Abstract
Introduction
NF-Kappa B (NF-κB) is a vital transcription factor that is activated by numerous inflammatory stimuli. Its activity is tightly regulated by a family of deubiquitinating enzymes (A20, Cezanne, CYLD) that function in a negative-feedback loop, a process that prevents chronic and systemic inflammation. This study seeks to characterize the expression and functional role of NF-κB-regulating deubiquitinases in the sinonasal epithelium.
Methods
Expression of A20, Cezanne, and CYLD was assessed in normal sinonasal tissue using immunohistochemistry. Cultured sinonasal epithelial cells were stimulated with pro-inflammatory cytokines (TNF-α, IL-4, IL-13) or LPS and changes in NF-κB activation and deubiquitinase expression were assessed using western blots and quantitative real-time PCR, respectively.
Results
NF-kB was activated in response to LPS and TNF-α, but not IL-4 or IL-13. A20, Cezanne, and CYLD were all expressed in sinonasal tissue, primarily along the apical surface of the epithelium. Pro-inflammatory mediators primarily affected expression of A20, with upregulation by LPS and TNF-α and downregulation by IL-4 and IL-13.
Conclusions
The NF-κB-regulating deubiquitinases A20, Cezanne, and CYLD are expressed in sinonasal tissue and are differentially induced by pro-inflammatory cytokines and microbial antigens. These results suggest an important role for NF-κB-regulating deubiquitinases in mucosal immunity and homeostasis.
Keywords: rhinosinusitis, A20, TNFAIP3, Cezanne, CYLD, deubiquitinase, epithelial cell, sinonasal, NF-κB, inflammation
INTRODUCTION
Chronic rhinosinusitis (CRS) is a persistent inflammatory condition associated with dysregulation of the innate and adaptive immune systems and bacterial colonization 1. The mechanism by which CRS persists remains unclear but likely involves alterations in immune system tolerance to microbial pathogens and other extracellular stimuli 2. Both cytokines and microbial antigens initiate inflammatory responses through cell-surface receptors, including the Toll-like receptors (TLRs) and interleukin receptors, respectively. Though individual pathway components vary substantially among different agonists and receptors, all such stimuli ultimately regulate inflammatory cascades by altering gene transcription. Nuclear factor-kappa B (NF-κB) is perhaps the most well characterized transcription factor in immune signaling, with potent activators including tumor necrosis factor-α (TNF-α) 3, bacterial lipopolysaccharide (LPS) 3,4, and interleukin-1β3,5. Ultimately, NF-κB alters the transcription of numerous stress-response and pro-inflammatory genes.
NF-κB is ubiquitously expressed and is actually a heterodimeric protein that consists of different combinations of subunits. Under resting conditions, NF-κB is sequestered in the cell cytoplasm by IκBα and other members of the family of IκB inhibitors. Inflammatory stimuli result in the phosphorylation and degradation of IκBα and the subsequent release and phosphorylation of NF-κB subunits. These transcriptionally active subunits, including p65/RelA and p50, are then translocated into the nucleus where they alter the expression of numerous pro-and anti-inflammatory mediators. This is a tightly regulated process, as unrestricted NF-κB activation and downstream gene transcription could otherwise result in persistent local or systemic inflammation. Consequently, several negative feedback loops serve to precisely control NF-κB activity and terminate its activation. Chief among these are a family of enzymes with deubiquitinating activity. Ubiquitin is a small molecule that covalently attaches to lysine residues of various protein substrates, and in doing so regulates cellular processes such as proteasomal degradation and protein trafficking 6,7. Signaling downstream of TLRs, as well as receptors for cytokines such as TNF-α and IL-1 requires the polyubiquitination of several signaling molecules at the cell surface. A20, Cezanne, and CYLD are deubiquitinases that play a central role in NF-κB signaling pathways by modulating the ubiquitination-dependent activity of these signaling molecules 8. As such, these proteins function as ‘molecular brakes’ on NF-κB signaling and help to maintain immune homeostasis.
