Abstract
Manganese (Mn) is an essential nutrient in humans, but excessive exposure to Mn may cause neurotoxicity. Despite homeostatic regulation, Mn concentrations in blood vary considerably among individuals. We evaluated if common single-nucleotide polymorphisms (SNPs) in SLC30A10, which likely encodes an Mn transporter, influence blood Mn concentrations and neurological function. We measured blood Mn concentrations by ICP-MS or atomic absorption spectroscopy and genotyped 2 SLC30A10 non-coding SNPs (rs2275707 and rs12064812) by TaqMan PCR in cohorts from Bangladesh (N = 406), the Argentinean Andes (N = 198), and Italy (N = 238). We also measured SLC30A10 expression in whole blood by TaqMan PCR in a sub-group (N = 101) from the Andean cohort, and neurological parameters (sway velocity and finger-tapping speed) in the Italian cohort. The rs2275707 variant allele was associated with increased Mn concentrations in the Andes (8%, P = .027) and Italy (10.6%, P = .012), but not as clear in Bangladesh (3.4%, P = .21; linear regression analysis adjusted for age, gender, and plasma ferritin). This allele was also associated with increased sway velocity (15%, P = .033; adjusted for age and sex) and reduced SLC30A10 expression (−24.6%, P = .029). In contrast, the rs12064812 variant homozygous genotype was associated with reduced Mn concentrations, particularly in the Italian cohort (−18.4%, P = .04), and increased finger-tapping speed (8.7%, P = .025). We show that common SNPs in SLC30A10 are associated with blood Mn concentrations in 3 unrelated cohorts and that their influence may be mediated by altered SLC30A10 expression. Moreover, the SNPs appeared to influence neurological functions independent of blood Mn concentrations, suggesting that SLC30A10 could regulate brain Mn levels.
Keywords: manganese exposure, manganese regulation, manganese neurotoxicity, SLC30A10, blood Mn concentrations, parkinsonism
Manganese (Mn), one of the more abundant metals on Earth, acts as an essential cofactor of multiple enzymes. Mn occurs naturally in most foods and Mn deficiency in humans is therefore rare; however, elevated levels of Mn can occur due to a rare inherited genetic disorder (hypermanganesemia) or as a consequence of environmental Mn exposure (Lucchini et al., 2015). Toxic exposure to Mn can occur in occupational settings such as in the welding, ferroalloy, and mining industries (Lucchini et al., 1999; Racette et al., 2001, 2012; Rodier, 1955), through exposure to contaminated soil and house dust in the vicinity of ferroalloy industries (Lucas et al., 2015; Lucchini et al., 2007; Pavilonis et al., 2015), or from ingestion of contaminated ground water (Roels et al., 2012). Elevated environmental exposure to Mn has been associated with cognitive, behavioral, and neuromotor deficits in children (Zoni and Lucchini, 2013). Mn can accumulate in the brain and cause neurological disturbances in adults (referred to as manganism), with symptoms resembling those of Parkinson’s disease (Chen et al., 2015).
Physiological Mn levels are regulated by homeostatic mechanisms. Epithelial cells in the intestine or lungs take up Mn, which enters the blood circulation; from there, it can cross the blood-brain barrier (Lucchini et al., 2015). Mn may also enter the brain directly through the olfactory pathways (Fechter et al., 2002; Tjälve et al., 1995). The liver clears excess Mn, which is excreted via the bile. Several metal transporters are involved in the transport of Mn across the cell membrane, including the divalent metal transporter 1 (Chen et al., 2014, 2015), but the system that regulates Mn homeostasis has not been entirely elucidated.
Recent research has linked mutations in the solute carrier family 30 member 10 (SLC30A10) on chromosome 1, which was originally identified as a zinc (Zn)-transporter (Seve et al., 2004), to inherited forms of hypermanganesemia, in which patients with adequate Mn intake accumulate Mn in the liver and brain (Quadri et al., 2012, 2015; Tuschl et al., 2012). The link between SLC30A10 and Mn levels was also highlighted in a recent genome-wide association study in which genetic variation associated with serum Mn levels was mapped to SLC30A10 (Ng et al., 2015). Moreover, Mn induced up-regulation of SLC30A10 protein in cell culture (Quadri et al., 2012) and expression of human wild-type SLC30A10 in yeast (Saccharomyces cerevisiae) allowed the cells to grow in high-Mn conditions (Tuschl et al., 2012), further supporting the role of SLC30A10 as a key regulator of Mn homeostasis. The SLC30A10 protein, which localizes at the cell surface, protects the cell against toxic Mn levels by functioning as an Mn efflux transporter (Leyva-Illades et al., 2014). SLC30A10 expression is high in liver, intestines, and the central nervous system (IST online; http://ist.medisapiens.com), all organs involved in Mn transport and regulation.
Blood Mn concentrations vary over the human lifespan (declining from birth into adulthood), and during periods of heightened nutritional demand, such as pregnancy; individuals also show substantial variation. We hypothesized that part of the observed individual variation in blood Mn concentrations is due to genetic variation in the gene encoding the SLC30A10 transporter. To explore this individual variation, we genotyped 2 common SLC30A10 single nucleotide polymorphisms (SNPs) in 3 unrelated cohorts of adults with different Mn exposures. We also evaluated the influence of SLC30A10 genotypic variance on neurological function.
MATERIALS AND METHODS
Study Cohorts
In this study we used data from 3 independent cohorts from different parts of the world to evaluate the impact of genotypic variation on Mn concentrations in blood.
Bangladesh cohort
The study cohort from Bangladesh consists of pregnant women living in the rural area of Matlab, 53 km southeast of Dhaka, where the International Centre for Diarrhoeal Disease Research in Bangladesh (icddr,b) has a well-established Health and Demographic Surveillance System. The study was nested into a randomized food and micronutrient supplementation trial (MINIMat) conducted during pregnancy. In this region, well water often contains elevated arsenic concentrations, and thus, studies concerning the potential health effects of arsenic exposure in early life were nested in the MINIMat trial (Vahter et al., 2006). To avoid the arsenic, deeper wells are constructed; however, the water from these wells often contains elevated concentrations of Mn [median Mn value in drinking water was 228 µg/l (range 10–6336)] (Ljung et al., 2009; Rahman et al., 2013). The study cohort and sampling procedures have been described in detail (Kippler et al., 2007, 2009). Blood samples were collected in 5.5-ml Li-heparin tubes at gestational week 14. Mn, Zn, and ferritin concentrations have been published previously (Kippler et al., 2009; Ljung et al., 2009). In this study, we used data from 406 women for which Mn concentrations and DNA samples were available.
Argentinean Andes cohort
The Andean cohort consists of 202 subjects (284 women and 18 men) from the San Antonio de los Cobres village and surrounding smaller villages in the northern Argentinean Andes (altitude around 4000 m). These subjects participated in a cross-sectional study of the health effects of toxic elements (primarily arsenic and lithium) in drinking water (Ameer et al., 2015; Broberg et al., 2011). This region has low levels of Mn in drinking water (<5 µg/l) (Concha et al., 2010). Peripheral blood samples were collected in vacutainers with EDTA anticoagulant for Mn analysis and DNA extraction for genotyping analysis. From the 202 subjects, 198 DNA samples were available for genotyping.
