Abstract
Background
The data on human rhinovirus (HRV), coronavirus (hCoV), bocavirus (hBoV), metapneumovirus (hMPV), Chlamydophila pneumoniae, Mycoplasma pneumoniae and Bordetella pertussis infections in children with cancer is limited.
Methods
We sought to determine prospectively the prevalence of respiratory pathogens in these children, using multiplexed-PCR.
Results
We enrolled 253 children with upper, or lower respiratory tract infection (URTI/LRTI) during a one year period. A respiratory virus was detected in 193 (76%) patients; 156 (81%) patients had URTI. Human rhinovirus was the most common virus detected in 97 (62%) and 24 (65%) patients with URTI and LRTI, respectively. Leukemia or lymphoma (LL) was the most common underlying diagnosis in 95 (49%) patients followed by solid tumor 47 (24%), post-hematopoietic stem cell transplant (HCT) 28 (15%), and brain tumor in 23 (12%) patients. By multiple logistic regression analysis hBoV was the most commonly detected respiratory virus in patients with LRTI (P = 0.008; odds ratio, 4.52; 95% confidence interval, 1.48-13.79). Co-infection with more than 1 virus was present in 47 (24%) patients, and did not increase the risk for LRTI. Two (0.7%) patients succumbed to LRTI from parainfluenza virus (PIV)-3 and respiratory syncytial virus/HRV infection, respectively. C.pneumoniae and M.pneumoniae were detected in 4 and 3 patients, respectively.
Conclusions
HRV was the most common virus detected in children with cancer and post-HCT hospitalized with an acute respiratory illness, and was not associated with increased morbidity. Prospective studies with viral load determination and asymptomatic controls are needed to study the association of these emerging respiratory viruses with LRTI in children with cancer and post-HCT.
Keywords: respiratory, infections, children, cancer
Human rhinovirus (HRV) and coronavirus (hCoV) infections are common in children, and associated with hospitalization for acute respiratory illness (ARI).1,2 However, little is known about the clinical impact of these infections in children with cancer, and those undergoing hematopoietic cell transplantation (HCT); populations where ARI from influenza,3 parainfluenza virus (PIV),4,5 respiratory syncytial virus (RSV),6 human metapneumovirus (hMPV),7 human adenovirus,8 human bocavirus (hBoV),9 human enterovirus,10 Chlamydophila pneumoniae11 and Mycoplasma pneumoniae12 and have been described, mostly in case reports or retrospective series.
We hypothesized HRV to be the most common respiratory virus detected in this population predominantly with an upper respiratory tract infection (URTI), coinfection with other viruses predisposing patients to lower respiratory tract infection (LRTI) and hospitalization. To evaluate this, we prospectively analyzed respiratory samples from children with URTI or LRTI, for HRV, hCoV, hBoV, human enterovirus, human adenovirus, hMPV, PIV, RSV, influenza, C. pneumoniae, M. pneumoniae and Bordetella pertussis using multiplexed–polymerase chain reaction (PCR) in children with leukemia/lymphoma (LL), solid tumors, brain tumors and those who had undergone HCT. Previous studies at our institution have shown increased morbidity and mortality from parainfluenza5,6 and respiratory syncytial13 virus infections in children post-HCT compared with those with hematologic malignancies. Children post-HCT are also prone to bacterial, fungal and other viral infections particularly late after transplant owing to prolonged immune suppression.14
METHODS
Children ≤18 years diagnosed with cancer or post-HCT, with URTI or LRTI, from the in-patient units and out-patient clinics at St. Jude Children’s Research Hospital were eligible for enrollment in this prospectively conducted study. The duration of the study was for 1 year from October 2010 to September 2011, both months inclusive. The study was approved by the St. Jude Children’s Research Hospital institutional review board.
Respiratory specimens including nasopharyngeal wash, tracheal aspirate and bronchoalveolar lavage samples were collected as ordered by the treating physician based on presence of symptoms of ARI at the time of initial presentation and follow-up as defined. Nasopharyngeal wash was obtained using a presaline-filled syringe aspiration kit (N-Pak, Annandale, MN). A minimum of 0.5 mL of aspirate was collected and transported immediately to the St. Jude Children’s Research Hospital microbiology laboratory on ice. Diagnostic studies were performed and available to the physicians after reporting. An aliquot from the sample remaining after completion of clinical diagnostic testing (aliquot A) was tested by multiplexed-PCR as previously described15 after removal of patient identifiers. Physicians were blinded to the results of testing on aliquot A.
