Abstract
Objective
This was a secondary data analysis of a cluster-randomized clinical trial that tested the efficacy of a 20-week Sun-style Tai Chi (TC) program in reducing pain in community-dwelling elders with cognitive impairment and knee osteoarthritis (OA). The study also examined whether elders’ level of cognitive function was related to the outcomes of the TC program.
Method
Elders (N=55) were recruited from 8 study sites. Each site was randomly assigned to participate in either a 20-week TC or an education program. Verbal report of pain was measured by a Verbal Descriptor Scale (VDS) at Weeks 1, 5, 9, 13, 17 and 21 (designated as Times 1-6). Pain behaviors and analgesic intake were also recorded at Times 1-6.
Results
At post-test, scores on the VDS and observed pain behaviors were significantly better in the TC group than in the control group (p=.008-.048). The beneficial effects of TC were not associated with cognitive ability.
Conclusion
These results suggest that TC can be used as an adjunct to pharmacological intervention to relieve OA pain in elders with cognitive impairment.
Keywords: Tai Chi, knee osteoarthritis, pain report, pain behavior, cognitive impairment
1. Introduction
Osteoarthritis (OA) is a painful musculoskeletal disorder. The prevalence of OA in elders with cognitive impairment is comparable to that in elders without cognitive impairment. Among people with cognitive impairment, 38.2% to 52% are reported to have OA, compared with 31.8% to 60% of people without cognitive impairment (1, 2). The knee is particularly affected because it is a major weight-bearing joint and is ranked 2nd in years lost to disability among all diseases and injuries (3). Pharmacological interventions for OA knee pain have shown limited efficacy (4), and in elders they can produce side effects such as impaired concentration, agitation, increased risk of hypertension and hip fracture, and decreased renal function (5-9). Alternative non-pharmacological interventions should therefore be considered to treat knee OA pain in this frail population.
Non-pharmacological interventions for elders with knee OA pain include land-based exercise, water-based exercise, strength training, self-management and education (10). Among these, land-based exercise and strength training have the largest effect sizes in treating pain associated with knee OA (land-based exercise: 0.34-0.63 vs. strength training: 0.38) and improving function (land-based exercise: 0.25 vs. strength training: 0.41) (10-12). Because of the pain, elders with knee OA tend to avoid activity, including land-based exercise such as walking and running (13). However, they may be willing to participate in mild exercise that does not worsen pain. Tai Chi (TC), a low-impact aerobic exercise, has shown promise in reducing OA knee pain in elders with an effect size of 0.72 (95% CI: 0.97, 0.47) (14-19). It is also recommended by the United States Arthritis Foundation for treating OA (20). However, studies examining the efficacy of TC have largely excluded elders with clinical cognitive impairment (15-19), even though cognitive impairment is common among elders.
If TC can reduce OA knee pain in elders with cognitive impairment, perhaps these elders can perform activities of daily living longer, thus delaying their institutionalization. In addition to reducing OA knee pain, benefits of TC have been shown to improve or maintain cognition in elders with very mild to moderate CI (21-23). However, without directly testing the efficacy of TC in the cognitively impaired, we cannot prescribe the right dose or appropriate strategies for teaching TC to this vulnerable population. Therefore, a randomized controlled trial was designed to test the efficacy of a TC program in reducing OA knee pain among elders with subtle-to-moderate cognitive impairment.
The trial investigated TC's effects on pain (primary outcome) and other secondary health outcomes (discussed elsewhere) (24). The analysis found that cognitively impaired elders with knee OA who attended a 20-week TC program reported less pain than an education attention control group (24). The elders verbally reported answers to the Western Ontario and MacMaster (WOMAC) pain scale, a 5-item OA-specific pain measurement, to a research assistant. However, it is not entirely clear whether the WOMAC pain scale is reliable with the cognitively impaired, because only one study has examined its reliability with this population (25).
Therefore, to substantiate our findings, this secondary analysis used additional results obtained with the Verbal Descriptor Scale (VDS) for pain. This tool has been recommended as a way to evaluate verbal self-report of pain intensity in elders with dementia (26, 27). It is a 1-item verbal report tool with a list of words from “no pain” to “the most intense pain imaginable,” to indicate the intensity of the pain experienced (28). Information about the psychometric properties of this tool is detailed in the methods section below.
Elders’ pain can also be manifested by both observable pain behaviors and analgesic intake (29). Without examining changes in these pain manifestations, we cannot confidently claim that TC reduces pain in elders. Furthermore, TC forms consist of a series of upper- and lower-extremity movements performed in a particular choreographic manner. If those with impaired cognition have less learning capability, then TC may be less useful in reducing OA pain among these elders. Therefore, Aim 1 of this secondary data analysis examined the effects of TC on VDS pain reports, observed pain behaviors and analgesic intake. Aim 2 explored the relationship of cognitive level to the observed effects.
2. MATERIALS AND METHODS
2.1. Design
The study was a secondary analysis of data from a previous cluster-randomized clinical trial. In that study, we tested the efficacy of TC in reducing OA knee pain and improving other health outcomes in community-dwelling elders with varying levels of cognitive impairment. The methods of that clinical trial have been reported elsewhere (24).
