Abstract
Background:
Endovascular aneurysm repair (EVAR) is one of the first-line therapies of abdominal aortic aneurysms. Postoperative endoleak is the most common complication of EVAR. Computed tomography angiography (CTA), which is routine for follow-up, has side effects (e.g., radiation) and also has a certain percentage of missed diagnosis. Preliminary studies on contrast-enhanced ultrasound (CEUS) have shown that the sensitivity of CEUS for detecting endoleak is no lower than that of CTA. To investigate the advantages of CEUS, we conducted CEUS examinations of post-EVAR cases in which CTA failed to detect endoleak or could not verify the type of endoleak.
Methods:
Post-EVAR patients, who were clinically considered to have endoleak and met the inclusion criteria were enrolled between March 2013 and November 2014. All of the patients underwent color Doppler flow imaging (CDFI) and a CEUS examination. Size, location, microbubble dispersion, and hemodynamic characteristics of leaks were recorded. Comparison between the diagnosis of CEUS and CDFI was conducted using Fisher's exact test and clinical outcomes of all patients were followed up.
Results:
Sixteen patients were enrolled, and 12 (75%) had endoleaks with verified types by CEUS. Among 12 cases of endoleaks were positive by CEUS, 10 were CDFI-positive, and the four CEUS-negative cases were all negative by CDFI. The diagnostic values of CEUS and CDFI were statistically different (P = 0.008). Six patients with high-pressure endoleaks received endovascular re-intervention guided by CEUS results. One patient with type III endoleak had open surgery when endovascular repair failed.
Conclusions:
CEUS is a new, safe, and effective means for detection of endoleaks post-EVAR. This technique can be used as a supplement for routine CTA follow-up to provide more detailed information on endoleak and its category.
Keywords: Abdominal Aortic Aneurysm, Color Doppler Flow Imaging, Contrast-enhanced Ultrasound, Endoleak, Endovascular Aneurysm Repair
INTRODUCTION
Large-scale epidemiological studies have shown that the incidence of abdominal aortic aneurysm (AAA) in senior citizens is as high as 4.9–8%, composing one of the major risks to the aged population.[1,2,3] Endovascular aneurysm repair (EVAR) is one of the first-line treatments for AAA. Because of the mini-invasiveness and efficacy of EVAR, it accounts for 42–50% of total surgeries on AAA.[4,5,6] Endoleak is the most common post-EVAR complication and is widely regarded by vascular surgeons as the Achilles's heel of EVAR, causing failure of EVAR. The incidence rate of endoleak post-EVAR is 15–23%.[7] Computed tomography angiography (CTA) is the most common method for detecting postsurgery endoleak, and is the gold standard for diagnosing endoleak. However, there are still some post-EVAR cases with increasing aneurysmal volume, which indicates endoleak, but this is missed by CTA. Additionally, some patients are diagnosed with endoleak by CTA without accurate typing, which is crucial for treatment options. Furthermore, because of shortcomings of CTA, such as radioactivity and allergies caused by iodine contrast agents, frequent use of CTA during follow-up has certain risks. Recently, some researchers have started to attempt contrast-enhanced ultrasound (CEUS) for detecting endoleaks. Comparative studies of CEUS and CTA have indicated that the sensitivity of CEUS in detecting endoleak is no lower than that of CTA.[8,9,10] However, these studies only focused on the ability to detect endoleak among the whole post-EVAR population. Few reports have focused on cases of a growing aneurysm, but with false negative results of CTA, which creates a dilemma for surgeons.
Therefore, this study aimed to examine the advantages of CEUS in these cases. We conducted CEUS examinations in post-EVAR patients from March 2013 to November 2014 in which CTA failed to detect endoleaks or could not verify the type of endoleaks.
METHODS
Ethics statement
The study was endorsed by Ethics Committee of Peking Union Medical College Hospital (PUMCH). The process and potential risks were explained before the examination, and all patients signed informed consent.
Patients
We studied post-EVAR patients from PUMCH who were clinically suspected of endoleak between March 2013 and November 2014.
Enrollment criteria were as follows: (1) Post-EVAR patients with AAA; (2) postsurgery patients with recurrence of obvious pulsation in a physical examination or patients whose imaging tests suggested an increase in the aneurysm body, which indicated endoleak; and (3) cases where CTA failed to detect endoleak or CTA suggested leakage of the contrast agent, while the type of endoleak could not be determined. Exclusion criteria were as follows: (1) Patients with abnormal cardio-pulmonary function; and (2) the interval between CTA and CEUS was more than 2 weeks.