Testing on aliquot A was performed using an automated broadly multiplexed PCR-system (FilmArray, Idaho Technology, Inc., Salt Lake City, UT), integrating specimen processing with nested multiplexed-PCR. This system enables simultaneous detection of HRV, hCoV-229E, hCoV-HKU1, hCoV-OC43, hCoV-NL63, hBoV, human enterovirus, PIV 1–4, RSV, influenza A, influenza AH1, influenza AH12009, influenza AH3, influenza B, human adenovirus, hMPV, M. pneumoniae, C. pneumoniae and B. pertussis.
Definitions
The day of infection onset was defined as the day when the first positive sample was collected. Patients were considered infected by a respiratory virus if it was detected by multiplexed-PCR. URTI was defined as the presence of 2 or more of the following symptoms: fever, rhinorrhea, nasal congestion, otitis media, pharyngitis or cough with a normal chest examination and chest radiograph. LRTI included any of the suggestive symptoms and signs with crepitation or rhonchi on lung auscultation, or a new pulmonary infiltrate seen on radiography. Duration of viral shedding was time in days from the first positive to the first negative test result. Mortality was considered attributable to the respiratory virus if the patient died of LRTI as a result of respiratory failure with detection of virus from a respiratory sample, and with no other ascertainable cause.
Clinical Analyses
Clinical data was abstracted from a prospectively collected database that included patient demographics, underlying disease, URTI/LRTI, use of systemic steroids in the 2 weeks before infection, radiological findings, copathogens, presence of fever, need for oxygen or mechanical ventilation, admission to the intensive care unit, mortality, absolute neutrophil count (ANC) and absolute lymphocyte count (ALC) at time of infection.
Statistical Analyses
The descriptive statistics of the cohort and prevalence of respiratory viruses in patients with URTI/LRTI were provided. Fischer exact test and t test were used to compare the prevalence of respiratory viruses by age, gender, race, sample source, symptoms and underlying disease. Exact test was used to test association of individual viruses and coinfections with URTI/LRTI. The first sample test positive for any virus was used for this purpose. The first episode of URTI or LRTI was used for analysis, whether or not a virus was isolated. Patients with both URTI and LRTI were classified as LRTI. Univariate logistic regression analysis was performed to determine the association between LRTI and clinical variables including age as a continuous variable, gender, race, ANC <500 cells/μL, ALC <100 cells/μL, use of steroids ≤2 weeks before infection, underlying disease (LL versus solid tumor versus brain tumor versus post-HCT), in-patient versus out-patient status and the 9 respiratory viruses and 3 bacterial pathogens detected. All factors that demonstrated a statistical association (P = 0.15) were included in the multiple logistic regression model. Potential confounders were assessed using exact test. Duration of shedding was analyzed in a descriptive manner. All reported P-values were 2-sided and considered significant if <0.05. SAS version 9.2 (SAS Institute, Cary, NC) and StatXact (Cytel Corporation, Cambridge, MA) Windows version 8 were used for statistical analyses.
RESULTS
Detection of Respiratory Virus
Of 253 patients with ARI who were eligible and enrolled on study, a respiratory virus was detected in 193 (76%) patients (Table 1). The mean age was 6.9 (median 5.9; range 0.1–17.9) years. Children <10 years of age comprised three quarter of patients in whom respiratory virus was detected. LL was the most common underlying diagnosis in 95 (49%) infected patients. Detection of respiratory virus was not significantly different in patients with LL, solid tumor, brain tumor and post-HCT (P = 0.45).
TABLE 1.
