Abstract
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily and have diverse functions during development and organogenesis. BMPs play a major role in skeletal development and bone formation, and disruptions in BMP signaling cause a variety of skeletal and extraskeletal anomalies. Several knockout models have provided insight into the mechanisms responsible for these phenotypes. Proper bone formation requires the differentiation of osteoblasts from mesenchymal stem cell (MSC) precursors, a process mediated in part by BMP signaling. Multiple BMPs, including BMP2, BMP6, BMP7 and BMP9, promote osteoblastic differentiation of MSCs both in vitro and in vivo. BMP9 is one of the most osteogenic BMPs yet is a poorly characterized member of the BMP family. Several studies demonstrate that the mechanisms controlling BMP9-mediated osteogenesis differ from other osteogenic BMPs, but little is known about these specific mechanisms. Several pathways critical to BMP9-mediated osteogenesis are also important in the differentiation of other cell lineages, including adipocytes and chondrocytes. BMP9 has also demonstrated translational promise in spinal fusion and bone fracture repair. This review will summarize our current knowledge of BMP-mediated osteogenesis, with a focus on BMP9, by presenting recently completed work which may help us to further elucidate these pathways.
Keywords: BMP, BMP9, Bone Regeneration, IGF, Osteogenesis, TGF-β, Wnt, Signal Transduction, Mesenchymal Stem Cells, MSCs
1. Introduction
Bone morphogenetic proteins (BMPs) are members of the transforming group factor-beta (TGF-β) superfamily. This group of homologous signaling proteins has a diverse number of functions and plays an important role in embryogenesis, organogenesis, cell proliferation and stem cell differentiation [1-5]. For example, BMP7 is involved in proper kidney, eye, and limb development; BMPs 4, 7 and 15 are important for proper reproductive tissue development; BMPs 2, 3 and 7 contribute to cartilage regeneration; and BMPs 12 and 13 are required for normal tendon healing [6-12]. BMPs also play a major role in skeletal development, bone formation and mesenchymal stem cell (MSC) differentiation [13,14].
MSCs are adult stem cells found in the bone marrow and like all other types of stem cells, have the unique ability to self-renew and to differentiate into various mesodermal cell lineages, osteoblastic, chondrocytic, myocytic and adipocytic, as well as non-mesodermal tissues, such as cardiac muscle and skin [11,13,15-18] (Figure 1). The differentiation of these multipotent stem cells towards an osteoblastic fate depends on numerous signaling pathways, including BMP transduction. The osteoinductive ability of BMPs was discovered when it was found that demineralized bone could induce de novo bone formation and that BMPs were responsible for this observed osteogenesis [19,20]. Disruptions in BMP signaling have subsequently been shown to result in a variety of skeletal and extraskeletal anomalies [21,22]. At least 15 different BMPs have been identified in humans to date.
Figure 1.
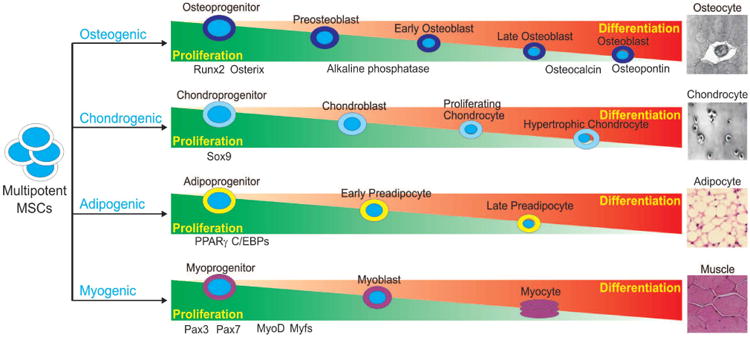
Schematic representation of lineage-specific differentiation of mesenchymal stem cells (MSCs). MSCs are pluripotent progenitor cells with the ability to differentiate along multiple lineages, including osteogenic, chondrogenic, adipogenic and myogenic lineages. Differentiation of MSCs along these unique lineages is an exquisitely coordinated process with critical regulators responsible for each lineage. Regulators and indicators of lineage-specific differentiation are depicted.
BMP9, also known as growth differentiation factor 2 or GDF-2, is a relatively poorly characterized member of the BMP family first isolated from fetal mouse liver cDNA libraries. BMP9 is expressed at high levels within the developing mouse liver and acts to stimulate hepatocyte proliferation [23]. It also acts to induce and maintain the cholinergic phenotype within basal forebrain neurons, inhibit hepatic glucose production, inhibit enzymes of lipid metabolism, maintain metabolic homeostasis of iron and synergize in the generation of hematopoietic progenitor cells [24-26].
BMP9 is among the most osteogenic BMPs and promotes the osteoblastic differentiation of mesenchymal stem cells (MSCs) both in vitro and in vivo [11,13,27-30]. We have demonstrated that BMP9 regulates a distinct set of downstream targets likely playing a role in osteoinduction, and these targets will be discussed later in this review [11,27-30]. While BMP9 has been demonstrated as one of the most osteogenic BMPs, little is known about the detailed mechanisms responsible for its functions. This review aims to summarize our current knowledge of BMP9-mediated osteogenesis, which may help us to further elucidate these pathways.
2. Axial Skeletal Development and MSCs
Mesenchymal stem cells undergo several stages of maturation during their proliferation and differentiation along the osteoblastic lineage. MSCs initially form preosteoblasts, which proliferate near the surface of bone and secrete alkaline phosphatase, an early marker of osteogenesis [11,31-33]. Preosteoblasts further mature into osteoblasts, which are involved in initial extracellular matrix maturation and mineralization. Osteoblasts ultimately form osteocytes, which are mature, terminally differentiated cells embedded in an extracellular matrix responsible for mechanical support and regulating the mineralization of bone [34,35]. These stages of osteogenic proliferation and differentiation are characterized by the expression of various markers, including cell-cycle associated genes during the proliferative phase, the early osteoblastic marker alkaline phosphatase and late markers osteocalcin and osteopontin [36].
From MSCs, bone can form in one of two ways, either by endochondral or intramembranous ossification [36]. The majority of bones in the human skeleton are formed via endochondral ossification, whereby MSCs first differentiate into chondrocytes and secrete a cartilaginous matrix. This matrix subsequently undergoes osteoblast-facilitated ossification to form bone [37-39]. Flat bones, which mainly comprise the axial skeleton, are formed by intramembranous ossification and do not have a cartilaginous precursor scaffold. Instead, MSCs differentiate directly into osteoblasts, which secrete an osteoid matrix to form bone [37,38]. The bone formed by both of these processes is a highly vascularized tissue which undergoes constant remodeling, necessitating a balance between hematopoietic-derived osteoclasts, which break down bone, and mesenchymal-derived osteoblasts, which rebuild bone [36,40,41]. Thus, bone maintenance and remodeling depends, in part, on the proper formation of osteoblasts from MSCs, a highly regulated and complex process in which the BMP signaling pathway plays a critical role.
3. BMP Knockout Phenotypes
The BMP signaling pathway plays many crucial roles in bone formation and is involved in multiple stages of the developmental process, including osteoblast differentiation, mesoderm patterning, bone formation, and craniofacial and limb development. Knockout of specific BMPs or mediators of BMP signal transduction often leads to phenotypes which demonstrate the critical importance of BMP signaling in skeletal development.
BMP signaling is required for the differentiation of multipotent mesenchymal cells into osteochondroprogenitor cells, which are capable of forming both chondrocytes and osteoblasts. BMP signal transduction is also necessary for the proper functioning of differentiated osteoblasts, enabling them to appropriately secrete the matrix upon which bone formation occurs [31]. This is especially important during development, when the axial skeleton forms from cellular condensations of mesenchymal cells, which proceed to form bone via the previously described process of endochondral ossification. The first genetic evidence that BMPs have a role in bone morphogenesis was natural loss-of-function mutations of BMP5, resulting in short ear phenotype and brachypodism [22,42]. Subsequent studies have supported the crucial role of BMP signaling in both cartilage and bone formation during endochondral ossification [43-46].
To investigate the functions of BMP2 and BMP4 in the growth plate, chondrocyte-specific BMP2 and BMP4 conditional knockout mice and BMP2/BMP4 conditional double knockout mice were developed [47]. Deletion of BMP2 and BMP4 or BMP2 alone resulted in a severe chondrodysplastic phenotype, while deletion of BMP4 alone had minor effects on cartilage development. Double knockout and BMP2 knockouts demonstrated disorganization of chondrocytes within the growth plate, decreased cell proliferation, poor differentiation and increased apoptosis. BMP2 up-regulated protein expression of the essential osteogenic regulator Runx2. These findings demonstrate that BMP2, but not BMP4, is critical in chondrocytic differentiation and hypertrophy within the growth plate during endochondral bone formation.
BMPs have been shown to regulate endochondral ossification by promoting chondrocyte proliferation and inducing chondrocyte hypertrophy [48-50]. Loss of the inhibitory Smad, Smad6, in mice causes defects in axial and appendicular skeletal development [48]. Smad6 double knockouts demonstrate posterior transformation of the cervical vertebrae, ossification centers within lumbar vertebrae and incomplete sternal band fusion. These mice feature delayed hypertrophic differentiation and mineralization during gestation. However, by late gestation, the hypertrophic zone within the growth plate demonstrated an increased pool of proliferating, hypertrophic chondrocytes likely caused by increased BMP responsiveness in the Smad6 knockout mutants. Smad6 thus appears to be necessary to limit BMP signaling during endochondral bone formation.
Smad1 is a critical immediate downstream mediator of BMP receptor transduction [51,52]. Chondrocyte-specific and osteoblast-specific Smad1 conditional knockout mice were bred to assess postnatal bone formation [51]. Chondrocyte-specific deletion of the Smad1 gene resulted in delayed calvarial bone development. Osteoblast-specific deletion resulted in partial inhibition of BMP signaling and an osteopenic phenotype, with impaired osteoblastic proliferation and differentiation. These findings demonstrate the critical role of Smad1 and BMP signal transduction in postnatal bone formation.
Several studies have also investigated mediators and inhibitors of BMP signal transduction. Noggin is a well-established antagonist of BMPs, and overexpression of noggin results in osteopenia [53-56]. Other studies have also shown that bone and skeletal development are decreased when BMP antagonists are overexpressed in osteoblasts [45,57]. While complete inactivation of noggin results in death in utero, noggin conditional knockout mice demonstrate decreased weight, shortened femoral length and osteopenia [53]. Although bone formation was found to be increased in 3-month-old mice, adult female mice did not exhibit increased bone formation or remodeling. Noggin-deficient mice also exhibit enlarged growth plates and joint fusions [48,58]. These findings indicate that either BMP excess can have a detrimental effect on bone or that noggin has BMP-independent roles in skeletal homeostasis.
In mice with conditional knockout of BMP receptor type IA (BMPRIA), an unexpected increase in bone mass was observed in mouse embryos, neonates and adults [59-61]. Adult bone demonstrated severely decreased resorption due to reduced RANKL-OPG-induced osteoclastogenesis [53]. Expression levels of both bone formation and resorption makers were decreased. In another study, mice with osteoblasts expressing a dominant-negative, non-functional BMP receptor (BMPRIB) exhibited a decrease in bone mineral density, bone volume, and bone formation [62]. Thus, BMP signaling is important both for the initial formation of chondrocytes and cartilage, as well as the subsequent formation of bone. These findings demonstrate the importance of BMP signaling in regulating the balance between bone formation and resorption.