A20 is perhaps the most well-studied NF-κB regulating deubiquitinase and serves as a prototypical member of this group. Also known as TNF-α inducible protein 3 (TNFAIP3), A20 was discovered more than 20 years ago as an NF-κB early response gene that can be activated by numerous cytokines 9. It is now recognized as a potent inhibitor of NF-κB activity and an important regulator of inflammation. A20 knockout mice die prematurely due to systemic inflammation, multi-organ failure, and sepsis 10. Polymorphisms at the A20 locus are now associated with multiple autoimmune and inflammatory diseases including rheumatoid arthritis 11,12, type 1 diabetes 13, and psoriasis 14. Polymorphisms within the A20 gene were also recently associated with CRS 15. Cezanne belongs to the A20 family of deubiquitinases and likewise can attenuate NF-κB activation and the transcription of pro-inflammatory cytokines downstream of the TNF-α receptor 16. CYLD was initially identified as a gene that confers susceptibility to familial cylindromatosis. In addition to its well-characterized regulatory function in NF-κB signaling, CYLD also has tumor suppressor properties 17.
The functional role of A20 and other deubiquitinases has not been previously characterized in sinonasal tissue. The purpose of the current study is to determine which deubiquitinating enzymes are expressed in sinonasal tissue and to evaluate the regulation of these factors by different inflammatory stimuli.
METHODS
Patients and Tissue specimens
Patients without a clinical history of chronic rhinosinusitis or allergic rhinitis were recruited from the Vanderbilt Asthma, Sinus and Allergy Program (ASAP) and Otolaryngology clinics. Most patients were undergoing either endoscopic endonasal pituitary surgery, endoscopic skull base tumor resection, endoscopic cerebrospinal fluid leak repair, or nasal septoplasty. Patients who had received oral corticosteroids within 4 weeks of surgery were excluded from the study. Patients with a diagnosis of cystic fibrosis, Churg-Strauss syndrome, immunodeficiency, or autoimmune disease were also excluded. Voluntary informed consent was obtained for all patients and the study protocol was approved by the Vanderbilt University Institutional Review Board. Sinonasal tissue was harvested from either the ethmoid sinus or sphenoid rostrum.
Cell Culture
SNECs were harvested from the middle meatus using a cytology brush (Andwin Scientific; Schaumburg, IL) and placed in RPMI medium supplemented with 100 units/mL penicillin/streptomycin, 50 μg/mL gentamicin, 0.25 mg/mL amphotericin B, and 100 units/mL nystatin. Cells were then centrifuged at 500X g for 5 min, washed with Hank’s balanced salt solution, and resuspended in Bronchial Epithelial Growth Media (BEGM) supplemented with bovine pituitary extract, hydrocortisone, EGF, epinephrine, transferrin, insulin, retinoic acid, triiodothyronine, gentamicin/amphotericin, and penicillin/streptomycin per the manufacturer’s instructions (Lonza; Basel, Switzerland). Cells were subsequently added to 6 well culture plates coated with Collagen I (Advanced Biomatrix; San Diego, CA) and cultured at 37°C in a humidified incubator (5% CO2). Media was changed every 48–72 hours until cells reached 90–95% confluence. Cells were then stimulated with either LPS (100 ng/mL), TNF-α (20 ng/mL, IL-4 (10 ng/mL), or IL-13 (10 ng/mL) for between 30 minutes and 4 hours.
Immunoblotting
Cultured SNECs were stimulated with pro-inflammatory mediators as described above, and then harvested by scraping directly into SDS loading buffer. Each sample was sonicated for 15 seconds and then centrifuged for 10 min at 14,000 x g. Clarified extracts were separated by SDS-PAGE. Separated proteins were transferred to PVDF and incubated with primary rabbit anti-human antibody to either phospho-p65/RelA or total p65/RelA (Cell Signaling Technology; Beverly, MA), followed by incubation with an alkaline-phosphatase-conjugated secondary goat anti-rabbit antibody (Santa Cruz Biotechnology, Dallas, TX). Proteins were detected using chemiluminescence by exposure to Kodak X-AR film.
