Abstract
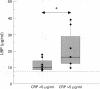
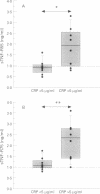
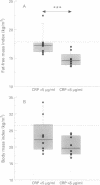
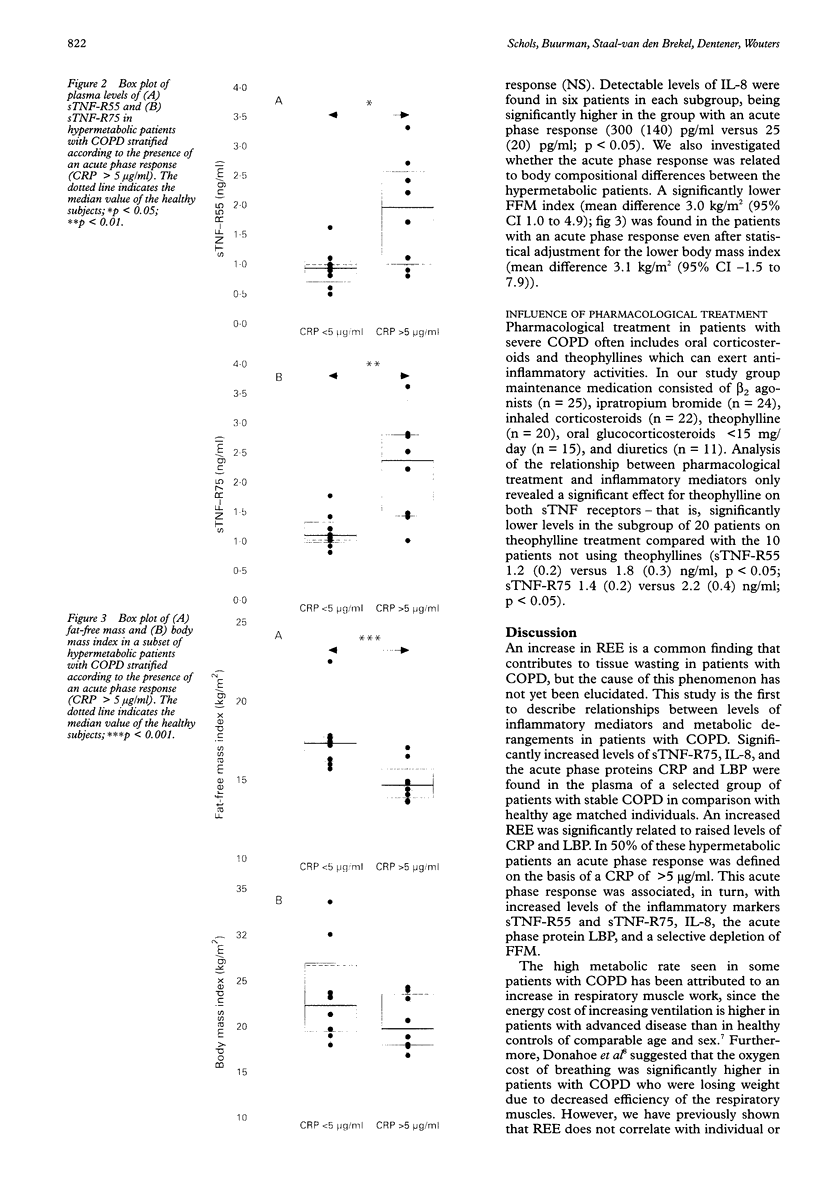
BACKGROUND: An increase in resting energy expenditure (REE) commonly occurs in patients with chronic obstructive pulmonary disease (COPD), the cause of which is as yet unknown. The objective of this study was to assess the relationship between REE, acute phase proteins, and inflammatory mediators in patients with COPD. METHODS: Thirty patients were studied and 26 healthy age-matched subjects served as controls. REE was measured by indirect calorimetry and adjusted for fat-free mass (FFM) by bioelectrical impedance analysis. Tumour necrosis factor alpha (TNF-alpha), soluble tumour necrosis receptor (sTNF-R)55 and sTNF-R75, interleukin (IL)-6, IL-8, and lipopolysaccharide binding protein (LBP) were measured by ELISA. RESULTS: Fourteen patients had a normal REE and in 16 it was raised. The mean body mass index and fat mass were significantly lower in the latter but pulmonary function data were similar in the two groups. In the 30 patients with COPD the mean (SD) sTNF-R75 was 1.7 (1.0) ng/ml compared with 1.1 (0.4) ng/ml in the controls; C-reactive protein (CRP) was detectable (> 5 micrograms/ml) in eight patients compared with none of the control subjects, and LBP was 13.2 (7.7) micrograms/ml compared with 8.6 (3.1) micrograms/ml in the controls. The patients with a raised REE had increased mean levels of CRP compared with the patients with a normal REE (median 5.5 micrograms/ml (range 5-193) and < 5 micrograms/ml, respectively); the same was true for LBP (median 12.4 micrograms/ml (range 8.1-39.1) and 9.5 micrograms/ml (range 5.0-16.6), respectively), but sTNF-R55 and R75 and IL-8 were similar in the two groups. Of the 16 patients with a raised REE, the CRP level was increased in eight and normal in eight. In those with an increased level of CRP the FFM was decreased and LBP, IL-8, and sTNF-R55 and R75 were increased compared with those with normal CRP levels. CONCLUSIONS: A subset of patients with COPD with an increased REE and decreased FFM have increased levels of acute phase reactant proteins and inflammatory cytokines in their serum; these phenomena may be causally related.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J., Pauwels R. A. Theophylline in the management of asthma: time for reappraisal? Eur Respir J. 1994 Mar;7(3):579–591. doi: 10.1183/09031936.94.07030579. [DOI] [PubMed] [Google Scholar]
- Bouma M. G., Stad R. K., van den Wildenberg F. A., Buurman W. A. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol. 1994 Nov 1;153(9):4159–4168. [PubMed] [Google Scholar]
- Brown M. A., Morgan W. J., Finley P. R., Scuderi P. Circulating levels of tumor necrosis factor and interleukin-1 in cystic fibrosis. Pediatr Pulmonol. 1991;10(2):86–91. doi: 10.1002/ppul.1950100209. [DOI] [PubMed] [Google Scholar]
- Dash A., Agrawal A., Venkat N., Moxham J., Ponte J. Effect of oral theophylline on resting energy expenditure in normal volunteers. Thorax. 1994 Nov;49(11):1116–1120. doi: 10.1136/thx.49.11.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentener M. A., Bazil V., Von Asmuth E. J., Ceska M., Buurman W. A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993 Apr 1;150(7):2885–2891. [PubMed] [Google Scholar]