Unlike the axial skeleton, which forms from cellular condensations of mesenchymal cells, the craniofacial skeleton and its associated cartilaginous elements are formed from the neural crest. This group of pluripotent cells forms in the dorsal neural tube and subsequently migrates to various areas of the developing embryo, including the skull, differentiating into bone, cartilage, and connective tissue of the head and neck. BMP signaling has been found to play a role in proper neural crest cell formation, migration, and differentiation, with BMP2, 4, and 7 specifically expressed during craniofacial skeletal development [63,64]. The importance of BMP signaling in craniofacial development is highlighted by multiple studies [65,66]. In one study, when Alk2, a type I BMP receptor, was conditionally deleted from a murine neural crest population, subsequent craniofacial malformations occurred, including cleft palate, hypotrophic mandible, and reduced ossification of the frontal bone [67].
BMPs are also expressed in the developing limb bud, where they play a role in proper limb formation and contribute to limb patterning along three different axes: anterior-posterior, dorsal-ventral, and proximal-distal. The apical ectodermal ridge is a structure located at the distal ectodermal tip of the limb bud and is especially important for proximal-distal limb patterning. It has been shown that BMP2, 4, and 7 are expressed in the AER, as well as in underlying mesenchymal cells, where these signaling cascades are likely involved in proper limb development [2,22]. Since BMP2 and BMP4 are embryonic lethal, conditional knockouts were created to remove BMP2 and BMP4 from the limb bud mesenchyme [68]. A threshold level of BMP signaling was found to be required for the onset of chondrogenesis, and chondrogenic condensations were not formed in limbs deficient in both BMP2 and BMP4. When condensations did form, however, chondrogenic differentiation proceeded normally in the absence of BMP2 and BMP7 or BMP2 and BMP4. Additionally, the combined knockdown of BMP2 and BMP4 from the limb bud resulted in lack of bone marrow cavity formation, as well as inability to form trabecular or cortical bone. Decreased expression of osterix, an osteoblast-specific gene, was also observed, showing that osteoblast differentiation was also decreased in this knockout model [68]. These findings demonstrate the importance of BMP2 and BMP4 in proper limb formation.
Other BMP knockout models also highlight the importance of this signaling cascade in proper bone and skeletal development. BMP7 mutants have multiple skeletal defects, as well as kidney and eye defects [69,70]. However, BMP6 mutations only result in minor sterna defects [71]. BMP11 mutations result in defects of anterior-posterior axial skeletal patterning [72]. Meanwhile, BMP3 inactivation results in increased bone density [73]. Although BMP2 and -4 mutant embryos die in utero before limb phenotypes can be determined, deletion of BMP7 embryos display polydactyly with incomplete penetrance and otherwise normal limbs [5,69,70,74]. These many studies highlight the critical importance of BMPs and mediators of BMP signal transduction in skeletal development and homeostasis (Table 1).
Table 1.
Examples of BMP-induced osteogenesis in MSCs.
| Treatment | Experiment setting | Experiment metric | Experimental results | Reference |
|---|---|---|---|---|
| BMP2 to BMP15 (14 BMPs) | In vitro | ALP activity | BMP2, 6, and 9 induced ALP activity in C3H10T1/2 pluripotent cells. | Cheng et al. 2003; Kang et al. 2004 |
| Osteocalcin expression | BMP2, 4, 6, 7, and 9 led to increased ALP activity in C2C12 preosteoblastic cells and TE-85 osteoblastic cells. | |||
| Alizarin Red S | BMP2, 6, and 9 were able to significantly induce osteocalcin expression and mineralization in C3H10T1/2 cells. | |||
| Mouse | Bone formation | BMP6 and BMP9-transduced C2C12 cells induced the most osteogenesis was not inhibited by BMP3, a negative regulator of bone formation. | Kang et al. 2004 | |
|
| ||||
| BMP2 | In vitro | ALP activity | Mesenchymal stem cells transduced with AdBMP2 showed increased osteoblastic differentiation and were able to form bone in vitro, with increased ALP activity, Alizarin Red S osteocalcin, COL1a1, and bone sialoprotein | Cheng et al. 2001 |
| Alizarin Red S | ||||
| Northern blot analysis | ||||
| Rabbit | Bone formation | AdBMP2-transduced cells were able to form bone when injected into the L5/6 intertransverse spinal space | ||
|
| ||||
| BMP6 | In vitro | ALP activity | AdBMP6-transduced equine mesenchymal stem cells showed increased ALP activity and mineralization. | Zachos et al. 2006 |
| Von Kossa staining mRNA microarray | AdBMP6 also induced increased expression of genes associated with osteoblastic differentiation. | |||
| Mouse | Bone formation | AdBMP6 subcutaneously injected into athymic nude was able to induce rapid ectopic bone formation. | Jane et al. 2002 | |
|
| ||||
| BMP7 | In vitro | ALP activity | C2C12 murine myoblast cells transduced with AdBMP7 differentiated into osteoblasts, displaying increased ALP activity and osteoblast-like morphology. These cells did not develop a myogenic phenotype. | Francheshi et al. 2000 |
| Cell morphology | ||||
| Mouse | Bone formation | Subcutaneous and intramuscular injections of AdBMP7 induced ectopic bone ossicle formation in mice. | ||
|
| ||||
| BMP9 + TGF-β1 | In vitro | ALP activity | Low concentrations of rhTGF-β1 synergistically increase ALP activity, matrix mineralization, gene expression and protein expression of osteopontin, osteocalcin and COL1a2 on BMP-9mediated osteogenic differentiation. | Li et al. 2012 |
| Alizarin Red S | ||||
| RT-PCR & Western blot | ||||
| Smad pathway activation | TGF-β1 combined with BMP9 exhibits lower BMPR-Smad receptor activity than BMP9 alone. | |||
|
| ||||
| BMP9 + GH | In vitro and ex vivo | ALP activity | GH potentiates BMP9-induced ALP activity, osteopontin/osteocalcin, expression and calcium deposition in MSCs | Huang et al. 2012 |
| Osteocalcin/Osteopontin expression | JAK/STAT inhibitors blunt BMP9-GH synergy | |||
| Alizarin Red S | GH enhances BMP9-induced endochondral ossification in cultured limb explants | |||
| Mouse | Ectopic bone formation | GH augments BMP9-induced ectopic bone formation with more mature bone | ||
4. BMPs and Osteogenesis
While the specific molecular mechanisms underlying BMP-mediated osteogenesis are not well-characterized, studies have demonstrated that BMPs play a critical role in osteogenic differentiation; overexpression of osteogenic BMPs with adenoviral, retroviral and recombinant systems have induced bone formation in animal studies [75-95] (Table 2). Exposure of MSCs to osteogenic BMPs results in increased expression of osteoblast-specific markers, including the early osteogenic marker alkaline phosphatase (ALP), the late osteogenic markers osteocalcin and osteopontin, connective tissue growth factor (CTGF), inhibitor of DNA binding (Id) and Cbfa1/Runx2 [11,18,28-30,96-98].
Table 2.
Skeletal phenotypes of several members of the BMP signaling pathway.
| Deleted gene | Cells affected | Skeletal phenotype | Reference |
|---|---|---|---|
| BMP2; BMP2 + 4 | Chondrocytes | Chondrodysplasia, disorganized chondrocytes in growth plate, defects in chondrocyte proliferation, differentiation, increased apoptosis | Shu et al. 2011 |
| BMP2 + 4 | Limb bud mesenchymal cells | Failure of chondrogenic condensations to form normally, no formation of bone marrow cavity, trabecular or cortical bone | Bandyopadhyay et al. 2006 |
| BMP3 | All cells | Increased bone density | Daluiski et al. 2001 |
| BMP5 | All cells | Short ear, brachypodism | King et al. 1994 |
| BMP6 | All cells | Minor sternal defects | Solloway et al. 1998 |
| BMP7 | All cells | Hindlimb polydactyly, defects in rib cage, skull | Luo et al. 1995 |
| BMP11 | All cells | Anterior-posterior axial skeletal patterning defects | McPherron et al. 1999 |
| Alk2 | Neural crest cells | Craniofacial malformations, including cleft palate, hypotrophic mandible, reduced ossification of frontal bone | Dudas et al. 2004 |
| BMPR1A | Osteoblasts | Increased bone mass, decreased bone resorption, reduced osteoclastogenesis | Kamiya et al. 2008 |
| BMPR1B | Osteoblasts | Decreased bone mineral density, bone volume, and bone formation | Zhao et al. 2002 |
| Smad6 | All cells | Axial and appendicular skeletal defects, posterior transformation of the cervical vertebrae, bilateral ossification centers in lumbar vertebrae, incomplete sternal band fusion, | Estrada et al. 2011 |
| Smad1 | Chondrocytes | Delayed calvarial bone development | |
| Smad1 | Osteoblasts | Osteopenia, impaired osteoblast proliferation and differentiation | Wang et al. 2011 |
| Noggin | Osteoblasts | Decreased weight, shortened femoral length, osteopenia | Canalis et al. 2012 |
Among the osteogenic BMPs, BMP2 and BMP7 were the first to be studied in depth. Adenoviral-mediated delivery of BMP2 to MSCs and other nonosteogenic cells increases osteogenic activity in vitro, lending support to the role of BMP2 in osteoblastic differentiation [76,86, 99,100]. C3H10T1/2 cells overexpressing AdBMP2 exhibited increased ALP activity, mineralization and mRNA expression of bone-specific proteins including type I collagen, osteopontin and osteocalcin [86,99]. AdBMP2-transfected bone marrow osteoprogenitor cells formed bone when seeded onto biodegradable polymer scaffolds as assessed by increased ALP activity, type I collagen production and mineralization [100]. BMP7 has also demonstrated the ability to induce osteogenesis. AdBMP7-transduced C2C12 myoblasts and muscle-derived progenitor cells differentiated into osteoblasts as assessed by ALP activity and matrix mineralization [81]. Furthermore, adipose-derived adult stem cells transduced with AdBMP7 differentiated into osteoblasts, eventually formed bone [101].
Both BMP2 and BMP7 have the ability to induce osteogenesis in vivo. Several studies have shown that MSCs and other progenitor cell types transduced with BMP2 or BMP7 induces bone formation in various animal models [75,76,78,79,81,83-86,88,89,91,99-102]. AdBMP2 injected directly into the thighs of rats led to bone formation at the injection sites as observed with CT, digital radiography and planar radionuclide scintigraphy [102]. When AdBMP7-transduced human and rat fibroblasts were subcutaneously implanted in mice, these normally non-osteogenic cells differentiated into osteoblasts and induced bone formation [83].
Non-adenoviral vectors, including recombinant BMP2 (rhBMP2) and rhBMP7, also induce bone formation [82, 95,103-108]. Treatment of C3H10T1/2 cells with rhBMP2 increased ALP activity [105]. In a canine ulnar segmental defect model, rhBMP7 treatment initiated new bone formation and led to defect repair and complete bony union [103]. rhBMP2 has shown a similar ability to repair bony defects in animal models [73].
