Abstract
Carbohydrate response element binding protein (ChREBP) is an important transcription factor that regulates a variety of glucose-responsive genes in hepatocytes. To date, only two natural isoforms, Chrebpα and Chrebpβ, have been identified. Although ChREBP is known to be expressed in pancreatic β cells, most of the glucose-responsive genes have never been verified as ChREBP targets in this organ. We aimed to explore the impact of ChREBP expression on regulating genes linked to accumulation of lipid droplets, a typical feature of β-cell glucotoxicity. We assessed gene expression in 832/13 cells overexpressing constitutively active ChREBP (caChREBP), truncated ChREBP with nearly identical amino acid sequence to Chrebpβ, or dominant negative ChREBP (dnChREBP). Among multiple ChREBP-controlled genes, ChREBP was sufficient and necessary for regulation of Eno1, Pklr, Mdh1, Me1, Pdha1, Acly, Acaca, Fasn, Elovl6, Gpd1, Cpt1a, Rgs16, Mid1ip1,Txnip, and Chrebpβ. Expression of Chrebpα and Srebp1c were not changed by caChREBP or dnChREBP. We identified functional ChREBP binding sequences that were located on the promoters of Chrebpβ and Rgs16. We also showed that Rgs16 overexpression lead to increased considerable amounts of lipids in 832/13 cells. This phenotype was accompanied by reduction of Cpt1a expression and slight induction of Fasn and Pklr gene in these cells. In summary, we conclude that Chrebpβ modulates its own expression, not that of Chrebpα; it also regulates the expression of several metabolic genes in β-cells without affecting SREBP-1c dependent regulation. We also demonstrate that Rgs16 is one of the ChREBP-controlled genes that potentiate accumulation of lipid droplets in β-cells.
Introduction
Expression of glycolytic and lipogenic genes, including L-type pyruvate kinase (Pklr), acetyl-CoA carboxylase alpha (Acaca), thyroid hormone responsive (Thrsp) and fatty acid synthase (Fasn), is known to be regulated by glucose [1–5]. Promoters of these genes contain carbohydrate response element (ChoRE or ChRE) which is crucial for their glucose regulation [4, 6–12]. The ChoRE consists of two E-box motifs with CACGTG sequence separated by five nucleotides. In 2001, a transcription factor which recognizes ChoRE was firstly isolated from rat liver extract [13]. This basic helix-loop-helix leucine zipper transcription factor, namely carbohydrate response element binding protein (ChREBP), is highly expressed in hepatocytes, adipocytes and pancreatic β-cells [13–15]. ChREBP is activated in response to high glucose. Prolonged high glucose induces its mRNA expression, translocation from the cytosol to the nucleus, transactivation activity and DNA binding activity [13, 16–19]. Several other target genes involved in glucose metabolism and lipogenesis as well as their ChoREs have been identified, i.e. glycerol-3-phosphate dehydrogenase 1 (Gpd1) [11, 20], G0/G1 switch 2 (G0S2) [11, 20], glucose-6-phosphatase catalytic subunit (G6pc) [11, 21], solute carrier family 2 (facilitated glucose transporter) member 4 (Slc2a4) [11, 20], glucokinase (hexokinase 4) regulator (Gckr) [11, 20], pyruvate carboxylase (Pc) [11, 22, 23], glyceraldehyde 3-phosphate dehydrogenase (Gapdh) [11, 24], fibroblast growth factor 21 (Fgf21) [11, 25], and deleted in esophageal cancer 1 (Dec1) [26]. It has been newly discovered that glucose also regulates expression of Chrebpβ, naturally occurring constitutively active ChREBP variant, through a ChoRE on the exon 1b [15].