[The CONSORT flow diagram was published online at http://www.sciencedirect.com/ as supplementary data.]
The current study used the complete sample from the previous clinical trial and focused on multiple pain outcomes, including the VDS, pain behaviors and analgesic intake. Additionally, the relationships between these outcomes and cognition were analyzed.
2.2. Participants
Recruitment was conducted in 8 study sites (6 retirement apartments and 2 senior centers) between January 2008 and February 2010. A total of 123 elders in the 8 study sites were recruited and screened for eligibility; 55 elders in the 8 study sites were eligible and each site was randomly assigned to a TC group (N=28) or an education control group (N = 27). Participants were aged ≥ 60 years; had moderate, mild or subtle cognitive impairment, defined as a Mini Mental State Exam (MMSE) score of 18-28; had a diagnosis of knee OA based on medical history reviewed with elders or family members/staff and confirmed by a health care provider; had self-report of knee OA pain ≥ 2 on the VDS or a pain score ≥ 3 on the WOMAC pain subscale; were able to speak English; had physician's/nurse practitioner's permission to participate; had not participated in a regular exercise program in the past month; could walk without assistance from staff or a walking device for 50 meters; and could stand and maintain balance for one minute without support.
We included only elders with MMSE scores of 18-28 to focus on those with less than optimum cognitive function. The MMSE score range used is consistent with several recent studies which categorized elders with MMSE scores equal to or less than 28 as having low cognitive function or symptomatic cognitive impairment (30, 31).
Elders were excluded if they had uncorrectable moderate or severe hearing or vision deficits; Parkinson's disease; cancer pain; chronic pain conditions, such as rheumatoid arthritis, fibromyalgia, or severe low back pain; diabetic neuropathy; arthroscopic surgery or total knee or hip replacement surgery in the past 6 months; fractures in the past 6 months; major psychiatric disorder or a positive screen for depressive symptoms (Geriatric Depression Scale-15 score ≥ 5) without taking medications; history of falls in the past 3 months; or vertigo in the past month. Approval by the University's Institutional Review Board was granted, and informed consent was obtained from all participants.
2.3. Randomization and Blinding
Research assistants (RAs) recruited participants at each site and screened potential participants for eligibility. Assessor 1 conducted a pre-test for the outcome measures. The statistician, who was blinded to the characteristics of the sites and the elders, then randomly allocated each site to either the TC or the control arm.
The two lead investigators on the study (Tsai and Chang) were involved closely in the fieldwork and thus were not blinded to participants’ group assignments. The RA who screened elders for eligibility, enrolled the elders, and collected data on site also could not be blinded to group assignment. The same instructors led both the TC and the control groups so they were not blinded. Assessor 1, who collected outcome data, could not be completely blinded because cognitively impaired participants revealed their group assignment during conversations with the assessor. The rest of the research team, including the three co-investigators and Assessor 2, who reviewed and coded pain behavior, were blinded to group assignment.
2.4. Power analysis
A sample of 40 per group (80 total) was required to provide 80% power to detect an effect size of 0.8 using a two-sided t-test with alpha=0.05. We were able to recruit 55 participants over 3 years for the study. However, as noted in Table 2, the intracluster correlation (ICC) for the observed pain behaviors and analgesic intake was ≤0.0001, and the ICCs for the measure of VDS were 0.181. Based on these data, we estimated the effect size from the design effect of our randomized cluster design (32). We had sufficient power to detect large effect sizes for observed behavior and the VDS measure (0.89 and 1.29 at 21 weeks, respectively). However, power to detect the effect of 0.28 for analgesic intake was below 50%.
2.5. Intervention
Tai Chi
The intervention used Sun-style TC (33), which includes 6 basic and 6 advanced forms. These forms can be completed by people of all ages seeking a joint-safe exercise routine (20). The TC intervention was modified based on pilot work (34-36), to ease the physical and cognitive requirements for learning TC. The TC group received three sessions of TC a week for 20 weeks, by certified instructors. The exercise started with 20 minutes per session and gradually increased to 40 minutes per session.
Attention control education group
To control for the attention that the TC group received, an “attention control education group” participated in instructor-led educational activities on site. The length of each session in the control group matched that of the TC intervention (24).
2.6. Outcome variables
Pain outcomes were measured at six time points [Time 1-6 (T1-6)]: Weeks 1 (pre-test), 5, 9, 13, 17 and 21 (post-test) on three non-intervention days. Elders who completed assessments received a $10 gift certificate at each time point, for a total of $60.
The Verbal Descriptive Scale (VDS)
The VDS (37), evaluated by Assessor 1, is recommended for measuring pain in elders with cognitive impairment (38). This one-item scale scores pain from 0 to 6, from no pain, slight pain, mild pain, moderate pain, severe pain, extreme pain, to the most intense pain imaginable. Elders with cognitive impairment were able to complete the VDS, and the score had a 0.99 correlation with the Present Pain Intensity Scale and a 0.79-0.84 correlation with the WOMAC pain scale (25). The VDS has good sensitivity, shown by its association with an increasingly painful thermal stimulus (F 6, 1038 =64.3, p<0.0001) (37). The VDS was collected for 3 days at each time point (T1-6), and the average of the three reports was used for the analysis.
