Abstract
Objective
Probiotics have proven beneficial in a number of immune-mediated and allergic diseases. Several human studies have evaluated the efficacy of probiotics in allergic rhinitis, however, evidence for their use has yet to be firmly established. The current systematic review seeks to synthesize the results of available randomized trials.
Study Design
Systematic review and meta-analysis.
Methods
The Medline, EMBASE, and Cochrane Library databases were reviewed and randomized controlled trials were extracted based on defined inclusion criteria. The effect of probiotics on Rhinitis Quality of Life (RQLQ) scores, Rhinitis Total Symptom Scores (RTSS), as well as total and antigen-specific serum IgE levels were evaluated by meta-analysis.
Results
A total of 23 studies with 1919 patients were identified, including 21 double-blind randomized controlled trials and 2 randomized crossover studies. Multiple probiotic strains, study populations, and outcome measures were utilized in individual trials. Seventeen studies showed a significant clinical benefit from the use of probiotics in at least one outcome measure when compared to placebo, while 6 trials showed no benefit. Among the trials eligible for meta-analysis, the use of probiotics resulted in significant improvement in RQLQ scores compared to placebo [standard mean difference (SMD) −2.23; p = 0.02]. Probiotics had no effect on RTSS [SMD −0.36; p = 0.13] or total IgE levels [SMD 0.01; p = 0.94], while there was a trend toward a reduction in antigen-specific IgE [SMD 0.20; p = 0.06] in the placebo group compared to probiotic.
Conclusions
Probiotics may be beneficial in improving symptoms and quality of life in patients with allergic rhinitis, however, current evidence remains limited due to study heterogeneity and variable outcome measures. Additional high-quality studies are needed to establish appropriate recommendations.
Keywords: Allergic rhinitis, probiotics, allergy, atopy, lactobacillus, randomized trial, meta-analysis
INTRODUCTION
Allergic rhinitis (AR) is a common disease estimated to affect between 10% and 30% of the general population.1 The disease process itself is initiated when an individual is exposed to an allergen that stimulates IgE-mediated inflammatory responses in the nasal mucosa. This leads to allergen sensitization and the development of an atopic reaction that symptomatically manifests as rhinorrhea, pruritus, sneezing, and nasal congestion. These symptoms can have major impacts on patient quality of life and result in significant economic burdens.2,3 Although typically a self-limited disease, medical intervention is often required for symptomatic relief, with current treatment regimens including allergen avoidance, antihistamines, decongestants, and intranasal corticosteroids. Unfortunately, complete symptom resolution of AR is typically very difficult to achieve with a recent prospective international survey finding adequate symptom control in as few as 38.8% of patients on varied regimens of antihistamines and intranasal corticosteroids.4
Probiotics are novel treatment options for AR and have recently generated considerable interest in the scientific community. At the writing of this manuscript, when the term ‘probiotic’ is queried in PubMed, 13,273 results are returned with over half of publications occurring in the past 5 years. Probiotics are living microorganisms that confer a physiologic benefit following host administration5 and are naturally found in foods such as yogurt, miso soup, sauerkraut, pickles, and dark chocolate.6 Probiotics have been utilized effectively in a number of immune and allergen-mediated conditions and recent evidence suggests that they may be preventative adjuvants for conditions such as atopic dermatitis, infectious and antibiotic-associated diarrhea, and vaginal infections during pregnancy.7–10
Numerous studies have evaluated the putative efficacy of probiotics for the treatment of AR, typically with mixed conclusions. Consequently, a consensus for or against the use of probiotics in AR has yet to be reached. Recent reviews have suggested that probiotics may have significant beneficial effects on AR management, with the potential to improve patient quality of life and reduce medication use.11,12 Additional randomized controlled trials have since been performed, however differences in study parameters and individual probiotics used has made synthesis of this data very difficult. The current study seeks to systematically review the role of probiotics as an adjuvant treatment for AR.
MATERIALS AND METHODS
A comprehensive systematic literature review was performed using the Medline, EMBASE, and Cochrane Library databases. The search was limited to articles published in the English language and studies performed on humans. Only randomized controlled trials were reviewed. The search criteria included the MESH terms ‘rhinitis’ and ‘probiotic’.
Retrieved titles and abstracts were reviewed by two study authors (A.Z., J.T.). A full text review was then performed on selected articles by both authors to confirm that inclusion and exclusion criteria were met. All randomized controlled trials that examined the effects of probiotic administration on the treatment of a population with AR – both seasonal and perennial - were considered eligible for inclusion. Studies with a treatment duration longer than 4 weeks were included. Only studies between the year 2000 and 2014 that included defined and comparable outcome measures, particularly the Rhinitis Quality of Life Questionnaire, Rhinitis Total Symptom Score, total IgE, and antigen-specific IgE, were included. Studies that analyzed prenatal data or had the mother ingest probiotics to determine effects on their child were excluded. Mixed populations where individual outcomes could not be extracted were excluded. RCTs that examined mixed AR, nonallergic AR, or rhinosinusitis were also excluded. No studies were excluded on the basis of participant gender/age.
Each included study was evaluated with the 5-point Jadad scale13 to assess the quality of included manuscripts. This scale assigns points in the following manner:
Was the study described as randomized? (0 = no; 1 = yes)
Was the study described as double blind? (0 = no; 1 = yes)
Was there a description of withdrawals and dropouts? (0 = no; 1 = yes)
Was the method of randomization well described and appropriate? (0 = no; 1 = yes)
Was the method of double blinding well described and appropriate? (0 = no; 1 = yes)
Deduct 1 point if methods for randomization or blinding were inappropriate.
Out of a maximum possible 5 point score, studies with a score ≥ 3 are considered to be of well-regarded quality and were included in this review.