In liver cells, ChREBP involved in hepatic de novo lipogenesis. Overexpression of ChREBP in liver induces the expression of Pklr, Fasn, Acaca, stearoyl-CoA desaturase-1 (Scd1), and ELOVL fatty acid elongase 6 (Elovl6), modifying lipid composition in the process [27]. Homozygous global ChREBP knockout mice exhibited significantly decreased de novo fatty acid synthesis and overall adiposity [28]. In addition, overexpression of dominant negative form of ChREBP dimerization partner Mlx (Max-like protein X) downregulates Pklr, Acaca, Fasn, and Elovl6 in hepatocytes and reduces intracellular triglyceride content [29].
Our previous study with pancreatic β-cells demonstrated that ChREBP deleteriously affects cell function and survival [30]. Constitutively active ChREBP (caChREBP) is a glucose-independent active mutant of ChREBP generated by deletion of the N-terminal low glucose inhibitory domain (the LID domain); its induced expression causes accumulation of neutral lipids in INS-1-derived 832/13 pancreatic β-cell line. Conversely, siRNA-mediated ChREBP silencing significantly reduces triglyceride in these cells [30]. Until now, only a few studies have explored this effect of ChREBP on accumulation of lipid droplets, an important characteristic of glucotoxicity, in pancreatic β-cells. The changes in the amount of intracellular lipid by ChREBP may be partially explained by up-regulated expression of Fasn, which encodes a key enzyme in de novo lipogenesis. ChREBP was shown to bind to both proximal and distal promoters of Fasn gene in β-cells [6, 31]. Microinjection of anti-ChREBP antibody in MIN6 mouse insulinoma cells blunted induction of its promoter activity by high glucose. Knockdown of ChREBP also inhibited high glucose-induced expression of Fasn gene. These findings have been corroborated by our previous work using 832/13 rat insulinoma cells that overexpression of caChREBP led to significant Fasn upregulation [30].
In this study, we aimed to further explore molecular mechanism of ChREBP-mediated lipid accumulation in pancreatic β-cells. We examined the effect of this transcription factor on expression of genes encoding enzymes of glucose metabolism and key lipogenic genes and isoforms of ChREBP itself as well.
Materials and Methods
Cell Culture
We cultured INS-1-derived 832/13 rat insulinoma cells (a generous gift of Dr. C. Newgard, Duke University, Durhanm, NC, USA) [32] in Roswell Park Memorial Institute (RPMI) medium (Life Technologies) supplemented with INS-1 solution, 10% fetal bovine serum (FBS) (Biochrom), 1X penicillin-streptomycin (Merck Millipore), at 37°C in a 5% CO2 humidified atmosphere [32].
Plasmid construction
For the Tet-on inducible system, we replaced AgeI-MluI fragment of pTRIPZ self-inactivating (SIN) lentiviral vector (Open Biosystems) with nuclear form of enhanced yellow fluorescent protein (eYFPnuc), N-terminal Myc tagged constitutively active ChREBP (caChREBP), N-terminal Myc tagged dominant negative ChREBP (dnChREBP), or N-terminal Myc tagged regulator of G-protein signaling 16 (Rgs16). For luciferase reporter constructs, we annealed oligonucleotides containing two copies of putative ChoREs from promoters of Chrebpβ, Rgs16 or Gpd1 and ligated to pGLuc-Basic vector (New England Biolabs) with minimal TATA promoter. Rgs16 promoter (-1519 to +159) and mutated Rsg16 promoter with ChoRE deletion were amplified from genomic DNA extracted from 832/13 cells and cloned into pTRIPZ containing eYFPnuc. We constructed inducible lentiviral vectors that contain a single copy of optimized microRNA-adapted short hairpin RNA [33]. The target sequence for rat Chrebpβ was CCCAAGCCCGGCTTTTAGA. All constructs were confirmed by sequencing.
Generation of stable tetracycline inducible cell lines
We transfect inducible lentiviral vector constructs, psPAX2 and pMD2.G vectors to human embryonic kidney 293T (HEK293T) cells using calcium phosphate method. Lentivirus were transduced into 832/13 cells in the presence of polybrene (Sigma). Infected cells were selected with puromycin (Sigma).