Enrolled patients were divided into two groups. Group A included patients whose aneurysms kept increasing, or pulsation recurred, but endoleak had not been detected yet by CTA. Group B included patients whose CTA examination suggested leakage of the contrast agent, but the type of endoleak could not be determined. All of the patients underwent a CEUS examination for detecting endoleak and were amenable to the assessment of treatment efficacy by CEUS and CTA.
Diagnostic criteria for endoleak
Endoleaks should be categorized into four types according to the reporting standards for endovascular aortic aneurysm repair that was published in 2002 [Table 1].[11] The treatment method was closely related to the type of endoleak. Concerning type II or type IV endoleak with low pressure, monitoring and close follow-up were the choices of surgeons. Treatment, such as embolization of the inferior mesenteric artery (IMA) or lumbar artery, was considered only when the pressure rose, and the aneurysmal volume rapidly increased. Types I and III endoleaks with the high-pressure required timely intervention. According to the characteristics of leaks, different methods could be used. These methods included adding a section of a graft, ligating the neck of the tumor, local embolization, and thrombin injection into the tumor cavity.
Table 1.
Diagnostic criteria for post-EVAR surgery[11]
| Types | Cause of perigraft flow |
|---|---|
| I | Inadequate seal at proximal end of endograft |
| Inadequate seal at distal end of endograft | |
| Inadequate seal at iliac occlude plug | |
| II | Flow from visceral vessel (LA, IMA, accessory renal, and hypogastric) without attachment site connection |
| III | Flow from module disconnection |
| Flow from fabric disruption | |
| IV | Flow from porous fabric (<30 days after graft placement) |
EVAR: Endovascular aneurysm repair; LA: Lumbar artery; IMA: Inferior mesenteric artery.
Ultrasound protocol
Ultrasound equipment (iU22; Philips, Bothell, WA, USA) and the convex array probes C5-2 (2–5 MHz) and C6-2 (2–6 MHz) were used. Patients were asked to fast the morning of the examination.
Color Doppler flow imaging
An examination was conducted under abdominal vascular conditions that were set in advance. Using gray-scale ultrasound, we observed the position, shape, and the internal echo of an aneurysm, as well as the position and shape of the stent. The size of an aneurysm was then measured and segmented into three parts (upper, middle, and lower), and the transverse section was segmented clockwise. The adjustment was made in line with patients’ body shapes. Blood flow within and around the stent, as well as the signal of blood flow from outside of an aneurysm to the inside, were detected by color Doppler flow imaging (CDFI), and the presence of an arterial spectrum was verified. With regard to defined leaks, their position, size, and direction of blood flow were recorded.
Contrast-enhanced ultrasound
The basic conditions of CEUS were machine-designed. Depth was adjusted to suit different body shapes and focus was set at the bottom of images. The mechanical index was 0.05–0.06, the thermal index was 0, and the dynamic range was 80 dB. Once these settings were obtained for each patient, they remained unchanged throughout the whole examination process. The contrast agent used was SonoVue (Braco, Milan, Italy). After routine disinfection, a bolus was injected in the ulnar vein. A bolus injection of 2.4 ml was performed, followed by 5 ml of 0.9% physiological saline.[8,12] First, we started horizontal and continuous dynamic scanning from the top to bottom of the stent. Detection was then focused on the upper and lower margins of the stent and its connection point to determine whether there were microbubbles leaking at the periphery of the stent's main body, as well as inside and outside of an aneurysm to determine whether a reflux branch artery entered an aneurysm. Once the position of suspicious microbubble outflow was identified, 2.4 ml of the contrast agent was injected as a bolus intravenously when the previous microbubbles disappeared. Observation then focused on the position of the suspicious endoleak. Phases of leaking were recorded. The width of leaks and reflux branches were measured at the point of microbubble outflow, and the diffusion range of microbubbles within aneurysms was measured. The above process was repeated once when there was difficulty in diagnosis.
All dynamic images were saved on a portable hard drive for later analysis. Doctors with more than 5 years of experience with CEUS performed ultrasound examinations. The doctors drew conclusions from all of the dynamic and static ultrasound images, while blinded to CTA results during the whole process.