Italian cohort
This cohort has previously been described in detail (Lucchini et al., 2014). It consists of 238 elderly people (107 men and 131 women) aged 65–76 years, residing in the province of Brescia, Italy, with varying degrees of environmental Mn exposure due to historic emissions from ferroalloy foundries in the area. The Valcamonica valley (n = 146 subjects), in which 3 ferroalloy plants operated for a century (until 2001), has been studied as an area of Mn exposure. The Garda Lake area (n = 92 subjects), with no history of ferroalloy industrial activity, has been used as a reference area. The median Mn level in soil for Valcamonica was 923 mg/kg (range 473–1724 mg/kg) and for the Garda Lake region was 410 mg/kg (range 313–549 mg/kg) (Lucchini et al., 2014). A significantly higher prevalence of Parkinsonism associated with Mn levels in settled dust was previously reported for Valcamonica (Lucchini et al., 2007). All subjects underwent extended neurological testing of motor, cognitive, sensory, and behavioral functions. None of the subjects was a confirmed patient or had a medically proven diagnosis of Parkinson’s disease or any similar symptoms. Blood samples were collected from study participants in EDTA vacutainers for analysis of Mn and subsequent DNA extraction and genotyping (Rentschler et al., 2012). From the 238 subjects, 232 DNA samples were available for genotyping.
Ethical Considerations
The studies were approved by the research and ethical review committees at the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) in Dhaka, the Ethical Committee of the Local Public Health Agency of Brescia and the Ethical Committee at Karolinska Institutet, Sweden. The procedures were in accordance with the Helsinki Declaration.
Analysis of Markers in Blood
Blood Mn concentrations were measured in erythrocyte preparations (Ery-Mn) from the Bangladeshi women and whole blood from the Andean cohort, using inductively coupled mass spectrometry (ICP-MS) (Agilent 7500ce, Agilent technologies, Tokyo, Japan) at the Karolinska Institutet, Stockholm. Prior to ICP-MS, samples were processed by microwave-assisted UltraClave to obtain carbon-free samples (Ljung et al., 2009). For the Italian cohort, Mn concentrations were measured in whole blood by atomic absorption spectroscopy (AAS) at the Laboratory of Industrial Toxicology of the University of Brescia. To enable comparison of Mn levels between cohorts, blood Mn concentrations in the Andean and Italian cohorts were converted to Ery-Mn. For this calculation, we estimated that 66% of Mn in blood is bound to erythrocytes (Milne et al., 1990) and that the density of the erythrocyte preparations was 1.055 g/ml. The volume fraction of erythrocytes in total blood for each individual was estimated by dividing their hemoglobin levels with the reference value of erythrocyte mean hemoglobin content of 340 g/l (León-Velarde et al., 2000; Lundh and Öhlin, 1991). Median hemoglobin levels were 155 g/l (131–183 g/l, 5–95 percentile) and 141 g/l (125–162 g/l, 5–95 percentile) in the Andean and Italian cohorts, respectively.
Plasma Zn was measured by spectrophotometry in the Andean samples (accredited method, LOD 0.6 µmol/l) and by AAS in the Bangladeshi samples (Kippler et al., 2009; Li et al., 2008). Plasma Zn was not measured in the Italian cohort.
Ferritin is the main iron (Fe) storage protein and plasma ferritin levels were measured as a proxy of nutritional iron status. Plasma ferritin was measured by immunoassay (Cobas e601; Roche Diagnostics, Mannheim, Germany) for the Andean samples, radioimmunoassay (Diagnostic Products, San Diego, California) for the Bangladeshi samples (Kippler et al., 2009; Li et al., 2008), and chemiluminescent microparticle immunoassay using the Architect SR 2000 Immunoassay Analyser (Abott Diagnostics, Illinois) for the Italian samples. Other Fe indicators included whole blood Fe in the Andean cohort measured by ICP-MS (Agilent), serum Fe in the Italian cohort measured using the Siemens Vista 500 (Siemens, Munich, Germany), and hemoglobin (Hb) measured in the Andean cohort using the Beckman Coulter LH 780 hematology analyzer (Beckman Coulter, Brea, California) and in the Italian using the HemoCue instrument (HemoCue, Ängelholm, Sweden).
Genotyping
SLC30A10 has no coding SNPs (GeneBank accession number: NG_032153.1) with sufficient minor allele frequencies for genotyping analysis. There was also insufficient genotyping data for SLC30A10 from the HapMap3 database (http://hapmap.ncbi.nlm.nih.gov/index.html.en) for the identification of tag SNPs. To select SNPs for the study, we therefore selected 6 non-coding SNPs in SLC30A10 (rs1776050, rs2275706, rs2275707, rs6663638, rs7525274, and rs12064812), with minor allele frequencies of >5% in Asian populations (NCBI, http://www.ncbi.nlm.nih.gov/) and genotyped them in the Bangladeshi cohort. SNPs for further analyses were selected from this data based on linkage disequilibrium (LD) analysis using the Haploview software (Barett et al., 2005) (Supplementary Figure 1) as well as association analysis between genotypes and blood Mn concentrations. All SNPs showed weak associations with blood Mn concentrations, except for rs2275706, which was therefore not selected for further analysis. Rs2275707 (A/C) showed strong linkage with rs1776050, rs6663638, and rs7525274, and was therefore selected as a tag SNP. Rs12064812 (T/C) was also chosen for further analysis since it could represent a different LD-block.
For genotyping, DNA was extracted from peripheral blood samples using the QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany). Genotyping was performed by allelic discrimination on the ABI 7900HT instrument using TaqMan SNP genotyping assays (Life Technologies, Carlsbad, California) and TaqMan Universal Mastermix (UNG free, no AmpErase; Life Technologies). Assay information is summarized in Supplementary Table 1. The following PCR conditions were applied: 10 min at 95°C followed by 45–50 cycles of 15 s at 92°C, and 90 s at 60°C. PCR amplifications were conducted in 96-well plates with negative controls (water) included on each plate. To ensure reproducibility and accuracy of genotyping data, 5% of samples were re-analyzed in a separate round of experiments. Deviation from the Hardy-Weinberg equilibrium was evaluated using chi-square analysis.
