Abstract
Increasing prostaglandin E2 by knocking out its inhibitor 15-hydroxyprostaglandin dehydrogenase (15-PDGH) or administering a compound that inhibits 15-PDGH was recently found to improve healing in hematopoietic stem cell transplants, colitis recovery, and hepatogenesis after transection in mice. These results are suggestive of pharmacologic therapies or even genetic therapy that could improve patient outcomes, especially since the excess PGE2 and the 15-PDGH inhibitor have proven to be non-toxic. However, elevated levels of PGE2 are associated with increased risk of cancer and blood clotting problems. It would be unacceptable to treat a cancer patient with chemotherapy and replenish the hematopoietic stem cells with the help of PGE2, only to have increased expression of PGE2 and induce another cancer. Therefore, to assess the most therapeutic aspects of PGE2, it is important to consider effects that could induce disease.
Keywords: Cancer, COX activity, Prostaglandin E2, Tissue injury, Tissue regeneration
Promoting healing by elevated PGE2
Prostaglandin E2 is a lipid signaling molecule that has diverse functions, ranging from fever mediation to vasodilation, uterine contractions during labor to stimulation of bone resorption. A recent article from Zhang et al. discovered that inhibiting 15-hydroxyprostaglandin dehydrogenase, an enzyme that physiologically oxidizes PGE2 to keep it from binding to prostaglandin receptors, leads to improvements in hematopoietic stem cell transplants, colitis recovery, and hepatogenesis after transection in mice. These results were consistent for both mice with the gene for 15-PDGH knocked out, as well as those that were administered with a pharmacologic dose of SW033291, an inhibitor of 15-PDGH that was discovered through high throughput screening.1
After chemical/genetic ablation of 15-PDGH, mice that received administration of oral dextran sodium sulfate (DSS) for seven days had a decrease in the number of colon ulcers, total area of ulcerated colon mucosa, mucosal inflammation, diarrhea, rectal bleeding, colon shortening, and inflammatory cytokines. On the other hand, wild type mice with 15-PDGH knockout bone marrow transplants did not see such effects. Observations of BrdU incorporation and presence of cleaved caspase 3 indicated that inhibition of 15-PDGH prevented DSS-induced colitis through increased cell proliferation, not by inhibiting apoptosis.1
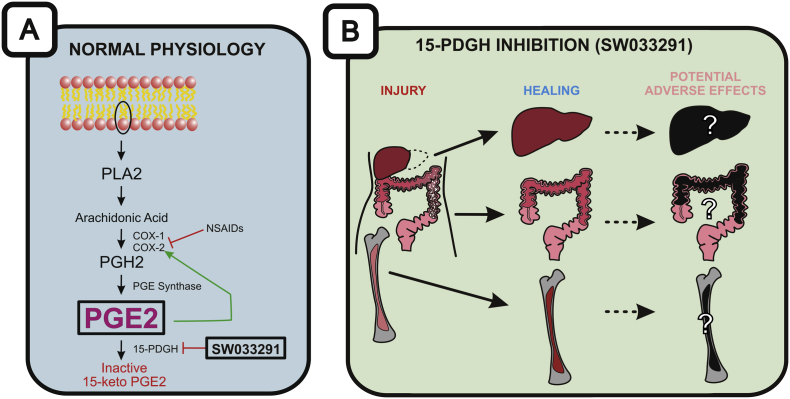
In addition, inhibiting 15-PDGH can aid in abnormal wound healing. Hypertrophic scars can form after severe burns or poor wound healing conditions lead to excessive proliferation of fibroblasts, producing excessive extracellular matrix. Administration of TD88, a 15-PDGH inhibitor, leads to increased Type IV collagen and decreased wound healing factors (PDGF, CTGF, TIMP-2) at the injury site, preventing the excessive wound scarring that occurs with suppression of PGE2.2 Inhibiting 15-PDGH allows for improved reepithelization on wounded surfaces (Fig. 1).
Fig. 1.
PGE2 production and its potential healing effect on tissue injury. (A) The making and breaking of PGE2. PGE2 synthesis begins with the conversion of a phospholipid from the membrane bilayer to PLA2. While most PGE2 studies have focused on inhibiting COX enzymes and PGE2 synthesis using NSAIDs, Zhang et al focused on inhibiting 15-PDGH by either knocking out its gene or delivering an inhibitor (SW03329). (B) Inhibition of PGE2 degradation may lead to improved healing for hepatectomies, ulcerative colitis models, and bone marrow transplantations although the potential for disease-causing adverse effects of high-dose and/or long-term uses of PGE2 should be considered.
Potential adverse effects of elevated levels of PGE2
Promoting tumorigenesis
Though Zhang et al. noted that the 15-PDGH inhibitor was not toxic, long-term effects of elevated PGE2 could lead to pathologies such as tumorigenesis or hemostatic perturbations. Lack of short-term toxicity does not indicate safety in the long term.