Computed tomography angiography protocol
Previous CTA was performed with a dual-source dual-energy computed tomography (CT) scanner (Somatom Definition Flash, Siemens Medical Solutions, Erlangen, Germany). A triple-phase CT protocol was carried out with unenhanced, arterial (with bolus-tracking), and portal phases at 65 s with 90 ml of nonionic contrast media Ultravist 370 (Schering AG, Berlin, Germany) at a flow rate of 4 ml/s. The acquisition thickness was 1 mm and reconstruction was performed at 1 and 7 mm using a soft kernel algorithm (B30), with 1 and 7 mm of recon increment. Precontrast and arterial phases scans were carried out with 120 kVp and 210 mAs. The portal phase was performed with a dual-energy mode of 100/140 kv and 210 mAs. An experienced vascular surgeon and a radiologist who specialized in vascular diseases made CTA diagnoses independently. When there was a difference in diagnosis, they discussed the diagnosis to make the final decision.
Statistical analysis
We analyzed data with the statistical software package SPSS 22.0 (IBM SPSS Statistics, Armonk, NY, USA). Data were shown as mean ± standard deviation (SD) for descriptive statistics and Fisher's exact test was used for calculation. P < 0.05 was the standard of statistical difference.
Follow-up
All of the patients were followed up from the date of examination to the time of writing this article. The clinical outcomes of patients, and CTA or ultrasound results during follow-up were recorded.
RESULTS
Baseline characteristics
We enrolled 16 patients who were clinically considered of having endoleaks based on the enrollment criteria for this study, aged from 53 to 84 years (mean, 71.2 ± 8.3 years). Among the 16 patients, 14 were men and 2 were women. The sizes of aneurysms ranged from 5.9 to 11.3 cm, with a mean of 8.3 ± 1.7 cm.
Diagnostic performance
The total dosage of SonoVue for each patient ranged from 4.8 to 9.6 ml. No side effects occurred during the CEUS process. Out of 16 patients, 12 had endoleaks detected with verified types using CEUS, including one type Ia [Figure 1], two type Ib [Figure 2], five type II [Figure 3], and five type IIIa endoleaks.
Figure 1.
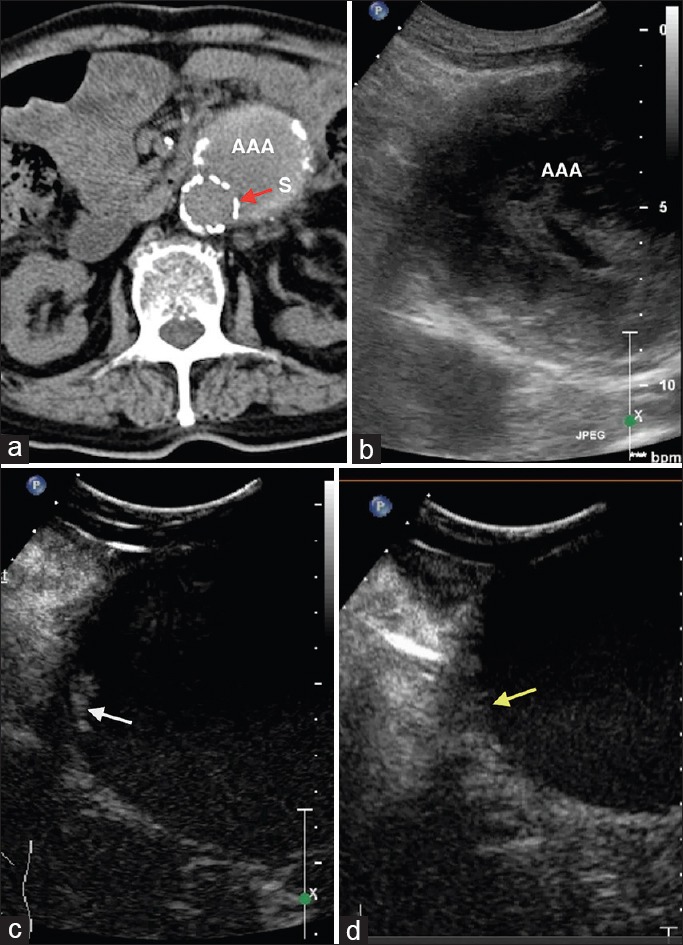
A 84-year-old male patient, postendovascular aneurysm repair. (a) Computed tomography angiography shows no endoleak. S: Stent (red arrow). (b) Gray-scale ultrasound. (c) Contrast-enhanced ultrasound shows a small endoleak lateral to the upper end of the stent (type Ia) (white arrow). (d) After re-intervention, contrast-enhanced ultrasound shows no endoleak (yellow arrow).
