Abstract
Background:
Sinonasal inverted papilloma (IP) is a rare benign tumor of the nasal cavities and paranasal sinuses. It is destructive or bone-remodeling, tends to recur after surgical resection, and has a significant malignant potential. The present study aimed to perform a retrospective analysis of patients with squamous cell carcinoma (SCC) arising from IP, including characteristics, survival outcome, and predictors of associated malignancy.
Methods:
The medical records of 213 patients diagnosed with IP from January 1970 to January 2014 were retrospectively reviewed. Eighty-seven patients were diagnosed with SCC/IP; their clinical characteristics, treatments, and survival outcomes were analyzed.
Results:
Of the 87 patients with SCC/IP, the 5- and 10-year overall survival outcomes were 39.6% and 31.8%, respectively. Twenty-nine of these patients received surgery and 58 received combined surgery and radiation. Of the patients with stages III–IV, the 5-year survival rate was 30.7% for those treated with surgery only and 39.9% for those given the combination treatment (P = 0.849). Factors associated with significantly poor prognosis were advanced-stage, metachronous tumors, or with cranial base and orbit invasion. Age, synchronous or metachronous tumors, and pathological stage were independent risk factors for mortality, shown by multivariate analysis.
Conclusion:
Patients with SCC/IP had low overall survival outcomes. Advanced age, stage, and metachronous tumors are the main factors affecting prognosis. Treatment planning should consider high-risk factors to improve survival outcome.
Keywords: Sinonasal Inverted Papilloma, Squamous Cell Carcinoma, Survival Outcome
INTRODUCTION
Sinonasal inverted papilloma (IP) or Schneiderian papilloma is a rare benign tumor of the nasal cavities and paranasal sinuses. Sinonasal IPs account for 0.5–4.0% of all nasal tumors and 2–3% of all nasal polyps.[1] An IP usually originates from the ectodermal Schneiderian mucosa of the nasal cavity, characteristically from the lateral nasal wall in the region of the middle turbinate or ethmoid recesses. Although pathologically benign, an IP can be destructive or bone-remodeling tends to recur after surgical resection and has a significant malignant potential.[2,3] IP is also associated with squamous cell carcinoma (SCC). In a report of IP comprising 162 patients, 17 (10.5%) were diagnosed with SCC.[4] SCC in IP (SCC/IP) may occur synchronously or develop after previous resection of IP (metachronous SCC); the rates of these were 7.1% and 3.6% of IP cases, respectively.[5]
Although there have been many studies of benign IP, few have focused on patients with SCC/IP. Because SCC/IP is so rare, there is relatively little information on its natural history or treatment outcomes, and survival outcomes have been considered only in some meta-analyses or reports with a small number of cases. A few reports have suggested that radical resection, with or without adjuvant radiation, can achieve an excellent result with long-term disease-free survival approaching 100%.[6] Another article reported that radiation, with or without surgery, resulted in a 5-year survival rate of 85%.[7] However, a pooled survival analysis showed that patients with SCC/IP have a poor prognosis and the 5-year survival rate was 61%.[8]
In the present study, we retrospectively reviewed medical records of Cancer Hospital, Chinese Academy of Medical Sciences from January 1970 to January 2014 to perform a retrospective analysis of patients with SCC/IP that included characteristics, survival outcome, and predictors of associated malignancy.
METHODS
Patient eligibility
This study was approved by Institutional Review Board of Cancer Hospital, Chinese Academy of Medical Sciences. We retrospectively reviewed the medical records of patients with IP of the sinonasal tract treated from January 1970 to January 2014 at the Department of Head and Neck Surgery of Cancer Hospital, Chinese Academy of Medical Sciences.
Of the 213 patients histologically confirmed with IP, 87 patients were diagnosed with SCC/IP. Eligibility criteria were: Patients primarily treated since malignant transformation, with pathologically confirmed SCC/IP, available pathologic specimen, no distant metastases, and complete medical records. Patients were excluded if they had synchronous active cancer or discontinued treatment.
Pathology specimens were re-examined by a head and neck pathologist. All medical data were reviewed. All deaths were followed up and verified by letters or phone calls.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Survival was estimated using the Kaplan-Meier method. Overall survival was calculated from the date of surgery, or the end day of radiation if radiation was undertaken after surgery, until the time of last follow-up. The Cox regression model was used for multivariate analysis. A P < 0.05 was considered as statistically significant.