Immunohistochemistry
Freshly harvested sinonasal tissue from a minimum of three different subjects was fixed in formalin, embedded in paraffin and routinely processed. Sections were then deparafinnized in graded ethanol baths followed by antigen retrieval with boiling citrate buffer (pH 6.6) for 10 minutes. Tissue endogenous peroxidases were quenched with 0.3% hydrogen peroxide and slides were then incubated with 3% BSA in PBS to prevent nonspecific binding. Slides were then incubated with primary rabbit anti-human A20/TNFAIP3, mouse anti-human Cezanne, or rabbit anti-human CYLD antibody (Abcam; Cambridge, UK) for 1h at room temperature, and then a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) for 1 h. Signal detection was performed using an avidin-peroxidase detection agent (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate (Thermo Scientific, Waltham, MA). The sections were then counterstained with hematoxylin for 10 min, mounted, and subsequently analyzed with a Nikon Eclipse Ti inverted microscope and a Nikon Digital Sight DS-Fi1 color camera.
RNA Isolation and Quantitative Real-time PCR (qRT-PCR)
RNA from cultured SNECS was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions and then purified using an RNeasy (QIAGEN, Valencia, CA) spin column. The quality and concentration of RNA was measured on a Nanodrop ND-1000 spectrophotometer and samples with an A260/A280 ratio > 1.8 were used for subsequent steps. 2 micrograms of purified RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Semi-quantitative real-time RT-PCR was performed on a MyIQ real-time PCR detection system (Bio-Rad) in a 96-well format. Primer sets for A20 (sense, 5′-GTGTGCTTTGTGGTTGCTGT-3′; antisense, 5′-GGGAAAAACTTAGGGGGCTCT-3′), Cezanne (sense, 5′-ACAATGTCCGATTGGCCAGT-3′; antisense, 5′-ACAGTGGGATCCACTTCACATTC-3′), CYLD (sense, 5′-GCGTTCCCACAATTCAGCAGT-3′; antisense, 5′-TCCGGATCGTCGTAGCATTCTC-3′), and glyceraldehyde 3-phosphate dehydrogenase (sense, 5′-AGAAGGCTGGGGCTCATTTG-3′; antisense, 5′-AGGGGCCATCCACAGTCTTC-3′) were designed using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Relative expression levels were determined using the ΔCT method with GAPDH as the reference gene.
Statistics
Data are reported as means ± SEM. Differences between experimental and control groups were analyzed using a student’s T-test and statistical significance was defined as a p value of less than 0.05.
RESULTS
NF-κB Activation in SNECs
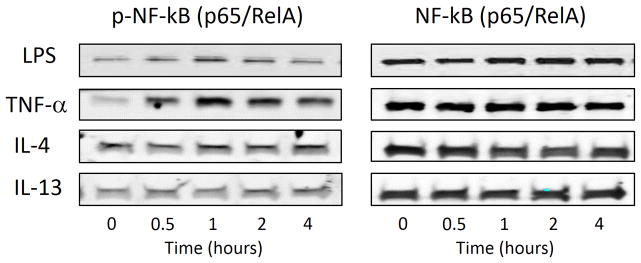
NF-κB is strongly activated by Th1-associated cytokines (TNF-α, IL-1β) and LPS in most tissues. Conversely, Th2 cytokines (IL-4, IL-13) are typically weak activators of NF-κB, and instead modulate inflammatory cascades through activation of STAT-mediated gene transcription 18,19. Activation of the NF-κB pathway in SNECs was assessed using phospho-specific antibodies. NF-κB activation requires phosphorylation of the inhibitory factor Iκκ, which typically sequesters NF-κB subunits in the cytoplasm. Phosphorylation of Iκκ releases NF-κB dimers (p65/RelA and p50) which are subsequently phosphorylated and translocated into the nucleus. We stimulated SNECs with LPS (100 ng/mL), TNF-α (20 ng/mL), IL-4 (10 ng/mL), or IL-13 (10 ng/mL) for between 0 and 4 hours and assessed the phosphorylation of p65/RelA via western blot. As shown in Figure 1, LPS and TNF-α result in the phosphorylation of p65/RelA with a maximum activation at 1 hour. No change in p65/RelA phosphorylation was noted for IL-4 or IL-13. These results confirm that NF-κB is activated in SNECs by LPS and TNF-α, but not by IL-4 or IL-13.