Gene Expression Analysis
For a sub-group of the Andean cohort (N = 101), all female and including no first-degree relatives, blood was also collected in PAX tubes (PreAnalytiX GmbH, Hombrechtikon, Switzerland) and stored at −20°C for RNA extraction and subsequent gene expression analysis. RNA was extracted using the PAXgene Blood RNA kit (PreAnalytiX GmbH, Hombrechtikon, Switzerland) and stored at −80°C. RNA concentrations and purities were evaluated on a Nanodrop spectrophotometer (Wilmington, Delaware). Adequate RNA integrity (RNA integrity number >7.5) was confirmed using a Bioanalyzer 2100 (Agilent, Santa Clara, California). RT-PCR was performed using the High-Capacity cDNA reverse transcription Kit (Life Technologies) in 20 µl reaction volumes with input RNA in the range of 150–600 ng. Expression analysis was performed by real-time PCR using TaqMan Gene Expression Assays (list of assay IDs are presented in Supplementary Table 2) and TaqMan Gene Expression Mastermix from Life Technologies. Reactions were run on the ABI 7900-HT Fast Real Time PCR instrument (Life Technologies) in a total volume of 10 µl using 1 µl of cDNA. Initially, a set of reference genes (2,4-Dienoyl CoA Reductase 1DECR1; Folylpolyglutamate Synthase; and Hypoxanthine Phosphoribosyltransferase 1) was evaluated for stable expression in 20 RNA samples extracted from blood. DECR1 showed the smallest variation of expression between samples [standard deviation of 0.32 cycle threshold (Ct)] for which the same RNA input had been used and was therefore selected as a reference gene for calculation of relative expression levels using the delta Ct method. DECR1 has previously been described as a suitable reference gene for gene-expression analysis in blood (Stamova et al., 2009). Due to low expression levels of SLC30A10 in blood, some samples did not generate useful data. Samples were selected for further analysis based on the criteria that 2 out of 3 triplicates had to generate a signal and that the replicates had to deviate by <1.5 Ct. Negative controls (water) did not generate any signal.
Analysis of Neurological Parameters (Italian Cohort Only)
To assess psychomotor speed, finger tapping was measured using the Finger Tapping test, computerized version from the SPES (Iregren et al., 1996). The task of the participant is to tap a push-button alternatively with the dominant and non-dominant hand over the 5-min test duration.
Body sway (motor-balance) was recorded by placing the subject on a balance plate that produces signals from 3 sensors to map the position of the force center, using the Catsys Tremor 7.0 by Danish Product Development (Després et al., 2000), as previously described (Lucchini et al., 2012). The center is defined as the center of equilibrium of the 3 vertical forces, which are recorded at the 3 supports of the sway plate. The sway plate is visualized on the computer screen and the journey of the force center can be observed in an X-Y coordinate system.
Bioinformatic Analysis of Gene-Regulatory Elements and Transcription
The potential effects of the 2 SNPs on regulatory elements were examined in relation to signatures of gene-regulatory elements available from the UCSC Genome Browser (www.genome.ucsc.edu), including H3K27Ac [Histone H3 acetylation at Lys27 (7 cell lines from ENCODE); indicative of active regulatory regions], DNaseI [DNase I hypersensitive sites (125 cell types from ENCODE); indicative of open and active chromatin], and TF [transcription factor binding sites (ChIP-Seq of 161 factors 91 cell types combined)]. Transcription factor analysis of different allelic variants was performed using MatInspector (Genomatix, Munich, Germany) with the vertebrate matrix and 75% matrix similarity filter. Non-coding RNA transcription and conserved miRNA binding sites were analyzed using data available from snoRNABase, miRBase, and TargetScanHuman 5.1 available via the UCSC Genome Browser. 3′UTR sequence motifs were searched for using UTRScan (http://itbtools.ba.itb.cnr.it/utrscan).
Statistical Analysis
All statistical analyses were performed using unconverted whole-blood Mn concentrations for the Andean and Italian cohorts and Ery-Mn concentrations for the Bangladeshi cohort. Correlations between subject characteristics and markers in blood were performed using Spearman correlation coefficients. Associations between genotypes and Ery-Mn (Bangladesh), whole-blood Mn (Andes and Italy), plasma Zn (Bangladesh and Andes), SLC30A10 expression levels in blood cells (Andes), and neurological parameters (sway velocity and finger tapping; Italy) were estimated using a multivariable-adjusted regression with the general linear model. Mn (dependent variable) was natural log (ln)-transformed to generate an improved distribution pattern, which was verified by Q-Q-plots. All analyses were performed with and without adjustments for age and sex, which were considered potential effect modifiers.
Iron status can influence Mn levels because Mn and Fe compete for the same cellular transporters (Au et al., 2008; Chen et al., 2014; Kippler et al., 2009). Blood Mn and Fe are therefore often inversely correlated (Ljung et al., 2009). Associations of genotypes with Mn levels and SLC30A10 expression levels were therefore also adjusted for Fe status. Ferritin (stored iron), which was measured in all 3 cohorts and showed the strongest correlations with Mn concentrations compared with other Fe indicators (see ‘Results’ section), was used as a proxy for Fe status in statistical analyses. However, since ferritin is upregulated in connection with inflammation (Kell and Pretorius, 2014), we also evaluated association analyses using other Fe indicators that were available in the Andean and Italian cohorts, including Hb (both cohorts), whole blood Fe (Andean cohort), and plasma Fe (Italian cohort). We also performed the analysis of genotypes in association with Mn concentrations with adjustments for Zn; however, including Zn in the model caused a change of <5% in the effect estimate and it was therefore excluded. Age and gender were also considered likely effect modifiers of neurological function due to the increase in Parkinson’s-related symptoms with age and higher prevalence in men (Kalia and Lang, 2015).
P-value < .05 denotes statistical significance. All statistical analyses were performed using SPSS (Version 20, Chicago, Illinois).
RESULTS
Cohort Characteristics and Correlations
Descriptive characteristics and data on relevant markers in blood are summarized in Table 1. Concentrations of Hb, serum Fe, and whole blood Fe are presented in Supplementary Table 3. Correlations between subject characteristics and markers in blood for all cohorts, as well as SLC30A10 gene-expression levels (Andean sub-group only) and neurological parameters (Italian cohort only), are presented in Supplementary Tables 4–7.
TABLE 1.
Characteristics of Study Cohorts
| Bangladesh |
Andes |
Italy |
||||||
|---|---|---|---|---|---|---|---|---|
| All |
Sub-groupa |
|||||||
| N | Median (5–95 percentile) | N | Median (5–95 percentile) | N | Median (5–95 percentile) | N | Median (5–95 percentile) | |
| Women | 406 | – | 184 | – | 78 | – | 131 | – |
| Men | 0 | – | 18 | – | – | – | 107 | – |
| Age | 404 | 26 (18–37) | 202 | 34 (18–65) | 78 | 32 (16–62) | 238 | 69 (65–76) |
| BMI | 394 | 19.8 (16.6–24.8) | 202 | 24.6 (18.8–35.0) | 78 | 23.8 (18.2–35.0) | – | – |
| Whole bloodb Mn (µg/l) | – | – | 202 | 16.3 (10.8–26.4) | 78 | 16.3 (10.8–30.0) | 238 | 8.5 (5.0–15.6) |
| Ery–Mnc (µg/kg) | 406 | 21.6 (13.3–35.1) | 202 | 22.4 (14.2–40.7) | 78 | 22.6 (14.5–41.3) | 238 | 12.8 (7.6–23.7) |
| Plasma ferritin (µg/l) | 402 | 29.7 (8.0–88.0) | 194 | 51.5 (8.0–336.0) | 77 | 51.0 (5.9–279.7) | 238 | 126 (23.9–372.6) |
| Plasma Zn (mg/l) | 402 | 0.56 (0.37–1.02) | 122 | 0.79 (0.60–1.05) | 51 | 0.78 (0.65–1.10) | – | – |
| MAFd rs2275707 (%) | 406 | C = 17.3 | 196 | C = 24.7 | 75 | C = 24.7 | 232 | C = 24.5 |
| MAFd rs12064812 (%) | 406 | C = 40.6 | 198 | C = 45.7 | 75 | C = 48.0 | 232 | C = 29.0 |
aSub-group with gene expression data.
bMeasured in the Andean and Italian cohorts only.
cMn concentrations in erythrocytes (µg/kg). For the Andean and Italian cohorts, the values were converted from total blood Mn (µg/l) based on the estimation that 66% of Mn in blood is bound to erythrocytes and that the density of the erythrocyte preparations were 1.055 g/ml. The volume fraction of erythrocytes in total blood for each individual was estimated by dividing their hemoglobin concentrations with the reference value of erythrocyte mean hemoglobin content of 340 g/l.
dMinor allele frequency for SNP.