RESULTS
Patient characteristics
Among 87 patients with SCC/IP, 64 were men, and 23 were women. The mean age of the entire group was 54 years (range, 17–80 years). Tumors were located at many different sites within the nasal cavities and paranasal sinuses; most (52/87, 59.7%) originated from the ethmoid region (12, 13.8%), maxillary sinus (21, 24.1%), or lateral nasal wall (19, 21.8%) rather than other sites.
According to the staging system of the American Joint Committee on Cancer (AJCC), 87 patients were in cancer stages I–IV, with the majority (85.1%) at stage III or IV. For the original primary tumors, most (83.9%) were at T3 or T4. Pathology results showed that almost half of the tumors (51.7%) were moderately differentiated while the others were well or poorly differentiated [Table 1].
Table 1.
Clinical characteristics of 87 patients with SCC/IP
| Characteristics | Values |
|---|---|
| Age, years, mean (range) | 54 (17–80) |
| Male/female, n | 64/23 |
| Primary site, n (%) | |
| Ethmoid region | 12 (13.8) |
| Maxillary sinus | 21 (24.1) |
| Lateral nasal wall | 19 (21.8) |
| Sphenoid sinus | 7 (8.1) |
| Frontal sinus | 4 (4.6) |
| Inferior turbinate | 5 (5.8) |
| Septum | 3 (3.4) |
| Unidentified | 16 (18.4) |
| Stage, n (%) | |
| I–II | 13 (14.9) |
| III | 30 (34.5) |
| IV | 44 (50.6) |
| T1 | 2 (2.3) |
| T2 | 12 (13.8) |
| T3 | 30 (34.5) |
| T4a | 29 (33.3) |
| T4b | 14 (16.1) |
| N0 | 57 (65.5) |
| N1 | 2 (2.3) |
| N2 | 2 (2.3) |
| N3 | 1 (1.15) |
| Pathology, n (%) | |
| Well differentiated | 20 (23.0) |
| Moderately differentiated | 45 (51.7) |
| Poorly differentiated | 22 (25.3) |
SCC: Squamous cell carcinoma; IP: Inverted papilloma.
Treatment
Of the 87 patients with SCC/IP, 29 received surgery as their initial treatment. Among these 29 patients, 7 (stages I–II) and 9 (stage III) had no indication for radiotherapy, and 2 (stage III) and 11 (stage IV) were reluctant to undergo radiotherapy.
Seven (8.0%) patients were resected with a purely endoscopic technique; 25 (28.7%) underwent lateral rhinotomy; 10 (11.5%) extended lateral rhinotomy; 16 (18.4%) subtotal maxillectomy; 9 (10.3%) radical maxillectomy; and 20 (23.0%) craniofacial resection. Six patients concurrently received neck dissection because of cervical lymph node metastasis (3, I–III dissection; 2, I–IV dissection; and 1, II–V dissection).
Fifty-eight patients received combined surgery and radiation treatment as their initial treatment. Of these patients, 32 received preoperative radiotherapy (mean, 50.84 Gy; range, 10–70 Gy), but one patient refused treatment after 10 Gy radiation. The remaining 26 patients received postoperative radiotherapy (mean, 54.37 Gy; range, 30–74 Gy). Six of the 26 patients who received postoperative radiotherapy also received concurrent chemotherapy (cisplatin weekly at 30 mg/m2 body surface, with a total dose of 200–350 mg).
After radical excision of the primary tumors, repair and constructive procedures were performed mostly with a pectoralis major musculocutaneous flap. Others included a submental flap, free forearm flap, or free rectus abdominis myocutaneous flap. The distribution of treatments by stage is summarized in Table 2.
Table 2.
Treatments according to stage of SCC/IP, n (%)
| Treatments | Stages I–II | Stage III | Stage IV | Total |
|---|---|---|---|---|
| Surgery | 7 (24.2) | 11 (37.9) | 11 (37.9) | 29 (33.3) |
| Preoperative radiotherapy + surgery | 4 (12.5) | 12 (37.5) | 16 (50.0) | 32 (36.8) |
| Surgery + postoperative radiotherapy | 0 (0) | 6 (30.0) | 14 (70.0) | 20 (23.0) |
| Surgery + postoperative radiotherapy + chemotherapy | 2 (33.3) | 1 (16.7) | 3 (50.0) | 6 (6.9) |
| Total | 13 (14.9) | 30 (34.5) | 44 (50.6) | 87 (100) |
SCC: Squamous cell carcinoma; IP: Inverted papilloma.
Survival outcome
The Kaplan-Meier estimates showed that the 5- and 10-year overall survival outcomes of patients with SCC/IP were 39.6% and 31.8%, respectively [Figure 1]. Univariate analysis revealed that the factors influencing the overall survival were pathological stage, synchronous or metachronous tumors, and the presence of the cranial base and orbit invasion [Table 3].