Observation of pain behavior
Assessor 2 evaluated 10-minute videotaped behavior samples while participants engaged in a series of daily tasks (sitting, standing, walking, and reclining), using Keefe's observational method for OA knee pain (39). Five pain related behaviors were observed: guarding (abnormally slow, stiff, interrupted or rigid movements when moving from one position to another or while walking); active rubbing of the knee or hip (hands moving to or holding affected knee or hip); rigidity (excessive stiffness of the affected knee or hip during activities other than walking); unloading the joint (shifting weight from one leg to the other while standing), and joint flexion (flexing the affected knee or hip while in a static position). Keefe et al found that inter-rater reliability was 0.93 using the k-statistic (40), and concurrent validity was evidenced by a strong correlation with patients’ self-report of pain (r=0.46, p<0.001); those with knee OA pain exhibited significantly more pain behaviors than patients without knee OA pain (t=2.82, p<0.01) (40). Assessor 2 then coded the five pain behaviors using a “20-second observe; 10-second record” interval (39). Each participant received one point for each pain behavior observed. The maximum score for each 20-second observation period was 5 points. With 20 observation periods, scores could range from 0 to 100. A total pain behavior score was computed at each time point (T1-6) and this was used for the analysis.
Analgesic intake
An additional way to assess pain was to examine changes in analgesic intake. The type and dosage of analgesics were collected by Assessor 1 at T1-T6. At T1, elders were asked the name of any analgesic they were taking, the dosage, and the frequency of use. Assessor 1 then did pill counts for each analgesic to provide a basis for comparison over the following months. The difference in the pill count of each analgesic between two time points, for example, T1 and T2, was calculated and then divided by the number of days between the two time points, to obtain the average dosage per day for a specific analgesic. Dosages of each analgesic per day were then standardized to acetaminophen equivalents (41). Finally, the acetaminophen equivalent dosages for each analgesic were added together to create a daily analgesic dosage at months 1-5, and this figure was used for the analysis.
2.7. Statistical analyses
Using either chi-square or t-tests, baseline demographics (gender, race, age in years, education in years), pain outcomes (VDS, pain behavior, analgesic intake) and other variables (physical function, stiffness, cognitive function and depression) were compared between the experimental and control groups. Data were also compared for participants who completed the study and those who dropped out. Missing data were imputed by the last observation carried forward; that is, the missing final values of the outcome were replaced by the last known value before the participant dropped out. An intent-to-treat analysis was used.
General linear mixed models, controlling for site effects (clusters), were used to analyze the data (24). The three-way interaction between group, MMSE score, and time was added to the mixed models to determine whether cognitive function was associated with the efficacy of TC for pain. In these analyses, MMSE score was categorized as ≤ 23 or ≥ 24.
3. Results
3.1. Descriptive results
The average age for both groups was 79 years, and the majority of participants were women and Caucasian. Demographics, physical function, stiffness, and depression scores were similar between the two groups at baseline (24). The TC group's average MMSE score (M = 26.04 ± 1.92) also did not significantly differ from that of the control group (M = 24.85 ± 2.64, p = 0.062). Pain measures (VDS, pain behaviors, analgesic intake) were also similar between the two groups at baseline (Table 1). Demographics, pain outcomes and other variables (physical function, stiffness, cognitive function and depression) were also similar at baseline between participants who completed the study and those who dropped out.
Table 1.
Study Variables at Baseline
| Variable | Tai Chi (N=28) | Control (N=27) |
|---|---|---|
| VDS | 1.32 (1.02) | 1.44 (0.96) |
| Pain behaviors | 4.61 (3.82) | 5.00 (4.61) |
| Analgesics intakea | 1,340 (2,603) | 1,580 (2,404) |
VDS = The Verbal Descriptor Scale
Data was the daily average of acetaminophen equivalence dosage (mg) during month 1.
3.2. Major study results
3.2.1. To determine the effects of TC on pain reports, observed pain behaviors and analgesic intake in elders with cognitive impairment
Effects of TC on VDS
The TC group's VDS score was 0.73 points (95% CI: −1.46; −0.01; p=0.048) lower than that of the control group at post-test (See Table 2, VDS, between group difference at Week 21). Trend analysis indicated that there was a significant increase in the difference between the two groups over time (p=0.032). Significant differences started at T4 (Week 13) and continued at T5 (Weeks 17) and T6 (week 21), with p=0.048, 0.036 and 0.048, respectively. These results suggest that the TC intervention yielded beneficial effects on pain relief, as measured by the VDS, after approximately 12 weeks.
Table 2.