PGE2's signaling through the Wnt pathway, first identified for its role in carcinogenesis, and its many effects that align with the hallmarks of cancer (e.g., increased cell proliferation, angiogenesis, etc.) indicate that complications due to over-expression of PGE2 must be considered.
Reduced expression of 15-PGDH leads to prolonged availability and action of PGE2 and has been linked to several cancers, including colorectal, bladder, pancreatic, and gastric adenocarcinomas. 15-PDGH knockout mice have been shown to have a 7.6-fold increase in colon tumors and confers carcinogen susceptibility to normally resistant mice, concomitant with a doubling of 15-PGDH. In familial adenomatous polyposis (FAP), there is a universal loss of 15-PGDH expression, including adenomas as small as a single crypt.3 In both human FAP and murine models of the disease, COX-2 is constitutively over-expressed in the colon.4 In fact, measuring the levels of PGE2 metabolites in urine, such as 13,14-dihydro-15-keto-PGE2, has been used to demonstrate the increased synthesis of PGE2 in individuals with colorectal and lung cancer.5, 6, 7 Treatment with NSAIDs, which inhibits PGE2 synthesis, prevents tumor formation in mouse models of FAP.4
Other cancers are also closely associated with 15-PDGH regulation. Given the already proliferative nature of hepatocytes, further inducing cellular growth could warrant cancerous growth, as has been indicated by previous studies.8 Partial hepatectomies of half the liver volume in humans need about 12 weeks to regain liver function.9 Whether this is enough time for excess PGE2 to induce neoplasms has yet to be determined.
PGE2 is associated with growth-stimulation in breast cancer, often with COX-2 overexpressed. Up-regulation of 15-PDGH decreases clonal growth and cellular ability to form tumors in vivo, while silencing 15-PDGH enhances cellular proliferation and tumorigenesis, suggesting that 15-PDGH could be a novel tumor suppressor gene in breast cancer.10 Lung tumors have shown increased expression COX-2 and a PGE2 synthesis enzyme, with a reciprocal relationship between COX-2 and 15-PDGH expression.11 Under-expression of 15-PDGH in lung tumors suggests that 15-PDGH might behave as a tumor suppressor in lung cancer.12
PGE2 also feeds back to positively increase COX-2 expression, further augmenting its own synthesis. In the microenvironment of the tumor, this creates a vicious cycle where tumor-related signaling increases COX-2 expression leading to PGE2 production, which not only drives angiogenesis while suppressing apoptosis and innate immunity but also heightens COX-2 expression and perpetuates PGE2 synthesis.13
Though the effect of PGE2 is local, as an autocrine and paracrine lipid mediator, the administration of SW033291 is systemic, its major effectors being bone marrow, the colon, and the liver. Knockout mice have a similarly systemic effect. Since PGE2 feeds back to increase COX2 expression, which is typically absent from most cell types until inflammatory mediators like TNF-α and IL-1β induce its expression, excess PGE2 from 15-PDGH inhibition could likely have serious carcinogenic effects.
Perturbing hemostasis
Inhibition of 15-PDGH would lead to decreased PGE2 degradation, thus disrupting the physiological levels of PGE2. Low concentrations of PGE2 enhance platelet aggregation, whereas high PGE2 levels inhibit aggregation.14, 15, 16, 17, 18 Among the four PGE2 receptors (EP1-EP4), activation of EP3 is sufficient to mediate the proaggregatory actions of low PGE2 concentration. In contrast, the prostacyclin receptor (IP) mediates the inhibitory effect of higher PGE2 concentrations. The relative activation of these two receptors, EP3 and IP, regulates the response of platelets to aggregating agents. PGE2 has been shown to facilitate arterial thrombosis by acting via EP316; loss of the EP3 receptor has been shown to prevent intravascular clots.15
Stimulation of receptors EP2 and EP4 usually increases intracellular cAMP levels through activation of Gas protein, resulting in decrease of intracellular calcium levels. This results in inhibition of platelet aggregation, calcium mobilization, and upregulation of P-selectin. Furthermore, EP4 activation enhances the inhibitory effect of aspirin,19 acting as a potent inhibitor of platelet function with respect to adhesion, aggregation, and thrombosis.20 While it may seem like activation of EP4 could balance out the pro-aggregatory effects of EP3, the exact effects of administration of PGE2 on the balance between thrombotic and anti-thrombotic effects is unknown. It would be prudent to consider blood clotting pathologies like disseminated intravascular coagulation as possible complications before moving forward with using 15-PDGH inhibitors as a therapeutic solution.