The results from these studies demonstrating the osteoinductive properties of BMP2 and BMP7 have increased the clinical utilization of recombinant BMPs. In degenerative lumbar disc disease patients, use of rhBMP2/collagen within a lumbar spine interbody fusion cage achieved better fusion rates than use of autologous bone graft [109]. Treatment with rhBMP2 at the fracture site improved healing of patients with open tibial fractures [110]. rhBMP2 and rhBMP7 have subsequently been used to augment bone healing with improved fusion rates compared to autografts and fewer associated complications [83,91,93,94,99,100,102,104,111-114].
While BMP2 and BMP7 were initially identified by their ability to promote osteogenesis, it was unknown then if they were the most osteogenic BMPs. Subsequent studies have shown that osteogenic BMPs include 2, 4, 6, 7 and 9 [1,3,11,16,18,37,39,115-117]. Previously, the recombinant form of each BMP was unavailable and the osteogenic activity of all BMPs could not be analyzed. However, using adenoviral-mediated gene delivery into MSCs, we conducted a comprehensive analysis of the in vitro and in vivo osteogenic activity of 14 BMPs [11,13, 15-18]. We demonstrated BMP2, BMP6 and BMP9 as the most osteogenic BMPs, with BMP9 inducing the most potent osteogenic activity both in vitro and in vivo [11,13,18].
The osteogenic potential of BMP6 has been described both in vitro and in vivo [118-128]. AdBMP6-transduced equine bone marrow MSCs demonstrated enhanced osteoblastic differentiation with increased ALP activity, matrix mineralization and expression of osteogenic marker genes similar to AdBMP2 [128]. In a rabbit model, autologous bone marrow-derived osteoprogenitor cells exposed to an rhBMP6-containing extracellular matrix induced bone formation and enhanced spinal fusion [125]. In a rabbit ulnar osteotomy model, AdBMP6 accelerated bone formation and mineralization, leading to orthotopic bone formation when injected into the calf muscles of mice [118,124].
BMP9 has demonstrated potent osteoinduction in many studies including our comprehensive analysis of the osteogenic capacity of BMPs [13,18], but it remains one of the least studied and most poorly characterized BMPs. Further investigation may show that BMP9 provides a better therapeutic avenue for the augmentation of bone regeneration than the BMPs currently used in the clinical setting.
5. BMP9 Induces Osteogenic Differentiation
Many investigations have described the osteogenic properties of BMP9, implicating its role in osteoblastic differentiation and bone regeneration. Adenoviral-mediated overexpression of BMP9 in C3H10T1/2 cells increased ALP activity and calcium deposition at significantly higher levels than BMP2 or BmP7 treatment groups. [11,45,66]. BMP3, a known inhibitor of BMP2 and BMP7-mediated osteogenesis, did not inhibit BMP9-mediated bone formation, suggesting that BMP9-mediated osteogenesis may occur via a distinct mechanism from other osteogenic BMPs [13].
Non-adenoviral delivery of BMP9 has also demonstrated potent osteoinduction of MSCs [129-131]. Direct sonoporation of rhBMP9 into mouse quadriceps muscles caused the formation of ectopic bone [131]. Nucleofection of human MSCs (hMSCs) with BMP9 caused bone formation at four weeks post-injection in vivo and significantly increased calcium deposition in vitro [129]. Treatment of MC3T3-E1 preosteoblastic cells with a peptide derived from BMP9 (pBMP-9) induced downstream phosphorylation of Smad uninhibited by noggin, a known extracellular antagonist of BMP2 and caused a dose-dependent increase in ALP activity, Runx2, Osterix, type 1 collagen and osteocalcin [132].
In vivo studies have confirmed BMP9 as a potent inducer of bone formation. BMP9-transduced C2C12 cells injected into the quadriceps muscles of mice demonstrated significant orthotopic bone formation [13,14,115]. AdBMP9 directly injected into the quadriceps muscles of mice and rats increased osteoid and mature lamellar bone formation in BMP9-treated group compared to BMP2-and 7-treated groups, demonstrating that that skeletal muscle may harbor multipotent MSCs or osteoblastic progenitor cells [79,84,133]. Furthermore, the BMP9-induced ectopic bone was histologically determined to be the result of normal physiologic endochondral mechanisms.
BMP9 has demonstrated efficacy in non-union bone fracture repair and inducing spinal fusion in animal models [134,135]. Percutaneous paraspinal injection of AdBMP9-transduced hMSCs resulted in successful spinal fusion [134]. Non-union fracture was created in the radii of mice and filled with a collagen sponge electroporated with BMP9 plasmid; new bone was formed bridging the defect gap [135]. A defect in the radius of rabbits filled with an implant consisting of bone cement and AdBMP9 demonstrated more rapid callus and more bone formation compared to control and BMP2 treatment groups [136].
BMP9-induced bone formation demonstrates a distinct ossification pattern from other BMPs. In mice injected with AdBMP2 and 9-transduced C2C12 cells, increased bone maturation and marrow elements were seen in the BMP9-treated group [13]. When AdBMP9 was injected into the quadriceps muscles of mice and rats, primitive chondroblasts secreting a loose extracellular matrix was seen by six days, chondroblasts by nine days, areas of hypertrophic chondrocytes histologically similar to the epiphyseal end plate by 12 days, woven bone between days 12 and 19 and mature lamellar bone by three months [94]. These studies illustrate that the process of BMP9-induced osteogenesis resembles the sequential physiologic phases of endochondral ossification which occur during the repair of bony fractures. While the specific mechanisms of BMP9-mediated osteogenesis remain to be defined, it appears that the BMP9-mediated osteogenic pathway is unique from that of other members of the BMP family and may provide a more effective, physiologic therapy for bone regeneration.
6. Canonical BMP Signaling Pathway
While the specific mechanisms governing for BMP9-mediated osteogenesis are largely unknown, much work has been done to elucidate the signaling pathways of the BMP family. And while many characteristics of BMP9 signaling are similar to other members of the BMP family, identification of the unique aspects of BMP9 signal transduction will allow us to better understand and utilize is potent osteogenic properties.
6.1. BMP Ligands, BMP Type I and Type II Receptors Are Required for BMP Signaling
Like other members of the TGF-β superfamily, mature BMPs are secreted as a long precursor protein containing N-terminal pro-region that is cleaved prior to secretion. However, the mature BMP9 protein retains this N-terminal pro-region, which does not inhibit the function of BMP9 and may actually stabilize it following secretion [5,21,22, 137-142].
The BMP signaling pathway is initiated upon BMP ligand binding to a heterodimeric Type I/Type II BMP transmembrane serine/threonine kinase receptor (BMPR1 and BMPR2); both must be active for signal transduction (Figure 2) [143,144]. Type II receptors are constitutively active and serve to phosphorylate and activate BMPR1 upon BMP ligand binding [145]. The phosphorylation site on Type I receptors is located near the C-terminal intracellular domain in a glycine-serine rich region termed the GS domain [3,143].
Figure 2.
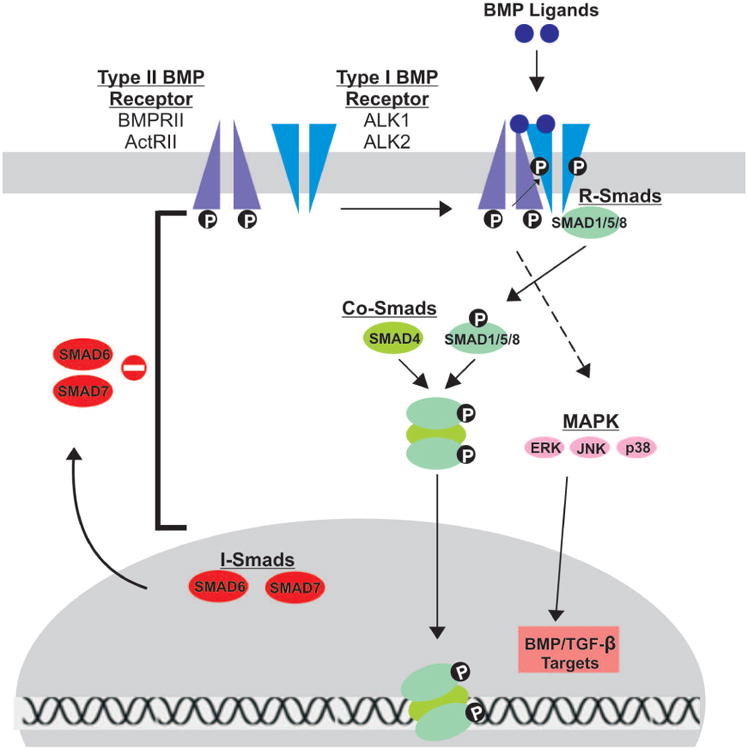
Schematic representation of BMP signal transduction. Upon BMP ligand binding to Type II BMP receptors, Type I BMP receptors are cross-phosphorylated, recruiting R-Smads (Smads 1/5/8) to the intracellular domain of the Type I receptor and initiating signal transduction via phosphorylation. Activated R-Smads then form a heteromeric complex with Co-Smads (Smad 4) before translocating to the nucleus to regulate gene expression. Inhibitory Smads (Smads 6/7) reside in the nucleus, migrate to the cytoplasm and negatively regulate BMP signaling by inhibiting signal transduction at several points along the pathway. The Type I and Type II receptors thought to be involved in BMP9 signal transduction are highlighted.
While seven different type I receptors have been identified, only three type I receptors, ALK1, ALK5 and endoglin, are considered potential BMP9 type I receptors [137,146-148]. In fact, BMP9 has a poor affinity for BMPR-IA, a receptor that generally transduces BMP signaling [137]. Dominant negative mutations of the seven type I receptors demonstrated that only ALK1 and ALK2 mutants effectively inhibit BMP9-mediated osteogenic differentiation and bone formation [66]. ALK1 and 2 directly interacted with BMP9, and silencing of ALK1 and 2 inhibited BMP9-induced osteogenic differentiation of MSCs both in vitro and in vivo. These results strongly suggest that ALK1 and ALK2 are the type I BMPRs responsible for BMP9-mediated osteogenic signaling.
Type II BMP receptors are thought to be largely responsible for the osteogenic activity of BMPs, and four have been identified [45]. Dominant negative (DN) mutations introduced in two of these receptors, BMPRII and ActRII, decreased BMP9-induced ALP activity, expression of downstream Smad 6 and Smad 7, bone mineralization in vitro and ectopic bone formation in vivo [45]. These results strongly suggest that BMPRII and ActRII are the type II BMP receptors responsible for BMP9-mediated osteogenic signaling.
6.2. Smad-Dependent Signaling Is Required for BMP Osteogenic Signaling
BMP binding to receptor heterodimers triggers one of two signaling pathways: Smad-dependent and Smad-independent. For the purpose of understanding the role of BMPs in osteogenesis, we will specifically focus on the Smad-dependent signaling pathway. There are 3 classes of Smads, all of which are important for BMP signal transduction [149]. Receptor-Smads (R-Smads) interact directly with activated type I receptors, while Common-Smads (co-Smads) form complexes with activated R-Smads to then regulate gene expression in the nucleus. Inhibitory Smads (I-Smads) negatively regulate BMP signaling, inhibiting signal transduction at several points in the pathway.
