami R, Ohashi M, Yamada Y, Abe H. Significantly higher incidence of carotid atherosclerosis found in Japanese type 2 diabetic patients with early nephropathy. Diabetes Res Clin Pract. 2004;66(Suppl 1):S161–3. doi: 10.1016/j.diabres.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Frauchiger B, Schmid HP, Roedel C, Moosmann P, Staub D. Comparison of carotid arterial resistive indices with intima-media thickness as sonographic markers of atherosclerosis. Stroke. 2001;32:836–41. doi: 10.1161/01.str.32.4.836. [DOI] [PubMed] [Google Scholar]
- 11.Landwehr P, Schulte O, Voshage G. Ultrasound examination of carotid and vertebral arteries. Eur Radiol. 2001;11:1521–34. doi: 10.1007/s003300100963. [DOI] [PubMed] [Google Scholar]
- 12.Nandalur KR, Baskurt E, Hagspiel KD, Phillips CD, Kramer CM. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. AJR Am J Roentgenol. 2005;184:295–8. doi: 10.2214/ajr.184.1.01840295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wintermark M, Jawadi SS, Rapp JH, Tihan T, Tong E, Glidden DV, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol. 2008;29:875–82. doi: 10.3174/ajnr.A0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streifler JY, Eliasziw M, Fox AJ, Benavente OR, Hachinski VC, Ferguson GG, et al. Angiographic detection of carotid plaque ulceration. Comparison with surgical observations in a multicenter study. North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1994;25:1130–2. doi: 10.1161/01.str.25.6.1130. [DOI] [PubMed] [Google Scholar]
- 15.Hinton DP, Cury RC, Chan RC, Wald LL, Sherwood JB, Furie KL, et al. Bright and black blood imaging of the carotid bifurcation at 3.0T. Eur J Radiol. 2006;57:403–11. doi: 10.1016/j.ejrad.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Anumula S, Song HK, Wright AC, Wehrli FW. High-resolution black-blood MRI of the carotid vessel wall using phased-array coils at 1.5 and 3 Tesla. Acad Radiol. 2005;12:1521–6. doi: 10.1016/j.acra.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. 2000;102:959–64. doi: 10.1161/01.cir.102.9.959. [DOI] [PubMed] [Google Scholar]
- 18.Cappendijk VC, Heeneman S, Kessels AG, Cleutjens KB, Schurink GW, Welten RJ, et al. Comparison of single-sequence T1w TFE MRI with multisequence MRI for the quantification of lipid-rich necrotic core in atherosclerotic plaque. J Magn Reson Imaging. 2008;27:1347–55. doi: 10.1002/jmri.21360. [DOI] [PubMed] [Google Scholar]