Figure 1. NF-κB activation in SNECs.
The NF-κB pathway is differentially regulated by pro-inflammatory mediators in SNECs. Cells were simulated with LPS (100ng/mL), TNF-α (20 ng/mL), IL-4 (10 ng/mL), or IL-13 (10 ng/mL) for between 0 and 4 hours and levels of phosphorylated and total p65/RelA were assessed via western blot. n≥3.
Expression of Deubiquitinases in Sinonasal Tissue
The expression of deubiquitinases such as A20, Cezanne, and CYLD have not been previously evaluated in sinonasal tissue. We assessed the expression of these factors using immunohistochemistry. A20 was expressed primarily in the cytoplasm of the apical epithelium but was also observed in submucosal glands and inflammatory cells (Figure 2). Cezanne was also expressed in the sinonasal epithelium in both a cytoplasmic and nuclear pattern (Figure 3). Very limited expression of CYLD was observed in epithelial cells.
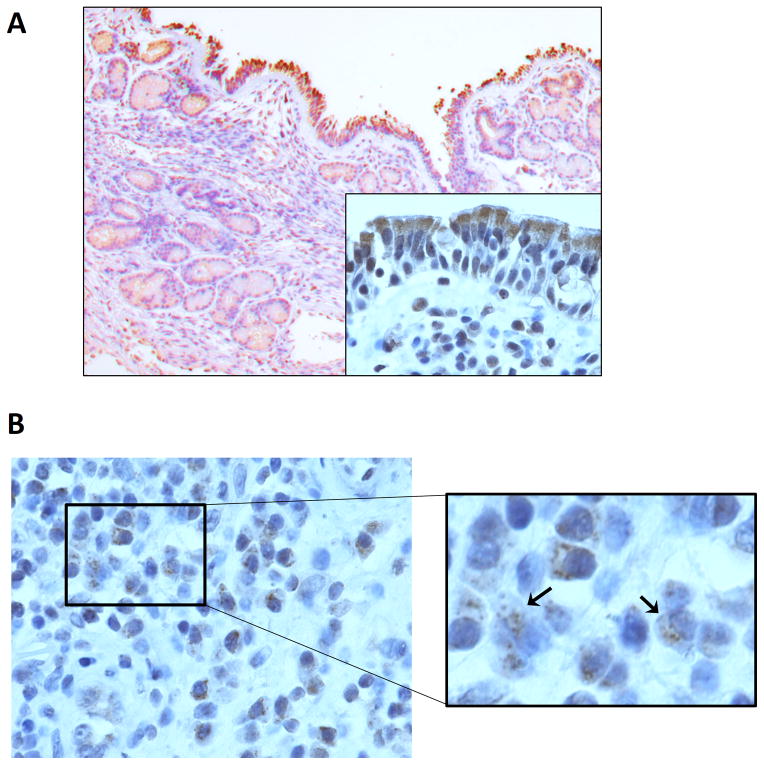
Figure 2. Expression of A20 in sinonasal tissue.
(A) Representative image of A20 expression in sinonasal tissue (100X magnification; inset image, 600X magnification). A20 staining is observed primarily in the apical epithelium and to a lesser extent in submucosal glandular tissue. (B) A20 is also expressed in submucosal inflammatory cells (600X magnification). n≥3.
