Abstract
Objective
Atrial fibrillation (AF) has been associated with increased mortality in critically-ill patients. We sought to determine whether AF in the intensive care unit (ICU) is an independent risk factor for death. A secondary objective was to determine if patients with new-onset AF have different risk factors or outcomes compared to patients with a previous history of AF.
Design
Prospective observational cohort study.
Setting
Medical and general surgical ICUs in a tertiary academic medical center.
Patients
1,770 critically-ill patients requiring at least 2 days in the ICU.
Interventions
None.
Measurements
Demographics, medical history, development of AF, fluid balance, echocardiographic findings, medication administration, and hospital mortality were collected during the first four days of ICU admission.
Main Results
AF occurred in 236 (13%) patients (Any AF). Of these, 123 patients (7%) had no prior AF (New-onset AF) while the remaining 113 (6%) had Recurrent AF. Any AF was associated with male gender, Caucasian race, increased age, cardiac disease, organ failures, and disease severity. Patients with Any AF had increased mortality compared to those without AF (31% vs. 17%, p <0.001) and Any AF was independently associated with death (OR 1.62, 95% CI 1.14-2.29, p=0.007) in multivariable analysis controlling for severity of illness and other confounders. The association of AF with death was magnified in patients without sepsis (OR 2.92, 95% CI 1.52-5.60, p=0.001). Treatment for AF had no effect on hospital mortality. New-onset AF and Recurrent AF were each associated with increased mortality. New-onset AF, but not Recurrent AF, was associated with increased diastolic dysfunction and vasopressor use and a greater cumulative positive fluid balance.
Conclusions
AF in critical illness, whether new-onset or recurrent, is independently associated with increased hospital mortality, especially in patients without sepsis.
Keywords: atrial fibrillation, intensive care unit, critical illness, mortality, fluid balance, vasopressor
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia in critically ill patients, affecting as many as 25% of patients admitted to non-cardiac surgery intensive care units (ICUs) (1-14). Several studies have identified risk factors associated with development of AF in these patients, but few have been large enough to test the independent contribution of AF to poor clinical outcomes. In addition, management of AF in this population is particularly challenging given that many patients have coincident hypotension that may limit use of both rate- and rhythm-controlling medications.
That the onset of AF during critical illness is associated with poor outcomes in a variety of patient populations is clear. Several previous reports have suggested that development of new-onset AF in the intensive care unit portends increased mortality (1, 3-7, 9, 11-13, 15-17), particularly in patients with sepsis. Despite these reports, it remains unclear whether the association between mortality and AF in critical illness is due to AF itself or due to AF simply being a marker of greater severity of illness. Additionally, limited data is available to examine the strength of association of new-onset AF versus recurrent or pre-existing AF in association with mortality. Finally, the effect of fluid management, vasopressor use, or therapeutic interventions for AF on its association with mortality has not been studied.
In order to address these uncertainties, we designed a large prospective cohort study of AF in critical illness that was done concurrently with a prospective study of biomarkers of acute lung injury. The primary goal of the AF study was to test the hypothesis that development of AF during critical illness is associated with increased mortality independent of underlying comorbidities and severity of illness. The secondary goal of this study was to determine whether new-onset AF and recurrent AF had similar risk factors and consequences in critically ill patients. We further hypothesized that a more positive fluid balance, greater requirement for vasopressors, and underlying cardiac disease would be associated with an increased risk of AF.
MATERIALS AND METHODS
Study Design
This study was a prospective observational cohort study of 1,770 critically ill adults (age ≥ 18 years) admitted to the Medical or general Surgical ICUs at Vanderbilt University Medical Center who were prospectively enrolled in the Validating Acute Lung Injury Markers for Diagnosis (VALID) study within 24 hours of ICU admission. Patients in the Cardiovascular/Cardiothoracic Surgical ICU and in the Trauma ICU were excluded from the current study. Patients were excluded from VALID if they did not remain in the ICU beyond 24 hours, if they had cardiac arrest prior to enrollment, had medication overdose, or had chronic lung disease requiring home oxygen therapy (18). The study protocol was approved by the Vanderbilt University Institutional Review Board (IRB# 051065). Informed consent was obtained from the patient or their designee whenever possible; in cases where neither individual was able to give consent, a waiver of consent was approved. On enrollment, clinical data including patient demographics, medical history including history of atrial fibrillation, cardiac history, cardiac risk factors, medications, vital signs, and laboratory values were extracted from the medical record. Additional data including the APACHE II score on enrollment (19), daily fluid balance, echocardiographic findings, vasopressor use, and evidence of organ failures according to Brussels definitions (20) were recorded for the first 3 days after enrollment in addition to the 24 hours before study enrollment, for a total of 4 study days. Development of AF was determined daily by documentation of any occurrence of the arrhythmia in a physician's progress note, electrocardiogram interpretation by a cardiologist, nursing vital sign flowsheet records. Patients who had any AF during the first 4 days in the ICU were defined as Any AF and were compared to patients who did not have AF during the study period in the ICU (No AF). Within the Any AF group, new-onset AF was defined as development of AF in the ICU in a patient with no prior history of AF by patient history or review of available medical records. Recurrent AF was defined as AF in the ICU in a patient with any previous history of AF, without distinction between those with chronic persistent AF and those with paroxysmal AF. All treatments administered for AF were recorded daily and categorized as rate-controlling (beta blockers, calcium channel blockers) or rhythm-controlling (amiodarone, digoxin) agents. Outcome data including hospital mortality, duration of mechanical ventilation, ICU length of stay, and hospital length of stay were also recorded.