RNA extraction and reverse transcription
We lysed cells after induction with 1 μg/mL doxycycline (Bio Basic). We followed manufacturer’s protocol to isolate total RNA from these lysates by RNeasy Plus Kit (Qiagen). Then, we assessed total RNA using FLUOstar Omega instrument with LVis Plate (BMG Labtech). We used Omniscript RT kit (Qiagen), random primers and 1 μg of total RNA to make cDNA in a volume of 20 μL, according to the manufacturer’s instruction.
Quantitative PCR
We quantitated gene expression using gene-specific primers and KAPA SYBR green PCR master mix (KAPAbiosystem) on Roter-GeneQ (Qiagen) or LightCycler 480 (Roche Diagnostics). We analyzed expression of 9 housekeeping genes, i.e. eukaryotic translation elongation factor 1 gamma (Eef1g), hydroxymethylbilane synthase (Hmbs), delta-aminolevulinate synthase 1 (Alas1), ribosomal protein L13a (Rpl13a), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta (Ywhaz), hypoxanthine phosphoribosyltransferase 1 (Hprt1), beta-actin (Actb), 18s ribosomal RNA (Rns18), and peptidylprolyl isomerase A (cyclophilin A) (Ppia). We selected three most stable housekeeping genes of each experimental setting for normalization of gene expression based on geNorm analysis [34]. Primer sequences used in this study are available upon request.
Sequence alignment
We aligned the amino acid sequence in MegAlign module of DNASTAR software (Lasergene). We identified multi-species conserved regulatory sequences by GenomeVISTA (http://genome.lbl.gov/vista/) for ChREBPβ and Gpd1, and UCSC Genome Browser (https://genome.ucsc.edu/) for Rgs16.
Luciferase assay
We transiently transfected three plasmid DNA constructs, Gaussia luciferase construct containing putative ChREBP binding elements, cytomegalovirus promoter-driven Cypridina luciferase construct, and caChREBP construct or empty vector, to 832/13 cells in 96-well plates by Lipofectamine2000 (Life Technologies). We collected culture media at 48 hours after transfection. We then sequentially detected secreted luciferases using Gaussia luciferase and Cypridina luciferase assay kits (New England Biolabs). Luciferase expressed from Gaussia luciferase reporter plasmid is normalized by the activity of Cypridina luciferase.
Oil Red O Staining
We washed with phosphate-buffered saline after induction by 1 μg/mL doxycycline, and fixed these cells in 10% neutral buffered formaldehyde for 2 hour. We then removed formaldehyde and washed the cells with water. We added 0.3% Oil Red O in isopropanol to the fixed cells. We removed Oil Red O solution and washed the cells with water. Stained neutral lipid droplets were visualized and photographed under the IX81 inverted microscope (Olympus). Areas of the lipid droplets were quantified using Adobe Photoshop 9.0 CS2.
Statistical analysis
We presented all data as the mean ± standard deviation (SD) between three biological replicates (expression) or standard error of the mean (SEM) of four determinations from two independent experiments (luciferase assay). We compared sample groups by Student’s two-tailed t-test. The p<0.05 was considered statistically significant.
Result
Chrebpβ stimulates its own expression via a feed-forward loop
We previously demonstrated that constitutively active ChREBP (caChREBP), mouse ChREBP with deletion of low glucose inhibitory domain, induces apparent β-cell apoptosis by upregulation of thioredoxin-interacting protein (Txnip) [30]. This caChREBP is highly similar to mouse ChREBPβ, which is 19 amino acids longer at the N-terminal end (Fig 1A), both being characterized by the absence of the LID domain conferring them constitutive activity. To overcome a problem with cell lost in stable transgene overexpressing cells, we adopted Tet-on system with reverse tetracycline transactivator 3 (rtTA3), which offers minimal leakiness and high inducibility [35]. We established inducible 832/13 pancreatic β-cell lines for caChREBP (caChREBP cells) and dominant negative ChREBP lacking transactivation domain (dnChREBP cells) using self-inactivating lentiviral vector (Fig 1B). Since both caChREBP and dnChREBP are located in the nucleus, nuclear form of enhanced yellow fluorescent protein (eYFPnuc cells) was chosen to establish control cells.