Figure 1.
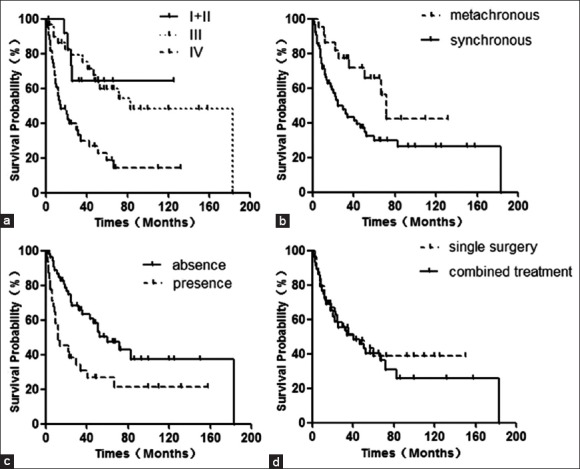
Kaplan–Meier curves for overall survival stratified by stage (a), synchronous or metachronous (b), with or without cranial base and orbit invasion (c), and treatments (d).
Table 3.
Prognostic factors of survival for 87 patients with SCC/IP
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OS (5-year rate), % | P | Relative risk (95% CI) | P | |
| Age (years) | 0.007 | |||
| ≤50 | 31.9 | |||
| >50 | 46.6 | 1.038 (1.010–1.066) | ||
| Gender | – | |||
| Male | 38.7 | 0.801 | ||
| Female | 44.8 | |||
| Tumor stage | 0.000 | |||
| I–II | 64.2 | 0.000 | 1 | |
| III | 59.7 | 0.179 (0.056–0.571) | ||
| IV | 19 | 0.211 (0.092–0.481) | ||
| Cranial base and orbit invasion | ||||
| Yes | 26.9 | 0.003 | ||
| No | 47.5 | |||
| Synchronous or metachronous | 0.000 | |||
| Metachronous | 29.7 | 0.020 | 4.34 (1.957–9.625) | |
| Synchronous | 65.7 | |||
| Differentiation | 0.448 | |||
| Well | 48.5 | 0.349 | 1 | |
| Moderately | 39.2 | 0.865 (0.375–1.996) | ||
| Poorly | 30.0 | 0.640 (0.314–1.305) | ||
| Treatments | 0.551 | |||
| Single surgery | 38.6 | 0.688 | 0.808 (0.402–1.627) | |
| Combined treatment | 40.2 | |||
SCC: Squamous cell carcinoma; IP: Inverted papilloma; OS: Overall survival; CI: Confidence interval.
The 5-year survival rates at stages I–II, III, and IV were 64.2%, 59.7%, and 19.0%, respectively [χ2 = 18.100, P = 0.000; Figure 1a]. The 5-year survival rate for patients who were not treated with surgery for IP was 65.7%; and the rate for those who had surgery was 29.7% [χ2 = 5.446, P = 0.020; [Figure 1b]. For patients who had no involvement of the cranial base and (or) orbit invasion, the 5-year survival rate was 47.5%; the rate for those with such involvement was 26.9% [χ2 = 9.095, P = 0.003; Figure 1c]. For patients with stages III–IV and treated with surgery only, the 5-year survival rate was 30.7%; the 5-year survival rate for those given surgery and radiotherapy was 39.9% [χ2 = 0.036, P = 0.849; Figure 1d].
There were 56 metachronous cases in our study. Of these, the median number of surgeries was 2 (range, 1–9), and there were statistically significant differences among the surgery times (χ2 = 18.805, P = 0.005).
Multivariate analysis showed that age, synchronous or metachronous tumors, and pathological stage were independent factors for mortality risk [Table 3].
The median follow-up time, beginning from the time of initial diagnosis of carcinoma, was 40 months (7–183 months). Six patients (6.9%) were lost during follow-up; the response rate was 93.1%. Among the 87 patients, 50 died from the disease, with mean survival time of 25.7 months (1–183 months). Of these, 27 had distant metastases (11 lung, 8 brain, 6 abdomen, and 2 bone), 7 had local recurrence, 2 had neck recurrence, 9 had uncontrolled loco-regional disease, and 5 died of natural or unknown causes.