Statistical Analysis
The primary outcome was in-hospital mortality in relation to the presence of Any AF or No AF during the VALID study period. Secondary analyses compared New-onset AF to Recurrent AF in relation to in-hospital mortality. Univariate analyses for categorical data were conducted using Chi-squared or Fisher's exact test. For continuous variables, comparisons were performed using Mann-Whitney U tests. P values of ≤ 0.05 were considered significant. Multivariate logistic regression models including variables known to be associated with both AF and poor clinical outcomes were created using the a priori selected variables of age, history of congestive heart failure, hypertension, APACHE II score, shock, and sepsis to determine the association between AF and in-hospital mortality. Logistic regression analysis for New AF and Recurrent AF were each performed comparing to patients with No AF. All statistical analysis was performed with SPSS version 22 for Macintosh (IBM, Armonk, NY).
RESULTS
Study population
The study population included 1,275 patients admitted to the Medical ICU and 495 patients admitted to the general Surgical ICU (Figure 1). The patient demographics and clinical characteristics are shown in Table 1. Patients with Any AF were significantly older, more likely to be male, and had increased severity of illness as measured by higher APACHE II scores and more organ failures. Known risk factors for AF including congestive heart failure, stroke, and hypertension were more frequent in the Any AF group.
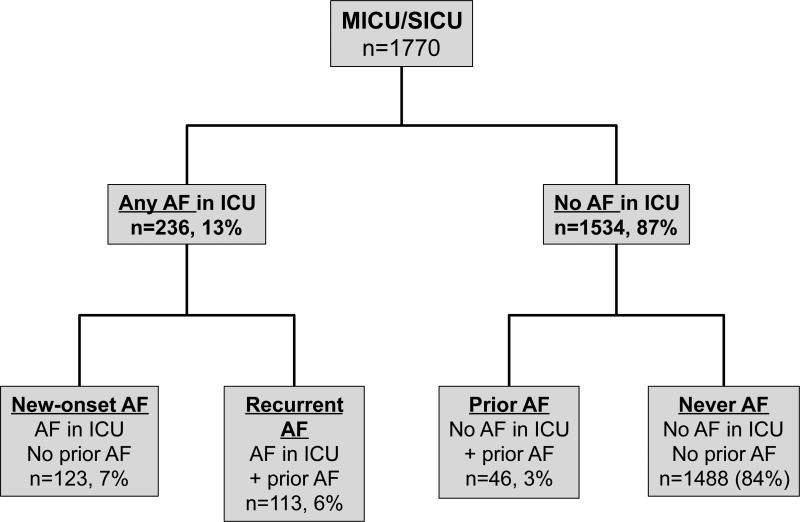
Figure 1. Study population.
Patients at risk for acute lung injury were enrolled on the morning of ICU day 2 and separated into groups based on the occurrence of AF in the ICU and history of prior AF. MICU, medical intensive care unit; SICU, surgical intensive care unit; AF, atrial fibrillation; ICU, intensive care unit
Table 1.
Demographics and baseline clinical characteristics of the study population.
| Characteristic | Any AF (n=236) | No AF (n=1534) | P value |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age (yrs) | 68 (61-77) | 56 (46-65) | <0.001 a |
| Male | 154 (65%) | 821 (54%) | 0.001 b |
| Caucasian | 215 (91%) | 1287 (84%) | 0.004 b |
| CARDIAC RISK FACTORS | |||
| Current Smoker | 50 (21%) | 495 (32%) | 0.001 b |
| Weight (kg) | 83 (69-100) | 77 (65-94) | 0.001 a |
| Dialysis | 17 (7%) | 96 (6%) | 0.580b |
| Diabetes | 84 (36%) | 458 (30%) | 0.075b |
| MI/Angina | 51 (22%) | 165 (11%) | <0.001 b |
| CHF | 60 (25%) | 159 (10%) | <0.001 b |
| CVA | 32 (14%) | 116 (8%) | 0.002 b |
| Hypertension | 177 (75%) | 749 (49%) | <0.001 b |
| Hyperlipidemia | 108 (46%) | 368 (24%) | <0.001 b |
| ICU RISK FACTORS | |||
| APACHE II | 28 (22-33) | 25 (20-31) | <0.001 a |
| Sepsis | 154 (65%) | 898 (59%) | 0.051b |
| Total # Organ Failures | 2 (1-2) | 2 (1-2) | <0.001 a |
| Respiratory Failure | 164 (70%) | 1038 (68%) | 0.576b |
| Shock | 141 (60%) | 663 (43%) | <0.001 b |
| Renal Failure | 124 (53%) | 567 (37%) | <0.001 b |
Data are presented as median (interquartile range) or n (%). P values determined by Mann-Whitney Ua or chi-squareb tests. AF, atrial fibrillation; yrs, years; kg, kilograms; MI, myocardial infarction; CHF, congestive heart failure; CVA, cerebrovascular accident; APACHE II, Acute Physiology and Chronic Health Evaluation II.