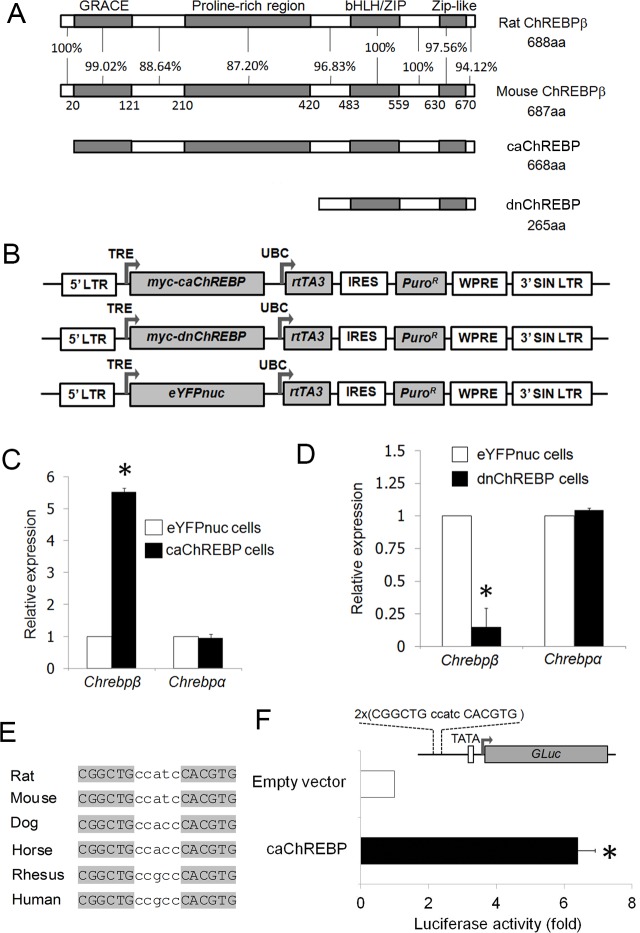
Fig 1. Exogenous ChREBP regulates Chrebpβ expression.
(A) Amino acid alignment among rat ChREBPβ, mouse ChREBPβ, caChREBP, and dnChREBP. GRACE, glucose response activation conserved element; bHLH, basic Helix-Loop-Helix-Leucine. (B) Schematic diagram of tetracycline-inducible vectors for overexpression of caChREBP, dnChREBP, and eYFPnuc. LTR, long terminal repeat; TRE, tetracycline-responsive promoter element; UBC, human ubiquitin C promoter; rtTA3, reverse tetracycline-transactivator 3; IRES, internal ribosomal entry site; PuroR, puromycin resistance gene; WPRE, Woodchuck hepatitis posttranscriptional regulatory element; SIN LTR, self-inactivating long terminal repeat. (C) caChREBP induces Chrebpβ expression. We incubated caChREBP cells for 48h in RPMI with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. We isolated the RNA and performed RT-qPCR using ChREBP isoform-specific primers. The histograms are the means of relative RNA levels normalized to Eef1g and Rpl13a and expressed as fold activation over the activity seen in eYFPnuc cells incubated in RPMI with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. *, p< 0.05 compared with eYFPnuc cells. (D) dnChREBP reduces the Chrebpβ expression. We preincubated dnChREBP cells for 24h in RPMI with 5.5 mmol/l D-glucose in the presence of doxycycline 1 μg/mL to minimize expression of endogenous nuclear ChREBP and let the induced dnChREBP occupy ChoREs and switched to in RPMI with 25 mmol/l D-glucose in the presence of doxycycline 1 μg/mL for 48h to induce strong expression and activity of endogenous nuclear ChREBP. We isolated the RNA and performed RT-qPCR using ChREBP isoform-specific primers. The histograms are the means of relative RNA levels normalized to Rns18 and Hprt1 and expressed as fold activation over the activity seen in eYFPnuc cells incubated under the same condition. *, p< 0.05 compared with eYFPnuc cells. (E) Alignment of ChoRE sequence presents in Chrebpβ promoter among rat (at the position -109 to -93), mouse, dog, horse, rhesus, and human. Sequence assemblies and coordinates are as follows: Rat: Mar.2012 Chr12(-): 26638089–26638073, Mouse: Jul.2007 Chr5(+): 135565651–135565667, Rhesus: Jan.2006 Chr3(-):51178915–51178899, Horse: Jan.2007 ChrUn(-):175282430–175282414, Dog: May 2005 Chr6(+): 9619423–9619439, Human: Mar.2006 Chr7(-):72700300–72700284. Color coding: light grey, identical residues; dark grey, unconserved residues. (F) Functional analysis of putative ChoRE sequences at the position -109 to -92 on Chrebpβ. We co-transfected luciferase reporter driven by two copies of rat Chrebpβ ChoRE upstream of minimal TATA promoter with caChREBP in 832/13 cells in RPMI with 5.5 mmol/l D-glucose. Gaussia luciferase activity was measured at 48h and normalized to Cypridina luciferase, and expressed as fold activation over the activity seen in cells transfected with two copies of ChoRE with minimal TATA promoter and empty vector. *, p< 0.05.