Figure 2.
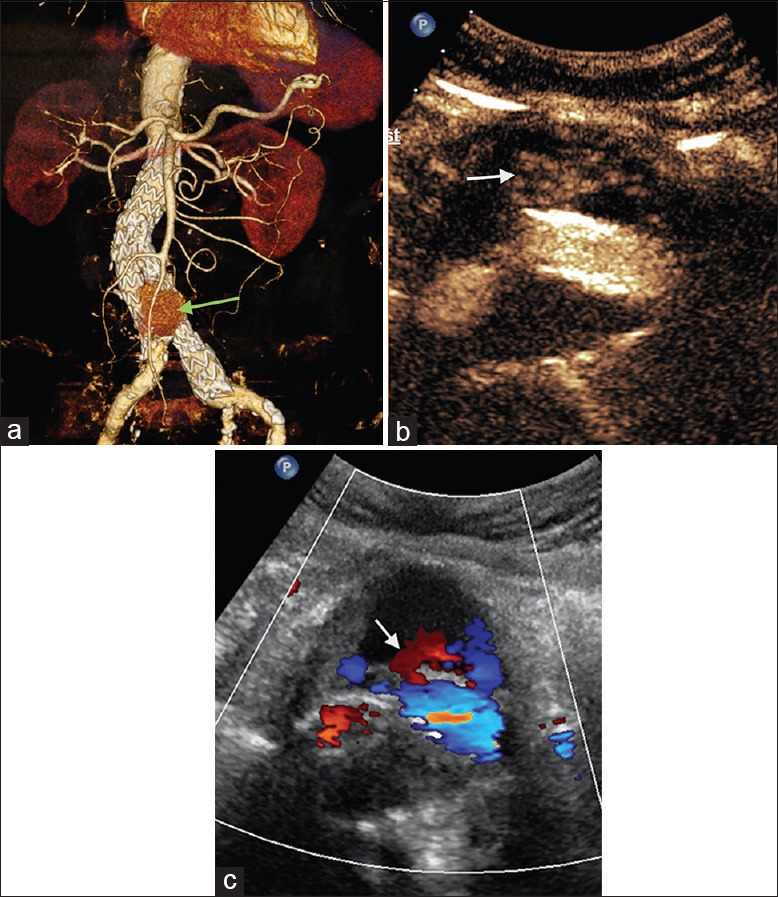
A 83-year-old male patient, postendovascular aneurysm repair. (a) Computed tomography angiography shows contrast agent around the stent with obscure leak site (green arrow). (b) Contrast-enhanced ultrasound shows leak at the lower part of the stent's right leg (type Ib) (white arrow). (c) Color Doppler flow imaging shows outflow from the stent (white arrow).
Figure 3.
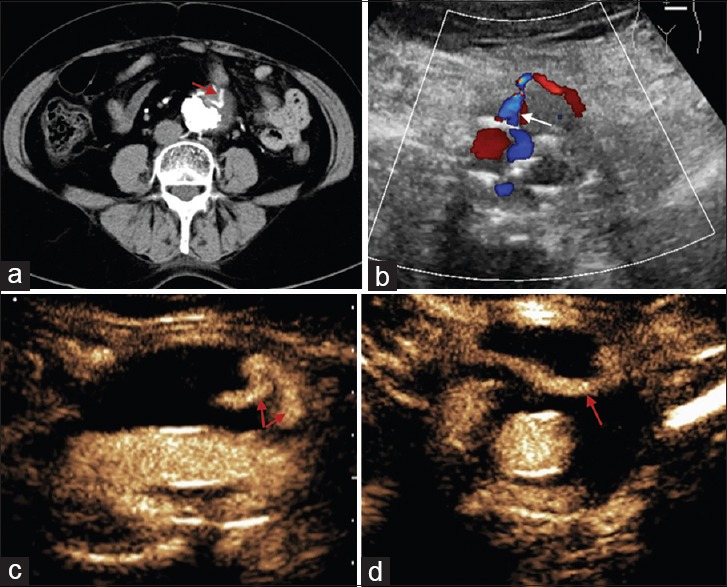
A 71-year-old female patient, postendovascular aneurysm repair. (a) Computed tomography angiography shows an endoleak in an aneurysm (red arrow). (b) Color Doppler flow imaging shows the leak's origin from an inferior mesenteric artery (white arrow). (c) Contrast-enhanced ultrasound demonstrates two reflux flow (red arrows), and (d) the flow unseen by color Doppler flow imaging and computed tomography angiography travels horizontally (red arrow).