- Di Francia M., Barbier D., Mege J. L., Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994 Nov;150(5 Pt 1):1453–1455. doi: 10.1164/ajrccm.150.5.7952575. [DOI] [PubMed] [Google Scholar]
- Engelberts I., Möller A., Schoen G. J., van der Linden C. J., Buurman W. A. Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res. 1991 Apr;10(1-2):69–76. [PubMed] [Google Scholar]
- Engelen M. P., Schols A. M., Baken W. C., Wesseling G. J., Wouters E. F. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J. 1994 Oct;7(10):1793–1797. doi: 10.1183/09031936.94.07101793. [DOI] [PubMed] [Google Scholar]
- Engelmann H., Aderka D., Rubinstein M., Rotman D., Wallach D. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. J Biol Chem. 1989 Jul 15;264(20):11974–11980. [PubMed] [Google Scholar]
- Falconer J. S., Fearon K. C., Plester C. E., Ross J. A., Carter D. C. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg. 1994 Apr;219(4):325–331. doi: 10.1097/00000658-199404000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Barber A., Manogue K., Tracey K. J., Kuo G., Fischman D. A., Cerami A. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol. 1989 Mar;256(3 Pt 2):R659–R665. doi: 10.1152/ajpregu.1989.256.3.R659. [DOI] [PubMed] [Google Scholar]
- Froon A. H., Bemelmans M. H., Greve J. W., van der Linden C. J., Buurman W. A. Increased plasma concentrations of soluble tumor necrosis factor receptors in sepsis syndrome: correlation with plasma creatinine values. Crit Care Med. 1994 May;22(5):803–809. doi: 10.1097/00003246-199405000-00015. [DOI] [PubMed] [Google Scholar]
- Froon A. H., Dentener M. A., Greve J. W., Ramsay G., Buurman W. A. Lipopolysaccharide toxicity-regulating proteins in bacteremia. J Infect Dis. 1995 May;171(5):1250–1257. doi: 10.1093/infdis/171.5.1250. [DOI] [PubMed] [Google Scholar]
- Goldstein S. A., Thomashow B. M., Kvetan V., Askanazi J., Kinney J. M., Elwyn D. H. Nitrogen and energy relationships in malnourished patients with emphysema. Am Rev Respir Dis. 1988 Sep;138(3):636–644. doi: 10.1164/ajrccm/138.3.636. [DOI] [PubMed] [Google Scholar]
- Larsen K. Creatinine assay by a reaction-kinetic principle. Clin Chim Acta. 1972 Oct;41:209–217. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg J. F., Dentener M. A., Buurman W. A. Lipopolysaccharide LPS-mediated soluble TNF receptor release and TNF receptor expression by monocytes. Role of CD14, LPS binding protein, and bactericidal/permeability-increasing protein. J Immunol. 1994 May 15;152(10):5070–5076. [PubMed] [Google Scholar]
- Leeuwenberg J. F., Jeunhomme T. M., Buurman W. A. Slow release of soluble TNF receptors by monocytes in vitro. J Immunol. 1994 Apr 15;152(8):4036–4043. [PubMed] [Google Scholar]
- Levine B., Kalman J., Mayer L., Fillit H. M., Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990 Jul 26;323(4):236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- Pfisterer M., Burkart F., Jockers G., Meyer B., Regenass S., Burckhardt D., Schmitt H. E., Müller-Brand J., Skarvan K., Stulz P. Trial of low-dose aspirin plus dipyridamole versus anticoagulants for prevention of aortocoronary vein graft occlusion. Lancet. 1989 Jul 1;2(8653):1–7. doi: 10.1016/s0140-6736(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Porteu F., Hieblot C. Tumor necrosis factor induces a selective shedding of its p75 receptor from human neutrophils. J Biol Chem. 1994 Jan 28;269(4):2834–2840. [PubMed] [Google Scholar]