We performed reverse transcription followed by the quantitative polymerase chain reaction (RT-qPCR) and found that expression of all ChREBP mutants were successfully induced by addition of doxycycline in culture media (data not shown). High levels of caChREBP and dnChREBP expression were reached after 48–72 hours of induction. We found that endogenous rat Chrebpβ expression was significantly increased in caChREBP cells by ~5.5–fold as compared to expression in eYFPnuc cells, while expression of endogenous Chrebpα did not change (Fig 1C). In contrast, Chrebpβ expression was decreased significantly by ~85% in dnChREBP cells, while Chrebpα expression also failed to show a change in this setting (Fig 1D).
Herman et al identified a ChoRE at the position +158 to +174 on exon1b of mouse Chrebp that mediates specific induction of Chrebpβ expression by Chrebpα [15]. This sequence is partially conserved from rat to human. In addition, a separate E-box at the position -98 to -93 also conferred glucose responsiveness of proximal mouse Chrebpβ promoter. We noted that deletion of this E-box obviously abolished high glucose-induced promoter activity in the presence of Chrebpα and Mlx in their luciferase assay data, whereas deletion of ChoRE had minor effect on enhancing promoter activity by glucose, a fact that is consistent with a crucial role of this E-box in regulation of Chrebpβ transcription. We observed the presence of another E-box-liked sequence separated from the first E-box by 5 base pairs (Fig 1E). The sequence of this newly identified putative ChoRE is highly conserved from rat to human, supporting a functionally important role for Chrebpβ expression. When this manuscript was under review, Zhang et al concomitantly found the same ChoRE sequence and demonstrated that ChREBP binds to this ChoRE [36]. This element is necessary for Chrebpβ promoter in response to glucose in 832/13 cells.
To further explore whether both ChoREs are sufficient for the activation by ChREBP and are equally preferred to caChREBP, we generated two luciferase reporter constructs driven by two copies of rat ChoRE located upstream of minimal TATA promoter and performed luciferase assay. We found that co-transfection of caChREBP construct and luciferase reporter construct with two copies of the rat sequence at the position +160 to +176 of Chrebpβ, that is conserved to previously identified mouse Chrebpβ ChoRE, did not change luciferase reporter compared with empty vector in 832/13 cells (data not shown). Interestingly, caChREBP gave 6.4-fold stimulation of activity of promoter containing two copies of rat Chrebpβ ChoRE sequence identified in this study (position -109 to -92) (Fig 1F). Given the fact that amino acid sequences of our caChREBP is almost identical to endogenous Chrebpβ in mouse and rat (Fig 1A) and caChREBP stimulates expression of endogenous Chrebpβ, our finding provides a clue to an autoregulation of Chrebpβ via recently recognized ChoRE sequence on its proximal promoter.