Data was then extracted from individual studies and assembled in a standardized database using Cochrane Review Manager 5.3 software. Mean values, standard deviations, and sample sizes were utilized for each comparable objective criterion. This data was then formatted into forest and funnel plots to illustrate the relative strength of treatment effects and assessment of publication bias, respectively. Quantitative assessment of publication bias using the Begg and Mazumdar’s Rank Correlation Test and Egger’s Regression were performed using Comprehensive Meta Analysis 2.2 software. When applicable, results are described in accordance with the PRISMA guidelines for reporting systematic reviews and meta-analyses,14 with 95% confidence intervals reported throughout. A P value of <0.05 was considered significant for all statistical tests.
RESULTS
Systematic Review
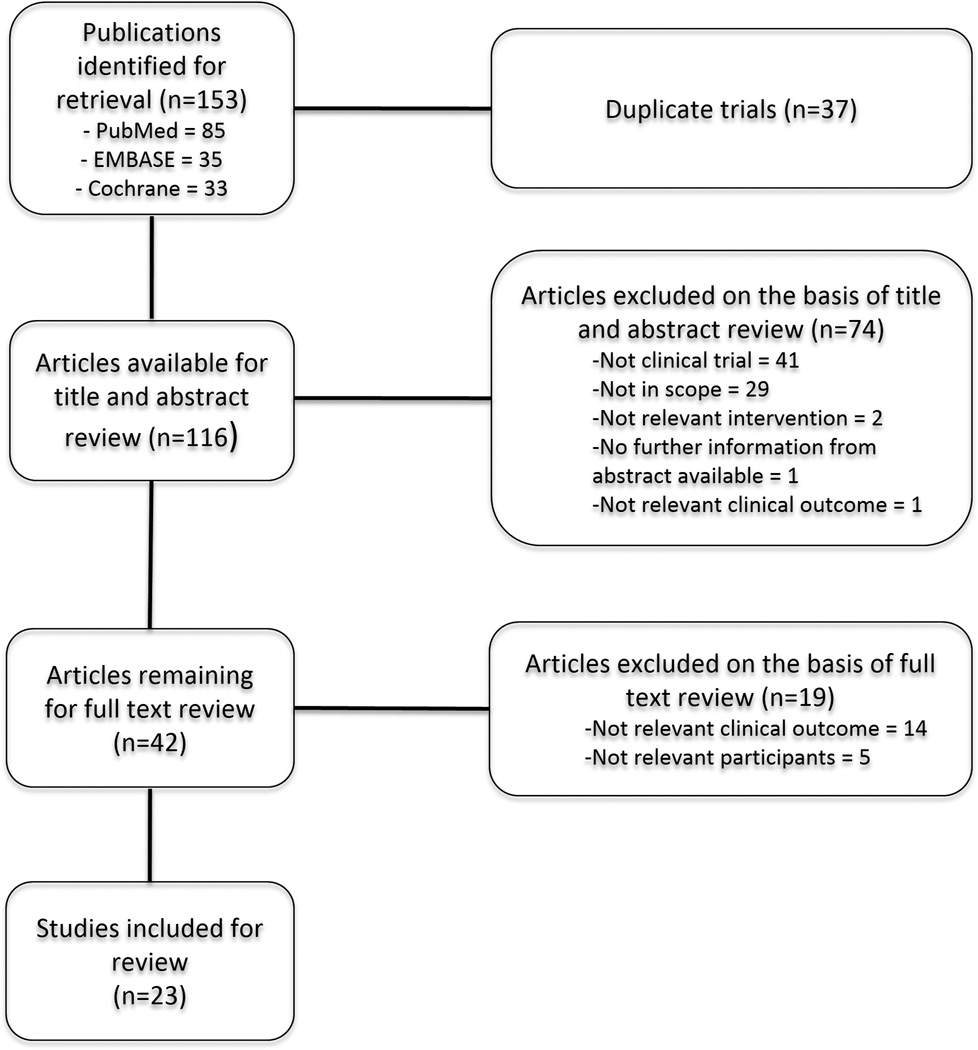
The literature search retrieved a total of 153 articles. A title and abstract review followed by exclusion of any duplicate publications resulted in 42 remaining articles for full text review. Twenty-three articles were ultimately included in the study, with the majority of studies being excluded due to a lack of quantifiable data or insufficient study description. The selection process is detailed in Figure 1. Studies identified during the systematic review included 21 double-blind randomized controlled trials and 2 randomized crossover studies.
Figure 1.
Article Selection Process for Systematic Literature Review
Study Details
Details regarding the individual studies identified during the systematic review can be found in Table 1. Sixteen studies used Lactobacillus strains while six studies used Bifidobacterium. Escherichia coli (Nissle 1917), Tetragenococcus halophilus (Th221), and Bacillus clausii were used in single studies. The duration of probiotic administration varied between studies and ranged from 4 weeks to 12 months. The most commonly used outcome measures were the Rhinitis Quality of Life Questionnaire (6 studies), Symptom Medication Score (5 studies) and Rhinitis Total Symptom Score (5 studies). Seventeen of 23 studies showed significant improvement in at least one measured outcome with the use of probiotics, while 6 studies showed no benefit. Measurement of total or antigen specific IgE was included in 8 and 7 studies, respectively. The quality of included studies was assessed using the 5-point Jadad scoring system. 9 (39.13%) trials had a total score of 3, while 13 (56.52%) trials had a total score of 4, and 1 (4.35%) trial had a total score of 5.
Table 1.