Compared with the Andean cohort (men and women), the Bangladeshi women were younger (median ages of 26 and 34 years, respectively) and had lower BMI (median 19.8 and 25.0 kg/m2, respectively). Whole-blood Mn values were higher in the Andean cohort than in the Italian cohort. Mn concentrations in erythrocytes (Ery-Mn; converted from whole blood concentrations for the Andean and Italian cohorts) were similar in the Bangladeshi and Andean cohorts and lower in Brescia. Plasma ferritin concentrations varied between cohorts with the lowest levels in the pregnant Bangladeshi women and the highest levels in the Italian cohort consisting of elderly men and women (Table 1).
For Bangladeshi women, as previously described (Ljung et al., 2009), Mn concentrations in erythrocytes (Ery-Mn) at gestational week 14 were weakly inversely correlated with age (rS = −0.13, P = .012) and plasma ferritin (rS = −0.18, P < .001) (Supplementary Table 4). Inverse correlations were observed between whole-blood Mn concentrations and Fe indicators (blood/plasma Fe, and plasma ferritin) in the Andean and Italian cohorts and Hb in the Andean cohort (men and women), with the strongest correlations observed for plasma ferritin (rS = −0.41, P < .001 and rS = −0.20, P = .002 in the Andes and Italy, respectively). There were no correlations for Mn and age in theses cohorts (Supplementary Tables 5 and 7).
Characteristics of SLC30A10 SNPs and Associated Sequences
Minor allele frequencies for rs2275707 and rs12064812 are presented in Table 1. Both SNPs were in Hardy-Weinberg equilibrium in all 3 cohorts (χ2 values are presented in Supplementary Table 1). Due to low subject numbers for the rs2275707 rare variant allele homozygotes in all cohorts (<7%), values for the variant homozygote (CC) and heterozygote (CA) genotypes were combined in the statistical analyses.
The rs2275707 (A/C) is located in the 3′UTR and rs12064812 (T/C) in the second intron of SLC30A10 and these SNPs are 9.8 kb apart (Figure 1A). LD plot analyses of the 2 SNPs showed R2 values of 0.12, 0.27, and 0.13 in the Bangladeshi, Andean, and Italian cohorts, respectively. The potential of the 2 SNPs to affect post-transcriptional regulation by altering non-coding RNA transcription and/or miRNA binding was investigated by bioinformatics, but we did not observe any RNA transcription or conserved miRNA binding sites at the SNPs. We also tested whether rs2275707 coincided with any known 3′UTR sequence motifs involved in post-transcriptional regulation, but no such sites were found at the SNP. The possibility of the 2 SNPs being situated in regions involved in transcriptional regulation was also evaluated in silico. Both SNPs coincided with weak histone acetylation (H3K27Ac) and rs12064812 also coincided with a DNase I hypersensitivity site (Figure 1B). Transcription factor recognition site analysis showed differences between alleles for both SNPs. We also observed that the rs12064812 variant allele introduces a CpG site. The results are summarized in Figure 1B and Supplementary Table 8, which also includes information on function and gene-expression patterns of relevant transcription factors.
FIG. 1.
(A) Location of the SNPs rs2275707 and 12064812, in the SLC30A10 gene and in relation to signatures of gene-regulatory elements available from the UCSC Genome Browser including Histone H3 acetylation at Lys27 (H3K27Ac), DNase I hypersensitive sites (DNase I), and transcription factor (TF) binding sites. (B) DNA sequences flanking the 2 SNPs, including changes of transcription factor binding sites between alleles (only TFs for which the SNP affected the core sequence are shown). For rs2275707, the rare variant (C) replaced a binding site for Hmx2/Nkx5-2 homeodomain transcription factor (HMX2) with an Ets variant 6 (ETV6) binding site. For rs12064812, the variant allele (C) introduced 2 TF recognition sites [GDNF-inducible zinc finger protein 1 (GZ1F) and E2F transcription factor 3 (E2F3)]. The CpG site for rs12064812 variant allele is marked with a box.
Association of SLC30A10 Genotypes with Mn and Zn Concentrations
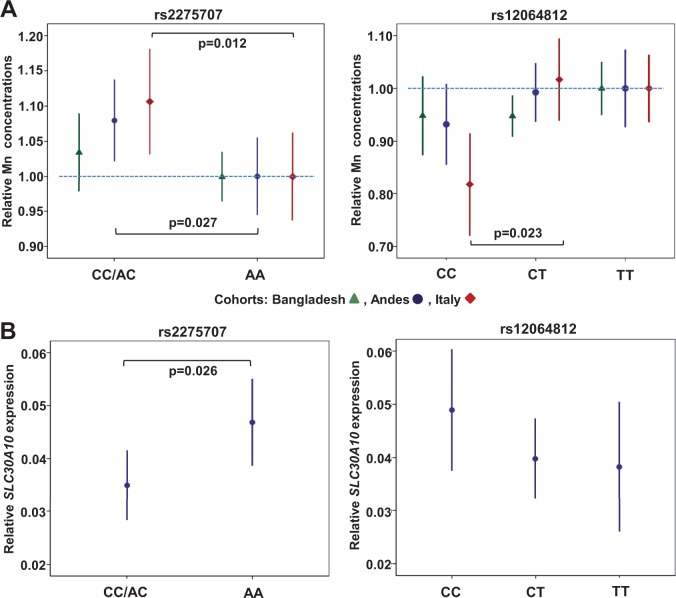
Both SNPs showed an association with whole-blood Mn concentrations (natural log(ln)-transformed) in the Andean and Italian cohorts and although the strength of associations differed between cohorts, the directions were consistent (Table 2, Figure 2A). The rs2275707 variant allele (C) was significantly associated with increased Mn concentrations. A similar but non-significant trend for the effect of genotype was observed for Ery-Mn in the Bangladeshi cohort (Figure 2A, Table 2). The average increase in Mn concentrations for the heterozygotes and variant allele homozygotes combined (AC/CC) relative to the common allele homozygotes (AA) was 3.4%, 8.0%, and 10.6% in the Bangladeshi, Andean, and Italian cohorts, respectively. In contrast, the rs12064812 variant allele (C) appeared to have a negative influence on Mn concentrations in all 3 cohorts, but the association was significant only in Italy. The average decrease in Mn concentrations for the variant allele homozygotes (CC) relative to the common allele homozygotes (TT) was 5.1%, 6.7%, and 18.4% in Bangladesh, Andes, and Italy, respectively (Table 2). The effect of both SNPs remained after adjusting for plasma ferritin, gender, and age (Table 2). The associations also remained after adjustments for other Fe indicators (Hb, serum Fe, and blood Fe) (Supplementary Table 9).