- Ravussin E., Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989 May;49(5 Suppl):968–975. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- Richman-Eisenstat J. B., Jorens P. G., Hébert C. A., Ueki I., Nadel J. A. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol. 1993 Apr;264(4 Pt 1):L413–L418. doi: 10.1152/ajplung.1993.264.4.L413. [DOI] [PubMed] [Google Scholar]
- Schols A. M., Fredrix E. W., Soeters P. B., Westerterp K. R., Wouters E. F. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991 Dec;54(6):983–987. doi: 10.1093/ajcn/54.6.983. [DOI] [PubMed] [Google Scholar]
- Schols A. M., Mostert R., Soeters P. B., Wouters E. F. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax. 1991 Oct;46(10):695–699. doi: 10.1136/thx.46.10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols A. M., Schoffelen P. F., Ceulemans H., Wouters E. F., Saris W. H. Measurement of resting energy expenditure in patients with chronic obstructive pulmonary disease in a clinical setting. JPEN J Parenter Enteral Nutr. 1992 Jul-Aug;16(4):364–368. doi: 10.1177/0148607192016004364. [DOI] [PubMed] [Google Scholar]
- Schols A. M., Soeters P. B., Dingemans A. M., Mostert R., Frantzen P. J., Wouters E. F. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993 May;147(5):1151–1156. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- Schols A. M., Soeters P. B., Mostert R., Saris W. H., Wouters E. F. Energy balance in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991 Jun;143(6):1248–1252. doi: 10.1164/ajrccm/143.6.1248. [DOI] [PubMed] [Google Scholar]
- Schols A. M., Wouters E. F., Soeters P. B., Westerterp K. R. Body composition by bioelectrical-impedance analysis compared with deuterium dilution and skinfold anthropometry in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991 Feb;53(2):421–424. doi: 10.1093/ajcn/53.2.421. [DOI] [PubMed] [Google Scholar]
- Seckinger P., Isaaz S., Dayer J. M. Purification and biologic characterization of a specific tumor necrosis factor alpha inhibitor. J Biol Chem. 1989 Jul 15;264(20):11966–11973. [PubMed] [Google Scholar]
- Sridhar M. K., Carter R., Lean M. E., Banham S. W. Resting energy expenditure and nutritional state of patients with increased oxygen cost of breathing due to emphysema, scoliosis and thoracoplasty. Thorax. 1994 Aug;49(8):781–785. doi: 10.1136/thx.49.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal-van den Brekel A. J., Schols A. M., ten Velde G. P., Buurman W. A., Wouters E. F. Analysis of the energy balance in lung cancer patients. Cancer Res. 1994 Dec 15;54(24):6430–6433. [PubMed] [Google Scholar]
- Strassmann G., Fong M., Kenney J. S., Jacob C. O. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992 May;89(5):1681–1684. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. O., Rogers R. M., Wright E. C., Anthonisen N. R. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989 Jun;139(6):1435–1438. doi: 10.1164/ajrccm/139.6.1435. [DOI] [PubMed] [Google Scholar]
- van der Poll T., Sauerwein H. P. Tumour necrosis factor-alpha: its role in the metabolic response to sepsis. Clin Sci (Lond) 1993 Mar;84(3):247–256. doi: 10.1042/cs0840247. [DOI] [PubMed] [Google Scholar]