Characteristics of Included Studies
| Study | Type | Patients | Ages | Intervention (Probiotic Strain) |
Outcomes | Results | Jadad Score |
|---|---|---|---|---|---|---|---|
| Lue 201230 | RC | 63 | 7–12 yrs | Lactobacillus johnsonii EM1 | Changes in RTSS and PRQLQ | Decreased RTSS; no change in PRQLQ | 3 |
| Wang 200431 | RCT-DB | 80 | 15.87±1.53 yrs (probiotic) and 14.00±1.90 yrs (placebo) | Lactobacillus paracasei-33 × 30 days | Change in modified PRQLQ | Decreased PRQLQ (decreased frequency, level of bother) | 4 |
| Peng 200532 | RCT-DB | 90 | 16.07±2.11 yrs (live-probiotic), 14.50±1.78 yrs (heat-killed probiotic), 16.60±2.02 yrs (placebo) | Lactobacillus paracasei × 30 days | Change in modified PRQLQ | Decreased PRQLQ (decreased frequency, level of bother) | 3 |
| Costa 201427 | RCT-DB | 425 | 18–60 yrs | Lactobacillus paracasei LP-33 × 5 weeks | Change in RQLQ, RTSS | Decreased RQLQ; no change in RTSS | 4 |
| Lin 201333 | RCT-DB | 199 | 6–12 yrs | Lactobacillus salivarius × 12 weeks | Change in SSS, SMS, total IgE | Reduction in nasal, eye, medication scores; no change in total IgE | 4 |
| Singh 201334 | RCT-DB | 20 | 20–65 yrs | Bifidobacterium lactis NCC2818 × 8 weeks | Change in TNSS | Reduction in total nasal symptom scores | 4 |
| Nishimura 200935 | RCT-DB | 45 | 33.8±2.0 yrs (high dose probiotic), 36.7±1.2 yrs (low dose probiotic), 36.5±2.8 yrs (placebo) | Tetragenococcus halophilus Th221 × 8 weeks | Change in disease severities, TNSS, total IgE, antigen-specific IgE | Decreased total nasal symptom scores at high dose only; no change in sneezing rhinorrhea, or antigen-specific IgE | 4 |
| Kawase 200936 | RCT-DB | 40 | 20–57 yrs | Lactobacillus GG and L. gasseri TMC0356 × 10 weeks | Change in mean symptom score, mean symptom-medication score, total IgE, antigen-specific IgE | Decreased nasal blockage and medication score for nasal blockage; no change in total or antigen-specific IgE | 4 |
| Giovannini 200737 | RCT-DB | 187 | 2–5 yrs | Lactobacillus casei × 12 months | Change in time free from and number of episodes of asthma/rhinitis, total IgE | Decrease in annual rhinitis episodes; no change in total IgE | 5 |
| Tamura 200738 | RCT-DB | 120 | 39.3±8.0 yrs (probiotic) and 39.5±10.9 yrs (placebo) | Lactobacillus casei Shirota × 8 weeks | Change in SMS | No benefit | 4 |
| Xiao 2006a39 | RCT-DB | 40 | 23–61 yrs (probiotic) and 24–55 yrs (placebo) | Bifidobacterium longum BB536 × 14 weeks | Change in subjective symptoms | Decreased eye symptoms; no benefit in other symptoms; | 4 |
| Xiao 2006b40 | RCT-DB | 44 | 26–57 yrs (probiotic) and 22–48 yrs (placebo) | Bifidobacterium longum BB536 × 13 weeks | Change in subjective symptoms | Decreased symptom scores for rhinorrhea, congestion, and composite scores | 4 |
| Helin 200241 | RCT-DB | 36 | 14–36 yrs | Lactobacillus rhamnosus × 5.5 months | Change in allergic nose, eye, lung, and RTSS | No benefit | 3 |
| Xiao 200742 | RC | 24 | 41.0±8.0 yrs (placebo 1st) and 37.6±7.5 yrs (probiotic 1st) | Bifidobacterium longum BB536 × 4 weeks | Change in subjective symptoms | No change in nasal symptom score; reduced throat and ocular symptoms | 3 |
| Dölle 201326 | RCT-DB | 34 | 19–54 yrs | Escherichia coli strain Nissle 1917 × 6 months | Change in SMS | No benefit | 4 |
| Nagata 201043 | RCT-DB | 55 | 18–27 yrs | Lactobacillus plantarum No. 14 × 6 weeks | Change in SMS, total IgE, antigen-specific IgE | Decreased SMS and itchy eyes; no effect on medication intake | 3 |