Changes in outcomes controlling for site effect
| Within group difference |
Between group difference |
Trendd |
ICCe |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tai Chi (n=28) | Control (n=27) | ||||||||
| Outcome | Estimatesb | 95% CI | Estimatesb | 95% CI | Estimatesc | 95% CI | p | p | r |
| VDSa | .032 | .181 | |||||||
| Week 1 | −0.25 | (−0.98, 0.47) | .497 | ||||||
| Week 5 | −0.43 | (−0.80, −0.06) | −0.07 | (−0.37, 0.24) | −0.61 | (−1.34, 0.11) | .098 | ||
| Week 9 | −0.30 | (−0.67, 0.06) | 0.03 | (−0.28, 0.33) | −0.58 | (−1.30, 0.15) | .117 | ||
| Week 13 | −0.54 | (−0.91, −0.18) | −0.06 | (−0.37, 0.25) | −0.73 | (−1.46, −0.01) | .048 | ||
| Week 17 | −0.66 | (−1.03, −0.29) | −0.14 | (−0.44, 0.17) | −0.78 | (−1.50, −0.05) | .036 | ||
| Week 21 | −0.54 | (−0.91, −0.18) | −0.06 | (−0.37, 0.25) | −0.73 | (−1.46, −0.01) | .048 | ||
| Pain Behaviora | .522 | .000 | |||||||
| Week 1 | −0.72 | (−2.85, 1.41) | .506 | ||||||
| Week 5 | 1.50 | (0.29, 2.71) | 1.52 | (0.23, 2.81) | −0.74 | (−2.87, 1.39) | .495 | ||
| Week 9 | 0.64 | (−0.57, 1.86) | 2.41 | (1.12, 3.70) | −2.49 | (−4.62, −0.35) | .023 | ||
| Week 13 | 2.00 | (0.79, 3.21) | 0.89 | (−0.40, 2.18) | 0.39 | (−1.74, 2.52) | .719 | ||
| Week 17 | 1.96 | (0.75, 3.18) | 1.89 | (0.60, 3.18) | −0.65 | (−2.78, 1.49) | .552 | ||
| Week 21 | −0.82 | (−2.03, 0.39) | 1.37 | (0.08, 2.66) | −2.91 | (−5.05, −0.78) | .008 | ||
| Analgesic Intakea | .062 | .000 | |||||||
| Month 1 | −239.85 | (−1561.03, 1081.33) | .721 | ||||||
| Month 2 | −105.44 | (−247.02, 36.15) | 238.68 | (3.09, 474.27) | −583.97 | (−1905.15, 737.21) | .385 | ||
| Month 3 | −69.77 | (−211.36, 71.82) | 228.01 | (−7.57, 463.60) | −537.63 | (−1858.81, 783.55) | .423 | ||
| Month 4 | −170.13 | (−311.71, −28.54) | 117.44 | (−118.15, 353.03) | −527.41 | (−1848.59, 793.77) | .432 | ||
| Month 5 | −222.97 | (−364.55, −81.38) | 221.70 | (−13.89, 457.29) | −684.52 | (−2005.70, 636.66) | .308 | ||
Lower scores indicate improvement
Mean difference between each follow-up time point and baseline based on model estimation controlling for site effect
Mean difference between Tai Chi and control groups at each time point based on model estimation controlling for site effect
Test linearly increasing trend on group difference over time by linear contrast
ICC= intracluster correlation
Effects of TC on pain behavior
The TC group's pain behavior score was 2.91 points lower than that of the control group at post-test (95% CI: −5.05; −0.78; p=0.008) (See Table 2, Pain Behavior, between group difference at Week 21). However, there was no clearer trend toward improvement in the TC group than in the controls (p=0.522). There was a significant difference at T3 (Week 9, p=0.023), but this difference disappeared at Weeks 13 and 17, then reappeared at the post-test at Week 21 (p=0.008).
Effects of TC on analgesic intake
As noted earlier, the study was under-powered for detecting changes in analgesic intake, and therefore the data must be interpreted with caution. The between group difference was not significant at post-test (p=0.308) (See Table 2, Analgesic Intake, between group difference at Month 5), though trend analyses indicated that there was an increase in the difference between groups over time (p=0.062).
3.2.2. Association of cognitive function with the effect of TC for pain
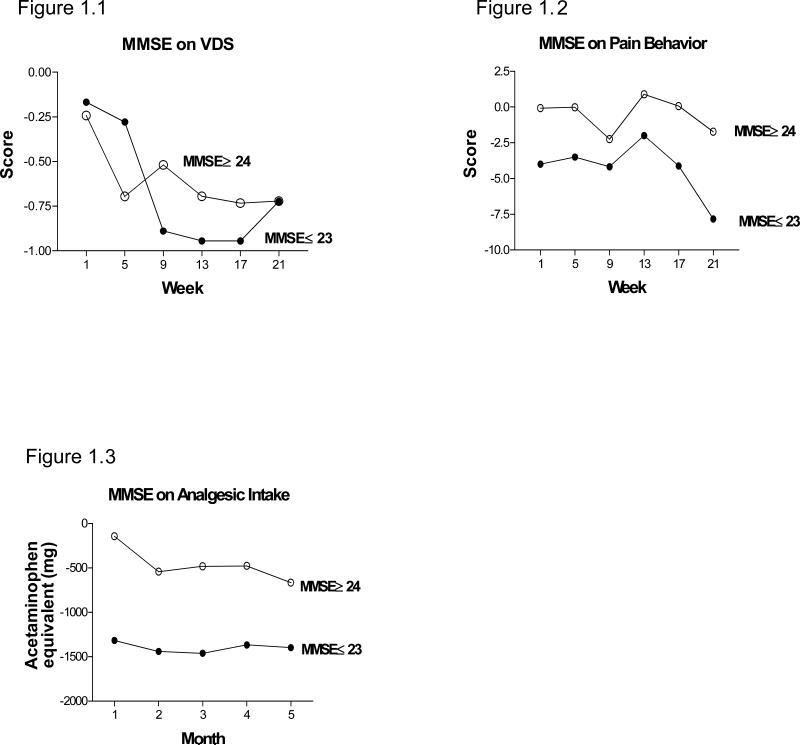
Overall, cognitive function was not related to the efficacy of TC for pain in these elders. Examination of the three-way interaction between group, time and MMSE score for each pain outcome over time showed p values between 0.734 and 0.892, indicating that MMSE score was not related to the effect of TC on any of the pain outcomes. However, again, these findings should be interpreted with caution since the study did not have enough power to test the three-way interaction. Figures 1.1-1.3 illustrates that for pain reports, pain behaviors, and analgesic intake, the differences between the TC and control groups over time were similar for participants with MMSE scores ≤ 23 and those with MMSE scores ≥ 24. However, at the last follow-up, the differences between the TC group and controls on most outcomes were larger for elders with MMSE ≤ 23, suggesting that elders with MMSE ≤ 23 had a greater response to the intervention.
