To the Editor: Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine that plays a crucial role in the pathogenesis of rheumatoid arthritis (RA), while the advent of anti-TNF-α agents brings better control and low side effects in RA. The most frequent side effects are infectious disease and drug-induced lupus. Herein, we present a case of Chinese man with a 10-year history of RA developing crescentic immunoglobulin A (IgA) nephritis and multiple autoantibodies during adalimumab treatment.
A 62-year-old Chinese man was admitted for new occurrence of hematuria and proteinuria for 20 days. He had a 10-year history of RA diagnosed according to the American College of Rheumatology Criteria.[1] Thereafter, a low dose of prednisone, oral methotrexate (15 mg/week) and intermittent administration of nonsteroidal anti-inflammatory drugs (NSAIDs) were initiated. Because of tenderness of multiple joints and new occurrence of rheumatoid nodules on the left knee on 16 February 2014, and erythrocyte sedimentation rate (ESR) increased to 70 mm/h and C-reactive protein (CRP) 16.3 mg/L, with a high disease activity score for 28 joints (DAS28) scored 5.44, adalimumab (40 mg subcutaneous every other week) was initiated. Antinuclear antibody (ANA) and antineutrophil cytoplasmic antibody (ANCA) were not detected, and renal parameters were normal before adalimumab treatment. After four doses of adalimumab therapy (2 months later), rheumatoid nodules resolved and ESR decreased to 13 mm/h, CRP was 1.7 mg/L, and DAS28 scored 2.0 suggestive of clinical remission; however, he presented with hematuria, proteinuria and oliguria.
On admission, bilateral pitting edema was found in the lower extremities. His hands showed deformities. Laboratory findings showed elevated white blood cell count, 15.72 × 109/L (normal range: 4.0–10.0 × 109/L); hemoglobin, 90 g/L (120–160); platelets, 232 × 109/L (normal range: 100–300 × 109/L); serum urea nitrogen, 31.84 mmol/L (normal range: 1.07–7.14 mmol/L); serum creatinine (Cr), 249 μmol/L (normal range: 45–84 μmol/L); urinary protein excretion was 5.41 g/24 h, and calculated eGFR was 23 ml·min−1·1.73 m−2. On serologic examination, ESR was 38 mm/h (normal range: 0–20 mm/h); CRP, 16.21 mg/L (normal range: 0–3 mg/L), ANA was 1:80 positive (homogeneous pattern); anticardiolipin (ACL) antibody, 24 pLIgG U/ml (normal range: 0–12 U/ml); perinuclear-ANCA, 1:80 positive; while myeloperoxidase-ANCA and proteinase 3-ANCA were negative. Anti-double-stranded DNA, anti-extractable nuclear antigen antibody, anti-glomerular basement membrane antibody and cryoglobulin were not detected. Rheumatoid factor (RF) was 95.8 U/ml (normal range: 0–20 U/ml); IgA, 6.13 g/L (normal range: 0.7–4.0 g/L); C3 complement fraction, 0.585 g/L (normal range: 0.7–1.4 g/L); IgG, IgM and C4 complement fraction values were normal. Hepatitis B surface antigen, hepatitis C virus antibody, human immunodeficiency virus antibody and anti-streptolysin O test were all negative. Computed tomography scan of chest and abdomen showed no evidence of pulmonary vasculitis and solid tumor. Renal ultrasound showed 10.8 cm of right kidney, 11.7 cm of left kidney.
Renal bioposy was performed. The specimen [Figure 1a and b] showed 21 glomeruli, with 9 globally sclerosed glomerulus. It showed proliferation of mesangial cells and matrix and 11 active cellular crescents were observed. There were red blood cell casts and lymphocytic infiltrates in the interstitium. On immunofluorescence (IF) examination [Figure 1c], IgA deposits of strong intensity and C3, κ and λ light chain deposits of weak intensity were detected in the mesangium. IF stainings for IgG, IgM, C4, C1q and fibrinogen were all negative. On electron microscopic examination, electron-dense deposits of high density were detected in the mesangium. Accordingly, crescentic IgA nephritis was diagnosed.
Figure 1.
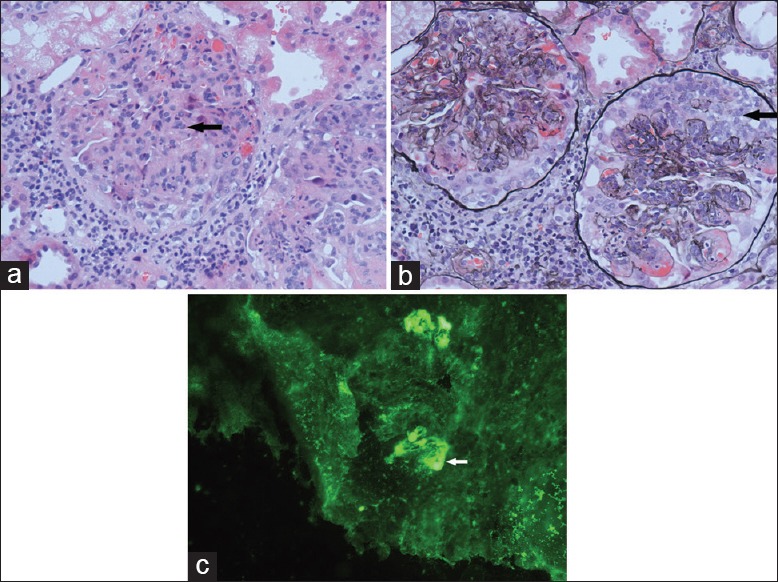
Renal pathology of the patient. (a) H and E staining (original magnification, ×400) of renal biopsy demonstrating proliferation of mesangial cells and matrix (arrow). (b) Periodic acid-silver metheramine staining (original magnification, ×400) of renal biopsy showed cellular crescents (arrow). (c) Immunofluorescence of renal biopsy showed immunoglobulin A deposits in the mesangium (arrow).