| Chen 201044 | RCT-DB | 105 | 6–12 yrs | Lactobacillus gasseri A5 × 8 weeks | Change in subjective symptoms, total IgE | Decreased nasal allergic symptoms | 4 |
| Ouwehand 200945 | RCT-DB | 47 | 4–13 yrs | Lactobacillus acidophilus NCFM and Bifidobacterium lactis BI-04 × 4 months | Change in subjective symptoms | No benefit | 4 |
| Lin 201446 | RCT-DB | 60 | 6–13 yrs | Lactobacillus paracasei HF.A00232 × 8 weeks | Change in RTSS and PRQLQ | No benefit thru 8 wks; lower PRQLQ scores and individual symptom scores (sneezing, itchy eyes, swollen eyes) at 12 wks | 3 |
| Yonekura 200947 | RCT-DB | 116 | 20–50 yrs | Lactobacillus paracasei KW3110 × 3 months | Change in RQLQ, antigen-specific IgE | Improved quality of life when pollen scattering low. | 4 |
| Ishida 200548 | RCT-DB | 49 | 34.0±3.4 yrs (probiotic) and 36.9±3.0 yrs (placebo) | Lactobacillus acidophilus L-92 × 8 weeks | Change in SMS, total IgE, antigen-specific IgE | Improvement in nasal symptom-medication scores, no change in total IgE or antigen-specific IgE | 3 |
| Ciprandi 200549 | RCT-DB | 20 | 12–15 yrs | Bacillus clausii × 3 weeks | Change in RTSS and medication use | No significant difference in RTSS; reduced medication use | 3 |
| Aldinucci 200250 | RCT-DB | 20 | 19–44 yrs | Lactobacillus acidophilus and Bifidobacterium × 4 months | Change in subjective symptoms | Decreased nasal symptoms | 3 |
| Total | 1919 |
PRQLQ = Pediatric Rhinoconjunctivitis Quality of Life Questionnaire
RQLQ = Rhinitis Quality of Life Questionnaire
RTSS = Rhinitis Total Symptom Score
SSS = Specific Symptoms Score
SMS = Symptom Medication Score
TNSS = Total Nasal Symptom Score
Rhinitis Quality of Life
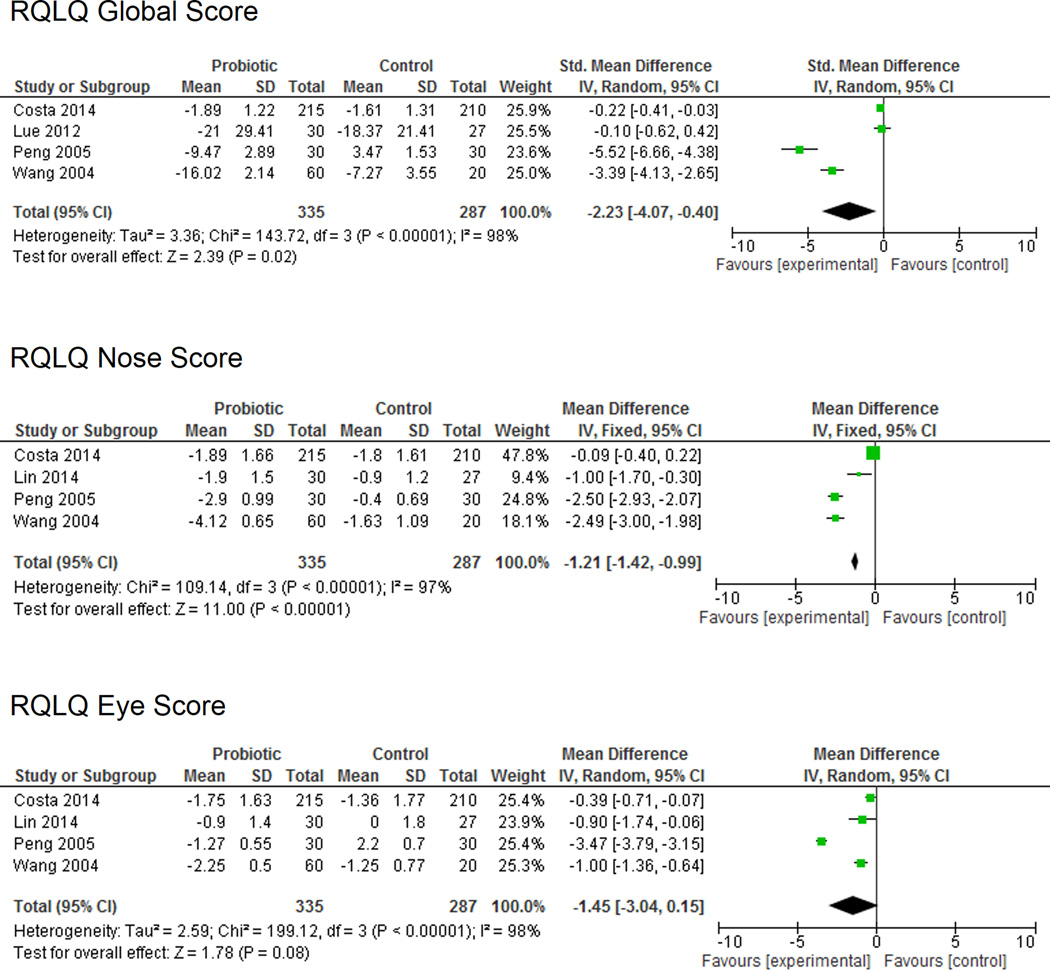
Of the 6 studies that utilized the RQLQ, four included descriptive data that allowed for direct comparison and meta-analysis. This particular metric was created to assess functional problems (physical, emotional, social, and occupational) associated with AR. Data from the four studies included a total of 335 patients treated with probiotics and 287 patients treated with placebo (Figure 2). The meta-analysis demonstrated a significant improvement in RQLQ global scores in the probiotic group compared to placebo (SMD −2.23 (95% CI −4.07, −0.40); P = 0.02) as well as in RQLQ nasal symptoms (SMD −1.21 (95% CI −1.42, −0.99); P <0.00001). There was a trend toward improvement in RQLQ eye symptoms (SMD –1.45 (95% CI –3.04, 0.15); P = 0.08), though this did not reach statistical significance. As a frame of reference, Juniper et al.15 showed that mean changes in RQLQ greater than 0.5 can generally be considered clinically significant. For example, Demoly et al.16 examined the effect of desloratadine on RQLQ in patients with AR and showed a change of −1.4. Of note, significant heterogeneity was observed with an I2 statistic of 97% or above for RQLQ global and symptom-specific scores. Risk of bias was quantitatively assessed using the Begg and Egger tests. Both tests were nonsignificant (p = 0.09 and p =0.16, respectively). However, the fairly low p-values and significant heterogeneity suggests that the effect identified in this meta-analysis may be at least partially due to confounding factors and differences between studies. This is highlighted by the fact that the two older, small studies showed a fairly significant difference between placebo and probiotic, while the two larger, more recent studies identified either a small difference or no difference between groups.
Figure 2.