Clinical characteristics of patients with and without a detectable respiratory virus
| Characteristic | All patients | RV detected | No RV detected | P |
|---|---|---|---|---|
| Number of patients | 253 | 193 (76) | 60 (24) | |
| Mean age years (SD) | 6.88 (4.81) | 6.53 (4.52) | 7.99 (5.52) | 0.12 |
| Age in years | ||||
| 0-<2 | 34 (13) | 27 (14) | 7 (12) | 0.15 |
| 2-<10 | 151 (60) | 120 (62) | 31 (52) | |
| > 10 | 68 (27) | 46 (24) | 22 (36) | |
| Male | 139 (55) | 106 (55) | 33 (55) | 1.00 |
| Race | 0.20 | |||
| White | 180 (71) | 139 (72) | 41 (68) | |
| AA | 46 (18) | 31 (16) | 15 (25) | |
| Other | 27 (11) | 23 (12) | 4 (7) | |
| Sample source | 0.09 | |||
| NPW | 243 (96) | 188 (97) | 55 (92) | |
| BAL | 4 (2) | 2 (1) | 2 (3) | |
| TA | 6 (2) | 3 (2) | 3 (5) | |
| Symptoms | < 0.0001 | |||
| URTI | 196 (77) | 156 (81) | 40 (67) | |
| LRTI | 57 (23) | 37 (19) | 20 (33) |
| Characteristic | All patients | RV detected | No RV detected | P |
|---|---|---|---|---|
| n=253 | n=193 | n=60 | ||
| Underlying disease | 0.45 | |||
| LL | 121 (48) | 95 (49) | 26 (44) | |
| ST | 61 (24) | 47 (24) | 14 (23) | |
| BT | 29 (11) | 23 (12) | 6 (10) | |
| Post-HCT | 42 (17) | 28 (15) | 14 (23) |
Data are no. (%) of patients, unless otherwise indicated. RV, respiratory virus; AA, African-American; NPW, nasopharyngeal wash; BAL, broncho-alveolar lavage; TA, tracheal aspirate; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; LL, leukemia or lymphoma; ST, solid tumor; BT, brain tumor; HCT, hematopoietic cell transplantation; SD, standard deviation.
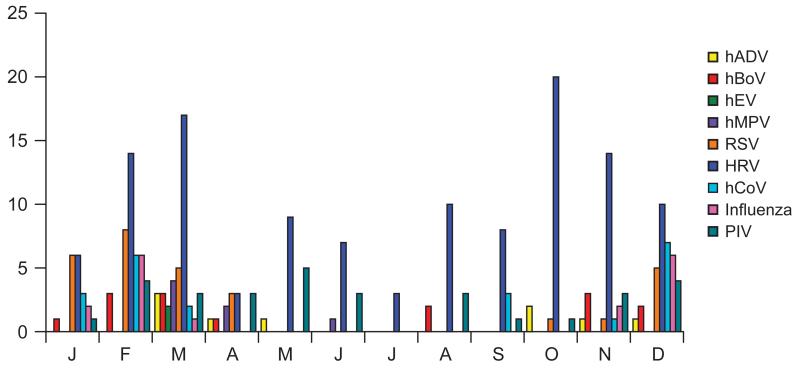
HRV was the most common respiratory virus detected in 121 (63%) infected patients (Table 2). Respiratory viruses were detected in 137 (71%) patients between October and March, and 56 (29%) patients between April and September (both months inclusive; Fig. 1). Of the 121 patients with HRV and 22 patients with hCoV infection, HRV and hCoV were detected in 81 (67%) and 19 (86%) patients, October–March, and in 40 (33%), and 3 (14%) patients, respectively, April–September. This difference in distribution was statistically significant for HRV (P = 0.0003) and hCoV (P = 0.001). HRV and PIV were distributed year around. The distribution was not significantly different among the 4 groups of patients for HRV (P = 0.11) and PIV (P = 0.39).
TABLE 2.
Respiratory viruses detected in patients with URTI and LRTI
| Virus | Overall | URTI | LRTI | P |
|---|---|---|---|---|
| n=193 | n=156 | n=37 | ||
| HRV | 121 (63) | 97 (62) | 24 (65) | 0.85 |
| hCoV | 22 (11) | 20 (13) | 2 (5) | 0.26 |
| hCoV-229e | 0 (0) | 0 (0) | 0 (0) | |
| hCoV-HKU1 | 2 (1) | 2 (1) | 0 (0) | |
| hCoV-OC43 | 13 (7) | 12 (8) | 1 (3) | |
| hCoV-NL63 | 9 (5) | 8 (5) | 1 (3) | |
| hBoV | 15 (8) | 8 (5) | 7 (19) | 0.01 |
| hEV | 2 (1) | 1 (1) | 1 (3) | 0.35 |
| PIV | 31 (16) | 26 (17) | 5 (14) | 0.80 |
| PIV1 | 6 (3) | 6 (4) | 0 (0) | |
| PIV2 | 6 (3) | 6 (4) | 0 (0) | |
| PIV3 | 18 (9) | 13 (8) | 5 (14) | |
| PIV4 | 1 (1) | 1 (1) | 0 (0) | |
| RSV | 29 (15) | 21 (13) | 8 (22) | 0.21 |
| Influenza | 17 (9) | 15 (10) | 2 (5) | 0.54 |
| Influenza A | 1 (1) | 1 (1) | 0 (0) | |
| Influenza AH1 | 0 (0) | 0 (0) | 0 (0) | |
| InfluenzaAH12009 | 3 (2) | 3 (2) | 0 (0) | |
| Influenza AH3 | 2 (1) | 2 (1) | 0 (0) | |
| Influenza B | 12 (6) | 10 (6) | 2 (5) | |
| hADV | 9 (5) | 8 (5) | 1 (3) | 1.00 |
| hMPV | 7 (4) | 4 (3) | 3 (8) | 0.13 |
Data are no. (%) of patients, unless otherwise indicated. URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; HRV, human rhinovirus; hCoV, human coronavirus; hBoV, human bocavirus; hEV, human enterovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; hADV, human adenovirus; hMPV, human metapneumovirus.