Incidence of AF in the ICU
Overall, 236 of 1,770 patients (13%) patients developed Any AF during the 4 day study period in the ICU (Figure 1). The incidence of Any AF was 13% in Medical ICU subjects and 15% in Surgical ICU subjects. Of the 236 patients with Any AF, 123 had New-onset AF (no prior history of AF) and 113 had Recurrent AF (prior history of AF). The majority of patients with a previous history of AF had recurrence in the ICU (113/159, 71%).
Any AF in the ICU is associated with increased mortality and prolonged duration of illness
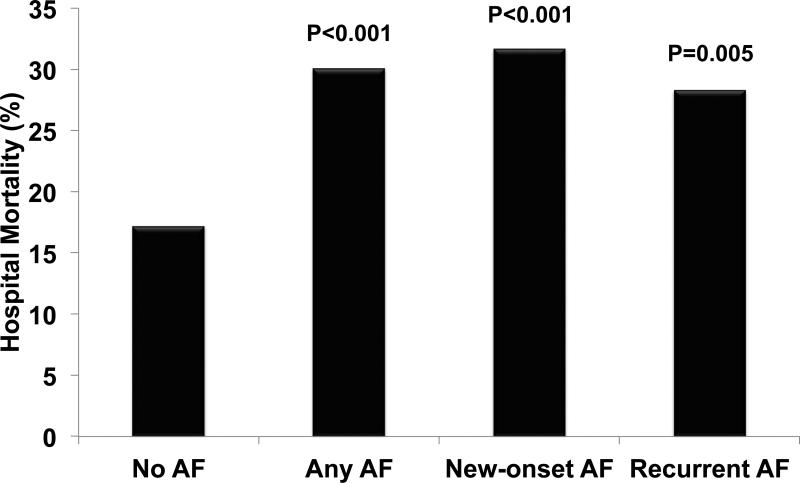
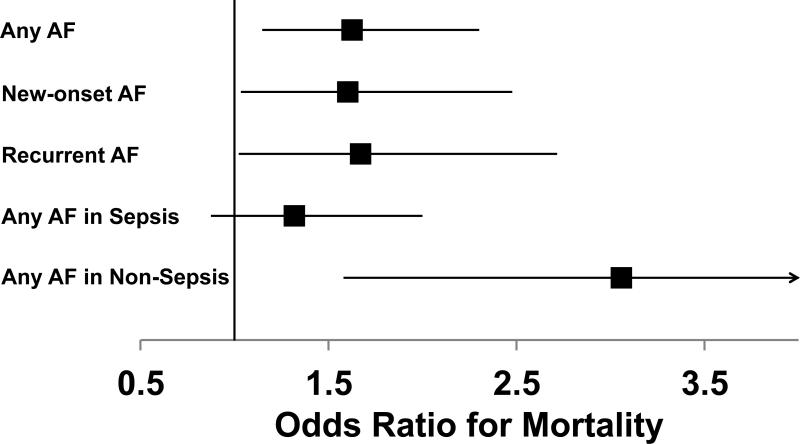
Of the patients who had Any AF during the 4-day study period, 30% died during hospitalization compared with 17% of patients with No AF (p<0.001) (Figure 2). Patients with Any AF also had increased lengths of stay in the ICU and in the hospital (Table 2). To determine whether the association between Any AF and hospital mortality was independent of differences in severity of illness and other potential confounders, we created a multivariable logistic regression model with in-hospital mortality as the outcome. We focused on variables that have been associated with increased risk for development of AF or with increased mortality. After controlling for these potential cofounding factors, Any AF remained significantly associated with increased risk of mortality (odds ratio [OR] 1.62, 95% confidence interval [CI] 1.14-2.29, p=0.007) (Figure 3, Supplemental Table 1).
Figure 2. AF during critical illness is associated with increased mortality.
P values shown are compared to No AF. AF, atrial fibrillation
Table 2.
Clinical outcomes of patients with Any AF or No AF in the ICU.
| Any AF (n=236) | No AF (n=1534) | P value | |
|---|---|---|---|
| ICU LOS (days) | 7 (4-13) | 5 (2-11) | <0.001 a |
| Duration of MV (days) | 3 (0-8) | 2 (0-5) | 0.062 a |
| Hospital LOS (days) | 14 (9-24) | 11 (6-19) | 0.001 a |
| Hospital Mortality (n, %) | 71 (30%) | 264 (17%) | <0.001b |
Values are median (interquartile range) or n (%). P values determined by Mann-Whitney Ua or chi-squareb tests. AF, atrial fibrillation; ICU, intensive care unit; LOS, length of stay; MV, mechanical ventilation.
Figure 3. Odds ratios for mortality depending on AF group.
Odds ratios for death were calculated by logistic regression controlling for age, congestive heart failure, hypertension, severity of illness, sepsis, and shock. P values shown are compared to No AF.