Rhinitis Quality of Life Questionnaire
Rhinitis Total Symptom Score
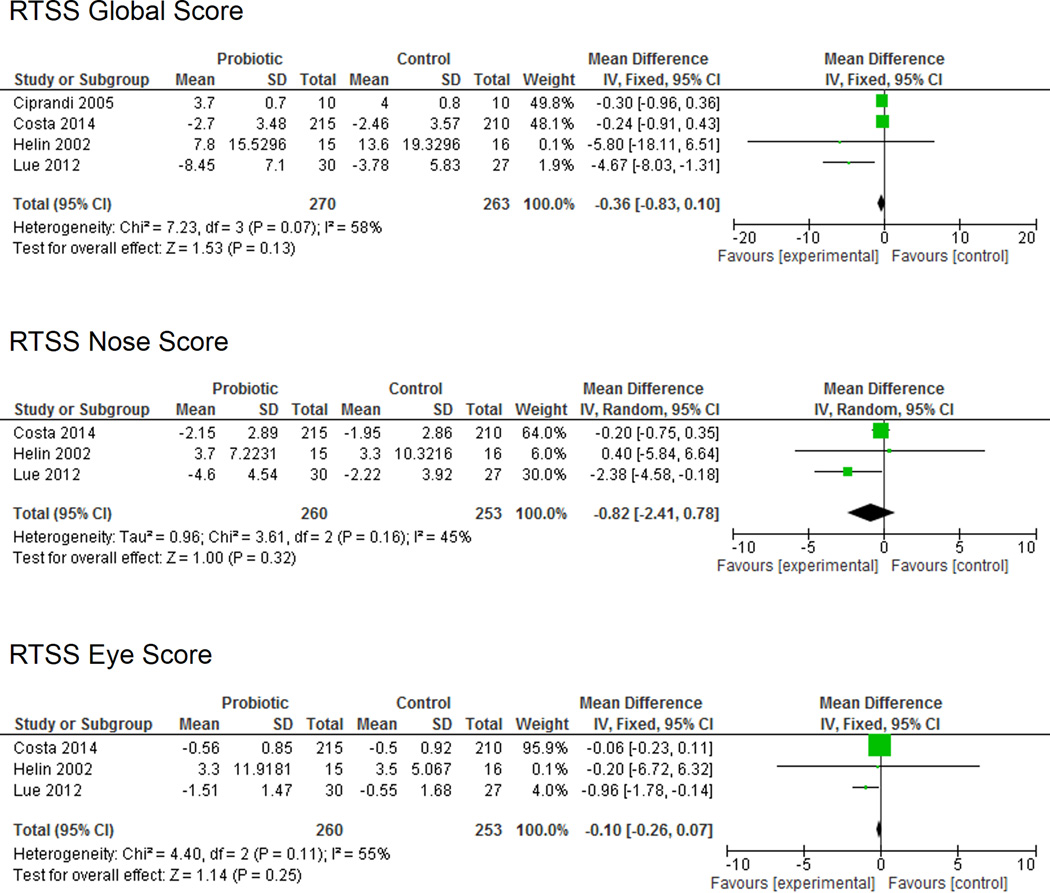
Of the six studies that assessed RTSS, four reported quantitative data that was sufficient for meta-analysis. The RTSS measures both nasal and non-nasal symptoms associated with AR. The eligible studies included 270 patients in the probiotic group and 263 patients in the placebo group (Figure 3). No significant differences in RTSS global scores (SMD −0.36 (95% CI −0.83, 0.10); P = 0.13) were identified between the probiotic and placebo groups. Likewise, RTSS eye symptoms (SMD −0.10 (95% CI −0.26, 0.07); P = 0.25) and RTSS nose symptoms (SMD −0.82 (95% CI −2.41, 0.78; P = 0.32) were not significantly different between groups. Moderate heterogeneity was noted among the study population (I2 = 45–58%) and no significant bias was identified using the Begg and Egger tests (p = 0.73 and p = 0.23, respectively).
Figure 3.
Rhinitis Total Symptom Score
Total and Antigen-Specific IgE
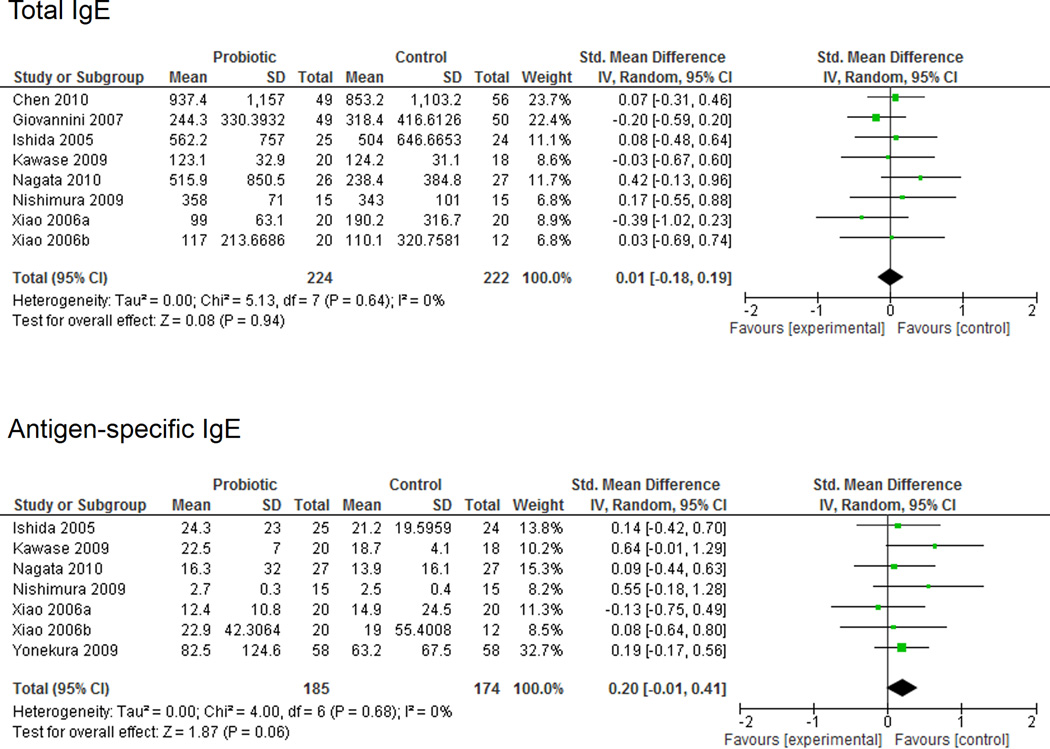
The effect of probiotics on total and antigen-specific IgE was assessed in 8 and 7 studies, respectively (Figure 4). A meta-analysis of included studies did not demonstrate any significant differences between the probiotic and placebo groups for total IgE (SMD 0.01 (95% CI −0.18, 0.19); P = 0.94). A trend toward a reduction in antigen-specific IgE levels was observed in the placebo group compared to the probiotic group (SMD 0.20 (95% CI −0.01, 0.41); P = 0.06). Minimal heterogeneity was identified (I2 = 0%) and no significant study bias was detected with the Begg and Egger tests (total IgE, p = 1.0 and p = 0.78; antigen-specific IgE, p = 0.76 and p = 0.63, respectively).