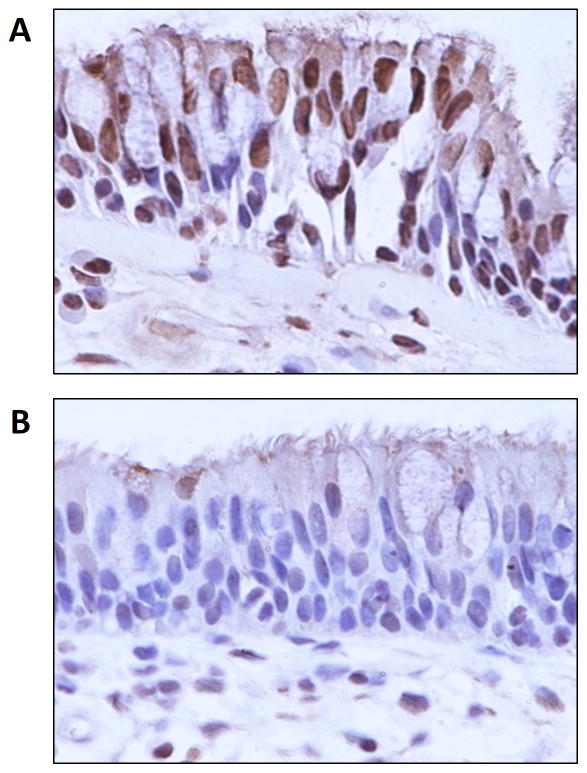
Figure 3. Expression of Cezanne and CYLD in sinonasal tissue.
(A) Cezanne is expressed in the sinonasal epithelium in both a cytoplasmic and nuclear pattern. (B) CYLD is expressed at low levels in the sinonasal epithelium. n≥3.
Regulation of Deubiquitinases by Inflammatory Cytokines
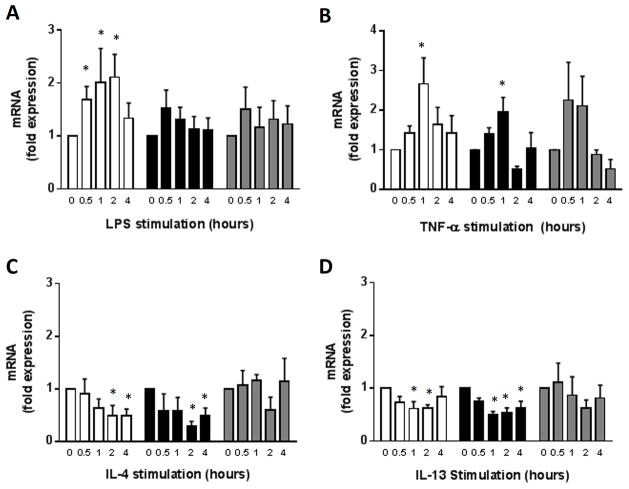
A20 and other deubiquitinases are NF-κB-dependent negative feedback proteins that are expressed downstream of several cell surface receptors. SNECs were stimulated with LPS for between 0 and 4 hours and deubiquitinase expression was assessed via quantitative real-time PCR (Figure 4A). LPS resulted in a rapid and significant increase in A20 expression that returned close to baseline levels after 4 hours. Only minimal increases in the expression of either Cezanne or CYLD was observed after LPS stimulation.
Figure 4. Effect of Inflammatory Stimuli on Expression of Deubiquitinases.
SNECs were stimulated with LPS (A), TNF-α (B), IL-4 (C), or IL-13 (D) for between 0 and 4 hours and expression of A20 (□), Cezanne (■), and CYLD (
 ) were assessed via qRT-PCR. Data shown is the mean ± SEM. n≥3. *, p < 0.05.
) were assessed via qRT-PCR. Data shown is the mean ± SEM. n≥3. *, p < 0.05.
The effect of pro-inflammatory cytokines on deubiquitinase expression were then assessed. SNECS were stimulated with either TNF-α (20 ng/mL), IL-4 (10 ng/mL), or IL-13 (10 ng/mL) for between 0 and 4 hours (Figure 4B–C). A20, Cezanne, and CYLD were all activated by TNF-α. In contrast, stimulation with the Th2 cytokines, IL-4 and IL-13, did not result in significant upregulation, and actually resulted in a decrease in deubiquitinase expression at most timepoints.