Comparison of New-onset AF to Recurrent AF
A subgroup analysis was done to determine whether there were clinically important differences between patients who had their first episode of AF in the ICU (New-onset AF) compared to those with a history of AF who developed AF in the ICU (Recurrent AF). The demographic and clinical characteristics of patients with New-onset AF and Recurrent AF are shown in Table 3. Compared to patients with New-onset AF, patients with Recurrent AF were older and more likely to have a history of congestive heart failure, hypertension, and hyperlipidemia. Patients with Recurrent AF had fewer organ failures and less shock than those with New-onset AF. Patients with Recurrent AF developed AF earlier during critical illness than those with New-onset AF, with 84% of Recurrent AF occurring within the first 2 study days compared to 51% of New-onset AF (p<0.001). The majority (65%) of New-onset AF resolved in less than 48 hours with no additional episodes of AF during the study period. In contrast, only 29% of Recurrent AF resolved in the same interval (p<0.001) and 52% of Recurrent AF persisted throughout the entire 4-day study period. Both New-onset AF and Recurrent AF were associated with increased hospital mortality (Figure 2). Logistic regression confirmed that New-onset AF and Recurrent AF were each independently associated with increased mortality after controlling for known AF risk factors and potential confounders (New-onset AF: OR 1.60, 95% CI 1.03-2.48, p=0.036; Recurrent AF: OR 1.66, 95% CI 1.02-2.71, p=0.042) (Figure 3, Supplemental Table 2). Those with Recurrent AF had longer hospital length of stay, but similar duration of mechanical ventilation and ICU length of stay compared to those with New-onset AF (Table 4).
Table 3.
Demographics and clinical characteristics of New-onset AF and Recurrent AF.
| Characteristic | New-onset AF (n=123) | Recurrent AF (n=113) | P value |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age (yrs) | 70 (63-78) | 66 (59-74) | 0.008 a |
| Male | 80 (65%) | 74 (66%) | 0.943b |
| Caucasian | 112 (91%) | 103 (91%) | 0.980b |
| CARDIAC RISK FACTORS | |||
| Current Smoker | 28 (23%) | 22 (20%) | 0.536b |
| Weight (kg) | 83 (70-101) | 83 (68-100) | 0.762 a |
| Dialysis | 9 (7%) | 8 (7%) | 0.944 b |
| Diabetes | 44 (36%) | 40 (35%) | 0.952 b |
| MI/Angina | 29 (24%) | 22 (20%) | 0.444 b |
| CHF | 19 (15%) | 41 (36%) | <0.001 b |
| CVA | 15 (12%) | 17 (15%) | 0.523 b |
| Hypertension | 78 (63%) | 99 (88%) | <0.001 b |
| Hyperlipidemia | 45 (37%) | 63 (56%) | 0.003 b |
| ICU RISK FACTORS | |||
| APACHE II | 27 (21-33) | 29 (22-34) | 0.185 a |
| Any Sepsis | 79 (64%) | 75 (66%) | 0.730 b |
| Total # Organ Failures | 2 (1-2) | 2 (1-3) | 0.006 a |
| Respiratory Failure | 90 (73%) | 74 (66%) | 0.200 b |
| Shock | 85 (69%) | 56 (50%) | 0.002 b |
| Renal Failure | 62 (50%) | 62 (55%) | 0.516 b |
Data are presented as median (interquartile range) or n (%). P values are determined by Mann-Whitney Ua or chi-squareb tests. AF, atrial fibrillation; yrs, years; kg, kilograms; MI, myocardial infarction; CHF, congestive heart failure; CVA, cerebrovascular accident; APACHE II, Acute Physiology and Chronic Health Evaluation II.
Table 4.
Clinical outcomes of patients with New-onset AF or Recurrent AF.
| New-onset AF (n=123) | Recurrent AF (n=113) | P value | |
|---|---|---|---|
| ICU LOS (days) | 6 (3-13) | 7 (4-14) | 0.067 a |
| Duration of MV (days) | 3 (0-7) | 3 (0-9) | 0.103 a |
| Hospital LOS (days) | 12 (7-22) | 15 (9-26) | 0.049 a |
| Hospital mortality (n, %) | 39 (32%) | 32 (28%) | 0.571 b |
Values are median (interquartile range) or n (%). P values determined by Mann-Whitney Ua or chi-squareb tests. AF, atrial fibrillation; ICU, intensive care unit; LOS, length of stay; MV, mechanical ventilation.
Treatment of AF in the ICU
Overall, the majority of patients with Any AF (209/236, 85%) received at least one form of rate- or rhythm-control treatment for arrhythmia during the study period. Patients with New-onset AF were more likely to be treated with direct current cardioversion or amiodarone, and less likely to receive the atrio-ventricular blocking agents digoxin or verapamil (Table 5). AF resolved during the study period in 39% of patients who received treatment for AF with a similar frequency of treatment success with rate-controlling or rhythm-controlling agents. Treatment of Any AF with either rate or rhythm control therapy was not associated with hospital mortality (untreated group 34% mortality, treated group 29% mortality, p=0.557), although there was a non-significant trend towards improved outcomes with rhythm control agents (mortality 34% with rate control agent vs. 25% with rhythm control agent, p=0.179). AF-directed therapy did not affect the minimum daily heart rate or the frequency of bradycardia (Supplemental Table 3). Patients receiving rhythm control agents had more severe hypotension on study days 2 and 3 compared to those receiving rate control agents.