TABLE 2.
Associations of Ery-Mn (Bangladeshi Cohort) and Whole-Blood Mn Concentrations (Andean and Italian Cohorts) with SLC30A10 Genotypes
| rs2275707 |
rs12064812 |
||||
|---|---|---|---|---|---|
| AA | AC/CC | TT | CT | CC | |
| Bangladesh | |||||
| N | 280 | 126 | 142 | 199 | 199 |
| % changea | – | 3.4 | – | −5.2 | −5.1 |
| Pb | 0.304 | 0.183 | |||
| Pc | 0.207 | 0.168 | |||
| Andes | |||||
| N | 110 | 86 | 61 | 93 | 44 |
| % changea | – | 8.0 | – | −0.8 | −6.7 |
| Pb | 0.021* | 0.356 | |||
| Pc | 0.027* | 0.269 | |||
| Italy | |||||
| N | 133 | 99 | 122 | 86 | 24 |
| % changea | – | 10.6 | – | 1.8 | −18.4 |
| Pb | 0.016* | 0.028* | |||
| Pc | 0.012* | 0.040* | |||
aPercentage change in Mn concentrations (average) compared with homozygote for common allele.
bP-value for unadjusted linear model with ln-transformed Mn levels.
cP-value for adjusted linear model (age, gender, and ferritin) with ln-transformed Mn levels.
*Statistical significance.
FIG. 2.
Associations of genotypes with Mn and SLC30A10 expression. Error bars represent 95% confidence intervals (CI). Due to the low subject number for the rs2275707 variant homozygote genotype (<5%), this was combined with the heterozygote genotype. (A) Associations of SLC30A10 genotypes with blood Mn concentrations (represented by Ery-Mn in Bangladesh and whole blood Mn in the Andes and Italy). To enable comparison between populations, Mn levels are presented relative to levels for the homozygote genotype of the common allele, which was set to 1. The dashed line marks the reference level. (B) Associations of SLC30A10 genotypes with gene-expression levels. SLC30A10 expression is presented as % expression relative to the reference gene DECR1. The P-values are derived from the adjusted linear model (age, gender, and ferritin) with ln-transformed Mn levels.
SLC30A10 genotypes were also evaluated for associations with plasma Zn concentrations in the Bangladeshi and Andean cohorts to evaluate the potential of SLC30A10 as a Zn transporter (Table 3). We did not observe any statistically significant associations between genotypes and plasma Zn concentrations in any of the cohorts before or after adjusting for age and gender.
TABLE 3.
Associations of Plasma Zinc Concentrations and SLC30A10 Genotypes in the Bangladeshi and Andean Cohorts
| rs2275707 |
rs12064812 |
|||||
|---|---|---|---|---|---|---|
| AA | AC/CC | TT | CT | CC | ||
| Bangladesh | ||||||
| N | 275 | 128 | 141 | 199 | 65 | |
| % changea | – | 3.6 | – | 1.0 | 8.3 | |
| Pb | 0.358 | 0.348 | ||||
| Pc | 0.351 | 0.471 | ||||
| Andes | ||||||
| N | 64 | 52 | 36 | 56 | 25 | |
| % changea | – | 0.6 | – | 0.14 | 2.1 | |
| Pb | 0.861 | 0.858 | ||||
| Pc | 0.835 | 0.851 | ||||
aPercentage change in Zn concentrations (average) compared with homozygote for common allele.
bP-value for unadjusted linear model.
cP-value for adjusted linear model (age and gender).
SLC30A10 Genotypes and Gene Expression in the Andean Cohort
Transcript levels of SLC30A10 in blood were very low with Ct values in the range of 35–38, and of the initial 101 mRNA samples, 78 generated data that could be used for further analysis. We did not observe any significant correlations between SLC30A10 expression and Mn concentrations; however, we did observe a weak indication that gene expression could be inversely correlated with blood Mn (rS = −0.18, P = .11) and the opposite trend was observed for ferritin (rS = 0.18, P = .12) (Supplementary Table 6).
The rs2275707 variant allele was significantly associated with reduced SCL30A10 expression levels and we observed an average reduction of expression levels of 25% between common allele homozygotes (AA) and the combined group (AC/CC) (Table 4, Figure 2B). In contrast, the rs12064812 variant allele showed a weak, non-significant trend toward increased SLC30A10 expression (Figure 2B).
TABLE 4.
Associations of SLC30A10 Expression and SLC30A10 Genotypes in the Andean Sub-Group
| rs2275707 |
rs12064812 |
||||
|---|---|---|---|---|---|
| AA | AC/CC | TT | CT | CC | |
| N | 41 | 34 | 17 | 38 | 20 |
| % changea | – | −24.6 | – | 3.9 | 26.8 |
| Pb | 0.029* | 0.329 | |||
| Pc | 0.029* | 0.334 | |||
aPercentage change in gene-expression (average) compared with homozygote for common allele.
bP-value for unadjusted linear model.
cP-value for adjusted linear model (age and ferritin).
*Statistical significance
SLC30A10 Genotypes and Markers of Neurotoxicity in the Italian Cohort
SLC30A10 genotypes were further analyzed for their potential influence on neurological markers that are associated with Parkinsonism, including sway velocity (open and closed eyes) and finger tapping (dominant and non-dominant hand). None of the described neurological parameters showed correlations with blood Mn concentrations (Supplementary Table 7).
The rs2275707 variant allele, which was associated with increased blood Mn, was also associated with increased sway velocity (average 15% increase) in the Italian cohort and the effect was significant for closed eyes (Table 5). The significance increased somewhat after adjustments were made for age and gender.
TABLE 5.
Associations of Neurological Parameters and SLC30A10 Genotypes in the Italian Population
| rs2275707 |
rs12064812 |
||||
|---|---|---|---|---|---|
| AA | AC/CC | TT | CT | CC | |
| Sway velocity (open eyes) | |||||
| N | 127 | 86 | 112 | 78 | 23 |
| Group meana | 11.4 | 12.5 | 11.5 | 12.3 | 11.7 |
| % changeb | – | 10.1 | – | 6.8 | 1.5 |
| Pc | 0.083 | 0.528 | |||
| Pd | 0.065 | 0.805 | |||
| Sway velocity (closed eyes) | |||||
| N | 127 | 86 | 112 | 78 | 23 |
| Group meana | 15.4 | 17.7 | 15.6 | 17.1 | 17.2 |
| % changeb | – | 15.0 | – | 9.7 | 10.0 |
| Pc | 0.050 | 0.424 | |||
| Pd | 0.033* | 0.745 | |||
| Finger tapping (dominant hand) | |||||
| N | 132 | 96 | 119 | 85 | 24 |
| Group meana | 47.7 | 47.8 | 46.0 | 49.3 | 51.0 |
| % changeb | – | 0.4 | – | 7.1 | 10.8 |
| Pc | 0.892 | 0.032* | |||
| Pd | 0.871 | 0.084 | |||
| Finger tapping (non-dominant hand) | |||||
| N | 132 | 96 | 119 | 85 | 24 |
| Group meana | 44.4 | 45.2 | 42.9 | 46.7 | 46.7 |
| % changeb | – | 1.93 | – | 8.9 | 8.7 |
| Pc | 0.505 | 0.011* | |||
| Pd | 0.444 | 0.025* | |||
aMean value for group
bPercentage change in Mn concentrations (average) compared with homozygote for common allele.
cP-value for unadjusted linear model.
dP-value for adjusted linear model (age, gender).