Future directions
The results of increased production of PGE2 in mice through the inhibition of 15-PDGH appear to have many promising effects for healing, specifically within bone marrow, the colon, and the liver. It would be interesting to investigate if such healing promoting effects of PGE2 can be extended to the injuries of other tissues, such as soft tissues, bone, and skeletal tissues. Considering the dysregulation that could potentially result, erring toward the side of cell proliferation, additional investigation into potentially carcinogenic or adverse thrombotic events after long-term use is needed before its merits can be considered as a part of standard therapies.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The research efforts of the authors' laboratories were supported in part by research grants from the National Institutes of Health (AR50142, AR054381, and AT004418 to RCH, HHL, and TCH), and Scoliosis Research Society (MJL). CS was a recipient of by the Pritzker Summer Research Program fellowship through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant #5T35 DK062719-28.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Tong-Chuan He, Email: tche@uchicago.edu.
Michael J. Lee, Email: mlee5@bsd.uchicago.edu.
References
- 1.Zhang Y., Desai A., Yang S.Y. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science. 2015;348:aaa2340. doi: 10.1126/science.aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo S.Y., Han S.-I., Bae C.S., Cho H., Lim S.C. Effect of 15-hydroxyprostaglandin dehydrogenase inhibitor on wound healing. Prostaglandins Leukot Essent Fat Acids PLEFA. 2015;97:35–41. doi: 10.1016/j.plefa.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Myung S.-J., Rerko R.M., Yan M. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boolbol S.K., Dannenberg A.J., Chadburn A. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556–2560. [PubMed] [Google Scholar]
- 5.Masrour Roudsari J., Mahjoub S. Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and paget's specimens. Casp J Intern Med. 2012;3:478–483. [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Q., Gao Y.-T., Chow W.-H. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24:5010–5016. doi: 10.1200/JCO.2006.06.4931. [DOI] [PubMed] [Google Scholar]
- 7.Murff H.J., Shu X.-O., Li H. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2009;18:2283–2291. doi: 10.1158/1055-9965.EPI-08-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menter D.G., DuBois R.N. Prostaglandins in cancer cell adhesion, migration, and invasion. Int J Cell Biol. 2012;2012:e723419. doi: 10.1155/2012/723419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lock J.F., Malinowski M., Seehofer D. Function and volume recovery after partial hepatectomy: influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg Dtsch Ges Für Chir. 2012;397:1297–1304. doi: 10.1007/s00423-012-0972-2. [DOI] [PubMed] [Google Scholar]
- 10.Wolf I., O'Kelly J., Rubinek T. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006;66:7818–7823. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 11.Tong M., Ding Y., Tai H.-H. Reciprocal regulation of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase expression in A549 human lung adenocarcinoma cells. Carcinogenesis. 2006;27:2170–2179. doi: 10.1093/carcin/bgl053. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y., Tong M., Liu S., Moscow J.A., Tai H.-H. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005;26:65–72. doi: 10.1093/carcin/bgh277. [DOI] [PubMed] [Google Scholar]
- 13.Greenhough A., Smartt H.J.M., Moore A.E. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 14.Shio H., Ramwell P.W., Jessup S.J. Prostaglandin E2: effects on aggregation, shape change and cyclic AMP of rat platelets. Prostaglandins. 1972;1:29–36. doi: 10.1016/0090-6980(72)90063-9. [DOI] [PubMed] [Google Scholar]
- 15.Salzman E.W., Kensler P.C., Levine L. Cyclic 3′,5′-adenosine monophosphate in human blood platelets. IV. Regulatory role of cyclic amp in platelet function. Ann N. Y Acad Sci. 1972;201:61–71. doi: 10.1111/j.1749-6632.1972.tb16287.x. [DOI] [PubMed] [Google Scholar]
- 16.Thierauch K.H., Prior G. Modulation of platelet activation by prostaglandin E2 mimics. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:383–386. [PubMed] [Google Scholar]
- 17.Vezza R., Roberti R., Nenci G.G., Gresele P. Prostaglandin E2 potentiates platelet aggregation by priming protein kinase C. Blood. 1993;82:2704–2713. [PubMed] [Google Scholar]
- 18.Weiss H.J., Willis A.L., Kuhn D., Brand H. Prostaglandin E2 potentiation of platelet aggregation induced by LASS endoperoxide: absent in storage pool disease, normal after aspirin ingestion. Br J Haematol. 1976;32:257–272. doi: 10.1111/j.1365-2141.1976.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 19.Kauskot A., Hoylaerts M.F. Platelet receptors. In: Gresele P., Born G.V.R., Patrono C., Page C.P., editors. Antiplatelet Agents. vol. 210. Springer Berlin Heidelberg; Berlin, Heidelberg: 2012. pp. 23–57.http://link.springer.com/10.1007/978-3-642-29423-5_2 Accessed 22.06.15. [Google Scholar]
- 20.Philipose S., Konya V., Sreckovic I. The prostaglandin E2 receptor EP4 is expressed by human platelets and potently inhibits platelet aggregation and thrombus formation. Arterioscler Thromb Vasc Biol. 2010;30:2416–2423. doi: 10.1161/ATVBAHA.110.216374. [DOI] [PubMed] [Google Scholar]