Figure 4.
Total and Antigen-Specific IgE
Adverse Events
Few adverse events were reported among the included studies. Complaints including diarrhea, abdominal pain, and flatulence were reported in select studies, but at rates that typically mirrored the placebo group. There were no serious/life-threatening adverse events and no patients required additional treatment or intervention. Among the 23 included studies, only one patient did not compete the study due primarily to an adverse event (flatulence).
DISCUSSION
The current systematic review and meta-analysis represents the most comprehensive analysis to date of the use of probiotics for the treatment of AR. A majority of studies resulted in at least some clinical benefit with the use of probiotics compared to placebo. A meta-analysis resulted in contrasting findings, with the probiotic group showing a statistically significant improvement in global and symptom-specific RQLQ scores, but no improvement in RTSS scores. Probiotics did not have any effect on either total or antigen-specific IgE levels.
Probiotic supplementation has been shown to improve clinical outcomes in a variety of inflammatory disorders. For example, a review article examining the therapeutic potential of probiotics in irritable bowel syndrome found that in roughly two-thirds of controlled clinical trials, probiotic supplementation lead to an improvement in symptoms.17 Delivery of oral probiotics has also shown benefit for the treatment of food allergy,18,19 and atopic dermatitis.20 Probiotics have even been proven to reduce the development of hepatic encephalopathy in patients with liver cirrhosis.21 As summarized in this review, multiple randomized controlled trials have now also demonstrated potential benefits of probiotics for the treatment of AR.
The mechanism by which probiotics may modulate atopic diseases has yet to be completely defined. In mouse models, probiotics have the potential to promote T helper type 1 (Th1) immunity while suppressing Th2 responses.22 Other evidence suggests that probiotics may increase the predominance of regulatory T cells (Tregs) by altering the composition of the gut microflora.23 Multiple animal studies have found that probiotics can modify levels of antigen-specific serum IgE levels.24,25 However, our meta-analysis showed no significant change in total or antigen-specific IgE levels between study participants receiving probiotics versus placebo. Collectively, these data suggest that probiotics may serve as immunomodulators that alter systemic innate and adaptive immune responses. Much about the role of probiotics in the human immune response remains poorly understood and additional translational studies will likely be needed to clarify this in the future.
The current study suggests that probiotics have the potential to alter disease severity, symptoms, and quality of life in patients with AR. Positive outcomes were reported in a majority of studies with no significant adverse events. However, several limitations prevent us from making generalized recommendations based on this data. Despite including 23 studies with almost 2000 patients, the overall cohort remained fairly heterogeneous. Furthermore, a lack of quantifiable data prevented inclusion of most studies in the meta-analysis, a fact that restricted the power of these analyses. The term ‘probiotic’ is an all encompassing term, but the efficacy of particular formulations is largely dependent on geography, dietary practices, and prevailing gut microflora. This is echoed in the current study, with certain strains (Lactobacillus paracasei 33) proving effective for treatment of grass pollen allergies, while others (Escherichia coli strain Nissle 1917) proved ineffective.26,27 Similar differences in efficacy have been noted in other atopic diseases, with one probiotic strain proving effective for the treatment of atopic dermatitis in a comparative randomized controlled trial, while another was completely ineffective.28
Despite these self-evident limitations, this study was able to synthesize current literature and report several important findings. First, the majority of randomized-controlled studies reported improvement in patient symptoms or quality of life in at least one measured outcome. This was despite variability in study design, probiotic formulation, and outcome measures. A meta-analysis demonstrated improvement in patient quality of life as assessed by the RQLQ. This is perhaps the most commonly used and accepted quality of life metric for assessing the symptomatic impact of AR, and has been validated in multiple studies.15,29 While a similar improvement was not noted for the RTSS, there was a trend toward improvement with probiotic compared to placebo. These particular meta-analyses were likely limited by study heterogeneity and the small number of incorporated patients in most of the included studies. In particular, significant heterogeneity and possible bias were identified in the meta-analysis of RQLQ scores, issues which limit any conclusions that can be reported based on these results. Finally, a meta-analysis assessing the impact of probiotics on total and allergen-specific IgE levels did not result in any significant differences between the probiotic and placebo groups. Interestingly, there was a trend toward a reduction in antigen-specific serum IgE in the placebo group, an unexpected finding in light of prior animal studies.24,25 This would suggest that the physiologic effects of probiotics in humans may be unrelated to their putative modulatory effect on IgE levels.