DISCUSSION
IP is well-known for its potential malignant transformation. Because malignant transformation takes about 63 months (6–153 months),[9] some patients are lost to follow-up and the transformation has usually reached an advanced-stage before it is diagnosed. In the present study, 85.1% of the patients were in stage III or IV, and most involved invasion of the orbit, cranial base, brain, or other vital organs. Tanvetyanon et al.[8] analyzed 76 cases of SCC/IP and found a median overall survival of 126 months, with 3- and 5-year survival rates of 63% and 61%, respectively. In the present study, the 3- and 5-year survival rates were only 51.3% and 39.6%, respectively.
At present, there are no classification systems for SCC/IP, and for this study, we used the AJCC staging system. For the patients who had surgery for IP, the AJCC staging system may not be accurate, as the assigned stage was based on preoperative computed tomography or magnetic resonance imaging data and the operative records.
The tumor-node-metastasis staging system is a key to predicting the prognosis of patients with cancer. In the current study, patients with early-stage cancer had a longer mean overall survival time compared with patients with advanced-stage cancer. Six patients had cervical lymph node metastasis (21, 13+, 19+, 51, 19, and 60+), with low metastasis rate (6.9%) and poor prognosis.
The presence or absence of cranial base or orbit invasion also influences the survival outcomes: Patients with cranial base or orbit invasion had significantly shorter overall survival time compared with those patients without cranial base and orbit invasion. The involvement of the cranial base and orbit may be the main cause of relapse or metastasis. Oikawa et al.[10] suggested that magnetic resonance imaging is indicated for defining the extent of tumor involvement. Additionally, because tumor metastases may occur in the neck, lung, and brain, Shojaku et al.[11] suggested that preoperative positron emission tomography was necessary. For the resection of tumors involving the cranial base or orbit, the craniofacial or rhinotomy approach is frequently used. The pedicled frontal muscle or galea skull split flap can be used to repair a skull defect after tumor resection. Once the tumor involves the cranial base or orbit, it is difficult for a single operative treatment to achieve a safe resection margin. For SCC/IP patients, comprehensive treatment is necessary to improve survival rate and quality of life, combining head and neck surgery with neurosurgery, reconstruction, radiotherapy, chemotherapy, and other specialist subjects.[12]
In previous reports, the number of synchronous cases exceeded the metachronous,[5] and the traditional risk of malignancy with IP is about 10%. However, in the present study, the ratio was inverted (56/31). It was perhaps the characteristic of the cancer hospital. Patients with small IP tumor were often treated at a community center or the comprehensive hospital, and the patients were sent to cancer hospital due to advanced disease, even they have already received treatments in the comprehensive hospital before. Of the 56 metachronous cases, the number of surgeries significantly affected the overall survival rate. It suggested that in the treatment of IP, the tumors should be removed with a safe resection margin as much as possible to reduce the total number of surgeries for IP although recurrence of IP resulting in malignant transformation is disputed.[13]
Recently, endoscopic-assisted surgical treatment options have been recommended to the otolaryngologist for this disease. Lawson and Patel[14] thought that IP should be addressed endoscopically when possible. In the present study, we made the following correlation analysis. Among the seven patients given endoscopic-assisted surgical treatment, all were synchronous; 5 in stages I–II (71, 109+, 70+, 110, and 144+) and 2 in stage III (45 + and 70). One (45+) was lost to follow-up, and two patients are alive and free of disease. It seems that the outcomes of patients with stages I–II were better than that of patients with stage III. Based on our experiences, SCC/IP at stages I–II and synchronous can be treated with endoscopic-assisted surgery, but further study with more subjects are needed to confirm our findings.
Most clinicians prefer to treat nasal sinus malignant tumor comprehensively. Rajapurkar et al.[15] suggested that adequate local control with preserved visual function could be obtained via surgery and adjuvant radiation, in appropriately selected malignant sinonasal tumors with orbital involvement. Gras-Cabrerizo et al.[16] claimed that surgery combined with postoperative radiotherapy permitted good local control in patients with ethmoid sinus carcinoma but did not recommend prophylactic neck dissection. For SCC/IP, Hug et al.[7] concluded that combined radiotherapy and surgery could enable permanent disease-free survival in patients who achieve local control. In the present study, it was found that for stages III–IV patients, those who received the combined treatment had a better 5-year survival rate than those who underwent only surgery (39.9% and 30.7%, respectively), but the difference was not significant (P > 0.05). Further studies with more samples are needed to confirm this finding.
There were several limitations in our study. The number of cases was limited, and a multicenter study with a large series of cases should be performed to confirm our finding. In addition, this study lacked a treatment group who received only radiation, so we could not evaluate the effect of radiotherapy alone on patients with SCC/IP. Finally, we only included patients who were initially treated in our hospital, for whom pathological specimens were available and who had complete medical records, which could result in selection bias.