The R-Smads, Smad1, Smad5 and Smad8, are activated upon binding and phosphorylation by the Type I receptor at a conserved sequence in the C-terminus termed the Ser-Ser-Val/Met-Ser sequence, or SSXS motif. Along with this conserved sequence, R-Smads have two additional regions of homology at the N and C-terminal ends, the Mad homology (MH) 1 and MH2 domains, both of which are crucial for proper signal transduction [3,150]. The MH1 domain directly interacts with DNA sequences, while the MH2 domain interacts with BMPR1. Additionally, the MH2 domain can bind to other Smads and has a role in transcriptional activation. These domains are conserved across co-Smads and I-Smads. Upon activation, the phosphorylated R-Smad dissociates from BMPR1 and forms a complex with Smad 4 [143,151,152]. Smad 4 is the sole Co-Smad and is common to all BMP signaling pathways. In the nucleus, the R-Smad/Co-Smad heterodimer complexes with various transcription factors, co-activators and corepressors to modulate gene expression.
The BMP signaling pathway is negatively regulated by I-Smads, Smad 6 and Smad 7. These Smads typically reside in the nucleus and migrate to the cytoplasm and plasma membrane upon BMP activation, acting at various points along the BMP pathway to inhibit signal transduction [1,151,153-157]. Smad7 binds to activated BMPR1, preventing R-Smads from becoming activated. Smad7 also interacts with E3 ubiquitin ligase proteins, Smurf1 and Smurf2, targeting the BMP receptor for proteasomal degradation. Unlike Smad 7, Smad 6 can bind directly to R-Smads, thus competing with Smad 4 and ultimately preventing proper heterodimer complex formation.
Activation of Smads was found to be necessary for BMP9-mediated osteogenic differentiation of MSCs [158]. Phosphorylated Smad 1/5/8 levels were simultaneously increased in BMP9-treated MSCs, while knockdown of Smad 4 resulted in reduced formation of Smad heterodimers and nuclear translocation of Smad 1/5/8; knockdown of Smad4 also inhibited BMP9-induced ALP activity and calcium deposition. The p38 inhibitor SB203580 decreased BMP9-induced Smad signaling in MSCs, while the ERK1/2 inhibitor PD98059 stimulated Smad signaling. Together, these findings suggest that activation of the Smads pathway is critical in BMP9-induced osteogenesis.
7. Mediators of BMP9-Induced Osteogenic Signaling
Recent studies have identified various mediators thought to contribute to the potent osteogenic effects of BMP9. Among these mediators are Id genes, connective tissue growth factor (CTGF) and Hey1. Each of these three genes is among the most up-regulated following BMP9 stimulation of MSCs [24,26,139].
Balanced regulation of Id expression is important in lineage-specific MSC differentiation
Id genes are inhibitors of the binding of basic helix-loop-helix (bHLH) transcription factors [159-161] and function by dimerizing with bHLH proteins; these heterodimers are unable to bind DNA and regulate transcription. Id-1, -2 and -3 are among the most significantly up-regulated genes upon BMP9 stimulation [30]. However, both knockdown and overexpression of these three Id genes diminished BMP9-induced osteogenic differentiation. BMP9-mediated Id expression was also shown to be dependent on Smad4 signaling.
Balanced regulation of CTGF expression is important in BMP9-induced osteogenic differentiation
Connective tissue growth factor (CTGF) is a member of the CCN (Cyr61, CTGF and Nov) family of secreted cysteine-rich multimodular proteins [162-167] and has a major role in bone formation and embryogenesis [168]. Upon BMP9-stimulation of MSCs, CTGF was among the most up-regulated genes, especially during early stages of differentiation [28]. Similar to Id genes, both knockdown and overexpression diminished BMP9-mediated osteogenic differentiation.
Hey 1 Expression Enhances BMP9-induced osteogenic differentiation via Runx2
Hey 1 (also known as Hesr1, HRT1, CHF2 and HERP2) is a nuclear protein of the Hairy/Enhancer of split-Related (HERP) family of basic helix-loop-helix transcriptional repressors and is a direct target of the Notch pathway [14]. Constitutive Hey 1 expression synergized with BMP9-induced osteogenic differentiation in vitro and in vivo [169], while Hey 1 silencing decreased osteogenic differentiation. Hey1 and the essential osteogenic transcription factor Runx2 synergized in BMP9-induced osteogenic differentiation, whereas Hey 1 silencing decreased Runx2 expression. Following knockdown of Hey 1, exogenous Runx2 expression rescued defective osteogenic signaling, strongly suggesting that Runx2 is a downstream mediator of Hey 1 signaling.
8. Major Signaling Pathways Crosstalk With BMP9 Signaling
Several major signaling pathways with wide-ranging functions participate in BMP9-mediated osteogenesis, and many of these pathways are critical in the differentiation of other cell lineages. The following section describes recent studies illustrating the crosstalk between BMP9 signaling and these other important pathways. A brief description of each of these signaling pathways will be followed by a summary of results from recent studies.
TGF-β1 is one of the most abundant members of the TGF-β superfamily, regulating bone formation, osteoblast proliferation and mineralization while increasing the strength and flexibility of bone [170-173]. TGF-β1 and BMPs both regulate the late phases of differentiation and mineralization of bone [138,174-176].
Growth hormone (GH) plays a critical role in postnatal growth [177-184]. GH signaling pathway begins when GH binds GHR, triggering receptor tyrosine kinase activity and activation of JAK/STAT and other pathways [178,179,184]. IGF-2 is a member of the IGF signaling system, playing a critical role in prenatal growth and development [185]. IGF-2 signaling activates the phosphatidylinositol-3-kinase (PI3K)/AKT pathway or the mitogen-activated protein kinase pathway (MAPK) [186].
Wnts are a family of secreted proteins critical in osteoblastic differentiation and skeletal development [16, 116,187-193]. Upon Wnt binding the Frizzled (Frz) and LRP-5/6 co-receptors, distinct signaling pathways including the canonical Wnt pathway are activated [194]; mutations in LRP-5 adversely affect skeletal development and bone mass deposition [195].
MAPKs are protein kinases critical in regulation of gene expression, mitosis, metabolism, motility, survival, apoptosis and differentiation [21,158,196-199]. Members of the MAPK family become activated by BMPs in response to a variety of extracellular stimuli leading to diverse effects in cellular responses [198-201]. Hypoxia inducible factor 1 Alpha (HIF1α) is a regulator of angiogenesis during many developmental processes, including skeletal development [64,202].
Peroxisome proliferator-activated receptor gamma (PPAR-γ) is a critical regulator of adipogenesis and osteogenesis [203,204]. PPAR-γ binds fatty acid derivatives to induce differentiation of preadipocytes into terminal adipocytes, and PPAR-γ2 is the predominant iso-form expressed in adipose tissue [81,159,161]. Retinoic acids (RAs) play a crucial role during embryonic development and in the maintenance of vital organs [38,39]. RAs are ligands for two families of receptors, the RA receptors (RAR) that bind all-trans-RA (ATRA) and the Retinoid X Receptors (RXR) that bind 9-cis-RA (9CRA) [205,206]. RA binding to RAR/RXR causes heterodimerization and eventual transcriptional regulation [206].
Effects of Crosstalk Pathways on BMP9-Induced Osteogenic Differentiation
TGF-β1 has a biphasic effect on BMP9-induced osteogenic differentiation of MSCs
In BMP9-transduced MSCs, low concentrations of recombinant TGF-β1 (rhTGF-β/1) synergistically induced expression of ALP and matrix mineralization, while high concentrations of TGF-β1 inhibited BMP9-induced osteogenic activity [175].
GH synergizes with BMP9 via activation of the JAK/STAT/IGF1 pathway to induce osteogenesis
After over-expression of BMP9 in MSCs, GH was one of the most up-regulated transcripts [158]. Exogenous GH synergized with BMP9 to induce early and late osteogenic markers. Co-stimulation of long-bone explants with GH and BMP9 resulted in significant expansion of the growth plate, and co-stimulation of MSCs with BMP9 and GH formed mature ectopic bone masses; these synergistic effects of BMP9 and GH were inhibited by JAK/STAT inhibitors.
BMP9 signaling crosstalks with IGF-2 via PI3K/AKT signaling\
While endogenous IGF-2 levels are relatively low in MSCs, exogenous expression of IGF-2 potentiates BMP9-induced expression of early and late osteogenic markers [207]. Conversely, PI3K inhibition diminished IGF-2 potentiation on BMP9-mediated osteogenesis. IGF-2 augmented BMP9-induced ectopic bone formation and BMP9-mediated endochondral ossification in perinatal limb explants.
The canonical Wnt/β-catenin pathway interacts with Runx2 as a critical mediator of BMP9-mediated osteogenic signaling
Wnt3a and BMP9 synergized to induce ALP activity in MSCs, while the Wnt antagonist FrzB inhibited BMP9-induced ALP activity [194]. BMP9 stimulation of MSCs recruits β-catenin and Runx2 to the osteocalcin promoter, whereas knockdown of β-catenin decreased expression of early and late osteogenic markers [194]. BMP9-induced ectopic bone formation and matrix mineralization in vivo were inhibited by FrzB overexpression or β-catenin knockdown [194].
p38 and ERK1/2 have opposing regulatory effects in BMP9-induced osteogenic differentiation of MSCs via Smad signaling
BMP9 simultaneously promotes phosphorylation and thus activation of Smads, p38 and ERK1/2 [158]. p38 and ERK1/2 acted in opposition to regulate BMP9-mediated osteogenic differentiation via interactions with Smads. In vivo, inhibition of p38 significantly decreased BMP9-induced osteogenic differentiation, while inhibition of ERK1/2 significantly increased BMP9-induced osteogenic differentiation [208].
HIF1α synergizes with BMP9-induced osteogenic differentiation of MSCs
Exogenous overexpression of HIF1α synergistically increased BMP9-induced osteogenic differentiation of MSCs. Conversely, inhibition of HIF1α diminished BMP9-induced osteogenic signaling. BMP9 directly induced HIF1α expression in MSCs via Smad1/5/8 signaling [209]. HIF1α activated both angiogenic and osteogenic signaling pathways in MSCs. Osteogenic factors, including BMP9, may induce the convergence of osteogenic and angiogenic signaling in MSC differentiation, thereby enhancing the efficiency of bone formation and development.
PPAR-γ is an important regulator of BMP9-mediated osteogenesis
Overexpression of PPAR-γ2 in BMP-9-stimulated MSCs promoted both osteogenic and adipogenic differentiation, with mutually exclusive commitment to either lineage [18]. Conversely, knockdown of PPAR-γ2 in BMP9-stimulated MSCs showed significant decreases in osteogenic differentiation and matrix mineralization.
Retinoic acids synergistically enhance BMP9-mediated osteoinduction of MSCs
Both ATRA and 9CRA induced expression of BMP9, activated BMPRSmad transcription activity and increased expression of early and late osteogenic markers; these effects were synergistic when combined with overexpression of BMP9 [210]. RAs combined with BMP9 promoted expansion of the hypertrophic chondrocyte zone in neonatal mouse limb explants, and RARs synergized with BMP9 to induce trabecular bone formation and osteoid matrix production in vivo.