FIGURE 1.
Seasonal distribution of respiratory virus infections by months of the year. hEV indicates human enterovirus; hADV, human adenovirus.
URTIs and LRTIs
Of the 193 patients with respiratory virus detected, URTI was diagnosed in 156 (81%) and LRTI in 37 (19%) patients. The majority of patients with URTI 91 (58%) were outpatients at the time of diagnosis. The majority of patients with LRTI 23 (62%) were inpatients at the time of diagnosis. Symptoms of URTI included rhinorrhea 69 (44%), cough 45 (29%) and fever in 34 (22%) patients. Symptoms of LRTI included cough 7 (19%), fever 13 (35%) and shortness of breath in 11 (30%) patients. Abnormal signs on lung auscultation were seen in 14 (38%) patients. Unilateral pulmonary infiltrates were seen in 13 (35%) patients, and bilateral interstitial infiltrates or peribronchial thickening in the remainder, on radiography. LRTI was accompanied by URTI in 23 (62%) patients.
There were 59 patients with severe neutropenia (ANC <500 cells/μL) in the cohort, 13 (22%) developed LRTI. There were 37 patients with severe lymphopenia (ALC <100 cells/μL); 8 (22%) developed LRTI. Patients with severe neutropenia (P = 0.54) or lymphopenia (P = 0.79) were not at increased risk for developing LRTI.
Of the 193 patients where a respiratory virus was detected, 47 (24%) were febrile; 34 (72%) patients had URTI, and 13 (28%) had LRTI. Febrile patients had an underlying diagnosis of LL 16 (34%), brain tumor 11 (23%), solid tumor 15 (32%) and post-HCT 5 (11%).
Of the 37 patients with LRTI, 10 (27%) patients required oxygen therapy. Two patients were admitted to the intensive care unit for mechanical ventilation, and succumbed to LRTI. One was a patient with acute lymphoblastic leukemia, who developed PIV3 infection while undergoing reduced-intensity conditioning for an allogeneic HCT. He became tachypneic on day +5 post-HCT, requiring mechanical ventilation, and died on day +14, from respiratory failure. The other was a patient with infant acute lymphoblastic leukemia, week 22 of reinduction, who was admitted with respiratory distress, an ANC of 1400 and ALC of 0 cells/μL. After detection of RSV, therapy was instituted with ribavirin and palivizumab. She improved for several days, subsequently her respiratory distress worsened, requiring mechanical ventilation 4 weeks into hospitalization, and she died 2 weeks later from respiratory failure. This patient was coinfected with HRV.
Virus Type and Underlying Disease in Patients With LRTI
The underlying diagnoses in 17 (46%), 11 (30%) and 3 (8%), patients with LRTI were LL, solid tumor and brain tumor, respectively. Six patients (16%) underwent HCT. Patients post-HCT were not more likely to develop LRTI compared with patients with malignancies (P = 0.95).
The distribution of the underlying viruses in patients with different underlying diseases is shown in Table 3. Patients with LL and solid tumors were at increased risk for developing LRTI from hBoV (P = 0.002) and HRV (P = 0.02), respectively. Multiple logistic regression showed hBoV infection to be commonly detected in patients with LRTI (P = 0.008; Table 4).
Table 3.