Table 5.
Treatment characteristics of New-onset AF and Recurrent AF in the ICU.
| New-onset AF (n=123) | Recurrent AF (n=113) | P value | |
|---|---|---|---|
| Any treatment | 105 (85%) | 96 (85%) | 0.929a |
| Cardioversion | 20 (16%) | 3 (0.03%) | <0.001 a |
| Beta blockers | 58 (47%) | 57 (50%) | 0.614 a |
| Diltiazem | 58 (47%) | 56 (50%) | 0.712 a |
| Verapamil | 0 (0%) | 6 (0.05%) | 0.011 b |
| Amiodarone | 49 (40%) | 27 (24%) | 0.009 a |
| Digoxin | 16 (13%) | 32 (28%) | 0.004 a |
Values are n (%). P values determined by chi-squarea or Fisher's exactb tests.
Fluid balance and echocardiographic features of patients with and without AF
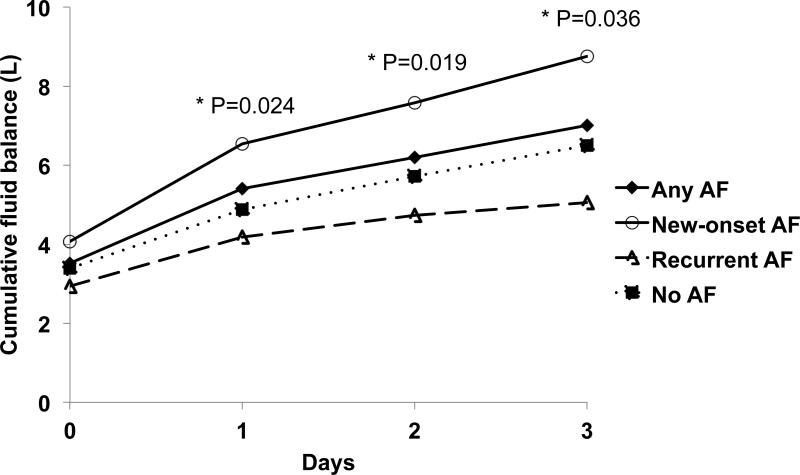
We hypothesized that greater net positive fluid balance is a surrogate marker of distension of the left atrium and would be associated with an increased risk of New-onset and Recurrent AF. During the first day in the ICU, all groups of patients had equivalent fluid resuscitation as measured by the net positive fluid balance (Figure 4). Through the 4-day study period, there were no differences in total fluid balance between Any AF and No AF (median total net fluid balance through entire study period of 4.93L and 5.4L, respectively, p=0.480). However, patients with New-onset AF, but not those with Recurrent AF, had significantly greater net positive cumulative fluid balance compared to those with No AF (median New-onset AF 6.1L, p=0.036 vs. No AF 5.4L; Recurrent AF 4.7L, p=0.250 vs. No AF 5.4L).
Figure 4. Cumulative fluid balance of patients with New-onset AF, Recurrent AF, and no AF.
P values shown are compared to No AF. AF, atrial fibrillation
In the subset of patients with Any AF who underwent echocardiography during the study hospitalization (143/236, 61%) (Table 6), the left atrium was larger in patients with New-onset AF and Recurrent AF compared to No AF, with significantly greater left atrial size in those with Recurrent AF. Diastolic dysfunction was more frequent in those with New-onset AF and less frequent in those with Recurrent AF compared to no AF. Mitral regurgitation was more frequently present in patients with Recurrent AF. There were no significant differences in plasma levels of troponin-I or brain natriuretic peptide (BNP) among groups (data not shown).
Table 6.
Echocardiographic characteristics of a subset of patients with New-onset AF or Recurrent AF in the ICU.
| New-onset AF (n=84) | Recurrent AF (n=59) | P value | |
|---|---|---|---|
| Left Atrial Size (cm) | 4.4 (3.8-5.0) | 4.0 (3.6-4.5) | 0.011a |
| Ejection Fraction (%) | 55 (40-55) | 55 (45-55) | 0.255 a |
| Diastolic dysfunction | 23 (27%) | 10 (17%) | 0.015 b |
| Mitral regurgitation | 53 (63%) | 42 (71%) | 0.025 b |
Values are median (interquartile range) or n (%). P values determined by Mann-Whitney Ua or chi-squareb tests. AF, atrial fibrillation
Patients with AF required more vasopressor and inotropic support
Because of the association of vasopressor use with development of atrial arrhythmias, the use of vasopressor and inotropic agents was assessed. More patients with Any AF were treated with vasopressors or inotropic agents compared to patients who did not develop AF in the ICU (59.7% vs. 43.2%, p<0.001). Compared to patients with Recurrent AF, patients with New-onset AF were more likely to be treated with vasoactive agents (69.1% vs. 49.6%, p=0.002) and were treated with vasopressors on more study days than those with Recurrent AF or No AF (1.7 ± 1.5 days in New-onset AF vs. 1.3 ± 1.5 days in Recurrent AF vs. 1.0 ± 1.3 days in No AF, p<0.001 for New-onset AF vs. No AF). Patients who developed New-onset AF on the second study day were more likely to have received vasopressors on the previous day than those with Recurrent AF (55% vs. 36%, p=0.018). The specific vasoactive agent chosen by the clinicians differed between AF groups (Table 7). Specifically, norepinephrine was used more frequently in patients who developed New-onset AF compared to those with Recurrent AF.