DISCUSSION
In the current study, we have characterized the sinonasal expression of different deubiquitinating proteins that negatively regulate activation of the NF-κB transcription factor. To our knowledge this is the first study to assess the expression and function of these proteins in sinus tissue and sinonasal epithelial cells. We found that A20, Cezanne, and CYLD are all expressed in the sinonasal epithelium, chiefly in the epithelial cells themselves. Expression of each deubiquitinase was stimulated by TNF-α and/or LPS, but not by the Th2 cytokines IL-4 and IL-13. These findings suggest that A20 and other deubiquitinases may play an essential role in chronic sinonasal inflammation and the response of sinonasal epithelial cells to microbial antigens and certain pro-inflammatory cytokines.
The role of A20 and other deubiquitinases in CRS is unclear but their essential role as modulators of immune system homeostasis lead to many interesting possibilities. Previous studies in the gastrointestinal tract have shown that A20 is essential for the maintenance of epithelial barrier integrity and homeostasis 20, and deletion of A20 in intestinal epithelial cells has been found to increase the susceptibility of mice to experimentally-induced colitis 21. In humans, several genome-wide association studies have likewise correlated single-nucleotide polymorphisms in the A20 locus to the development of inflammatory bowel disease 22.
The role of deubiquitinases in the sinonasal tract has not been previously evaluated, however, several studies have confirmed the importance of these factors in the lower airways. For example, A20 is required for the termination of TLR2- and TLR4-mediated immune signaling pathways in primary airway epithelial cells 23. Likewise, intratracheal delivery of A20 cDNA was able to inhibit airway inflammation in an allergic mouse model 24. Interestingly, studies of airway epithelial cells from patients with asthma or cystic fibrosis have noted drastic alterations in both the expression and function of A20, and lung function in CF patients has been found to closely correlate with A20 expression 25,26. These findings suggest that alterations in the expression or activity of specific deubiquitinases may also play important roles in sinonasal inflammation and CRS.
The results of the current study suggest that A20 and other deubiquitinases may be important regulators of inflammation in CRS. In the current study, deubiquitinase expression was preferentially stimulated by TNF-α, a key driver of sinonasal inflammation. This particular cytokine is primarily associated with Th1-mediated sinonasal inflammation in both mice and humans 27–30. Future studies will focus on comparing the expression of individual deubiquitinases in sinonasal tissue from CRS and healthy control subjects. We anticipate that the chronic inflammatory state in CRS may result in alterations in deubiquitinase expression. Likewise, we hypothesize that the chronic elevation of pro-inflammatory cytokines in CRS may result in a desensitization of this important negative feedback pathway, a consequence that could impair the sinonasal innate immune response to extracellular pathogens.
Very few studies have examined the role of NF-κB in sinonasal inflammation and immune signaling. Xu et al. found that both total and nuclear expression of the NF-κB monomers, p50 and p65, were elevated in CRS and allergic rhinitis 31. This elevated expression correlated with increases in levels of IL-6 and IL-8. However, NF-κB activity is largely independent of protein expression levels and no direct measures of NF-κB activity were included. A subsequent study by Lee et al. measured NF-κB activity in human nasal epithelial cells using phospho-specific antibodies and assessment of nuclear translocation. TNF-α rapidly stimulated NF-κB activation, a necessary step for the subsequent expression of secreted mucins 32. Finally, changes in NF-κB nuclear translocation have been associated with elevated levels of IL-8, IL-16, and eotaxin, as well as an increase in the recruitment of eosinophils within nasal polyp tissue. These authors hypothesized that elevated NF-κB activity in nasal polyp tissue may reflect hypersensitivity to microbial and inflammatory stimuli 33. In future studies, we plan to compare the expression and activity of NF-κB in control and CRS patients, and compare NF-κB activity with the expression of the deubiquitinases evaluated in this study.
The current study confirms that the deubiquitinases A20, Cezanne, and CYLD are expressed in sinonasal tissue and are differentially regulated by various cytokines and microbial antigens. The vital importance of these factors in immune signaling suggests that they may also contribute to the regulation of inflammation in CRS. Additional studies that further characterize the function of these regulatory factors and that assess differences in expression between CRS phenotypes and healthy controls are necessary to confirm their importance in the sinonasal tract.