Among 12 missed cases by CTA in Group A, eight were verified as having endoleak by CEUS and seven were verified by CDFI. For four cases with unknown type by CTA in Group B, four were verified and typed by CEUS and three by CDFI [Table 2].
Table 2.
Clinically suspected endoleaks of AAA post-EVAR as assessed by CEUS and CDFI
| Patient number | Group by CTA* | CEUS | CDFI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leak type† | Leak site‡ | Width of leak (cm) | Time phase (vs. in-stent) | Range of bubbles (cm × cm) | Flow shape | Flow direction | Flow velocity (cm/s) | ||
| 1 | A | Ia | U, 3 | 0.2 | Sync.§ | 0.7×0.2 | – | – | – |
| 2 | A | N | – | – | – | – | – | – | – |
| 3 | A | II, LA | M, 5 | 0.4 | 2 s delay | 1.8×1.0 | Thick, curved | Out to aneurysm | 25 |
| 4 | A | II, IMA | M,3 | 0.5 | 1.5 s delay | 4.1×2.3 | Thick short | Out to aneurysm | 30 |
| 5 | A | N | – | – | – | – | – | – | – |
| 6 | A | IIIa | M, 5 | 0.2 | Sync. | 1.1×0.6 | Thin, short | Stent to aneurysm | 120 |
| 7 | A | II, IMA | M, 1 | 0.4 | 1.5 s delay | 3.6×2.2 | Thick, short | Out to aneurysm | 35 |
| 8 | A | N | – | – | – | – | – | – | – |
| 9 | A | N | – | – | – | – | – | – | – |
| 10 | A | IIIa | M, 2 | 0.1 | Sync. | 0.9×0.1 | Thin, short | Stent to aneurysm | 103 |
| 11 | A | II, IMA | M, 2 | 0.2 | 1.5 s delay | 1.0×0.5 | Thin, short | Out to aneurysm | 36 |
| 12 | A | IIIa | M, 12 | 0.2 | Sync. | 3.8×2.4 | Thin, short | Stent to aneurysm | 52 |
| 13 | B | Ib | L, 4 | 0.1 | Sync. | 0.7×0.2 | – | – | – |
| 14 | B | Ib | L, 12 | 1.0 | Sync. | 2.9×1.5 | Large flake | Stent to aneurysm | 65 |
| 15 | B | II, IMA | M, 1 | 0.4 | 1 s delay | Branchs through, no dispersion | Large, long, curved | Out to aneurysm | 60 |
| 16 | B | IIIa | L, 12 | 0.5 | Sync. | 0.8×0.5 | Thick, short | Stent to aneurysm | 91 |
*Group by CTA: A, Aneurysms kept increasing or pulsation recurred, but endoleaks were not detected by CTA; B, CTA suggested leakage of the contrast agent but did not verify the type; †Leak type: N: None; IMA: Inferior mesenteric artery; LA: Lumbar artery; ‡Leak site: U: Upper part of the aneurysm; M: Middle; L: Lower; digitals after represent the position clockwise on the transverse section; §Sync.: Synchronized (i.e., the time that microbubbles leaking out were synchronized with emergence of microbubbles in the stent). AAA: Abdominal aortic aneurysm; EVAR: Endovascular aneurysm repair; CEUS: Contrast-enhanced ultrasound; CDFI: Color Doppler flow imaging; CTA: Computed tomography angiography.
Among 12 CEUS-positive cases, 10 were positive by CDFI. The four CEUS-negative cases were all negative by CDFI. The diagnostic values of CEUS and CDFI in assessing post-EVAR endoleak in our cases were statistically different (P = 0.008). Additionally, for case no. 15, CDFI showed that there was only one reflux branch artery entering the aneurysm, but CEUS showed that there was still another parallel branch of blood flow [Figure 3].
Clinical outcome
The time span of follow-up was 5–25 months, with a mean of 17.3 ± 6.4 months. Six patients with high-pressure endoleaks (three cases of type I and three cases of type III) received endovascular re-intervention guided by CEUS results. One patient with type III endoleak received open surgery for excising an aneurysm combined with implanting artificial blood vessels when endovascular repair failed for the 2nd time. At the time of writing this article, another patient with type III endoleak was waiting to be admitted for endoluminal re-treatment [Table 3].