Table 7.
Vasopressor and inotrope use in patients New-onset AF or Recurrent AF.
| New-onset AF (n=123) | Recurrent AF (n=113) | P value | |
|---|---|---|---|
| Any vasopressor or inotrope treatment | 85 (69%) | 56 (50%) | 0.002a |
| Norepinephrine | 73 (59%) | 47 (42%) | 0.006 a |
| Vasopressin | 35 (29%) | 20 (18%) | 0.051 a |
| Phenylephrine | 21 (17%) | 17 (15%) | 0.672 a |
| Epinephrine | 1 (1%) | 1 (1%) | 1.000 b |
| Dobutamine | 1 (1%) | 3 (2%) | 0.352 b |
| Dopamine | 13 (11%) | 10 (9%) | 0.656 a |
Values are presented as n (%). P values determined by chi-squarea or Fisher's exactb tests.
Comparison of AF in sepsis and non-sepsis patients
Because of presumed differences in pro-inflammatory stimuli in patients with sepsis compared to those without, we hypothesized that AF would be more common in patients with sepsis. Any AF occurred in 13% of patients with sepsis and 10% of those without sepsis (p=0.05). Occurrence of Any AF was associated with increased mortality regardless of the presence of sepsis (sepsis: 33% mortality with Any AF vs. 22% without AF, p=0.004; non-sepsis: 24% mortality with AF vs. 10% without AF, p<0.001). The independent association between Any AF and mortality was magnified in the absence of sepsis. In non-sepsis patients, Any AF was associated with an OR for death of 2.92 (95% CI 1.52-5.60, p=0.001). In contrast, in patients with sepsis, Any AF was not significantly associated with death in the multivariable analysis (OR 1.29, 95% CI 0.85-1.94, p=0.228), although this analysis may be underpowered.
DISCUSSION
In this large prospective observational study of a diverse population of critically ill patients admitted to medical and surgical ICUs, AF during critical illness is associated with an increased risk of in-hospital mortality that is independent of the severity of critical illness, underlying cardiac risk factors, or presence of sepsis. These results are consistent with, and build upon, several previous reports that development of AF in the ICU is associated with increased mortality, which are summarized in Supplemental Table 4 (1, 3-5, 7, 9, 11-13). Our study provides important new information compared to prior studies because the large cohort of patients with AF allowed us to determine that the association of AF with mortality in critical illness is not simply due to AF being a marker of increased disease severity. Furthermore, given the large sample size, we were able to compare the clinical characteristics and outcomes of New vs. Recurrent AF in critical illness.
AF was consistently associated with higher mortality in the multivariable logistic regression analyses, regardless of whether the AF was new-onset or occurred in the setting of a prior history of AF. Overall, the development of Any AF during the first 4 days in the ICU was associated with a 62% increased risk of in-hospital mortality. This effect size for a common and potentially modifiable risk factor for death is clinically significant and intervention to reduce this risk could have clinical benefit. Because of its independent association with hospital mortality, development of AF in the ICU warrants close clinical attention and further studies are needed to not only define the underlying pathophysiology of the arrhythmia but to also determine whether prevention or treatment of AF would improve clinical outcomes.
In addition to demonstrating the importance of AF during critical illness, we sought to determine whether there were differences between New-onset AF and Recurrent AF in the ICU. While both New-onset AF and Recurrent AF were independently associated with increased mortality, to our knowledge, the association of Recurrent AF during critical illness with hospital mortality has not previously been reported, since most prior studies actually excluded patients with a history of prior AF. Patients with Recurrent AF, but not New-onset AF were more likely to have underlying cardiac risk factors of congestive heart failure, hypertension, and hyperlipidemia. Despite having similar severity of illness, patients with New-onset AF more frequently had hypotension and had more organ failures compared to patients with Recurrent AF. New-onset AF was also more likely to be associated with positive fluid balance and antecedent vasopressor use. One interpretation of these differences could be that the development of New-onset AF occurs in the setting of prolonged hypotension and inadequate oxygen delivery while Recurrent AF is more likely related to underlying structural heart disease and traditional cardiac risk factors.