CONCLUSIONS
The deubiquitinating enzymes A20, Cezanne, and CYLD are expressed in normal sinonasal tissue and are differentially regulated by cytokines and microbial antigens. These proteins are preferentially expressed in sinonasal epithelial cells and likely serve to regulate inflammation due to extracellular pathogens and other inflammatory stimuli at the epithelial surface.
Acknowledgments
This project was supported by a New Investigator Award from the American Rhinologic Society (J.H.T.) and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Presented at the 60th Annual Meeting of the American Rhinologic Society; September 20, 2014
Financial disclosures: No relevant disclosures
Conflicts of interest: None
References
- 1.Lane AP, Turner JH. Etiologic Factors in Chronic Rhinosinusitis. In: Kennedy PHHaDW., editor. Diseases of the Nose, Sinuses, and Skull Base. New York, NY: Thieme Medical Publishers, Inc; 2012. pp. 171–181. [Google Scholar]
- 2.van Drunen CM, Mjosberg JM, Segboer CL, Cornet ME, Fokkens WJ. Role of innate immunity in the pathogenesis of chronic rhinosinusitis: progress and new avenues. Current allergy and asthma reports. 2012;12:120–126. doi: 10.1007/s11882-012-0249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shembade N, Harhaj NS, Liebl DJ, Harhaj EW. Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. The EMBO journal. 2007;26:3910–3922. doi: 10.1038/sj.emboj.7601823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mabilleau G, Chappard D, Sabokbar A. Role of the A20-TRAF6 axis in lipopolysaccharide-mediated osteoclastogenesis. The Journal of biological chemistry. 2011;286:3242–3249. doi: 10.1074/jbc.M110.150300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croston GE, Cao Z, Goeddel DV. NF-kappa B activation by interleukin-1 (IL-1) requires an IL-1 receptor-associated protein kinase activity. The Journal of biological chemistry. 1995;270:16514–16517. doi: 10.1074/jbc.270.28.16514. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Kinch LN, Brautigam CA, et al. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malynn BA, Ma A. Ubiquitin makes its mark on immune regulation. Immunity. 2010;33:843–852. doi: 10.1016/j.immuni.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renner F, Schmitz ML. Autoregulatory feedback loops terminating the NF-kappaB response. Trends in biochemical sciences. 2009;34:128–135. doi: 10.1016/j.tibs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends in immunology. 2014;35:22–31. doi: 10.1016/j.it.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Lee EG, Boone DL, Chai S, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson W, Barton A, Ke X, et al. Rheumatoid arthritis association at 6q23. Nature genetics. 2007;39:1431–1433. doi: 10.1038/ng.2007.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plenge RM, Cotsapas C, Davies L, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nature genetics. 2007;39:1477–1482. doi: 10.1038/ng.2007.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung EY, Smyth DJ, Howson JM, et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes and immunity. 2009;10:188–191. doi: 10.1038/gene.2008.99. [DOI] [PubMed] [Google Scholar]
- 14.Musone SL, Taylor KE, Nititham J, et al. Sequencing of TNFAIP3 and association of variants with multiple autoimmune diseases. Genes and immunity. 2011;12:176–182. doi: 10.1038/gene.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cormier C, Bosse Y, Mfuna L, Hudson TJ, Desrosiers M. Polymorphisms in the tumour necrosis factor alpha-induced protein 3 (TNFAIP3) gene are associated with chronic rhinosinusitis. Journal of otolaryngology - head & neck surgery = Le Journal d’oto-rhino-laryngologie et de chirurgie cervico-faciale. 2009;38:133–141. [PubMed] [Google Scholar]
- 16.Enesa K, Zakkar M, Chaudhury H, et al. NF-kappaB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. The Journal of biological chemistry. 2008;283:7036–7045. doi: 10.1074/jbc.M708690200. [DOI] [PubMed] [Google Scholar]