9. Other Functions Of BMP9
BMP9 is known to be a potent osteogenic factor, but it also influences several other pathways including cancer development and angiogenesis. While some studies have shown BMP9 to restrict tumor growth through diverse mechanisms [211-213], other studies have shown BMP9 to promote cancer progression [214]. BMP9 inhibited cell migration and induce apoptosis of osteosarcoma via the PI3K/ALT pathway [212] slowed tumor growth of colon adenocarcinoma via inhibitory effects on angiogenesis via the ALK1 receptor and endoglin coreceptor [211]. Conversely, BMP9 induced in vivo angiogenesis within pancreatic tumors [214]. The effects of BMP9 on angiogenesis remain controversial as well, with some studies demonstrating a pro-angiogenic effect [215,216] and others an anti-angiogenic effect [146,217-219]. It is evident that the effects of BMP9 on angiogenesis and cancer progression remain to be fully elucidated.
BMP9 also modulates neurogenesis, hepatocellular regeneration, adipogenesis, chondrogenesis and myogenesis. Several studies have demonstrated BMP9 to promote the cholinergic phenotype neurologically [220-223]. BMP9 also acts as a hepatic insulin-sensitizing substance and may play a role in hepatocellular regeneration [224,225]. BMP9 promotes adipogenesis [18] and also upregulates Sox9 expression to induce chondrogenic differentiation [226,227]. While BMP9 promotes MSC differentiation along osteogenic, adipogenic and chondrogenic lines to varying degrees, it inhibits the myogenic phenotype [29]. Overall, BMP9 has diverse effects beyond osteogenesis.
10. Concluding Remarks and Future Directions
The investigations discussed here demonstrate the critical role of BMPs, particularly BMP9, in the osteogenic differentiation of MSCs. The findings discussed here strongly support the notion that BMP9 may provide a more effective clinical strategy for the augmentation of bone regeneration and healing than other BMPs. Furthermore, studies demonstrating that BMP9-mediated osteogenesis resembles the physiologic phases of bone healing occurring during fracture repair make the prospect of clinical translation quite promising. With several diverse signaling pathways enhancing BMP9-mediated osteogenesis, further elucidation of these specific pathways will allow for the development of improved therapies. Over the last decade, there has been a substantial increase in the therapeutic use of recombinant proteins and medications targeting small molecules acting in various signaling pathways. With this, it is imperative that the mechanisms underlying BMP9-mediated osteogenesis become fully elucidated to allow for the development of much-needed clinical therapies.
Acknowledgments
The reported work was supported in part by research grants from the OREF and the National Institutes of Health (RCH, TCH and HHL). JDL was the recipient of the Pritzker Research Fellowship from The University of Chicago Pritzker School of Medicine and the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 2.Hogan BL. Bone morphogenetic proteins in development. Current Opinion in Genetics & Development. 1996;6:432–438. doi: 10.1016/S0959-437X(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 4.Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24:5713–5721. doi: 10.1038/sj.onc.1208919. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Li L. BMP signaling and stem cell regulation. Developmental Biology. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Christiansen JH, Coles EG, Wilkinson DG. Molecular control of neural crest formation, migration and differentiation. Current Opinion in Cell Biology. 2000;12:719–724. doi: 10.1016/S0955-0674(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 7.Derynck R, Feng XH. TGF-beta receptor signaling. Biochimica et Biophysica Acta. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 8.Di Cesare PE, Frenkel SR, Carlson CS, Fang C, Liu C. Regional gene therapy for full-thickness articular cartilage lesions using naked DNA with a collagen matrix. Journal of Orthopaedic Research. 2006;24:1118–1127. doi: 10.1002/jor.20143. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Chen YX, Wang D, Qi X, Li TG, Hao J, Mishina Y, Garbers DL, Zhao GQ. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Developmental Biology. 2004;276:158–171. doi: 10.1016/j.ydbio.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis and Cartilage. 2006;14:1126–1135. doi: 10.1016/j.joca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, He TC. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. Journal of Orthopaedic Research. 2007;25:665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 12.Mitu G, Hirschberg R. Bone morphogenetic protein-7 (BMP7) in chronic kidney disease. Frontiers in Bioscience. 2008;13:4726–4739. doi: 10.2741/3035. [DOI] [PubMed] [Google Scholar]
- 13.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Therapy. 2004;11:1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 14.Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J, Bi Y, Luo X, Luo J, Teven C, Shi Q, Kim SH, Gao JL, Huang E, Yang K, Rames R, Liu X, Li M, Hu N, Liu H, Su Y, Chen L, He BC, Zuo GW, Deng ZL, Reid RR, Luu HH, Haydon RC, He TC. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: Molecular mechanism and therapeutic potential. Current Gene Therapy. 2011;11:229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 15.Aubin JE. Regulation of osteoblast formation and function. Reviews in Endocrine and Metabolic Disorders. 2001;2:81–94. doi: 10.1023/A:1010011209064. [DOI] [PubMed] [Google Scholar]
- 16.Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC. Regulation of osteogenic differentiation during skeletal development. Frontiers in Bioscience. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 17.He BC, Chen L, Zuo GW, Zhang W, Bi Y, Huang J, Wang Y, Jiang W, Luo Q, Shi Q, Zhang BQ, Liu B, Lei X, Luo J, Luo X, Wagner ER, Kim SH, He CJ, Hu Y, Shen J, Zhou Q, Rastegar F, Deng ZL, Luu HH, He TC, Haydon RC. Synergistic antitumor effect of the activated PPARgamma and retinoid receptors on human osteosarcoma. Clinical Cancer Research. 2010;16:2235–2245. doi: 10.1158/1078-0432.CCR-09-2499. [DOI] [PubMed] [Google Scholar]
- 18.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK, Jiang W, Tang Y, Huang J, Su Y, Zhu GH, He Y, Yin H, Hu Z, Wang Y, Chen L, Zuo GW, Pan X, Shen J, Vokes T, Reid RR, Haydon RC, Luu HH, He TC. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differenttiation of mesenchymal progenitor cells. Stem Cells and Development. 2009;18:545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 20.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 21.Hogan BL. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes & Development. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 22.Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 23.Song JJ, Celeste AJ, Kong FM, Jirtle RL, Rosen V, Thies RS. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136:4293–4297. doi: 10.1210/en.136.10.4293. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Grzegorzewski KJ, Barash S, Zhao Q, Schneider H, Wang Q, Singh M, Pukac L, Bell AC, Duan R, Coleman T, Duttaroy A, Cheng S, Hirsch J, Zhang L, Lazard Y, Fischer C, Barber MC, Ma ZD, Zhang YQ, Reavey P, Zhong L, Teng B, Sanyal I, Ruben SM, Blondel O, Birse CE. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nature Biotechnology. 2003;21:294–301. doi: 10.1038/nbt795. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Coviella I, Berse B, Krauss R, Thies RS, Blusztajn JK. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289:313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- 26.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) The Journal of Bone and Joint Surgery American Volume. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. The Journal of Biological Chemistry. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 29.Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W, Luu HH, Park JY, Haydon RC, He TC. Transcriptional characterization of bone morpho-genetic proteins (BMPs)-mediated osteogenic signaling. Journal of Cellular Biochemistry. 2003;90:1149–1165. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- 30.Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, Luu HH, Park JK, Li X, Luo J, Montag AG, Hay-don RC, He TC. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. The Journal of Biological Chemistry. 2004;279:32941–32949. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 31.Karsenty G. The genetic transformation of bone biology. Genes & Development. 1999;13:3037–3051. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- 32.Lian JB, Stein GS, Stein JL, van Wijnen AJ. Transcriptional control of osteoblast differentiation. Biochemical Society Transactions. 1998;26:14–21. doi: 10.1042/bst0260014. [DOI] [PubMed] [Google Scholar]
- 33.Reddi AH. Bone morphogenetic proteins: An unconventional approach to isolation of first mammalian morphogens. Cytokine & Growth Factor Reviews. 1997;8:11–20. doi: 10.1016/S1359-6101(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 34.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. Journal of Cellular Biochemistry. 2011;112:3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcellini S, Henriquez JP, Bertin A. Control of osteogenesis by the canonical Wnt and BMP pathways in vivo: Cooperation and antagonism between the canonical Wnt and BMP pathways as cells differentiate from osteochondroprogenitors to osteoblasts and osteocytes. Bioessays. 2012;34:953–962. doi: 10.1002/bies.201200061. [DOI] [PubMed] [Google Scholar]
- 36.Olsen BR, Reginato AM, Wang W. Bone development. Annual Review of Cell and Developmental Biology. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 37.Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney International. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 38.Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes & Development. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nature Biotechnology. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 40.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 41.Ralston SH, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes & Development. 2006;20:2492–2506. doi: 10.1101/gad.1449506. [DOI] [PubMed] [Google Scholar]
- 42.King JA, Marker PC, Seung KJ, Kingsley DM. BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Developmental Biology. 1994;166:112–122. doi: 10.1006/dbio.1994.1300. [DOI] [PubMed] [Google Scholar]
- 43.Hall PA, Watt FM. Stem cells: The generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 44.Tsumaki N, Yoshikawa H. The role of bone morphogenetic proteins in endochondral bone formation. Cytokine & Growth Factor Reviews. 2005;16:279–285. doi: 10.1016/j.cytogfr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Wu N, Zhao Y, Yin Y, Zhang Y, Luo J. Identification and analysis of type II TGF-beta recaptors in BMP-9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. Acta Biochimica et Biophysica Sinica. 2010;42:699–708. doi: 10.1093/abbs/gmq075. [DOI] [PubMed] [Google Scholar]
- 46.Wu X, Shi W, Cao X. Multiplicity of BMP signaling in skeletal development. Annals of the New York Academy of Sciences. 2007;1116:29–49. doi: 10.1196/annals.1402.053. [DOI] [PubMed] [Google Scholar]
- 47.Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O'Keefe RJ, Hilton MJ, Wang Y, Chen D. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. Journal of Cell Science. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Estrada KD, Retting KN, Chin AM, Lyons KM. Smad6 is essential to limit BMP signaling during cartilage development. Journal of Bone and Mineral Research. 2011;26:2498–2510. doi: 10.1002/jbmr.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan Q, Yu Y, Chen Q, Li C, Wu H, Wan Y, Ma J, Sun F. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. Journal of Cellular Physiology. 2008;217:228–241. doi: 10.1002/jcp.21496. [DOI] [PubMed] [Google Scholar]
- 50.Zehentner BK, Dony C, Burtscher H. The transcription factor Sox9 is involved in BMP-2 signaling. Journal of Bone and Mineral Research. 1999;14:1734–1741. doi: 10.1359/jbmr.1999.14.10.1734. [DOI] [PubMed] [Google Scholar]