Respiratory viruses detected in patients with URTI and LRTI diagnosed with leukemia/lymphoma, solid tumor, brain tumor and post-HCT
| Virus | LL | n=95 | ST | n=47 | BT | n=23 | HCT | n=28 |
|---|---|---|---|---|---|---|---|---|
| URTI | LRTI | URTI | LRTI | URTI | LRTI | URTI | LRTI | |
| n= 78 | n=17 | n=36 | n=11 | n=20 | n=3 | n=22 | n=6 | |
| HRV | 51 (65) | 10 (59) | 14 (39) | 9 (82) | 15 (75) | 2 (67) | 17 (77) | 3 (50) |
| hCoV | 10 (13) | 1 (6) | 7 (19) | 1 (9) | 2 (10) | 0 (0) | 1 (5) | 0 (0) |
| hBoV | 2 (3) | 5 (29) | 4 (11) | 1(9) | 0 (0) | 0 (0) | 2 (9) | 1 (17) |
| hEV | 1(1) | 0 (0) | 0 (0) | 1(9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PIV | 15 (19) | 1 (6) | 7 (19) | 1(9) | 1 (5) | 0 (0) | 3 (14) | 3 (50) |
| RSV | 11(14) | 6 (35) | 7 (19) | 1(9) | 3 (15) | 1 (33) | 0 (0) | 0 (0) |
| Influenza | 8 (10) | 2 (12) | 3 (8) | 0 (0) | 3 (15) | 0 (0) | 1 (5) | 0 (0) |
| hADV | 3 (4) | 1 (6) | 2 (6) | 0 (0) | 1 (5) | 0 (0) | 2 (9) | 0 (0) |
| hMPV | 4 (5) | 1 (6) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 (0) | 1 (17) |
Data are no. (%) of patients, unless otherwise indicated. URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; HRV, human rhinovirus; hCoV, human coronavirus; hBoV, human bocavirus; hEV, human enterovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; hADV, human adenovirus; hMPV, human metapneumovirus; LL, leukemia/lymphoma; ST, solid tumor; BT, brain tumor; HCT, hematopoietic cell transplant.
TABLE 4.
Logistic regression analysis of clinical variables associated with LRTI
| Characteristic | OR | 95% CI | P |
|---|---|---|---|
| Univariate logistic regression | |||
| Age | 0.95 | (0.9-1.03) | 0.23 |
| Gender | 1.09 | (0.53-2.26) | 0.80 |
| Race | 0.86 | ||
| Underlying disease | 0.71 | ||
| ANC <500 cells / μL | 1.38 | (0.63-3.02) | 0.41 |
| ALC <100 cells / μL | 1.26 | (0.51-3.08) | 0.62 |
| Steroids 2 weeks prior infection | 0.80 | (0.21-0.30) | 0.74 |
| In-patient status | 2.30 | (1.10-4.80) | 0.03 |
| hBoV | 4.32 | (1.45-12.80) | 0.008 |
| Multiple logistic regression | |||
| In-patient status | 2.37 | (1.11-5.05) | 0.025 |
| hBoV | 4.52 | (1.48-13.79) | 0.008 |
OR, odds ratio; CI, confidence interval; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; hBoV, human bocavirus.
Of the 17 patients with LL and LRTI, 8 (47%) had LRTI diagnosed during the maintenance phase of chemotherapy, 6 (35%) during induction and 3 (18%) before diagnosis of the underlying disease. Patients diagnosed with solid tumor had LRTI both during and off-therapy.
There were 4 patients who underwent a bronchoalveolar lavage. Of these 2 were post-HCT, and the others were diagnosed with a solid tumor. Respiratory viruses were only detected in post-HCT patients; 1 had HRV and hBoV, and the other had PIV.
Respiratory Virus Coinfection
Coinfection with at least 1 additional respiratory virus was seen in 47 (24%) infected patients, and was not significantly higher in patients with LRTI (P = 0.28; Table 5), or in patients with LL (P = 0.69), solid tumor (P = 0.33) and post-HCT (P = 0.58). Of the 10 patients with LRTI who required oxygen therapy, coinfection with >1 virus was seen in 4 patients. This included a patient with acute lymphoblastic leukemia, who had coinfection with HRV, hBoV, hMPV and C. pneumoniae, detected at time of initial presentation. His respiratory symptoms resolved within a week. He started induction therapy for leukemia, and tolerated it well without any respiratory complications.
TABLE 5.