Our results demonstrate that a greater net positive fluid balance and increased vasopressor use were associated with development of New-onset AF or Any AF, suggesting that clinical management of critically ill patients may modulate the risk of developing AF in the ICU. Increased fluid administration and vasopressor use in patients with New-onset AF may have been in response to more frequent hypotension in this population. Conversely, patients with a history of AF may have received less fluid resuscitation due to the attendant risks of precipitating heart failure. One possible mechanism by which increased positive cumulative fluid balance may increase susceptibility to New-onset AF is by increasing atrial stretch acutely. This concept is supported by echocardiographic data showing that patients with AF had increased left atrial dimensions compared to those without AF. Vasoactive medications, particularly those with beta-adrenergic activity, may also directly influence AF. A potential causal role for vasopressors in development of AF is supported by recent data showing an increased frequency of AF in patients with septic shock who had high blood pressure targets compared to those with low blood pressure targets (21).
As most previous studies of AF in the critically-ill have focused specifically on patients with sepsis (7, 9, 11, 16), we tested whether the impact of AF differed in the presence or absence of sepsis. As anticipated, patients with sepsis were more likely to develop AF in the ICU than those without sepsis. AF during critical illness is associated with higher hospital mortality regardless of whether sepsis was present. Surprisingly, the association of Any AF with mortality was magnified in patients without sepsis (OR 2.92 for non-sepsis patients vs. 1.29 for sepsis patients), after controlling for other confounding variables including age, disease severity, shock, heart failure, and hypertension. In sepsis patients, AF in the ICU did not carry an independent risk for death. These data point to the possibility that the etiology and consequences of AF may be modulated by the underlying pathophysiology of the acute illness.
It remains unclear why some patients in the ICU develop New-onset AF and others do not. One hypothesis is that some patients have an underlying susceptibility to atrial arrhythmias that is unmasked by the complex pathophysiology of critical illness. Such a predisposition for AF may be genetic or related to subclinical structural abnormalities in the heart. Recently, a “two-hit” model for development of ambulatory AF has been proposed (22). This model states that a genetic risk for AF in the setting of an acquired risk factor such as systemic inflammation that is common in critical illness together function as a trigger for AF. In support of this hypothesis, C-reactive protein levels have also been shown to increase prior to onset of arrhythmias in patients with sepsis in the ICU (9). A recent meta-analysis showed the prophylactic use of the anti-inflammatory agent N-acetylcysteine in post-operative patients resulted in a decreased risk of developing New-onset AF (OR 0.56) or death (OR 0.40) (23). In addition, there are increasing data supporting genetic predisposition to development of new-onset AF that is not clinically apparent until an acute stressor occurs. Several genome wide association studies in the general population have identified common AF susceptibility alleles in genes encoding cardiac ion channels, cellular structure, intracellular signaling proteins, and inflammation that are associated with development of AF (24-26). However, none of these have been studied in critical illness. A greater understanding of the underlying pathophysiology of AF during critical illness is warranted in order to identify novel therapeutic targets and direct therapy to underlying mechanisms (22).
This study has several strengths. First, to our knowledge, it is the largest prospective study of AF in critical illness and includes a broad group of both medical and surgical critically ill patients. The large study population with extensive prospective clinical data collection provided sufficient power for us to determine that the association of AF with mortality was not simply due to higher severity of illness in those with AF. Previous smaller studies have not addressed this question. Furthermore, the large patient cohort allowed analysis of differences between New-onset AF and Recurrent AF which have not been previously explored. We were also able to compare patients with and without sepsis as an underlying diagnosis. There are also some limitations. In this prospective observational cohort study, we are unable to determine whether AF plays a causative role in increased mortality. There may also be additional unmeasured confounding variables that could influence risk for AF and for mortality which were not included in our regression analysis. Determining the specific contribution of AF to clinical outcomes would be challenging, even in a prospective study. It is possible that that the prior history of AF may be inaccurate as many patients have asymptomatic AF. Because we only studied AF during the first four ICU days, the implications of AF developing after ICU day 4 are unknown. Since the majority of patients with AF in this study received at least one medication or therapy aimed at rate or rhythm control, we were unable to detect a benefit of AF-directed therapy on mortality. However, the finding that treatment for AF did not worsen bradycardia may be valuable for designing a randomized trial of AF management in the critically ill.
In conclusion, AF in the ICU is associated with an increased mortality risk that is independent of other clinical risk factors such as severity of illness or pre-existing cardiac disease and is strongest in patients without sepsis. Furthermore Recurrent AF, which has not been previously studied, carries the same risk of mortality as new-onset AF during critical illness. Taken together with the existing literature, this study provides the framework for design of additional studies aimed at prevention and treatment of AF in critically ill patients with an ultimate goal to reduce patient mortality.