- 51.Wang M, Jin H, Tang D, Huang S, Zuscik MJ, Chen D. Smad1 plays an essential role in bone development and postnatal bone formation. Osteoarthritis and Cartilage. 2011;19:751–762. doi: 10.1016/j.joca.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. Journal of Cellular Biochemistry. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- 53.Canalis E, Brunet LJ, Parker K, Zanotti S. Conditional inactivation of noggin in the postnatal skeleton causes osteopenia. Endocrinology. 2012;153:1616–1626. doi: 10.1210/en.2011-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 55.Valenzuela DM, Economides AN, Rojas E, Lamb TM, Nunez L, Jones P, Lp NY, Espinosa R, 3rd, Brannan CI, Gilbert DJ, et al. Identification of mammalian noggin and its expression in the adult nervous system. The Journal of Neuroscience. 1995;15:6077–6084. doi: 10.1523/JNEUROSCI.15-09-06077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/S0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 57.Devlin RD, Du Z, Pereira RC, Kimble RB, Economides AN, Jorgetti V, Canalis E. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144:1972–1978. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- 58.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 59.Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. Journal of Bone and Mineral Research. 2009;25:200–210. doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. Journal of Bone and Mineral Research. 2008;23:2007–2017. doi: 10.1359/jbmr.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801–3811. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, Chen D. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. Journal of Cellular Biochemistry. 2002;157:1049–1060. doi: 10.1083/jcb.200109012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Francis-West PH, Tatla T, Brickell PM. Expression patterns of the bone morphogenetic protein genes Bmp-4 and Bmp-2 in the developing chick face suggest a role in outgrowth of the primordia. Developmental Dynamics. 1994;201:168–178. doi: 10.1002/aja.1002010207. [DOI] [PubMed] [Google Scholar]
- 64.Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, Schipani E, Clemens TL. Role of HIF-1alpha in skeletal development. Annals of the New York Academy of Sciences. 2010;1192:322–326. doi: 10.1111/j.1749-6632.2009.05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–1104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- 66.Luo J, Tang M, Huang J, He BC, Gao JL, Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Bi Y, Luo X, Jiang W, Su Y, Shen J, Kim SH, Huang E, Gao Y, Zhou JZ, Yang K, Luu HH, Pan X, Haydon RC, Deng ZL, He TC. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. The Journal of Biological Chemistry. 2010;285:29588–29598. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mechanisms of Development. 2004;121:173–182. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genetics. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes & Development. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 70.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes & Development. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 71.Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Developmental Genetics. 1998;22:321–339. doi: 10.1002/(SICI)1520-6408(1998)22:4<321∷AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 72.McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nature Genetics. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- 73.Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM. Bone morphogenetic protein-3 is a negative regulator of bone density. Nature Genetics. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 74.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes & Development. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 75.Alden TD, Pittman DD, Beres EJ, Hankins GR, Kallmes DF, Wisotsky BM, Kerns KM, Helm GA. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. Journal of Neurosurgery. 1999;90:109–114. doi: 10.3171/spi.1999.90.1.0109. [DOI] [PubMed] [Google Scholar]
- 76.Alden TD, Pittman DD, Hankins GR, Beres EJ, Engh JA, Das S, Hudson SB, Kerns KM, Kallmes DF, Helm GA. In vivo endochondral bone formation using a bone morphogenetic protein 2 adenoviral vector. Human Gene Therapy. 1999;10:2245–2253. doi: 10.1089/10430349950017220. [DOI] [PubMed] [Google Scholar]
- 77.Baltzer AW, Lattermann C, Whalen JD, Ghivizzani S, Wooley P, Krauspe R, Robbins PD, Evans CH. Potential role of direct adenoviral gene transfer in enhancing fracture repair. Clinical Orthopaedics and Related Research. 2000:S120–S125. doi: 10.1097/00003086-200010001-00016. [DOI] [PubMed] [Google Scholar]
- 78.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: Healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Therapy. 2000;7:734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 79.Bosch P, Musgrave D, Ghivizzani S, Latterman C, Day CS, Huard J. The efficiency of muscle-derived cell-mediated bone formation. Cell Transplant. 2000;9:463–470. doi: 10.1177/096368970000900403. [DOI] [PubMed] [Google Scholar]
- 80.Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: Applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Annals of Plastic Surgery. 1999;42:488–495. doi: 10.1097/00000637-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: In vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. Journal of Cellular Biochemistry. 2000;78:476–486. doi: 10.1002/1097-4644(20000901)78:3<476∷AID-JCB12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 82.Gazit D, Turgeman G, Kelley P, Wang E, Jalenak M, Zilberman Y, Moutsatsos I. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: A novel cell-mediated gene therapy. The Journal of Gene Medicine. 1990;1:121–133. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<121∷AID-JGM26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 83.Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Human Gene Therapy. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 84.Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A, Robbins P, Fu FH, Huard J. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. The Journal of Bone and Joint Surgery American Volume. 2001;83-A:1032–1039. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 85.Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, Stevenson S. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. Journal of Orthopaedic Research. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 86.Lou J, Xu F, Merkel K, Manske P. Gene therapy: Adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. Journal of Orthopaedic Research. 1999;17:43–50. doi: 10.1002/jor.1100170108. [DOI] [PubMed] [Google Scholar]
- 87.Mason JM, Grande DA, Barcia M, Grant R, Pergolizzi RG, Breitbart AS. Expression of human bone morphogenic protein 7 in primary rabbit periosteal cells: Potential utility in gene therapy for osteochondral repair. Gene Therapy. 1998;5:1098–1104. doi: 10.1038/sj.gt.3300703. [DOI] [PubMed] [Google Scholar]
- 88.Musgrave DS, Bosch P, Ghivizzani S, Robbins PD, Evans CH, Huard J. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24:541–547. doi: 10.1016/S8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 89.Okubo Y, Bessho K, Fujimura K, Iizuka T, Miyatake SI. Osteoinduction by bone morphogenetic protein-2 via adenoviral vector under transient immunosuppression. Biochemical and Biophysical Research Communications. 2000;267:382–387. doi: 10.1006/bbrc.1999.1975. [DOI] [PubMed] [Google Scholar]
- 90.Oyama M, Tatlock A, Fukuta S, Kavalkovich K, Nishimura K, Johnstone B, Robbins PD, Evans CH, Niyibizi C. Retrovirally transduced bone marrow stromal cells isolated from a mouse model of human osteogenesis imperfecta (oim) persist in bone and retain the ability to form cartilage and bone after extended passaging. Gene Therapy. 1999;6:321–329. doi: 10.1038/sj.gt.3300839. [DOI] [PubMed] [Google Scholar]
- 91.Riew KD, Wright NM, Cheng S, Avioli LV, Lou J. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcified Tissue International. 1998;63:357–360. doi: 10.1007/s002239900540. [DOI] [PubMed] [Google Scholar]
- 92.Ripamonti U, Ramoshebi LN, Matsaba T, Tasker J, Crooks J, Teare J. Bone induction by BMPs/OPs and related family members in primates. The Journal of Bone and Joint Surgery American Volume. 2001;83-A:S116–S127. [PubMed] [Google Scholar]
- 93.Sandhu HS, Khan SN, Suh DY, Boden SD. Demineralized bone matrix, bone morphogenetic proteins, and animal models of spine fusion: An overview. European Spine Journal. 2001;10:S122–S131. doi: 10.1007/s005860100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Varady P, Li JZ, Cunningham M, Beres EJ, Das S, Engh J, Alden TD, Pittman DD, Kerns KM, Kallmes DF, Helm GA. Morphologic analysis of BMP-9 gene therapy-induced osteogenesis. Human Gene Therapy. 2001;12:697–710. doi: 10.1089/104303401300057423. [DOI] [PubMed] [Google Scholar]
- 95.Whang K, Tsai DC, Nam EK, Aitken M, Sprague SM, Patel PK, Healy KE. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. Journal of Biomedical Materials Research. 1998;42:491–499. doi: 10.1002/(SICI)1097-4636(19981215)42:4<491∷AID-JBM3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 96.He TC. Distinct osteogenic activity of BMPs and their orthopaedic applications. Journal of Musculoskeletal & Neuronal Interactions. 2005;5:363–366. [PubMed] [Google Scholar]
- 97.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Reviews in Endocrine and Metabolic Disorders. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 98.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocrine Reviews. 2000;21:393–411. doi: 10.1210/er.21.4.393. [DOI] [PubMed] [Google Scholar]
- 99.Cheng SL, Lou J, Wright NM, Lai CF, Avioli LV, Riew KD. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcified Tissue International. 2001;68:87–94. doi: 10.1007/BF02678146. [DOI] [PubMed] [Google Scholar]
- 100.Partridge K, Yang X, Clarke NM, Okubo Y, Bessho K, Sebald W, Howdle SM, Shakesheff KM, Oreffo RO. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: In vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochemical and Biophysical Research Communications. 2002;292:144–152. doi: 10.1006/bbrc.2002.6623. [DOI] [PubMed] [Google Scholar]
- 101.Yang M, Ma QJ, Dang GT, Ma K, Chen P, Zhou CY. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy. 2005;7:273–281. doi: 10.1080/14653240510027244. [DOI] [PubMed] [Google Scholar]
- 102.Varady P, Li JZ, Alden TD, Kallmes DF, Williams MB, Helm GA. CT and radionuclide study of BMP-2 gene therapy-induced bone formation. Academic Radiology. 2002;9:632–637. doi: 10.1016/S1076-6332(03)80307-0. [DOI] [PubMed] [Google Scholar]
- 103.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC. Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clinical Orthopaedics and Related Research. 1994;301:302–312. [PubMed] [Google Scholar]
- 104.Gerhart TN, Kirker-Head CA, Kriz MJ, Holtrop ME, Hennig GE, Hipp J, Schelling SH, Wang E. Healing segmental femoral defects in sheep using recombinant human bone morphogenetic protein. Clinical Orthopaedics and Related Research. 1993;293:317–326. [PubMed] [Google Scholar]
- 105.Katagiri T, Yamaguchi A, Ikeda T, Yoshiki S, Wozney JM, Rosen V, Wang EA, Tanaka H, Omura S, Suda T. The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochemical and Biophysical Research Communications. 1990;172:295–299. doi: 10.1016/S0006-291X(05)80208-6. [DOI] [PubMed] [Google Scholar]
- 106.Valentin-Opran A, Wozney J, Csimma C, Lilly L, Riedel GE. Clinical evaluation of recombinant human bone morphogenetic protein-2. Clinical Orthopaedics and Related Research. 2002;395:110–120. doi: 10.1097/00003086-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 107.Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. Journal of Cell Biology. 1991;113:681–687. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. The Journal of Bone and Joint Surgery American Volume. 1992;74:659–670. [PubMed] [Google Scholar]
- 109.Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: A preliminary report. Spine. 2000;25:376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 110.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. The Journal of Bone and Joint Surgery American Volume. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 111.Bostrom MP, Camacho NP. Potential role of bone morphogenetic proteins in fracture healing. Clinical Orthopaedics and Related Research. 1998;355S:S274–S282. doi: 10.1097/00003086-199810001-00028. [DOI] [PubMed] [Google Scholar]