In summary, our retrospective study comprehensively reviewed the clinical features and prognosis of patients with SCC/IP. Our results showed that 5- and 10-year overall survival rates were 39.6% and 31.8%, respectively, which was lower than those reported in previous studies. The main factors that affected prognosis were advanced age, stage, and metachronous tumors. Treatment planning should consider high-risk factors to improve survival outcome. Although IP is a rare benign tumor, it has a poor prognosis once it is malignantly transformed, long-term periodic follow-up is necessary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Myers EN, Fernau JL, Johnson JT, Tabet JC, Barnes EL. Management of inverted papilloma. Laryngoscope. 1990;100:481–90. doi: 10.1288/00005537-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald C, Franzmann MB, Tos M. Sinonasal papillomas: A report of 82 cases in Copenhagen County, including a longitudinal epidemiological and clinical study. Laryngoscope. 1995;105:72–9. doi: 10.1288/00005537-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Lawson W, Kaufman MR, Biller HF. Treatment outcomes in the management of inverted papilloma: An analysis of 160 cases. Laryngoscope. 2003;113:1548–56. doi: 10.1097/00005537-200309000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Hong SL, Kim BH, Lee JH, Cho KS, Roh HJ. Smoking and malignancy in sinonasal inverted papilloma. Laryngoscope. 2013;123:1087–91. doi: 10.1002/lary.23876. [DOI] [PubMed] [Google Scholar]
- 5.von Buchwald C, Bradley PJ. Risks of malignancy in inverted papilloma of the nose and paranasal sinuses. Curr Opin Otolaryngol Head Neck Surg. 2007;15:95–8. doi: 10.1097/MOO.0b013e3280803d9b. [DOI] [PubMed] [Google Scholar]
- 6.Thorp MA, Oyarzabal-Amigo MF, du Plessis JH, Sellars SL. Inverted papilloma: A review of 53 cases. Laryngoscope. 2001;111:1401–5. doi: 10.1097/00005537-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Hug EB, Wang CC, Montgomery WW, Goodman ML. Management of inverted papilloma of the nasal cavity and paranasal sinuses: Importance of radiation therapy. Int J Radiat Oncol Biol Phys. 1993;26:67–72. doi: 10.1016/0360-3016(93)90174-t. [DOI] [PubMed] [Google Scholar]
- 8.Tanvetyanon T, Qin D, Padhya T, Kapoor R, McCaffrey J, Trotti A. Survival outcomes of squamous cell carcinoma arising from sinonasal inverted papilloma: Report of 6 cases with systematic review and pooled analysis. Am J Otolaryngol. 2009;30:38–43. doi: 10.1016/j.amjoto.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002;15:279–97. doi: 10.1038/modpathol.3880524. [DOI] [PubMed] [Google Scholar]
- 10.Oikawa K, Furuta Y, Oridate N, Nagahashi T, Homma A, Ryu T, et al. Preoperative staging of sinonasal inverted papilloma by magnetic resonance imaging. Laryngoscope. 2003;113:1983–7. doi: 10.1097/00005537-200311000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Shojaku H, Fujisaka M, Yasumura S, Ishida M, Tsubota M, Nishida H, et al. Positron emission tomography for predicting malignancy of sinonasal inverted papilloma. Clin Nucl Med. 2007;32:275–8. doi: 10.1097/01.rlu.0000257334.65253.cc. [DOI] [PubMed] [Google Scholar]
- 12.Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–70. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 13.Dahl M, Schaffer S, Wisdom GS. Inverting papilloma of the nose and paranasal sinuses. J La State Med Soc. 2003;155:235–7. [PubMed] [Google Scholar]
- 14.Lawson W, Patel ZM. The evolution of management for inverted papilloma: An analysis of 200 cases. Otolaryngol Head Neck Surg. 2009;140:330–5. doi: 10.1016/j.otohns.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Rajapurkar M, Thankappan K, Sampathirao LM, Kuriakose MA, Iyer S. Oncologic and functional outcome of the preserved eye in malignant sinonasal tumors. Head Neck. 2013;35:1379–84. doi: 10.1002/hed.23137. [DOI] [PubMed] [Google Scholar]
- 16.Gras-Cabrerizo JR, Montserrat-Gili JR, León-Vintró X, Massegur-Solench H, de Vega JM, Virós-Porcuna D. Treatment results for ethmoid sinus carcinoma. J Laryngol Otol. 2009;123:1120–4. doi: 10.1017/S0022215109990752. [DOI] [PubMed] [Google Scholar]