- 17.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell death and differentiation. 2010;17:25–34. doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada A, Sheikh F, Niimi T, et al. Induction of uteroglobin-related protein 2 (Ugrp2) gene expression by the Th2 cytokines IL-4 and IL-13. J Immunol. 2005;175:5708–5715. doi: 10.4049/jimmunol.175.9.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi T, Shively JD, Foley JS, Drazen JM, Tschumperlin DJ. Differentiation-dependent responsiveness of bronchial epithelial cells to IL-4/13 stimulation. American journal of physiology Lung cellular and molecular physiology. 2004;287:L119–126. doi: 10.1152/ajplung.00365.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kolodziej LE, Lodolce JP, Chang JE, et al. TNFAIP3 maintains intestinal barrier function and supports epithelial cell tight junctions. PloS one. 2011;6:e26352. doi: 10.1371/journal.pone.0026352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vereecke L, Sze M, Mc Guire C, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. The Journal of experimental medicine. 2010;207:1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nature reviews Immunology. 2012;12:774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gon Y, Asai Y, Hashimoto S, et al. A20 inhibits toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. American journal of respiratory cell and molecular biology. 2004;31:330–336. doi: 10.1165/rcmb.2003-0438OC. [DOI] [PubMed] [Google Scholar]
- 24.Kang NI, Yoon HY, Lee YR, et al. A20 attenuates allergic airway inflammation in mice. J Immunol. 2009;183:1488–1495. doi: 10.4049/jimmunol.0900163. [DOI] [PubMed] [Google Scholar]
- 25.Kelly C, Williams MT, Elborn JS, Ennis M, Schock BC. Expression of the inflammatory regulator A20 correlates with lung function in patients with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2013;12:411–415. doi: 10.1016/j.jcf.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Kelly C, Williams MT, Mitchell K, Elborn JS, Ennis M, Schock BC. Expression of the nuclear factor-kappaB inhibitor A20 is altered in the cystic fibrosis epithelium. The European respiratory journal. 2013;41:1315–1323. doi: 10.1183/09031936.00032412. [DOI] [PubMed] [Google Scholar]
- 27.Turner JH, May L, Reed RR, Lane AP. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. American journal of rhinology & allergy. 2010;24:192–196. doi: 10.2500/ajra.2010.24.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karosi T, Csomor P, Sziklai I. Tumor necrosis factor-alpha receptor expression correlates with mucosal changes and biofilm presence in chronic rhinosinusitis with nasal polyposis. The Laryngoscope. 2012;122:504–510. doi: 10.1002/lary.23190. [DOI] [PubMed] [Google Scholar]
- 30.Anand VK, Kacker A, Orjuela AF, Huang C, Manarey C, Xiang J. Inflammatory pathway gene expression in chronic rhinosinusitis. American journal of rhinology. 2006;20:471–476. doi: 10.2500/ajr.2006.20.2891. [DOI] [PubMed] [Google Scholar]
- 31.Xu R, Xu G, Shi J, Wen W. A correlative study of NF-kappaB activity and cytokines expression in human chronic nasal sinusitis. The Journal of laryngology and otology. 2007;121:644–649. doi: 10.1017/S0022215106001824. [DOI] [PubMed] [Google Scholar]
- 32.Lee SN, Ryu JH, Joo JH, et al. alpha-Melanocyte-stimulating hormone inhibits tumor necrosis factor alpha-stimulated MUC5AC expression in human nasal epithelial cells. American journal of respiratory cell and molecular biology. 2011;44:716–724. doi: 10.1165/rcmb.2009-0420OC. [DOI] [PubMed] [Google Scholar]
- 33.Takeno S, Hirakawa K, Ueda T, Furukido K, Osada R, Yajin K. Nuclear factor-kappa B activation in the nasal polyp epithelium: relationship to local cytokine gene expression. The Laryngoscope. 2002;112:53–58. doi: 10.1097/00005537-200201000-00010. [DOI] [PubMed] [Google Scholar]