Figure 1.1-1.3. Effect of cognition on pain outcomes.
Participants were divided in to a higher cognitive functioning (MMSE ≥ 24) and a lower cognitive functioning group (MMSE ≤ 23) to determine whether the level of cognitive functioning could affect the outcomes of TC intervention on VDS (Fig. 1.1), Pain Behavior (Fig. 1.2) or Analgesic Intake (Fig. 1.3). Examination of the three-way interaction (group, time and MMSE score) for each pain outcome between groups over time showed cognitive levels had no effect on these pain outcomes. See text for more detailed discussion of the analyses.
a. Difference in scores or dosages between two intervention groups.
4. Discussion
This was the first study to explore TC's effects on pain in elders with cognitive impairment and knee OA, and examine TC's effects on pain outcomes using multiple pain indicators. Strengths of the study include the use of rigorous methods to study a population that is often excluded from non-pharmacological intervention trials; exploration of an alternative exercise option to treat knee OA pain for this vulnerable population, and the use of multiple indicators to measure pain.
Scores on the VDS and observed pain behaviors were significantly better with the TC group than the control group at post-test. Further, the beneficial effects of TC were not associated with cognitive ability. These group comparisons suggest that 20 weeks of TC practice led to significant reductions in OA knee pain in cognitively impaired elders. Analgesic intake did not differ significantly between the experimental and control groups. However, over time, there was a trend toward lower analgesic intake in the TC group. The experimental group's results on verbal reports of pain, pain behaviors and analgesic intake at the post-test were consistent. This indicates that relief of pain resulting from TC was not due to attention or other psychosocial factors but was a beneficial effect of TC. Future studies should look for the mechanism(s) underlying the beneficial effects of TC in reducing OA knee pain.
It should be noted that the control group also showed some improvement in pain reports. This group had the same intervention duration, same staff/instructor and a similar number of group members as the TC group. Decreases in pain scores in the control group could have resulted from the Hawthorne effect, since participants knew they were under observation (42). Alternatively, the decrease in pain scores found in the control group might have been related to social interaction/attentional factors that affected members’ pain perceptions and pain report. Further, by participating in the study, activity levels of the control group participants might have increased since they had to get out of their beds/chairs, dress, leave their apartments, and walk/drive to the intervention site. Thus, by simply increasing their physical activities, elders in the control group might have “felt better”.
Interestingly, the VDS appeared to be less sensitive than the WOMAC pain instrument. Beneficial effects of TC measured by WOMAC pain scores appeared in Week 9 (p= 0.026) as reported previously (24). Based on this, one would expect that positive results from the VDS would also appear in Week 9 (2 months after beginning the TC intervention). However, positive effects of the VDS appeared only in Week 13 (3 months after beginning the TC intervention). Osteoarthritic knee pain is a chronic health problem and elders may have already adapted to it. Thus, elders may not be able to precisely identify the level of pain using the VDS scale without reminders. The WOMAC pain scale has items that remind elders about levels of pain caused by various daily activities, which may lead to more precise results. In addition, the WOMAC pain scale, a 5-item scale, has a wide range of possible scores (0-20), while the VDS has a single item score range from 0 to 6. A scale with a wide score range should better detect change in response to an intervention than a scale with a narrow score range. Whatever the reason for the observed delay, it is clear that TC practice was beneficial in relieving OA knee pain, based on the VDS.
While at post-test pain behaviors were significantly lower in the TC group than in the control group, there was not a clear trend toward reduction over the study period. This is not consistent with the results we obtained on the VDS, which indicated that pain improved over time. It is possible that, pain report and pain behaviors are manifestations of different constructs (29, 43). It is also possible that because these elders had suffered OA knee pain for years, they had developed a particular way of walking, rising from a chair and rising from a bed, the activities assessed in this study. Thus, they might have continued to walk or rise in a “guarded” way even though they actually perceived less pain in these activities.