- 112.Heckman JD, Boyan BD, Aufdemorte TB, Abbott JT. The use of bone morphogenetic protein in the treatment of non-union in a canine model. The Journal of Bone and Joint Surgery American Volume. 1991;73:750–764. [PubMed] [Google Scholar]
- 113.Helm GA, Alden TD, Beres EJ, Hudson SB, Das S, Engh JA, Pittman DD, Kerns KM, Kallmes DF. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. Journal of Neurosurgery. 2000;92:191–196. doi: 10.3171/spi.2000.92.2.0191. [DOI] [PubMed] [Google Scholar]
- 114.Lee AR, Wilkins AC, Leather C, Brenton AG. Translational energy spectra for single-electron capture by O2+ in He, Ne, and Ar. Physical Review A. 1994;50:1149–1154. doi: 10.1103/PhysRevA.50.1149. [DOI] [PubMed] [Google Scholar]
- 115.Luo J, Sun MH, Kang Q, Peng Y, Jiang W, Luu HH, Luo Q, Park JY, Li Y, Haydon RC, He TC. Gene therapy for bone regeneration. Current Gene Therapy. 2005;5:167–179. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 116.Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differrentiation. Clinical Orthopaedics and Related Research. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wagner ER, He BC, Chen L, Zuo GW, Zhang W, Shi Q, Luo Q, Luo X, Liu B, Luo J, Rastegar F, He CJ, Hu Y, Boody B, Luu HH, He TC, Deng ZL, Haydon RC. Therapeutic implications of PPARgamma in human osteosarcoma. PPAR Research. 2010;2010:956427. doi: 10.1155/2010/956427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bertone AL, Pittman DD, Bouxsein ML, Li J, Clancy B, Seeherman HJ. Adenoviral-mediated transfer of human BMP-6 gene accelerates healing in a rabbit ulnar osteotomy model. Journal of Orthopaedic Research. 2004;22:1261–1270. doi: 10.1016/j.orthres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 119.Boden SD, Hair G, Titus L, Racine M, McCuaig K, Wozney JM, Nanes MS. Glucocorticoid-induced differentiation of fetal rat calvarial osteoblasts is mediated by bone morphogenetic protein-6. Endocrinology. 1997;138:2820–2828. doi: 10.1210/en.138.7.2820. [DOI] [PubMed] [Google Scholar]
- 120.Boskey AL, Paschalis EP, Binderman I, Doty SB. BMP-6 accelerates both chondrogenesis and mineral maturation in differentiating chick limb-bud mesenchymal cell cultures. Journal of Cellular Biochemistry. 2002;84:509–519. doi: 10.1002/jcb.10032. [DOI] [PubMed] [Google Scholar]
- 121.Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. Journal of Cell Science. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- 122.Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. Journal of Cellular Biochemistry. 2006;98:538–554. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- 123.Hughes FJ, Collyer J, Stanfield M, Goodman SA. The effects of bone morphogenetic protein-2, -4, and -6 on differentiation of rat osteoblast cells in vitro. Endocrinology. 1995;136:2671–2677. doi: 10.1210/en.136.6.2671. [DOI] [PubMed] [Google Scholar]
- 124.Jane JA, Jr, Dunford BA, Kron A, Pittman DD, Sasaki T, Li JZ, Li H, Alden TD, Dayoub H, Hankins GR, Kallmes DF, Helm GA. Ectopic osteogenesis using adenoviral bone morphogenetic protein (BMP)-4 and BMP-6 gene transfer. Molecular Therapy. 2002;6:464–470. doi: 10.1006/mthe.2002.0691. [DOI] [PubMed] [Google Scholar]
- 125.Valdes M, Moore DC, Palumbo M, Lucas PR, Robertson A, Appel J, Ehrlich MG, Keeping HS. rhBMP-6 stimulated osteoprogenitor cells enhance posterolateral spinal fusion in the New Zealand white rabbit. The Spine Journal. 2007;7:318–325. doi: 10.1016/j.spinee.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 126.Visser R, Arrabal PM, Santos-Ruiz L, Becerra J, Cifuentes M. Basic fibroblast growth factor enhances the osteogenic differentiation induced by bone morphogenetic protein-6 in vitro and in vivo. Cytokine. 2012;58:27–33. doi: 10.1016/j.cyto.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 127.Yamaguchi A, Ishizuya T, Kintou N, Wada Y, Katagiri T, Wozney JM, Rosen V, Yoshiki S. Effects of BMP-2, BMP-4, and BMP-6 on osteoblastic differentiation of bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochemical and Biophysical Research Communications. 1996;220:366–371. doi: 10.1006/bbrc.1996.0411. [DOI] [PubMed] [Google Scholar]
- 128.Zachos TA, Shields KM, Bertone AL. Gene-mediated osteogenic differentiation of stem cells by bone morphogenetic proteins-2 or -6. Journal of Orthopaedic Research. 2006;24:1279–1291. doi: 10.1002/jor.20068. [DOI] [PubMed] [Google Scholar]
- 129.Aslan H, Zilberman Y, Arbeli V, Sheyn D, Matan Y, Liebergall M, Li JZ, Helm GA, Gazit D, Gazit Z. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Engineering. 2006;12:877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 130.Santos JL, Pandita D, Rodrigues J, Pego AP, Granja PL, Tomas H. Non-viral gene delivery to mesenchymal stem cells: Methods, strategies and application in bone tissue engineering and regeneration. Current Gene Therapy. 2011;11:46–57. doi: 10.2174/156652311794520102. [DOI] [PubMed] [Google Scholar]
- 131.Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasound-based non-viral gene delivery induces bone formation in vivo. Gene Therapy. 2008;15:257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 132.Bergeron E, Senta H, Mailloux A, Park H, Lord E, Faucheux N. Murine preosteoblast differenttiation induced by a peptide derived from bone morphogenetic proteins-9. Tissue Engineering Part A. 2009;15:3341–3349. doi: 10.1089/ten.tea.2009.0189. [DOI] [PubMed] [Google Scholar]
- 133.Lee JY, Peng H, Usas A, Musgrave D, Cummins J, Pelinkovic D, Jankowski R, Ziran B, Robbins P, Huard J. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Human Gene Therapy. 2002;13:1201–1211. doi: 10.1089/104303402320138989. [DOI] [PubMed] [Google Scholar]
- 134.Dumont RJ, Dayoub H, Li JZ, Dumont AS, Kallmes DF, Hankins GR, Helm GA. Ex vivo bone morphogenetic protein-9 gene therapy using human mesenchymal stem cells induces spinal fusion in rodents. Neurosurgery. 2002;51:1239–1244. doi: 10.1097/00006123-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 135.Kimelman-Bleich N, Pelled G, Zilberman Y, Kallai I, Mizrahi O, Tawackoli W, Gazit Z, Gazit D. Targeted gene-and-host progenitor cell therapy for nonunion bone fracture repair. Molecular Therapy. 2011;19:53–59. doi: 10.1038/mt.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li X, Chen L, Ke ZY, Yin LJ, Deng ZL. BMP9-induced osteogenic differentiation and bone formation of muscle-derived stem cells. Journal of Biomedicine and Biotechnology. 2012;2012:1. doi: 10.1155/2012/610952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L, Singh M, Tsareva T, Parice Y, Mahoney A, Roschke V, Sanyal I, Choe S. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. The Journal of Biological Chemistry. 2005;280:25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 138.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocrine Reviews. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 139.Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: A novel protein with protease inhibitor and follistatin domains. Molecular Endocrinology. 2003;17:1144–1154. doi: 10.1210/me.2002-0366. [DOI] [PubMed] [Google Scholar]
- 140.Li JZ, Li H, Sasaki T, Holman D, Beres B, Dumont RJ, Pittman DD, Hankins GR, Helm GA. Osteogenic potential of five different recombinant human bone morphogenetic protein adenoviral vectors in the rat. Gene Therapy. 2003;10:1735–1743. doi: 10.1038/sj.gt.3302075. [DOI] [PubMed] [Google Scholar]
- 141.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine & Growth Factor Reviews. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 142.Zhou L, An N, Jiang W, Haydon R, Cheng H, Zhou Q, Breyer B, Feng T, He TC. Fluorescence-based functional assay for Wnt/beta-catenin signaling activity. Biotechniques. 2002;33:1126–1128. 1130–1132. doi: 10.2144/02335dd07. passim. [DOI] [PubMed] [Google Scholar]
- 143.Massague J. TGF-beta signal transduction. Annual Review of Biochemistry. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 144.Yamashita H, Ten Dijke P, Heldin CH, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/S8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- 145.Yamashita H, Miyazono K. Bone morphogenetic protein (BMP) receptors and signal transduction. Nihon Rinsho. 1999;57:220–226. [PubMed] [Google Scholar]
- 146.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. Journal of Cell Science. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 147.Shao ES, Lin L, Yao Y, Bostrom KI. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Upton PD, Davies RJ, Trembath RC, Morrell NW. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. The Journal of Biological Chemistry. 2009;284:15794–15804. doi: 10.1074/jbc.M109.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes & Development. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 150.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cellular Signalling. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 151.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 152.Wrana JL. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/S0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 153.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/S0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 154.Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-beta family members through Smad proteins. European Journal of Biochemistry. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- 155.Miyazono K, ten Dijke P, Heldin CH. TGF-beta signaling by Smad proteins. Advances in Immunology. 2000;75:115–157. doi: 10.1016/S0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- 156.Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. Journal of Cellular Physiology. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 157.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & Growth Factor Reviews. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 158.Xu DJ, Zhao YZ, Wang J, He JW, Weng YG, Luo JY. Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Reports. 2011;45:247–252. doi: 10.5483/bmbrep.2012.45.4.247. [DOI] [PubMed] [Google Scholar]
- 159.Kreider BL, Benezra R, Rovera G, Kadesch T. Inhibition of myeloid differentiation by the helix-loop-helix protein Id. Science. 1992;255:1700–1702. doi: 10.1126/science.1372755. [DOI] [PubMed] [Google Scholar]
- 160.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. Journal of Cell Science. 2000;113:3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 161.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends in Cell Biology. 2003;13:410–418. doi: 10.1016/S0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 162.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: New targets for antifibrotic therapy? Matrix Biology. 2002;21:473–482. doi: 10.1016/S0945-053X(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 163.Brigstock DR. The CCN family: A new stimulus package. Journal of Endocrinology. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 164.Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H. Proposal for a unified CCN nomenclature. Molecular Pathology. 2003;56:127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lau LF, Lam SC. The CCN family of angiogenic regulators: The integrin connection. Experimental Cell Research. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 166.Moussad EE, Brigstock DR. Connective tissue growth factor: What's in a name? Molecular Genetics and Metabolism. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- 167.Planque N, Perbal B. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell International. 2003;3:15. doi: 10.1186/1475-2867-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development and Disease. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sharff KA, Song WX, Luo X, Tang N, Luo J, Chen J, Bi Y, He BC, Huang J, Li X, Jiang W, Zhu GH, Su Y, He Y, Shen J, Wang Y, Chen L, Zuo GW, Liu B, Pan X, Reid RR, Luu HH, Haydon RC, He TC. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. The Journal of Biological Chemistry. 2009;284:649–659. doi: 10.1074/jbc.M806389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Massague J, Chen YG. Controlling TGF-beta signaling. Genes & Development. 2000;14:627–644. [PubMed] [Google Scholar]