Respiratory virus co-infection in patients with URTI and LRTI
| Viruses | URTI | LRTI | P |
|---|---|---|---|
| n=156 | n=37 | ||
| 1 virus | 121(78) | 25 (68) | 0.21 |
| HRV | 70 (58) | 15 (60) | |
| hCoV | 11 (9) | 0 (0) | |
| hBoV | 0 (0) | 3 (12) | |
| PIV | 13 (11) | 2 (8) | |
| RSV | 13 (11) | 3 (12) | |
| Influenza | 7 (6) | 1 (4) | |
| hADV | 4 (3) | 0 (0) | |
| hMPV | 3 (2) | 1 (4) | |
|
| |||
| Co-infection¶ 2 viruses |
27 (17) | 9 (24) | 0.35 |
| HRV+PIV | 8 (30) | 2 (22) | |
| HRV+ hBoV | 2 (7) | 2 (22) | |
| HRV+ Influenza | 3 (11) | 0 (0) | |
| HRV+ hCoV | 3 (11) | 1 (11) | |
| HRV +RSV | 3 (11) | 1 (11) | |
| Influenza + hBoV | 2 (7) | 0 (0) | |
| Co-infection ¶ >2 viruses |
8 (5) | 3 (8) | 0.44 |
| hBoV+hCoV+RSV | 1 (<1) | 1 (<1) | |
| hBov+HRV+hMPV | 0 (0) | 1 (<1) | |
Data are no. (%) of patients, unless otherwise indicated. ARI, acute respiratory illness; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; HRV, human rhinovirus; hCoV, human coronavirus; hBoV, human bocavirus; hEV, human enterovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; hADV, human adenovirus; hMPV, human metapneumovirus.
Only combinations with more than one virus in each group are shown. No bacterial or fungal pathogens were identified with the co-infections.
Bacterial Infections
There were 2 other patients where C. pneumoniae was detected, with LRTI. One patient had coinfection with HRV. LRTI resolved in both patients without oxygen therapy. M. pneumoniae was detected in 4 patients. Three had coinfection with HRV. Two patients with HRV coinfection developed LRTI, which resolved without complications. B. pertussis was not detected in any patient. No other bacterial or fungal pathogens were detected in patients with LRTI.
Duration of Viral Shedding
There were 119 patients who had repeated samples collected based on persistence of symptoms. A median of 3 (range 2–13) samples were collected on these patients. The median duration and range of viral shedding for the 17, 25, 27 and 46 patients with influenza, PIV, RSV and HRV was (21; 7–224), (21; 4–55), (17; 6–71) and (31; 6–203) days, respectively. There were 2 and 6 patients who shed influenza and HRV for >100 days. Owing to small numbers, comparison could not be made between patients with different underlying diseases, or with other viruses.
DISCUSSION
HRV was the most common virus detected in patients with URTI and LRTI in this prospective study of children with cancer and post-HCT. Recently, HRV was noted to be associated with hospitalizations for respiratory illness in young children.1 A prospective study using molecular methods by Milano et al in adult allogeneic HCT recipients detected HRV in 45 of 215 (21%) patients. There was no seasonality of detection, median duration of shedding was 3 (range 0–49) weeks and 3 (1%) patients had LRTI.16 HRV showed a seasonal pattern and was detected in 65% of children with LRTI in our series. Progression to LRTI with HRV infection has been noted in immunocompetent children <5 years of age, in association with a high viral load.17
In a retrospective hospital-based study, hCoV was detected using molecular methods, in 66 of 1043 (6.3%) children. Of 32 children infected with hCoV as the sole pathogen, 13 (41%) had LRTI. Six of the 32 patients were immunocompromised, and 2 had LRTI.2 Milano et al detected hCoV in 22 (10%) adult HCT patients. Cases were more common in winter, median duration of shedding was 3 (range 0–22) weeks and 1 (0.5%) patient had LRTI.16 hCoV showed a seasonal distribution, and was seen with LRTI in 5% of children in our series. However, the prevalence of hCoV in 1 series was not significantly higher in symptomatic immunocompetent children, compared with asymptomatic controls.18
Similarly, others have shown similar rates of detection of hBoV in immunocompetent patients with pneumonia who did not have coinfection with other viruses, compared with controls.19 Although hBoV was significantly associated with pneumonia requiring hospitalization in that study, coinfections with other viruses were common. Of the 7 patients with LRTI where hBoV was detected in our series, 4 had coinfections with other viruses, only 1 patient required oxygen therapy and all resolved without any complications. Further studies to assess the role of hBoV in asymptomatic immunocompromised controls are needed. The role of hBoV as a respiratory pathogen presently remains unclear.
Lethal hMPV infection has been primarily described in adults with hematologic malignancies, where it was detected in 16 of 126 (13%) patients. Of 9 patients with LRTI, 3 died due to hMPV infection.20 Of the 3 patients with hMPV and LRTI, in our study, all required oxygen therapy but recovered without complications.