Table 3.
Clinical follow-up outcomes post-CEUS
| Patient number | Endoleak type* | Treatment | Follow-up (months) | Outcome |
|---|---|---|---|---|
| 1 | Ia | Endovascular re-intervention | 25 | CEUS: Endoleak disappeared after re-intervention |
| 2 | N | Observation | 23 | CTA (−) |
| 3 | II, LA | Observation | 23 | CTA (−) |
| 4 | II, IMA | Observation | 21 | CTA: Slight increase of aneurysm without detected endoleak |
| 5 | N | Observation | 21 | CTA (−) |
| 6 | IIIa | Endovascular re-intervention | 21 | CEUS (−) |
| 7 | II, IMA | Observation | 20 | CTA (−) CEUS: II, IMA, same as before |
| 8 | N | Observation | 20 | CTA (−) |
| 9 | N | Observation | 18 | CTA (−) |
| 10 | IIIa | Endovascular re-intervention | 13 | CEUS: Type II endoleak detected |
| 11 | II, IMA | Observation | 6 | CEUS: Same as before without increasing size of aneurysm |
| 12 | IIIa | Open surgery after failed endovascular re-intervention | 5 | CDFI (−) |
| 13 | Ib | Endovascular re-intervention | 21 | CEUS and CTA (−) |
| 14 | Ib | Endovascular re-intervention | 18 | CTA and CEUS (−) |
| 15 | II, IMA | Observation | 16 | CEUS: Endoleak same as before without increasing size of aneurysm |
| 16 | IIIa | Observation | 6 | CEUS (+), CTA (+), size of aneurysm increases, and waiting in line for endovascular surgery |
*Endoleak type: N: None; IMA: Inferior mesenteric artery; LA: Lumbar artery; CEUS: Contrast-enhanced ultrasound; CTA: Computed tomography angiography; CDFI: Color Doppler flow imaging.
DISCUSSION
We investigated the performance of CEUS on post-EVAR endoleaks not correctly diagnosed by CTA, and also compared the diagnostic value of CEUS and CDFI in these cases. There were two notable features in our study: (1) We focus on the cases misdiagnosed by CTA and find CEUS make accurate diagnosis on most of them, suggest that CEUS do provide more diagnostic information on endoleaks when confronted by suspected false negative results of CTA. (2) We report CEUS and CDFI imaging characteristics on different types of endoleaks thoroughly, not just comparing statistically as most previous studies have done. This will be more practical for clinical reference.
Monitoring post-endovascular aneurysm repair
Since 1991, an endoluminal intervention was applied to place stents in the vessel to separate blood flow from an aneurysm and relieve pressure on the wall of an aneurysm.[13] Owing to EVAR's effectiveness, minimal invasiveness, greatly shortened hospital stay, and reduction of short-term mortality after surgery, an increasing amount of surgeons have used this technology as the first alternative in treating AAA.[4,5,6,14]
Because of the risk of endoleak after EVAR, regular radiological monitoring after EVAR needs to be performed. At present, most vascular surgeons resort to periodic postsurgery review by CTA, with a frequency of 1, 6, and 12 months, and yearly afterward.[15] However, there is a certain level of false negatives in the detection of endoleak by CTA.[11,16,17,18] All of our 16 missed or untyped cases by CTA had clinical evidence of endoleak. Without a clear imaging diagnosis using CEUS, the surgeons could not have made treatment decisions.
More importantly, because these patients are subject to long-term radiological follow-up, CEUS could be a safer choice. The main contents of the contrast agent are microbubbles, which can be removed through the respiratory system. Therefore, CEUS is free from not only the cellular damage of X-rays, but also renal toxicity. And in our study, none of the patients had any allergies or discomfort during the CEUS examination.