Probiotics appear to have beneficial effects in a number of inflammatory and immunologic diseases. The current systematic review suggests that they may be similarly effective in AR, though the mechanism and duration of this effect remains unclear. Future studies will need to address the limitations of randomized trials to date, specifically the variability in study design and probiotic formulations, both of which make comparison between individual studies difficult. While the use of probiotics as a stand-alone therapy cannot be advised at this point, they may ultimately prove to be an effective adjuvant therapy for the treatment of recalcitrant AR in select populations.
CONCLUSIONS
Currently available trials evaluating the efficacy of probiotics for the treatment of AR suffer from variability in probiotic formulations, study designs, and outcome measures. Despite these shortcomings, current evidence suggests that probiotics may have some beneficial effects in this patient population. Additional randomized controlled trials using specific probiotic strains and consistent outcome measures are needed to confirm this putative efficacy and allow for evidence-based recommendations.
Footnotes
Financial disclosures: None
Conflicts of interest: None
REFERENCES
- 1.Pawankar R, Canonica GW, Holgate ST, Lockey RF. White Book on Allergy 2011–2012 Executive Summary [Google Scholar]
- 2.Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J. Allergy Clin. Immunol. 2001;107:3–8. doi: 10.1067/mai.2001.112262. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, et al. Sleep, quality of life, and productivity impact of nasal symptoms in the United States: findings from the Burden of Rhinitis in America survey. Allergy Asthma Proc. 2009;30:244–254. doi: 10.2500/aap.2009.30.3230. [DOI] [PubMed] [Google Scholar]
- 4.Schatz M. A survey of the burden of allergic rhinitis in the USA. Allergy. 2007;62(Suppl 85):9–16. doi: 10.1111/j.1398-9995.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 5.Reid G, et al. New scientific paradigms for probiotics and prebiotics. J. Clin. Gastroenterol. 2003;37:105–118. doi: 10.1097/00004836-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Piedmont Healthcare | Piedmont Living Better | Should you take a probiotics supplement? at< http://piedmont.org/patient-tools/living-better1/should-you-take-a-probiotics-supplement-588.aspx>. [Google Scholar]
- 7.Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J. Allergy Clin. Immunol. 2008;121:116.e11–121.e11. doi: 10.1016/j.jaci.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003048.pub2. CD003048. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324:1361. doi: 10.1136/bmj.324.7350.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Othman M, Neilson JP, Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005941.pub2. CD005941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vliagoftis H, Kouranos VD, Betsi GI, Falagas ME. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann. Allergy Asthma Immunol. 2008;101:570–579. doi: 10.1016/S1081-1206(10)60219-0. [DOI] [PubMed] [Google Scholar]
- 12.Das RR, Singh M, Shafiq N. Probiotics in treatment of allergic rhinitis. World Allergy Organ J. 2010;3:239–244. doi: 10.1097/WOX.0b013e3181f234d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, Guyatt GH, Griffith LE, Ferrie PJ. Interpretation of rhinoconjunctivitis quality of life questionnaire data. J. Allergy Clin. Immunol. 1996;98:843–845. doi: 10.1016/s0091-6749(96)70135-5. [DOI] [PubMed] [Google Scholar]
- 16.Demoly P, Dreyfus I, Dhivert-Donnadieu H, Mesbah K. Desloratadine for the treatment of cypress pollen-induced allergic rhinitis. Ann. Allergy Asthma Immunol. 2009;103:260–266. doi: 10.1016/S1081-1206(10)60191-3. [DOI] [PubMed] [Google Scholar]
- 17.Santos AR, Whorwell PJ. Irritable bowel syndrome: the problem and the problem of treating it - is there a role for probiotics? Proc Nutr Soc. 2014:1–7. doi: 10.1017/S0029665114000706. [DOI] [PubMed] [Google Scholar]
- 18.Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J. Allergy Clin. Immunol. 1997;99:179–185. doi: 10.1016/s0091-6749(97)70093-9. [DOI] [PubMed] [Google Scholar]
- 19.Kirjavainen PV, Gibson GR. Healthy gut microflora and allergy: factors influencing development of the microbiota. Ann. Med. 1999;31:288–292. doi: 10.3109/07853899908995892. [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeldt V, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J. Allergy Clin. Immunol. 2003;111:389–395. doi: 10.1067/mai.2003.389. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, et al. Effects of probiotic therapy on hepatic encephalopathy in patients with liver cirrhosis: an updated meta-analysis of six randomized controlled trials. HBPD INT. 2014;13:354–360. doi: 10.1016/s1499-3872(14)60280-0. [DOI] [PubMed] [Google Scholar]
- 22.Masuda S, et al. Immunomodulatory effect of halophilic lactic acid bacterium Tetragenococcus halophilus Th221 from soy sauce moromi grown in high-salt medium. Int. J. Food Microbiol. 2008;121:245–252. doi: 10.1016/j.ijfoodmicro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Torii A, et al. Lactobacillus Acidophilus strain L-92 regulates the production of Th1 cytokine as well as Th2 cytokines. Allergol Int. 2007;56:293–301. doi: 10.2332/allergolint.O-06-459. [DOI] [PubMed] [Google Scholar]
- 24.Shida K, et al. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin. Exp. Allergy. 2002;32:563–570. doi: 10.1046/j.0954-7894.2002.01354.x. [DOI] [PubMed] [Google Scholar]
- 25.Murosaki S, et al. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J. Allergy Clin. Immunol. 1998;102:57–64. doi: 10.1016/s0091-6749(98)70055-7. [DOI] [PubMed] [Google Scholar]