While there were no significant differences between groups in analgesic intake, detailed examination of the data showed that over time there was a reduction of about 223 mg/day acetaminophen equivalent dosage in the TC group, while there was an increase of 221.7 mg/day acetaminophen equivalent dosage in the control group. That is, by the end of the intervention, the difference between two groups in consumption of analgesic medication reached the equivalent of one regular strength Tylenol pill. It also suggested that it might take longer than 20 weeks for TC to exhibit beneficial effects on pain as measured by analgesic intake. Together with the pain report and pain behaviors, this suggests that TC reduced pain and possibly analgesic intake. Given the serious adverse events that may occur in patients taking acetaminophen, it is important to consider TC as an adjunct or alternative to pharmacological intervention to treat OA knee pain in the frail elderly.
As noted above, our measure of cognitive impairment (the MMSE) was not related to change in pain outcomes in these cognitively impaired elders. Elders with either higher or lower cognitive functioning were able to learn the TC exercise and gain the benefits of this exercise. In addition, they appeared to enjoy TC practice very much, as we have noted in prior reports (34, 35).
There were some limitations to the study. First, we identified elders with cognitive impairment using MMSE scores and identified elders with knee OA using self-report, with confirmation from medical providers. With this approach, we might have included some false-positive cases in the sample. Second, our study did not have sufficient power to detect changes in analgesic intake. In addition, we were able to blind only one of the assessors completely to group assignment. This might have led to bias toward favorable outcomes in the experimental group. However, Assessor 2, who evaluated and coded pain behaviors, was completely blinded to group assignment and since all pain measures suggested that TC was effective in reducing pain, assessor bias was probably limited. The percentages of Caucasians and females in this study were higher than those in the U.S population. Finally, our eligibility criteria were strict; it is possible that comorbidities are common in cognitively impaired elderly with OA, and thus some of them may have been excluded from the study.
TC is an effective non-pharmacological intervention option for treating OA knee pain even in elders with cognitive impairment (15, 24). In addition to drug treatment for pain, offering non-pharmacological interventions such as TC gives patients more choices which should promote patients’ autonomy and self-efficacy. In addition, giving options for treatment may improve adherence and lead to better health outcomes. Finally, TC has other health benefits, ranging from improving quality of life to potential reductions in healthcare costs (15, 44, 45). Policy makers should thus be informed of the benefits of TC and encouraged to advocate for reimbursement for a TC program.
There are unanswered questions. First, although this study showed a tendency to decrease analgesic intake in the intervention arm and a tendency to have better pain outcomes in elders with lower cognitive functions, the lack of power prevents us from providing definitive answers. In addition, as a group, elders who received the TC intervention had better pain outcomes than those who received the attention control intervention. However, it is not known if this result can be reproduced in clinical settings or in the community. Finally, the impact of patients’ treatment preference on the research results was not evaluated and no tailored intervention option was provided. Future studies should focus on comparative effectiveness evaluation of treatment options, including non-pharmacological interventions, such as TC, for elders with cognitive impairment and OA knee pain. This should also include development of a method to provide tailored treatment based on patients’ characteristics. Thus, patients can achieve optimum results by tailored treatment. In addition, research to develop and evaluate an algorithm for choosing treatment options based on patients’ preference should also be encouraged. All of these will assist in improving patients’ adherence and thus improving treatment outcomes.
Supplementary Material
Acknowledgments
The study described here was supported by Award Number R21NR010003 from the National Institute of Nursing Research. The study was also partially supported by funding from Alzheimer's Arkansas and a grant (1IL1RR029884) from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the views of Alzheimer's Arkansas, the National Institute of Nursing Research or the National Institutes of Health. The authors thank Elizabeth Tornquist for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration
ClinicalTrials.gov Identifier: NCT01528566
Author Contributions
All authors made substantial contributions to the conceptualization and design, acquisition of data, analysis and interpretation of data, preparation and revision of the article, and final approval of the version to be published. All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals.
Conflict of Interest
| Elements of Financial/Personal Conflicts | Pao-Feng Tsai | Jason Y. Chang | Cornelia Beck | Yong-Fang Kuo | Francis J. Keefe | Karl Rosengren | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | ||||||
| Grants/Funds | X | X | X | X | X | X | ||||||
| Honoraria | X | X | X | X | X | X | ||||||
| Speaker Forum | X | X | X | X | X | X | ||||||
| Consultant | X | X | X | X | X | X | ||||||
| Stocks | X | X | X | X | X | X | ||||||
| Royalties | X | X | X | X | X | X | ||||||
| Expert Testimony | X | X | X | X | X | X | ||||||
| Board Member | X | X | X | X | X | X | ||||||
| Patents | X | X | X | X | X | X | ||||||
| Personal Relationship | X | X | X | X | X | X | ||||||
There is no conflict of interest in this study for any authors.