Contrast-enhanced ultrasound versus color Doppler flow imaging for detection of endoleak
CDFI could be an alternative to CEUS for detecting blood flow within a stent or from outside of an aneurysm to the inside. Some studies have shown that CDFI is capable of capturing the vast majority of clinically meaningful endoleaks and it may safely replace CTA for post-EVAR surveillance.[16,19,20] There was also scholar suggested that because the majority of endoleaks requiring intervention have CTA-positive results or related symptoms in the early stage after surgery, CDFI could be used in follow-up if the CTA results do not show any abnormalities.[21] In our patients, the detection rate of endoleak by CEUS was higher than that by CDFI. There were two types of situations as follows: (1) There were no signals of blood flow by CDFI at the same sites that CEUS detected endoleak; and (2) when CEUS and CDFI detected endoleak, CEUS provided more information on endoleaks. This information included the dispersion mode of microbubbles and the time phase of microbubble outflow, which are helpful for identifying the type of endoleak and its severity. This advantage may be caused by the higher resolution of CEUS and exemption from the effect of sound beam angles, which cannot be avoided by CDFI. Examples of this advantage in our patients are as follows. In case 1, endoleak was caused by the loose isolation between an upper stent and an aneurysm, and CDFI failed to detect signals of blood flow because of the limited flow volume and angle of blood flow. However, continuous leakage of a small amount of microbubbles was observed at the site during CEUS [Figure 1]. In case 15, endoleak was detected by CDFI, but it only displayed blood flow moving towards the rear of an aneurysm. However, by CEUS, another branch of the reflux blood flow coursing horizontally to the right side of an aneurysm was also detected. The reason why CDFI failed to detect this branch is because blood flow could not be demonstrated vertical to the sound beam [Figure 3]. Another weakness of CDFI is that it is susceptible to disturbances of stents, especially metal stents, or calcified plaques of aneurysms, and these form artifacts. Furthermore, the detection ability of CDFI is limited when blood flow has a low velocity.[22]
Diagnostic advantages of contrast-enhanced ultrasound over computed tomography angiography
Our study showed that CEUS was able to be used to identify the type of endoleak in 75% of patients with a failed diagnosis by CTA, half of which with a high risk received surgical intervention guided by CEUS results. Based on our findings, the advantages of CEUS over CTA are as follows: (1) One advantage is the use of a second-generation microbubble contrast agent where microbubbles are 2–5 μm in diameter and have good vibration and echoing, which indicate great improvement in imaging resolution. (2) Microbubbles can remain as long as 5–6 min in the blood pool, which enables physicians to search for small endoleaks. However, with CTA, a lengthened time during the process means an increase in radiation dosage and involves ethics issues. (3) CTA can be used to identify local accumulation of a contrast agent, but sometimes layer thickness of CTA scan may lead to missing a small site of leakage. CEUS is real-time and its dynamic nature can help doctors better understand the hemodynamics of blood flow. (4) Because of the safety of microbubbles, in some difficult cases, repeated injection can be applied for more thorough observation.
Several studies have shown that the general sensitivity of CEUS in detecting endoleak is equal to CTA.[8,10] A large-sample meta-analysis indicated that when CTA was used as the gold standard in detecting endoleaks, there were false positive results comparing CEUS with CTA.[17] The authors believed that this issue resulted from the false negativity of CTA.[17]
In our 16 patients, there were still four patients in whom endoleak could not be detected by CEUS, but clinically, growing aneurysms were present. One limitation of CEUS is its operator-dependence for making an accurate diagnosis because skilled experience in general ultrasound and CEUS is required. And because abdominal gas will affect image quality, strict fasting in the morning is required for the examination. Obesity in patients is also challenging in CEUS.
This study was a preliminary study with a relatively small sample. Therefore, further studies are required to provide more supportive information for CEUS as a routine surveillance after EVAR for AAA.