*Statistical significance.
The rs12064812 showed no significant associations with sway velocity. Instead, the variant allele, which was associated with lower blood Mn concentrations, was associated with increased finger-tapping performance. The strongest association observed for the non-dominant hand with an average increase in finger-tapping performance of 9% between the rs12064812 common and variant allele homozygotes.
Combined Analyses
Since rs2275707 and rs12064812 influence Mn levels, and are not in LD, we also investigated the possibility of an interaction between the 2 SNPs. To address this, we performed association analysis combining genotypes for the 2 SNPs in the Italian cohort, where the associations were the strongest, and for which neurological variables were available. SNP interactions were evaluated for those outcome variables that were significantly associated with SLC30A10 genotypes, ie, blood Mn concentrations, sway velocity (closed eyes), and finger tapping (non-dominant hand). To evaluate potential interactions, we assessed if combining genotypes would significantly affect the influence of genotypes on outcomes. For combined genotypes, the largest difference in blood Mn concentrations (2.3 µg/l) was observed between the combinations CC:TT to AA:CC (rs2275707:rs12064812) (Supplementary Figure 2). The difference in Mn concentrations between the corresponding genotypes uncombined was 1.1 µg/l (rs2275707; CC to AA) and 1.7 µg/l (rs12064812; TT to CC). Since combining genotypes did not change the directions of associations, and the influence of combined genotypes on Mn concentrations was only marginally different compared with the additive effect of the corresponding genotypes uncombined, we concluded that that it is unlikely that the SNPs interact on blood Mn concentrations. Similar results were observed from combined genotype analysis for sway velocity and finger tapping.
DISCUSSION
The SLC30A10 protein was recently implicated as an important and potentially specific Mn transporter based on the identification of mutations in SLC30A10 as the causal factor of heritable neurological disorders, including hypermanganesemia, without any known excessive exposure to Mn (Quadri et al., 2012, 2015; Tuschl et al., 2012). These individuals carried rare mutations in the coding region of SLC30A10 that led to a truncation of the protein or amino acid substitutions which in turn resulted in impaired protein trafficking and severe failure of protein function (Quadri et al., 2012; Tuschl et al., 2012). This is the first study in which common genetic polymorphisms in SLC30A10 were studied in relation to blood Mn concentrations and Mn-related neurological outcomes in healthy individuals. In contrast to previously described functional mutations in SLC30A10, which are very rare and cause dramatic effects on protein function as well as internal Mn levels, we studied genetic variants that are present in the general populations and are associated with subtle changes in Mn blood concentrations. This is one of few studies to date investigating the influence of genetic polymorphisms on Mn regulation and transportation; while previous studies have focused mainly on genes in iron-related pathways (Claus Henn et al., 2011; Haynes et al., 2010), we here target a pathway of Mn homeostasis that appears to be independent of iron transport.
We found that the SLC30A10 rs2275707 variant allele was associated with increased Mn and the rs12064812 variant allele was associated with decreased Mn concentrations. Although all cohorts did not show statistically significant associations between genotypes and Mn concentrations, the results are strengthened by the consistency in the directions of associations between the 3 cohorts, which are from very different ethnic backgrounds, have very different Mn exposures, and represent a wide range of internal Mn status. The Bangladeshi women showed the weakest associations of SLC30A10 genotypes with Mn concentrations. Since Mn was measured in erythrocytes instead of whole blood in these women, the lower associations may reflect differences in Mn transport in different compartments of the blood. It is also possible that the weaker influence of SLC30A10 genotypes in the Bangladeshi cohort is due to the lower nutritional status of members of this group, clearly indicated by their markedly lower BMI, and to pregnancy. These conditions may cause a general up-regulation of several transporters involved in Mn uptake (Leazer et al., 2002), which might diminish the individual effect of SLC30A10.
The rs2275707 variant allele (C), which was significantly associated with increased blood Mn concentrations, was also associated with reduced SLC30A10 expression levels. This is consistent with the findings of Quadri et al., who showed that a mutation that removed a large part of the SLC30A10 protein and nearly depleted SLC30A10 immunoreactivity in the liver was associated with higher blood Mn levels (Quadri et al., 2012). In contrast to rs2275707, the rs12064812 variant allele was associated with lower Mn blood concentrations. In line with this, we also observed a weak trend toward increased SLC30A10 expression for this allele. Expression levels of SLC30A10 in blood were very low and the effect of SLC30A10 genotypic variance on gene expression levels would most likely be more pronounced in a tissue in which SLC30A10 is highly expressed (eg, liver); however, liver samples were not available in this study. It should also be noted that the observed associations of genotypes with SLC30A10 expression levels could be a secondary effect of their influence on Mn concentrations, which in turn could affect SLC30A10 expression.
These SNPs are non-coding and appeared to influence SLC30A10 transcript levels, indicating that, instead of affecting SLC30A10 protein function, they may be situated in gene regulatory elements and influence gene expression. Regulatory elements, eg, enhancers, are commonly found within intronic regions, where they can influence promoter activity via physical interactions mediated by transcription factors (Bulger and Groudine, 2011). SNPs within such intronic regulatory elements can alter the binding affinity of transcription factors and affect promoter activity (Pound et al., 2011; Visser et al., 2015). Although elements involved in the regulation of transcription have also have been identified in 3′UTRs (Jash et al., 2012), these sequences more commonly affect mRNA stability and post-translational regulation, eg, by the interaction with miRNAs. In silico analysis of these SNPs and immediate flanking regions did not reveal any non-coding RNA transcription, conserved miRNA binding sites, or 3′UTR sequence motifs that would indicate that they influence post-transcriptional regulation. However, publically available experimental data on the sequences nearest the 2 SNPs showed some regulatory activity of these regions, as observed by weak histone acetylation and DNase I hypersensitivity (rs12064812 only). In silico analysis revealed that for both SNPs, the 2 alleles generated different recognition sites for factors involved in transcriptional regulation. This is, however, merely an indication that the SNPs may affect transcriptional regulation, and should be investigated experimentally. It is also possible that the 2 selected SNPs in this study are not functional themselves, but instead tag functional SNPs by capturing genetic variation in a larger genetic region.
We further evaluated SLC30A10 genotypes in association with neurological parameters related to Parkinsonism. We observed that the rs2275707 variant allele, which was associated with increased blood Mn concentrations, was also associated with increased sway velocity. The rs12064812 variant allele, which showed reduced Mn concentrations in blood, was associated with increased finger tapping speed. This is consistent with previous findings showing that higher blood Mn levels are associated with reduced finger-tapping speed (Lucchini et al., 1995) and impaired postural balance (increased sway) (Rugless et al., 2014). Despite showing associations with SLC30A10 genotypes, none of these parameters of neuromotor function were correlated with blood Mn concentrations, which may suggest that these associations are linked to a second level of SLC30A10-mediated Mn regulation that prevents excessive amounts of Mn from entering the brain. The SLC30A10 transporter is highly expressed in the CNS, particularly in the basal ganglia, where it likely acts to regulate brain Mn levels and thereby protect against the neurotoxic effects of Mn (Quadri et al., 2012). SLC30A10 mutations that caused a dysfunctional SLC30A10 protein were also associated with Mn accumulation in the brain (Tuschl et al., 2012).