Adalimumab was discontinued. Pulse intravenous methylprednisolone (500 mg daily) for 3 consecutive days and followed by oral prednisone (1 mg·kg−1·d−1) and oral cyclophosphamide (1 mg/kg every other day) were given. Consequently, proteinuria decreased to 1.42 g/24 h, but the serum Cr increased to 709 μmol/L, and renal replacement therapy with hemodialysis was started 3 times/week. Unfortunately, Cytomegalovirus infection and pulmonary infection happened after 1 week of immunosuppressive therapy; Considering that the infection complications may be potentially life-threatening, we choose to reduce the doses of prednisone and cyclophosphamide and add anti-infection treatment. Followed up for 3 months, he still needed hemodialysis since his renal function was not restored.
RA is an autoimmune systemic disease, and the most common renal disorders associated with RA or drugs used in treatment such as NSAIDs and disease-modifying antirheumatic drugs (DMARDs) are secondary amyloidosis, rheumatoid vasculitis, analgesic nephropathy and drug-induced membranous nephropathy, especially gold salt and penicillamine;[2] however, IgA nephritis associated with RA was quite rare and there are no reports of crescentic IgA nephritis secondary to the use of NSAIDs or DMARDs. Though our case presented with multiple autoantibodies including ANA, ACL and perinuclear-ANCA, lupus nephritis and pauci-immune crescentic glomerulonephritis associated with ANCA cannot be diagnosed since there is no features suggestive of “full-house” and this patient showed the classic immunohistological features of IgA nephritis with C3 activation, the absence of other Igs and C1q. Accordingly, crescentic IgA nephritis should be diagnosed.
Adalimumab, a fully humanized monoclonal antibody that binds both soluble and membrane-bound TNF-α, has brought RA patients better disease control and was suggested for moderate–severe active RA patients. There were reports about new onset of lupus nephritis and ANCA-associated crescentic glomerulonephritis during anti-TNF agent therapy, but crescentic IgA nephritis was quite rarely reported. The probable pathogenic role of adalimumab in this case is suggested by: (1) The improvement of hematuria and proteinuria after adalimumab withdrawal and initiation of corticosteroids and immunosuppressive therapy, (2) the temporal relation of new-onset acute renal injury to use of adalimumab with long-standing RA of many years and renal function and urinalysis were normal before adalimumab treatment and (3) no signs of rheumatoid vasculitis by taking into account low titer of RF, the improvement of rheumatoid nodules and the absence of other signs of vasculitis.
A previous report[3] showed that renal damage could resolve completely or partially after anti-TNF agent withdrawal and subsequent use of immunosuppressants. Why did our case progress to end-stage renal disease? In our opinion, the co-existence of multiple autoantibodies including ANA, ACL antibody, and ANCA may prompt the immune complex deposition and activate complement unlike the reported cases before. In addition, the infectious complications might contribute to the deterioration of renal function through mechanisms such as molecular mimicry or epitope spreading[4] which could promote autoimmune reactions.
Taken together, we suggest that RA patients especially those with high risk of developing renal disorders be prescribed anti-TNF agents cautiously, and baseline of ANA, anti-dsDNA and ANCA should be screened and renal parameters be monitored closely during anti-TNF agent therapy. Once glomerulonephritis occurs, withdrawal of anti-TNF agents is recommended, and corticosteroid and/or immunosuppresants might be used according to clinical manifestations and kidney biopsy findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Clegg DO, Ward JR. Diagnostic criteria in rheumatoid arthritis. Scand J Rheumatol Suppl. 1987;65:3–11. doi: 10.3109/03009748709102172. [DOI] [PubMed] [Google Scholar]
- 2.Helin HJ, Korpela MM, Mustonen JT, Pasternack AI. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum. 1995;38:242–7. doi: 10.1002/art.1780380213. [DOI] [PubMed] [Google Scholar]
- 3.Piga M, Chessa E, Ibba V, Mura V, Floris A, Cauli A, et al. Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: Systematic literature review and analysis of a monocentric cohort. Autoimmun Rev. 2014;13:873–9. doi: 10.1016/j.autrev.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Prinz JC. Autoimmune-like syndromes during TNF blockade: Does infection have a role? Nat Rev Rheumatol. 2011;7:429–34. doi: 10.1038/nrrheum.2011.35. [DOI] [PubMed] [Google Scholar]