In conclusion, based on advantages of CEUS, some scholars suggest using CEUS as the preferred alternative for follow-up of post-EVAR AAA.[8,23,24] In addition to the safety and diagnostic accuracy of CEUS, there are still other advantages, such as lower price compared with CTA, and it is portable and convenient for conducting ongoing surveillance of surgery. CEUS provides a new, safe, effective, economic, and noninvasive follow-up method for post-EVAR patients with AAA. Our preliminary study suggests that CEUS can be combined with CTA to detect endoleaks after EVAR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649–56. doi: 10.1002/bjs.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 3.Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):S1–58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT, Jr, Kohler TR, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367:1988–97. doi: 10.1056/NEJMoa1207481. [DOI] [PubMed] [Google Scholar]
- 5.Mehta M, Byrne J, Darling RC, 3rd, Paty PS, Roddy SP, Kreienberg PB, et al. Endovascular repair of ruptured infrarenal abdominal aortic aneurysm is associated with lower 30-day mortality and better 5-year survival rates than open surgical repair. J Vasc Surg. 2013;57:368–75. doi: 10.1016/j.jvs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Reimerink JJ, Hoornweg LL, Vahl AC, Wisselink W, van den Broek TA, Legemate DA, et al. Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: A multicenter randomized controlled trial. Ann Surg. 2013;258:248–56. doi: 10.1097/SLA.0b013e31828d4b76. [DOI] [PubMed] [Google Scholar]
- 7.Grande W, Stavropoulos SW. Treatment of complications following endovascular repair of abdominal aortic aneurysms. Semin Intervent Radiol. 2006;23:156–64. doi: 10.1055/s-2006-941446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantisani V, Ricci P, Grazhdani H, Napoli A, Fanelli F, Catalano C, et al. Prospective comparative analysis of colour-Doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2011;41:186–92. doi: 10.1016/j.ejvs.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Lin PH, et al. Contrast-enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: Improved efficacy using a continuous infusion technique. J Vasc Surg. 2006;43:259–64. doi: 10.1016/j.jvs.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 10.Perini P, Sediri I, Midulla M, Delsart P, Mouton S, Gautier C, et al. Single-centre prospective comparison between contrast-enhanced ultrasound and computed tomography angiography after EVAR. Eur J Vasc Endovasc Surg. 2011;42:797–802. doi: 10.1016/j.ejvs.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–60. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 12.Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2009;49:552–60. doi: 10.1016/j.jvs.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–9. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 14.Paravastu SC, Ghosh J, Murray D, Farquharson FG, Serracino-Inglott F, Walker MG. A systematic review of open versus endovascular repair of inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2009;38:291–7. doi: 10.1016/j.ejvs.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Stavropoulos SW, Clark TW, Carpenter JP, Fairman RM, Litt H, Velazquez OC, et al. Use of CT angiography to classify endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2005;16:663–7. doi: 10.1097/01.RVI.0000152386.97448.F1. [DOI] [PubMed] [Google Scholar]
- 16.Bakken AM, Illig KA. Long-term follow-up after endovascular aneurysm repair: Is ultrasound alone enough? Perspect Vasc Surg Endovasc Ther. 2010;22:145–51. doi: 10.1177/1531003510382664. [DOI] [PubMed] [Google Scholar]
- 17.Mirza TA, Karthikesalingam A, Jackson D, Walsh SR, Holt PJ, Hayes PD, et al. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: Systematic review and bivariate meta-analysis. Eur J Vasc Endovasc Surg. 2010;39:418–28. doi: 10.1016/j.ejvs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 18.White SB, Stavropoulos SW. Management of Endoleaks following Endovascular Aneurysm Repair. Semin Intervent Radiol. 2009;26:33–8. doi: 10.1055/s-0029-1208381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning BJ, O’Neill SM, Haider SN, Colgan MP, Madhavan P, Moore DJ. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: A comparison with computed tomography aortography. J Vasc Surg. 2009;49:60–5. doi: 10.1016/j.jvs.2008.07.079. [DOI] [PubMed] [Google Scholar]
- 20.Chaer RA, Gushchin A, Rhee R, Marone L, Cho JS, Leers S, et al. Duplex ultrasound as the sole long-term surveillance method post-endovascular aneurysm repair: A safe alternative for stable aneurysms. J Vasc Surg. 2009;49:845–9. doi: 10.1016/j.jvs.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 21.Patel MS, Carpenter JP. The value of the initial post-EVAR computed tomography angiography scan in predicting future secondary procedures using the Powerlink stent graft. J Vasc Surg. 2010;52:1135–9. doi: 10.1016/j.jvs.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Millen A, Canavati R, Harrison G, McWilliams RG, Wallace S, Vallabhaneni SR, et al. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg. 2013;58:18–23. doi: 10.1016/j.jvs.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 23.Abbas A, Hansrani V, Sedgwick N, Ghosh J, McCollum CN. 3D contrast enhanced ultrasound for detecting endoleak following endovascular aneurysm repair (EVAR) Eur J Vasc Endovasc Surg. 2014;47:487–92. doi: 10.1016/j.ejvs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Motta R, Rubaltelli L, Vezzaro R, Vida V, Marchesi P, Stramare R, et al. Role of multidetector CT angiography and contrast-enhanced ultrasound in redefining follow-up protocols after endovascular abdominal aortic aneurysm repair. Radiol Med. 2012;117:1079–92. doi: 10.1007/s11547-012-0809-x. [DOI] [PubMed] [Google Scholar]