Although SLC30A10 was originally identified as a Zn-transporter (Seve et al., 2004), the structural features of this protein suggest that it could be specific to Mn transport (Quadri et al., 2012). This was further supported by the observation that patients with SLC30A10 mutations have normal Zn levels (Quadri et al., 2012; Tuschl et al., 2012). Also, the introduction of an SLC30A10-wt expression construct in HeLa cells caused reduced intracellular Mn levels but had no effect on Zn levels (Leyva-Illades et al., 2014). In this study, we did not observe any associations of SLC30A10 genotypes on plasma Zn concentrations that would contradict the notion that the SCL30A10 protein functions as a specific Mn transporter.
In conclusion, this study shows that common non-coding genetic variation in the Mn transporter SLC30A10 influences blood Mn concentrations in healthy individuals and that the association is likely to be mediated by altered levels of SLC30A10 expression. We also show that genetic variants of SLC30A10 influence neurological function independent of blood Mn concentrations, which indicates that SLC30A10 may play a role in the regulation of brain Mn levels. The hypothesis generated by this study is that a genetic predisposition caused by deficits in the SLC30A10 transporter in the brain and liver may contribute to an increased risk of Parkinsonism in populations environmentally exposed to Mn.
FUNDING
The Swedish Research Council FORMAS, the Swedish Research Council for Health, Working Life and Welfare; and the Karolinska Institutet. The study was also supported by funding from the European Union through its Sixth Framework Program for RTD (contract no. FOOD-CT-2006-016253). It reflects only the authors’ views, and the European Commission is not liable for any use that may be made of the information contained therein. The project was also supported by the National Institute of Environmental Health Sciences (NIEHS) (Award Number R01ES019222). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Ameer S. S., Engström K., Harari F., Concha G., Vahter M., Broberg K. (2015). The effects of arsenic exposure on blood pressure and early risk markers of cardiovascular disease: evidence for population differences. Environ. Res. 140, 32–36. [DOI] [PubMed] [Google Scholar]
- Au C., Benedetto A., Aschner M. (2008). Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology 29, 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barett J. C., Fry B., Maller J., Daly M. J. (2005). Haploview: analysis and visualization of LD and haplotypes maps. Bioinformatics 21, 263–265. [DOI] [PubMed] [Google Scholar]
- Broberg K., Concha G., Engström K., Lindvall M., Grandér M., Vahter M. (2011). Lithium in drinking water and thyroid function. Environ. Health. Perspect., 119, 827–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M., Groudine M. (2011). Functional and mechanistic diversity of distal transcription enhancers. Cell 144, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Chakraborty S., Mukhopadhyay S., Lee E., Paoliello M. M., Bowman A. B., Aschner M. (2015). Manganese homeostasis in the nervous system. J. Neurochem. 134, 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Parmalee N., Aschner M. (2014). Genetic factors and manganese-induced neurotoxicity. Front. Genet. 5, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B., Kim J., Wessling-Resnick M., Téllez-Rojo M. M., Jayawardene I., Ettinger A. S., Hernández-Avila M., Schwartz J., Christiani D. C., Hu H., et al. (2011). Associations of iron metabolism genes with blood manganese levels: a population-based study with validation data from animal models. Environ. Health 10, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha G., Broberg K., Grandér M., Cardozo A., Palm B., Vahter M. (2010). High-level exposure to lithium, boron, cesium, and arsenic via drinking water in the Andes of northern Argentina. Environ. Sci. Technol. 44, 6875–6880. [DOI] [PubMed] [Google Scholar]
- Després C., Lamoureux D., Beuter A. (2000). Standardization of a neuromotor test battery: the CATSYS system. Neurotoxicology 21, 1–11. [PubMed] [Google Scholar]
- Fechter L. D., Johnson D. L., Lynch R. A. (2002). The relationship of particle size to olfactory nerve uptake of a non-soluble form of manganese into brain. Neurotoxicology 23, 177–183. [DOI] [PubMed] [Google Scholar]
- Haynes E. N., Heckel P., Ryan P., Roda S., Leung Y. K., Sebastian K., Succop P. (2010). Environmental manganese exposure in residents living near a ferromanganese refinery in Southeast Ohio: a pilot study. Neurotoxicology 31, 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iregren A., Gamberale F., Kjellberg A. (1996). SPES: a psychological test system to diagnose environmental hazards. Neurotoxicol. Teratol. 18, 485–491. [DOI] [PubMed] [Google Scholar]
- Jash A., Yun K., Sahoo A., So J.-S., Im S.-H. (2012). Looping mediated interaction between the promoter and 3′ UTR regulates type II collagen expression in chondrocytes. PLoS One 7, e40828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia L. V., Lang A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. [DOI] [PubMed] [Google Scholar]
- Kell D. B., Pretorius E. (2014). Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 6, 748–773. [DOI] [PubMed] [Google Scholar]
- Kippler M., Ekström E. C., Lönnerdal B., Goessler W., Åkesson A., El Arifeen S., Persson L. A., Vahter M. (2007). Influence of iron and zinc status on cadmium accumulation in Bangladeshi women. Toxicol. Appl. Pharmacol. 222, 221–226. [DOI] [PubMed] [Google Scholar]
- Kippler M., Goessler W., Nermell B., Ekström E. C., Lönnerdal B., El Arifeen S., Vahter M. (2009). Factors influencing intestinal cadmium uptake in pregnant Bangladeshi women—a prospective cohort study. Environ. Res. 109, 914–921. [DOI] [PubMed] [Google Scholar]
- Leazer T. M., Liu Y., Klaassen C. D. (2002). Cadmium absorption and its relationship to divalent metal transporter-1 in the pregnant rat. Toxicol. Appl. Pharmacol. 185, 18–24. [DOI] [PubMed] [Google Scholar]
- León-Velarde F., Gamboa A., Chuquiza J. A., Esteba W. A., Rivera-Chira M., Monge C. C. (2000). Hematological parameters in high altitude residents living at 4,355, 4,660, and 5,500 meters above sea level. High. Alt. Med. Biol. 1, 97–104. [DOI] [PubMed] [Google Scholar]
- Leyva-Illades D., Chen P., Zogzas C. E., Hutchens S., Mercado J. M., Swaim C. D., Morrisett R. A., Bowman A. B., Aschner M., Mukhopadhyay S. (2014). SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci. 34, 14079–14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ekström E. C., Goessler W., Lönnerdal B., Nermell B., Yunus M., Rahman A., El Arifeen S., Persson L. A., Vahter M. (2008). Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ. Health Perspect. 116, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K. S., Kippler M. J., Goessler W., Grandér G. M., Nermell B. M., Vahter M. E. (2009). Maternal and early life exposure to manganese in rural Bangladesh. Environ. Sci. Technol. 43, 2595–2601. [DOI] [PubMed] [Google Scholar]