C. pneumoniae and M. pneumoniae were detected infrequently in immunocompromised children in our series. Serological evidence of chlamydial infection has been observed in patients with malignant lymphoma.21 Both patients with C. pneumoniae coinfection in our series had acute lymphoblastic leukemia, and a mild disease course. One patient was neutropenic. Severe C. pneumoniae infection was previously documented in 3 patients with acute lymphoblastic leukemia and therapy-related neutropenia.11 M. pneumoniae as the causative agent for pneumonia in the immunocompromised host has been described in isolated case reports.12 Infrequent detection of these pathogens in this prospective study is a novel observation and may have implications for empiric therapy of patients with fever and a pulmonary infiltrate.
We have previously reported on the increased risk of LRTI from respiratory virus infection in children with severe neutropenia and lymphopenia after treatment for hematologic malignancies.5 This was not seen in the present study, perhaps because the majority of patients were not neutropenic or lymphopenic.
Studies of ARI in children with leukemia have focused on febrile patients.22 Only a quarter of patients in our study were febrile. Hence ARI may be underestimated if only febrile episodes are studied. A higher incidence of positive tests for respiratory viruses and LRTI has been seen in children with hematological malignancies compared with immunocompetent controls.23
Our study had the advantage of being prospective, using multiplexed molecular diagnostics to detect newly described respiratory viruses in a unique population. Healthy asymptomatic patients can shed HRV,1 and hCoV,18 hence inferences on causality should be made with caution in symptomatic patients. Receiver operating characteristic analysis for quantitative HRV detection have shown that cutoff values for clinical relevance in symptomatic and asymptomatic patients are feasible.24 This could have implications for infection control. Currently all patients with symptoms of ARI are placed in respiratory isolation at our institution. Furthermore, accurate diagnosis of HRV infection in these patients may have future treatment implications. The antiviral pleconaril inhibits in vitro replication of most rhinoviruses. Double-blind randomized, placebo-controlled trials have shown that early pleconaril treatment was well tolerated, and led to significant reduction in symptom severity and frequency of recovery of HRV within 1 day after initiation of therapy.25
A major limitation of our study is a lack of asymptomatic immunocompromised control patients in each disease subset, for case-control analysis. Nasopharyngeal washes although preferred for respiratory sample collection are not well tolerated by children due to the unpleasant sensation of saline in the nasopharynx. This precluded inclusion of an adequate number of control subjects for case-control comparison. Furthermore, there is absence of viral load data, and sampling was based on presence of symptoms of ARI with no follow-up in patients who later became asymptomatic. The number of repeated samples per patient was likely not often enough to capture true duration of shedding. Because bronchoalveolar lavage was not performed in the majority of patients with LRTI, copathogens may have either not been detected or overrepresented.
HRV was the most common virus detected in patients with cancer. The course of LRTI was not severe in the majority of patients. HRV infections have been shown to contribute to the development of invasive pneumococcal disease in young children.26 PCR-based assays to detect HRV and other emerging respiratory viruses have only recently become commercially available, and may be of value in immunocompromised children. Multiviral infection, also increasingly recognized with the use of newer diagnostic methods, was not shown here to be associated with LRTI. A larger, prospective study with viral load determination is needed to study the association of these emergent respiratory viruses with LRTI, in children with cancer and post-HCT.
ACKNOWLEDGMENTS
The authors thank Harry R. McKeon from the Cancer Center Administration and Susan Jones from the Clinical Research Office, Bone Marrow Transplantation and Cellular Therapy, at St. Jude Children’s Research Hospital, Memphis, TN, for assistance in data collection. Testing reagents were kindly provided by Idaho Technology (Salt Lake City, UT).
Grant funding: This work was supported by National Cancer Institute Cancer Center CORE Support Grant P30 CA 21765, American Lebanese Syrian Associated Charities, and by the Anderson Foundation.
Footnotes
Conflict of interest statement: None of the authors have a commercial or other association that might pose a conflict of interest.