References
- 1.Samus QM, Mayer L, Onyike CU, Brandt J, Baker A, McNabney M, et al. Correlates of functional dependence among recently admitted assisted living residents with and without dementia. J Am Med Dir Assoc. 2009;10(5):323–9. doi: 10.1016/j.jamda.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Canadian Study of Health and Aging The Canadian Study of Health and Aging: risk factors for Alzheimer's disease in Canada. Neurology. 1994;44(11):2073–80. doi: 10.1212/wnl.44.11.2073. [DOI] [PubMed] [Google Scholar]
- 3.Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, et al. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;4:11. doi: 10.1186/1478-7954-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buffum MD, Sands L, Miaskowski C, Brod M, Washburn A. A clinical trial of the effectiveness of regularly scheduled versus as-needed administration of acetaminophen in the management of discomfort in older adults with dementia. J Am Geriatr Soc. 2004;52(7):1093–7. doi: 10.1111/j.1532-5415.2004.52305.x. [DOI] [PubMed] [Google Scholar]
- 5.Shorr RI, Griffin MR, Daugherty JR, Ray WA. Opioid analgesics and the risk of hip fracture in the elderly: codeine and propoxyphene. J Gerontol. 1992;47(4):M111–5. doi: 10.1093/geronj/47.4.m111. [DOI] [PubMed] [Google Scholar]
- 6.Forman JP, Stampfer MJ, Curhan GC. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension. 2005;46(3):500–507. doi: 10.1161/01.HYP.0000177437.07240.70. [DOI] [PubMed] [Google Scholar]
- 7.Curhan GC, Willett WC, Rosner B, Stampfer MJ. Frequency of analgesic use and risk of hypertension in younger women. Arch Intern Med. 2002;162(19):2204–8. doi: 10.1001/archinte.162.19.2204. [DOI] [PubMed] [Google Scholar]
- 8.Curhan GC, Knight EL, Rosner B, Hankinson SE, Stampfer MJ. Lifetime nonnarcotic analgesic use and decline in renal function in women. Arch Intern Med. 2004;164(14):1519–24. doi: 10.1001/archinte.164.14.1519. [DOI] [PubMed] [Google Scholar]
- 9.Desai AK, Chibnall JT. Propoxyphene use in the elderly. J Am Geriatr Soc. 2004;52(7):1227. doi: 10.1111/j.1532-5415.2004.52327_13.x. [DOI] [PubMed] [Google Scholar]
- 10.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–88. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Jansen MJ, Viechtbauer W, Lenssen AF, Hendriks EJ, de Bie RA. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review. J Physiother. 2011;57(1):11–20. doi: 10.1016/S1836-9553(11)70002-9. [DOI] [PubMed] [Google Scholar]
- 12.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2008;(4):CD004376. doi: 10.1002/14651858.CD004376.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Steultjens MP, Dekker J, Bijlsma JW. Avoidance of activity and disability in patients with osteoarthritis of the knee: the mediating role of muscle strength. Arthritis Rheum. 2002;46(7):1784–8. doi: 10.1002/art.10383. [DOI] [PubMed] [Google Scholar]
- 14.Bannuru R, Abariga S, Wang C. How effective is tai chi mind-body therapy for knee osteoarthritis (KOA)? A systematic review and meta-analysis Osteoarthritis Cartilage. 2012;20:S281–S282. [Google Scholar]
- 15.Wang C, Schmid CH, Hibberd PL, Kalish R, Roubenoff R, Rones R, et al. Tai Chi is effective in treating knee osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2009;61(11):1545–53. doi: 10.1002/art.24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brismee JM, Paige RL, Chyu MC, Boatright JD, Hagar JM, McCaleb JA, et al. Group and home-based tai chi in elderly subjects with knee osteoarthritis: a randomized controlled trial. Clin Rehabil. 2007;21(2):99–111. doi: 10.1177/0269215506070505. [DOI] [PubMed] [Google Scholar]
- 17.Fransen M, Nairn L, Winstanley J, Lam P, Edmonds J. Physical activity for osteoarthritis management: a randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis Rheum. 2007;57(3):407–14. doi: 10.1002/art.22621. [DOI] [PubMed] [Google Scholar]
- 18.Song R, Lee EO, Lam P, Bae SC. Effects of Tai Chi exercise on pain, balance, muscle strength, and perceived difficulties in physical functioning in older women with osteoarthritis: A randomized clinical trial. J Rheumatol. 2003;30(9):2039–2044. [PubMed] [Google Scholar]
- 19.Lee HY. [Comparison of effects among Tai-Chi exercise, aquatic exercise, and a self-help program for patients with knee osteoarthritis]. Taehan Kanho Hakhoe Chi. 2006;36(3):571–80. doi: 10.4040/jkan.2006.36.3.571. [DOI] [PubMed] [Google Scholar]
- 20.Arthritis Foundation. Tai Chi program. In: Arthritis Foundation. 2010 [Google Scholar]