- Lucas E. L., Bertrand P., Guazzetti S., Donna F., Peli M., Jursa T. P., Lucchini R., Smith D. R. (2015). Impact of ferromanganese alloy plants on household dust manganese levels: implications for childhood exposure. Environ. Res. 138C, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R. G., Albini E., Benedetti L., Borghesi S., Coccaglio R., Malara E. C., Parrinello G., Garattini S., Resola S., Alessio L. (2007). High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am. J. Ind. Med. 50, 788–800. [DOI] [PubMed] [Google Scholar]
- Lucchini R., Apostoli P., Perrone C., Placidi D., Albini E., Migliorati P., Mergler D., Sassine M. P., Palmi S., Alessio L. (1999). Long-term exposure to ‘low levels’ of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology 20, 287–297. [PubMed] [Google Scholar]
- Lucchini R. G., Aschner M., Kim Y., Šarić M. (2015). Manganese. In Handbook on the Toxicology of Metals (Nordberg G. F., Fowler B. A., Nordberg M., Eds.), 4th ed., pp. 975–1011. Amsterdam, Academic Press. [Google Scholar]
- Lucchini R. G., Guazzetti S., Zoni S., Benedetti C., Fedrighi C., Peli M., Donna F., Bontempi E., Borgese L., Micheletti S., et al. (2014). Neurofunctional dopaminergic impairment in elderly after lifetime exposure to manganese. Neurotoxicology 45, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R. G., Guazzetti S., Zoni S., Donna F., Peter S., Zacco A., Salmistraro M., Bontempi E., Zimmerman N. J., Smith D. R. (2012). Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology 33, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini A., Selis L., Folli D., Apostoli P., Mutti A., Vanoni O., Iregren A., Alessio L. (1995). Neurobehavioral effects of manganese in workers from a, ferroalloy plant after temporary cessation of exposure. Scand. J. Work Environ. Health 21, 143–150. [DOI] [PubMed] [Google Scholar]
- Lundh B., Öhlin A.-K. (1991). Erytronet. In Laurells Klinisk kemi i praktisk medicin (Fernlund P., Fex G., Hanson A., Stenflo J., Lundh B., Eds.), Part 6, pp. 291–349. Studentlitteratur, Lund; [in Swedish] [Google Scholar]
- Milne D. B., Sims R. L., Ralston N. V. (1990). Manganese content of the cellular components of blood. Clin. Chem. 36, 450–452. [PubMed] [Google Scholar]
- Ng E., Lind M., Lindgren C., Ingelsson E., Mahajan A., Morris A., Lind L. (2015). Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet. 24, 4739–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavilonis B. T., Lioy P. J., Guazzetti S., Bostick B. C., Donna F., Peli M., Zimmerman N. J., Bertrand P., Lucas E., Smith D. R., et al. (2015). Manganese concentrations in soil and settled dust in an area with historic ferroalloy production. J. Expo. Sci. Environ. Epidemiol. 25, 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound L. D., Sarkar S. A., Cauchi S., Wang Y., Oeser J. K., Lee C. E., Froguel P., Hutton J. C., O’Brien R. M. (2011). Characterization of the human SLC30A8 promoter and intronic enhancer. J. Mol. Endocrinol. 47, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M., Federico A., Zhao T., Breedveld G. J., Battisti C., Delnooz C., Severijnen L. A., Di Toro Mammarella L., Mignarri A., Monti L., et al. (2012). Mutations in SLC30A10 cause Parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 90, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M., Kamate M., Sharma S., Olgiati S., Graafland J., Breedveld G. J., Kori I., Hattiholi V., Jain P., Aneja S., Kumar A., et al. (2015). Manganese transport disorder: Novel SLC30A10 mutations and early phenotypes. Mov. Disord. 30, 996–1001. [DOI] [PubMed] [Google Scholar]
- Racette B. A., Criswell S. R., Lundin J. I., Hobson A., Seixas N., Kotzbauer P. T., Evanoff B. A., Perlmutter J. S., Zhang J., Sheppard L., et al. (2012). Increased risk of Parkinsonism associated with welding exposure. Neurotoxicology 33, 1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette B. A., Mcgee-Minnich L., Moerlein S. M., Mink J. W., Videen T. O., Perlmutter J. S. (2001). Welding-related Parkinsonism: clinical features, treatment, and pathophysiology. Neurology 56, 8–13. [DOI] [PubMed] [Google Scholar]
- Rahman S. M., Akesson A., Kippler M., Grander M., Hamadani J. D., Streatfield P. K., Persson L. Å., El Arifeen S., Vahter M. (2013). Elevated manganese concentrations in drinking water may be beneficial for fetal survival. PLoS One 8, e74119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentschler G., Covolo L., Haddad A. A., Lucchini R. G., Zoni S., Broberg K. (2012). ATP13A2 (PARK9) polymorphisms influence the neurotoxic effects of manganese. Neurotoxicology 33, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier J. (1955). Manganese poisoning in Moroccan miners. Br .J. Ind. Med. 12, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H. A., Bowler R. M., Kim Y., Claus Henn B., Mergler D., Hoet P., Gocheva V. V., Bellinger D. C., Wright R. O., Harris M. G., et al. (2012). Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology 33, 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugless F., Bhattacharya A., Succop P., Dietrich K. N., Cox C., Alden J., Kuhnell P., Barnas M., Wright R., Parsons P. J., et al. (2014). Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in Southeastern Ohio. Neurotoxicol. Teratol. 41, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seve M., Chimienti F., Devergnas S., Favier A. (2004). In silico identification and expression of SLC30 family genes: An expressed sequence tag data mining strategy for the characterization of zinc transporters’ tissue expression. BMC Genomics 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamova B. S., Apperson M., Walker W. L., Tian Y., Xu H., Adamczy P., Zhan X., Liu D. Z., Ander B. P., Liao I. H., et al. (2009). Identification and validation of suitable endogenous reference genes for gene expression studies in human peripheral blood. BMC Med. Genom. 2, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjälve H., Mejare C., Borg-Neczak K. (1995). Uptake and transport of manganese in primary and secondary olfactory neurones inpike. Pharmacol. Toxicol. 77, 23–31. [DOI] [PubMed] [Google Scholar]
- Tuschl K., Clayton P. T., Gospe S. M., Jr, Gulab S., Ibrahim S., Singhi P., Aulakh R., Ribeiro R. T., Barsottini O. G., Zaki M. S., et al. (2012). Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum Genet. 90, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. E., Li L., Nermell B., Rahman A., El Arifeen S., Rahman M., Persson L. A., Ekström E. C. (2006). Arsenic exposure in pregnancy: a population-based study in Matlab, Bangladesh. J. Health Popul. Nutr. 24, 236–245. [PubMed] [Google Scholar]
- Visser M., Palstra R. J., Kayser M. (2015). Allele-specific transcriptional regulation of IRF4 in melanocytes is mediated by chromatin looping of the intronic rs12203592 enhancer to the IRF4 promoter. Hum. Mol. Genet. 24, 2649–2661. [DOI] [PubMed] [Google Scholar]
- Zoni S., Lucchini R. G. (2013). Manganese exposure: cognitive, motor and behavioral effects on children: a review of recent findings. Curr. Opin. Pediatr. 25, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.