- 26.Dölle S, Berg J, Rasche C, Worm M. Tolerability and clinical outcome of coseasonal treatment with Escherichia coli strain Nissle 1917 in grass pollen-allergic subjects. Int. Arch. Allergy Immunol. 2014;163:29–35. doi: 10.1159/000356328. [DOI] [PubMed] [Google Scholar]
- 27.Costa DJ, et al. Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: a double-blind, randomized, placebo-controlled trial (GA2LEN Study) Eur J Clin Nutr. 2014;68:602–607. doi: 10.1038/ejcn.2014.13. [DOI] [PubMed] [Google Scholar]
- 28.Wickens K, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2008;122:788–794. doi: 10.1016/j.jaci.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J. Allergy Clin. Immunol. 1999;104:364–369. doi: 10.1016/s0091-6749(99)70380-5. [DOI] [PubMed] [Google Scholar]
- 30.Lue K-H, et al. A trial of adding Lactobacillus johnsonii EM1 to levocetirizine for treatment of perennial allergic rhinitis in children aged 7–12 years. Int. J. Pediatr. Otorhinolaryngol. 2012;76:994–1001. doi: 10.1016/j.ijporl.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Wang MF, Lin HC, Wang YY, Hsu CH. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr Allergy Immunol. 2004;15:152–158. doi: 10.1111/j.1399-3038.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 32.Peng G-C, Hsu C-H. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr Allergy Immunol. 2005;16:433–438. doi: 10.1111/j.1399-3038.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 33.Lin T-Y, Chen C-J, Chen L-K, Wen S-H, Jan R-H. Effect of probiotics on allergic rhinitis in Df, Dp or dust-sensitive children: a randomized double blind controlled trial. Indian Pediatr. 2013;50:209–213. doi: 10.1007/s13312-013-0068-2. [DOI] [PubMed] [Google Scholar]
- 34.Singh A, et al. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: an exploratory, randomized, placebo-controlled clinical trial. Eur J Clin Nutr. 2013;67:161–167. doi: 10.1038/ejcn.2012.197. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura I, et al. Clinical efficacy of halophilic lactic acid bacterium Tetragenococcus halophilus Th221 from soy sauce moromi for perennial allergic rhinitis. Allergol Int. 2009;58:179–185. doi: 10.2332/allergolint.O-08-548. [DOI] [PubMed] [Google Scholar]
- 36.Kawase M, et al. Effect of fermented milk prepared with two probiotic strains on Japanese cedar pollinosis in a double-blind placebo-controlled clinical study. Int. J. Food Microbiol. 2009;128:429–434. doi: 10.1016/j.ijfoodmicro.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Giovannini M, et al. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr. Res. 2007;62:215–220. doi: 10.1203/PDR.0b013e3180a76d94. [DOI] [PubMed] [Google Scholar]
- 38.Tamura M, et al. Effects of probiotics on allergic rhinitis induced by Japanese cedar pollen: randomized double-blind, placebo-controlled clinical trial. Int. Arch. Allergy Immunol. 2007;143:75–82. doi: 10.1159/000098318. [DOI] [PubMed] [Google Scholar]
- 39.Xiao JZ, et al. Effect of probiotic Bifidobacterium longum BB536 [corrected] in relieving clinical symptoms and modulating plasma cytokine levels of Japanese cedar pollinosis during the pollen season. A randomized double-blind, placebo-controlled trial. J Investig Allergol Clin Immunol. 2006;16:86–93. [PubMed] [Google Scholar]
- 40.Xiao J-Z, et al. Probiotics in the treatment of Japanese cedar pollinosis: a double-blind placebo-controlled trial. Clin. Exp. Allergy. 2006;36:1425–1435. doi: 10.1111/j.1365-2222.2006.02575.x. [DOI] [PubMed] [Google Scholar]
- 41.Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy. 2002;57:243–246. doi: 10.1034/j.1398-9995.2002.1s3299.x. [DOI] [PubMed] [Google Scholar]
- 42.Xiao J, et al. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergol Int. 2007;56:67–75. doi: 10.2332/allergolint.O-06-455. [DOI] [PubMed] [Google Scholar]
- 43.Nagata Y, Yoshida M, Kitazawa H, Araki E, Gomyo T. Improvements in seasonal allergic disease with Lactobacillus plantarum No. 14. Biosci. Biotechnol. Biochem. 2010;74:1869–1877. doi: 10.1271/bbb.100270. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y-S, Jan R-L, Lin Y-L, Chen H-H, Wang J-Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010;45:1111–1120. doi: 10.1002/ppul.21296. [DOI] [PubMed] [Google Scholar]
- 45.Ouwehand AC, et al. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J. Gastroenterol. 2009;15:3261–3268. doi: 10.3748/wjg.15.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin W-Y, Fu L-S, Lin H-K, Shen C-Y, Chen Y-J. Evaluation of the effect of Lactobacillus paracasei (HF.A00232) in children (6–13 years old) with perennial allergic rhinitis: a 12-week, double-blind, randomized, placebo-controlled study. Pediatr Neonatol. 2014;55:181–188. doi: 10.1016/j.pedneo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Yonekura S, et al. Effects of daily intake of Lactobacillus paracasei strain KW3110 on Japanese cedar pollinosis. Allergy Asthma Proc. 2009;30:397–405. doi: 10.2500/aap.2009.30.3256. [DOI] [PubMed] [Google Scholar]
- 48.Ishida Y, et al. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: a double-blind, placebo-controlled study. J. Dairy Sci. 2005;88:527–533. doi: 10.3168/jds.S0022-0302(05)72714-4. [DOI] [PubMed] [Google Scholar]
- 49.Ciprandi G, Vizzaccaro A, Cirillo I, Tosca MA. Bacillus clausii effects in children with allergic rhinitis. Allergy. 2005;60:702–703. doi: 10.1111/j.1398-9995.2005.00722.x. [DOI] [PubMed] [Google Scholar]
- 50.Aldinucci C, et al. Effects of dietary yoghurt on immunological and clinical parameters of rhinopathic patients. Eur J Clin Nutr. 2002;56:1155–1161. doi: 10.1038/sj.ejcn.1601465. [DOI] [PubMed] [Google Scholar]