- 21.Lam LC, Chau RC, Wong BM, Fung AW, Tam CW, Leung GT, et al. A 1-year randomized controlled trial comparing mind body exercise (Tai Chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Am Med Dir Assoc. 2012;13(6):568, e15–20. doi: 10.1016/j.jamda.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Burgener SC, Yang Y, Gilbert R, Marsh-Yant S. The effects of a multimodal intervention on outcomes of persons with early-stage dementia. Am J Alzheimers Dis Other Demen. 2008;23(4):382–94. doi: 10.1177/1533317508317527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng ST, Chow PK, Song YQ, Yu EC, Chan AC, Lee TM, et al. Mental and Physical Activities Delay Cognitive Decline in Older Persons With Dementia. Am J Geriatr Psychiatry. 2014;22(1):63–74. doi: 10.1016/j.jagp.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 24.Tsai PF, Chang JY, Beck C, Kuo YF, Keefe FJ. A pilot cluster-randomized trial of a 20-week Tai Chi program in elders with cognitive impairment and osteoarthritic knee: effects on pain and other health outcomes. J Pain Symptom Manage. 2013;45(4):660–669. doi: 10.1016/j.jpainsymman.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai PF, Richards K. Using an osteoarthritis-specific pain measure in elders with cognitive impairment: a pilot study. J Nurs Manag. 2006;14(2):90–5. doi: 10.1111/j.1365-2934.2006.00560.x. [DOI] [PubMed] [Google Scholar]
- 26.Hadjistavropoulos T, Herr K, Turk DC, Fine PG, Dworkin RH, Helme R, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain. 2007;23(1 Suppl):S1–43. doi: 10.1097/AJP.0b013e31802be869. [DOI] [PubMed] [Google Scholar]
- 27.Hadjistavropoulos T, Herr K, Prkachin KM, Craig KD, Gibson SJ, Lukas A, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216–27. doi: 10.1016/S1474-4422(14)70103-6. [DOI] [PubMed] [Google Scholar]
- 28.Herr KA, Mobily PR. Comparison of selected pain assessment tools for use with the elderly. Appl Nurs Res. 1993;6(1):39–46. doi: 10.1016/s0897-1897(05)80041-2. [DOI] [PubMed] [Google Scholar]
- 29.Waters SJ, Dixon KE, Keefe FJ. Pain assessment. In: Ayers S, Baum A, McManus C, Newman S, Wallston K, Weinman J, et al., editors. editors. Cambridge handbook of psychology, health and medicine. Cambridge University Press; Cambridge, UK: 2007. pp. 300–3. [Google Scholar]
- 30.Skoog I, Lithell H, Hansson L, Elmfeldt D, Hofman A, Olofsson B, et al. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE). Am J Hypertens. 2005;18(8):1052–9. doi: 10.1016/j.amjhyper.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Friedman TW, Yelland GW, Robinson SR. Subtle cognitive impairment in elders with Mini-Mental State Examination scores within the 'normal' range. Int J Geriatr Psychiatry. 2011;27(5):463–71. doi: 10.1002/gps.2736. [DOI] [PubMed] [Google Scholar]
- 32.Snijdres TAB, Bosker RJ. An introduction to basic and advanced multilevel modeling. SAGE Publication Ltd.; Thousand Oaks, CA: 1999. [Google Scholar]
- 33.Lam P. Tai Chi for Arthritis. East Action Publishing Pty Ltd; Narwee, Australia: 2004. [Google Scholar]
- 34.Tsai PF, Beck C, Chang JY, Hagen J, Kuo YF, Roberson PK, et al. The effect of tai chi on knee osteoarthritis pain in cognitively impaired elders: pilot study. Geriatr Nurs. 2009;30(2):132–9. doi: 10.1016/j.gerinurse.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang JY, Tsai PF, Beck C, Hagen JL, Huff DC, Anand KJ, et al. The effect of tai chi on cognition in elders with cognitive impairment. Medsurg Nurs. 2011;20(2):63–9. quiz 70. [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai P, Beck C, Chang JY, Hagen J, Anand S, Kuo Y, et al. The feasibility of implementing Tai Chi for nursing home residents with knee osteoarthritis and cognitive impairment. Activities Directors' Quarterly for Alzheimer's & Other Dementia Patients. 2009;10(1):9–17. [PMC free article] [PubMed] [Google Scholar]
- 37.Herr KA, Spratt K, Mobily P, Richardson G. Pain intensity assessment in older adults: Use of Experimental Pain to Compare Psychometric Properties and Usability of Selected Scales with younger adults. Clin J Pain. 2004;20(4):207–219. doi: 10.1097/00002508-200407000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Horgas AL. Assessing pain in persons with dementia. Medsurg Nurs. 2007;16(3):207–8. [PubMed] [Google Scholar]
- 39.Keefe FJ, Caldwell DS, Queen K, Gil KM, Martinez S, Crisson JE, et al. Osteoarthritic knee pain: a behavioral analysis. Pain. 1987;28(3):309–21. doi: 10.1016/0304-3959(87)90066-2. [DOI] [PubMed] [Google Scholar]
- 40.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87(3):325–34. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 41.Horgas AL, Tsai PF. Analgesic drug prescription and use in cognitively impaired nursing home residents. Nurs Res. 1998;47(4):235–42. doi: 10.1097/00006199-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Roethlisberger FJ, Dickson WJ. Management and the Worker. Harvard University Press; Cambridge, Massachusetts: 1939. [Google Scholar]
- 43.Fordyce WE. Behavioral methods for chronic pain and illness. St. Louis: C.V Mosby Company. 1976 [Google Scholar]
- 44.Taylor-Piliae R. Tai Chi as an Adjunct to Cardiac Rehabilitation Exercise Training Journal of Cardiopulmonary Rehabilitation. 2003;23:90–96. doi: 10.1097/00008483-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Harmer P, McAuley E, Duncan TE, Duncan SC, Chaumeton N, et al. An evaluation of the effects of Tai Chi exercise on physical function among older persons: a randomized controlled trial. Ann Behav Med. 2001;23(2):139–46. doi: 10.1207/S15324796ABM2302_9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.