- 171.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. The EMBO Journal. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nature Reviews Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 173.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8430–8435. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. The EMBO Journal. 2001;20:2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li RD, Deng ZL, Hu N, Liang X, Liu B, Luo J, Chen L, Yin L, Luo X, Shui W, He TC, Huang W. Biphasic effects of TGFbeta1 on BMP9-induced osteogenic differentiation of mesenchymal stem cells. BMB Reports. 2012;45:509–514. doi: 10.5483/bmbrep.2012.45.9.053. [DOI] [PubMed] [Google Scholar]
- 176.Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. The EMBO Journal. 2004;23:552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huang E, Zhu G, Jiang W, Yang K, Gao Y, Luo Q, Gao JL, Kim SH, Liu X, Li M, Shi Q, Hu N, Wang L, Liu H, Cui J, Zhang W, Li R, Chen X, Kong YH, Zhang J, Wang J, Shen J, Bi Y, Statz J, He BC, Luo J, Wang H, Xiong F, Luu HH, Haydon RC, Yang L, He TC. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. Journal of Bone and Mineral Research. 2012;27:1566–1575. doi: 10.1002/jbmr.1622. [DOI] [PubMed] [Google Scholar]
- 178.Blair JC, Savage MO. The GH-IGF-I axis in children with idiopathic short stature. Trends in Endocrinology & Metabolism. 2002;13:325–330. doi: 10.1016/S1043-2760(02)00631-8. [DOI] [PubMed] [Google Scholar]
- 179.Brooks AJ, Waters MJ. The growth hormone receptor: Mechanism of activation and clinical implications. Nature Reviews Endocrinology. 2010;6:515–525. doi: 10.1038/nrendo.2010.123. [DOI] [PubMed] [Google Scholar]
- 180.Hull KL, Harvey S. Growth hormone: Roles in male reproduction. Endocrine. 2000;13:243–250. doi: 10.1385/ENDO:13:3:243. [DOI] [PubMed] [Google Scholar]
- 181.Hull KL, Harvey S. Growth hormone: A reproductive endocrine-paracrine regulator? Reviews of Reproduction. 2000;5:175–182. doi: 10.1530/ror.0.0050175. [DOI] [PubMed] [Google Scholar]
- 182.Park P, Cohen P. Insulin-like growth factor I (IGF-I) measurements in growth hormone (GH) therapy of idiopathic short stature (ISS) Growth Hormone & IGF Research. 2005;15:S13–S20. doi: 10.1016/j.ghir.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 183.Tanaka H. Growth hormone and bone diseases. Endocrine Journal. 1998;45:S47–S52. doi: 10.1507/endocrj.45.Suppl_S47. [DOI] [PubMed] [Google Scholar]
- 184.Thomas MJ. The molecular basis of growth hormone action. Growth Hormone & IGF Research. 1998;8:3–11. doi: 10.1016/S1096-6374(98)80316-X. [DOI] [PubMed] [Google Scholar]
- 185.Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Molecular Genetics and Metabolism. 2005;86:84–90. doi: 10.1016/j.ymgme.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 186.O'Dell SD, Day IN. Insulin-like growth factor II (IGF-II) The International Journal of Biochemistry & Cell Biology. 1998;30:767–771. doi: 10.1016/S1357-2725(98)00048-X. [DOI] [PubMed] [Google Scholar]
- 187.Bergwitz C, Wendlandt T, Kispert A, Brabant G. Wnts differentially regulate colony growth and differentiation of chondrogenic rat calvaria cells. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2001;1538:129–140. doi: 10.1016/S0167-4889(00)00123-3. [DOI] [PubMed] [Google Scholar]
- 188.Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes & Development. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 189.Fischer L, Boland G, Tuan RS. Wnt signaling during BMP-2 stimulation of mesenchymal chondrogenesis. Journal of Cellular Physiology. 2002;84:816–831. doi: 10.1002/jcb.10091. [DOI] [PubMed] [Google Scholar]
- 190.Glass DA, 2nd, Karsenty G. Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway. Current Topics in Developmental Biology. 2006;73:43–84. doi: 10.1016/S0070-2153(05)73002-7. [DOI] [PubMed] [Google Scholar]
- 191.Glass DA, 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007;148:2630–2634. doi: 10.1210/en.2006-1372. [DOI] [PubMed] [Google Scholar]
- 192.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Developmental Cell. 2002;2:389–406. doi: 10.1016/S1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 193.Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Current Opinion in Genetics & Development. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 194.Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, Su YX, Jiang W, Tang M, He Y, Wang Y, Chen L, Zuo GW, Shen J, Pan X, Reid RR, Luu HH, Haydon RC, He TC. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. Journal of Cellular and Molecular Medicine. 2009;13:2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/S0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 196.Celil AB, Campbell PG. BMP-2 and insulin-like growth factor-I mediate osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. The Journal of Biological Chemistry. 2005;280:31353–31359. doi: 10.1074/jbc.M503845200. [DOI] [PubMed] [Google Scholar]
- 197.Chang SF, Chang TK, Peng HH, Yeh YT, Lee DY, Yeh CR, Zhou J, Cheng CK, Chang CA, Chiu JJ. BMP-4 induction of arrest and differenttiation of osteoblast-like cells via p21CIP1 and p27KIP1 regulation. Molecular Endocrinology. 2009;23:1827–1838. doi: 10.1210/me.2009-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491–498. doi: 10.1016/S8756-3282(01)00415-X. [DOI] [PubMed] [Google Scholar]
- 199.Noth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ, Hickok NJ, Tuan RS. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Experimental Cell Research. 2003;291:201–211. doi: 10.1016/S0014-4827(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 200.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. The Journal of Biochemistry. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 201.Verheyen EM. Opposing effects of Wnt and MAPK on BMP/Smad signal duration. Developmental Cell. 2007;13:755–756. doi: 10.1016/j.devcel.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 202.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Molecular Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundamental & Clinical Pharmacology. 2007;21:231–244. doi: 10.1111/j.1472-8206.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 204.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cellular and Molecular Life Sciences. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 206.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annual Review of Pharmacology and Toxicology. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 207.Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, Luo J, Tang M, Wietholt C, Luo X, Bi Y, Su Y, Liu B, Kim SH, He CJ, Hu Y, Shen J, Rastegar F, Huang E, Gao Y, Gao JL, Zhou JZ, Reid RR, Luu HH, Haydon RC, He TC, Deng ZL. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. Journal of Bone and Mineral Research. 2010;25:2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhao Y, Song T, Wang W, Wang J, He J, Wu N, Tang M, He B, Luo J. P38 and ERK1/2 MAPKs act in opposition to regulate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2012;7:e43383. doi: 10.1371/journal.pone.0043383. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 209.Hu N, Jiang D, Huang E, Liu X, Li RD, Liang X, Stephanie H, Kim XC, Gao JL, Zhang HY, Zhang WW. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. Journal of Cell Science. 2013;126:532–541. doi: 10.1242/jcs.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang W, Deng ZL, Chen L, Zuo GW, Luo Q, Shi Q, Zhang BQ, Wagner ER, Rastegar F, Kim SH, Jiang W, Shen J, Huang E, Gao Y, Gao JL, Zhou JZ, Luo J, Huang J, Luo X, Bi Y, Su Y, Yang K, Liu H, Luu HH, Haydon RC, He TC, He BC. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2010;5:e11917. doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Castonguay R, Werner ED, Matthews RG, Presman E, Mulivor AW, Solban N, Sako D, Pearsall RS, Underwood KW, Seehra J, Kumar R, Grinberg AV. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. The Journal of Biological Chemistry. 2011;286:30034–30046. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li B, Yang Y, Jiang S, Ni B, Chen K, Jiang L. Adenovirus-mediated overexpression of BMP-9 inhibits human osteosarcoma cell growth and migration through downregulation of the PI3K/AKT pathway. Internation Journal of Oncology. 2012;41:1809–1819. doi: 10.3892/ijo.2012.1617. [DOI] [PubMed] [Google Scholar]
- 213.Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Molecular Cancer Research. 2008;6:1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- 214.van Meeteren LA, Thorikay M, Bergqvist S, Pardali E, Stampino CG, Hu-Lowe D, Goumans MJ, ten Dijke P. Anti-human activin receptor-like kinase 1 (ALK1) antibody attenuates bone morphogenetic protein 9 (BMP9)-induced ALK1 signaling and interferes with endothelial cell sprouting. The Journal of Biological Chemistry. 2012;287:18551–18561. doi: 10.1074/jbc.M111.338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, Watabe T. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. Journal of Cell Science. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 217.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circulation Research. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Park JE, Shao D, Upton PD, Desouza P, Adcock IM, Davies RJ, Morrell NW, Griffiths MJ, Wort SJ. BMP-9 induced endothelial cell tubule formation and inhibition of migration involves Smad1 driven endothelin-1 production. PLoS One. 2012;7:e30075. doi: 10.1371/journal.pone.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, Bostrom KI. Cross-veinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119:5037–5047. doi: 10.1182/blood-2011-10-385906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lopez-Coviella I, Follettie MT, Mellott TJ, Kova-cheva VP, Slack BE, Diesl V, Berse B, Thies RS, Blusztajn JK. Bone morphogenetic protein 9 induces the transcriptome of basal forebrain cholinergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6984–6989. doi: 10.1073/pnas.0502097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lopez-Coviella I, Mellott TJ, Schnitzler AC, Blusztajn JK. BMP9 protects septal neurons from axotomy-evoked loss of cholinergic phenotype. PLoS One. 2011;6:e21166. doi: 10.1371/journal.pone.0021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schnitzler AC, Lopez-Coviella I, Blusztajn JK. Differential modulation of nerve growth factor receptor (p75) and cholinergic gene expression in purified p75-expressing and non-expressing basal forebrain neurons by BMP9. Brain Research. 2008;1246:19–28. doi: 10.1016/j.brainres.2008.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schnitzler AC, Mellott TJ, Lopez-Coviella I, Tallini YN, Kotlikoff MI, Follettie MT, Blusztajn JK. BMP9 (bone morphogenetic protein 9) induces NGF as an autocrine/paracrine cholinergic trophic factor in developing basal forebrain neurons. Journal of Neuroscience. 2010;30:8221–8228. doi: 10.1523/JNEUROSCI.5611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Caperuto LC, Anhe GF, Cambiaghi TD, Akamine EH, do Carmo Buonfiglio D, Cipolla-Neto J, Curi R, Bordin S. Modulation of bone morphogenetic protein-9 expression and processing by insulin, glucose, and glucocorticoids: Possible candidate for hepatic insulin-sensitizing substance. Endocrinology. 2008;149:6326–6335. doi: 10.1210/en.2008-0655. [DOI] [PubMed] [Google Scholar]
- 225.Sosa I, Cvijanovic O, Celic T, Cuculic D, Crncevic-Orlic Z, Vukelic L, Zoricic Cvek S, Dudaric L, Bosnar A, Bobinac D. Hepatoregenerative role of bone morphogenetic protein-9. Medical Science Monitor. 2011;17:33–35. doi: 10.12659/MSM.882108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Blunk T, Sieminski AL, Appel B, Croft C, Courter DL, Chieh JJ, Goepferich A, Khurana JS, Gooch KJ. Bone morphogenetic protein 9: A potent modulator of cartilage development in vitro. Growth Factors. 2003;21:71–77. doi: 10.1080/0897719031000148822. [DOI] [PubMed] [Google Scholar]
- 227.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. Journal of Cellular Physiology. 2001;189:275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]