Supplementary Material
Acknowledgments
Sources of Funding: This work is supported by NIH T32 HL087738 to CMS and DRJ, NIH HL092217 to DD, NIH HL103836 to LBW, and an American Heart Association Established Investigator Award to LBW.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Annane D, Sebille V, Duboc D, Le Heuzey JY, Sadoul N, Bouvier E, Bellissant E. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am J Respir Crit Care Med. 2008;178(1):20–25. doi: 10.1164/rccm.200701-031OC. [DOI] [PubMed] [Google Scholar]
- 2.Artucio H, Pereira M. Cardiac arrhythmias in critically ill patients: Epidemiologic study. Crit Care Med. 1990;18(12):1383–1388. doi: 10.1097/00003246-199012000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Brathwaite D, Weissman C. The new onset of atrial arrhythmias following major noncardiothoracic surgery is associated with increased mortality. Chest. 1998;114(2):462–468. doi: 10.1378/chest.114.2.462. [DOI] [PubMed] [Google Scholar]
- 4.Christian SA, Schorr C, Ferchau L, Jarbrink ME, Parrillo JE, Gerber DR. Clinical characteristics and outcomes of septic patients with new-onset atrial fibrillation. J Crit Care. 2008;23(4):532–536. doi: 10.1016/j.jcrc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Goodman S, Shirov T, Weissman C. Supraventricular arrhythmias in intensive care unit patients: Short and long-term consequences. Anesth Analg. 2007;104(4):880–886. doi: 10.1213/01.ane.0000255759.41131.05. [DOI] [PubMed] [Google Scholar]
- 6.Kanji S, Williamson DR, Yaghchi BM, Albert M, McIntyre L, Canadian Critical Care Trials G Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care. 2012;27(3):326, e321–328. doi: 10.1016/j.jcrc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Kindem IA, Reindal EK, Wester AL, Blaasaas KG, Atar D. New-onset atrial fibrillation in bacteremia is not associated with c-reactive protein, but is an indicator of increased mortality during hospitalization. Cardiology. 2008;111(3):171–180. doi: 10.1159/000121600. [DOI] [PubMed] [Google Scholar]
- 8.Knotzer H, Mayr A, Ulmer H, Lederer W, Schobersberger W, Mutz N, Hasibeder W. Tachyarrhythmias in a surgical intensive care unit: A case-controlled epidemiologic study. Intensive Care Med. 2000;26(7):908–914. doi: 10.1007/s001340051280. [DOI] [PubMed] [Google Scholar]
- 9.Meierhenrich R, Steinhilber E, Eggermann C, Weiss M, Voglic S, Bogelein D, Gauss A, Georgieff M, Stahl W. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: A prospective observational study. Crit Care. 2010;14(3):R108. doi: 10.1186/cc9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinelt P, Karth GD, Geppert A, Heinz G. Incidence and type of cardiac arrhythmias in critically ill patients: A single center experience in a medical-cardiological icu. Intensive Care Med. 2001;27(9):1466–1473. doi: 10.1007/s001340101043. [DOI] [PubMed] [Google Scholar]
- 11.Salman S, Bajwa A, Gajic O, Afessa B. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med. 2008;23(3):178–183. doi: 10.1177/0885066608315838. [DOI] [PubMed] [Google Scholar]
- 12.Seguin P, Laviolle B, Maurice A, Leclercq C, Malledant Y. Atrial fibrillation in trauma patients requiring intensive care. Intensive Care Med. 2006;32(3):398–404. doi: 10.1007/s00134-005-0032-2. [DOI] [PubMed] [Google Scholar]
- 13.Seguin P, Signouret T, Laviolle B, Branger B, Malledant Y. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. Crit Care Med. 2004;32(3):722–726. doi: 10.1097/01.ccm.0000114579.56430.e0. [DOI] [PubMed] [Google Scholar]
- 14.Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, Benjamin EJ. Atrial fibrillation among medicare beneficiaries hospitalized with sepsis: Incidence and risk factors. Am Heart J. 2013;165(6):949–955. e943. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora S, Lang I, Nayyar V, Stachowski E, Ross DL. Atrial fibrillation in a tertiary care multidisciplinary intensive care unit--incidence and risk factors. Anaesth Intensive Care. 2007;35(5):707–713. doi: 10.1177/0310057X0703500508. [DOI] [PubMed] [Google Scholar]
- 16.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146(5):1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Neal HR, Jr., Koyama T, Koehler EA, Siew E, Curtis BR, Fremont RD, May AK, Bernard GR, Ware LB. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39(6):1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache ii: A severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 20.American college of chest physicians/society of critical care medicine consensus conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 21.Asfar P, Teboul JL, Radermacher P. High versus low blood-pressure target in septic shock. N Engl J Med. 2014;371(3):283–284. doi: 10.1056/NEJMc1406276. [DOI] [PubMed] [Google Scholar]
- 22.Darbar D, Roden DM. Genetic mechanisms of atrial fibrillation: Impact on response to treatment. Nature reviews Cardiology. 2013;10(6):317–329. doi: 10.1038/nrcardio.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu XH, Xu CY, Fan GH. Efficacy of n-acetylcysteine in preventing atrial fibrillation after cardiac surgery: A meta-analysis of published randomized controlled trials. BMC cardiovascular disorders. 2014;14:52. doi: 10.1186/1471-2261-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Muller-Nurasyid M, Krijthe BP, Lubitz SA, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44(6):670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andalib A, Brugada R, Nattel S. Atrial fibrillation: Evidence for genetically determined disease. Current opinion in cardiology. 2008;23(3):176–183. doi: 10.1097/HCO.0b013e3282fa7142. [DOI] [PubMed] [Google Scholar]
- 26.Darbar D. Genetics of atrial fibrillation: Rare mutations, common polymorphisms, and clinical relevance. Heart Rhythm. 2008;5(3):483–486. doi: 10.1016/j.hrthm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.