REFERENCES
- 1.Iwane MK, Prill MM, Lu X, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 2.Kuypers J, Martin ET, Heugel J, et al. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119:e70–e76. doi: 10.1542/peds.2006-1406. [DOI] [PubMed] [Google Scholar]
- 3.Shah DP, El Taoum KK, Shah JN, et al. Characteristics and outcomes of pandemic 2009/H1N1 versus seasonal influenza in children with cancer. Pediatr Infect Dis J. 2012;31:373–378. doi: 10.1097/INF.0b013e3182481ef8. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan A, Wang C, Yang J, et al. Parainfluenza virus infections in children with hematologic malignancies. Pediatr Infect Dis J. 2011;30:855–859. doi: 10.1097/INF.0b013e31821d190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan A, Wang C, Yang J, et al. Symptomatic parainfluenza virus infections in children undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1520–1527. doi: 10.1016/j.bbmt.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall CB, Powell KR, MacDonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 7.Debur MC, Vidal LR, Stroparo E, et al. Human metapneumovirus infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2010;12:173–179. doi: 10.1111/j.1399-3062.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 8.Hale GA, Heslop HE, Krance RA, et al. Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplant. 1999;23:277–282. doi: 10.1038/sj.bmt.1701563. [DOI] [PubMed] [Google Scholar]
- 9.Schenk T, Maier B, Hufnagel M, et al. Persistence of human bocavirus DNA in immunocompromised children. Pediatr Infect Dis J. 2011;30:82–84. doi: 10.1097/INF.0b013e3181f12fcf. [DOI] [PubMed] [Google Scholar]
- 10.González Y, Martino R, Badell I, et al. Pulmonary enterovirus infections in stem cell transplant recipients. Bone Marrow Transplant. 1999;23:511–513. doi: 10.1038/sj.bmt.1701605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinemann M, Kern WV, Bunjes D, et al. Severe Chlamydia pneumoniae infection in patients with neutropenia: case reports and literature review. Clin Infect Dis. 2000;31:181–184. doi: 10.1086/313905. [DOI] [PubMed] [Google Scholar]
- 12.Perez CR, Leigh MW. Mycoplasma pneumoniae as the causative agent for pneumonia in the immunocompromised host. Chest. 1991;100:860–861. doi: 10.1378/chest.100.3.860. [DOI] [PubMed] [Google Scholar]
- 13.El Saleeby CM, Somes GW, DeVincenzo JP, et al. Risk factors for severe respiratory syncytial virus disease in children with cancer: the importance of lymphopenia and young age. Pediatrics. 2008;121:235–243. doi: 10.1542/peds.2007-1102. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan A, Flynn P, Gu Z, et al. Detection of respiratory viruses in asymptomatic children undergoing allogeneic hematopoietic cell transplantation. Pediatr Blood Cancer. 2013;60:149–151. doi: 10.1002/pbc.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden RT, Gu Z, Rodriguez A, et al. Comparison of two broadly multiplexed PCR systems for viral detection in clinical respiratory tract specimens from immunocompromised children. J Clin Virol. 2012;53:308–313. doi: 10.1016/j.jcv.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milano F, Campbell AP, Guthrie KA, et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115:2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piralla A, Lilleri D, Sarasini A, et al. Human rhinovirus and human respiratory enterovirus (EV68 and EV104) infections in hospitalized patients in Italy, 2008-2009. Diagn Microbiol Infect Dis. 2012;73:162–167. doi: 10.1016/j.diagmicrobio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Prill MM, Iwane MK, Edwards KM, et al. New Vaccine Surveillance Network. Human coronavirus in young children hospitalized for acute respiratory illness and asymptomatic controls. Pediatr Infect Dis J. 2012;31:235–240. doi: 10.1097/INF.0b013e31823e07fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fry AM, Lu X, Chittaganpitch M, et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JV, Martino R, Rabella N, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anttila TI, Lehtinen T, Leinonen M, et al. Serological evidence of an association between chlamydial infections and malignant lymphomas. Br J Haematol. 1998;103:150–156. doi: 10.1046/j.1365-2141.1998.00942.x. [DOI] [PubMed] [Google Scholar]
- 22.Koskenvuo M, Möttönen M, Rahiala J, et al. Respiratory viral infections in children with leukemia. Pediatr Infect Dis J. 2008;27:974–980. doi: 10.1097/INF.0b013e31817b0799. [DOI] [PubMed] [Google Scholar]
- 23.Fazekas T, Eickhoff P, Rauch M, et al. Prevalence and clinical course of viral upper respiratory tract infections in immunocompromised pediatric patients with malignancies or after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2012;34:442–449. doi: 10.1097/MPH.0b013e3182580bc8. [DOI] [PubMed] [Google Scholar]
- 24.Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayden FG, Herrington DT, Coats TL, et al. Pleconaril Respiratory Infection Study Group Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36:1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peltola V, Heikkinen T, Ruuskanen O, et al. Temporal association between rhinovirus circulation in the community and invasive pneumococcal disease in children. Pediatr Infect Dis J. 2011;30:456–461. doi: 10.1097/INF.0b013e318208ee82. [DOI] [PubMed] [Google Scholar]