ChREBP regulates metabolic enzyme genes and lipogenic genes in β-cells independent of SREBP-1c
We, and Da Silva Xavier et al, have shown that ChREBP controls Pklr and Fasn expression and intracellular triglyceride levels in rat and mouse pancreatic β-cell lines [30, 31]. In order to get a more comprehensive picture on ChREBP-regulated genes in β-cells, we first performed RT-qPCR analysis of expression of metabolic pathway gene panels involved in glycolysis, lipogenesis, and lipid oxidation in control and caChREBP 832/13 cells (Fig 2A). We found that not only were the expression levels of Pklr and Fasn upregulated in caChREBP cells, but expression of many glycolytic and lipogenic genes were also significantly increased, i.e. solute carrier family 2 (facilitated glucose transporter), member 2 (Slc2a2), glucose-6-phosphate isomerase (Gpi), phosphofructokinase (Pfk), Triosephosphate Isomerase 1 (Tpi1), Gapdh, phosphoglycerate kinase1 (Pgk1), enolase1 (Eno1), Pc, malate dehydrogenase 1 (Mdh1), malic enzyme1 (Me1), pyruvate dehydrogenase (lipoamide) alpha 1 (Pdha1), citrate synthase (Cs), ATP citrate lyase (Acly), Acaca, Elovl6, Scd1, Gpd1, and diacylglycerol o-acyltransferase 2 (Dgat2). Carnitine palmitoyltransferase 1 (Cpt1a), a gene associated with lipid oxidation, was reduced significantly.
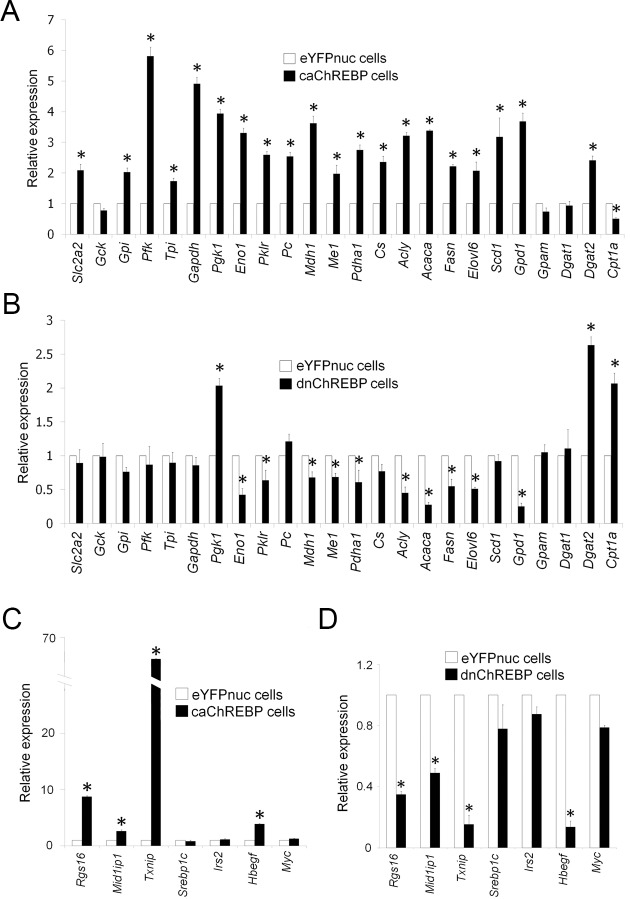
Fig 2. ChREBP regulates the expression of metabolic genes in 832/13 β-cells.
(A-B) Expression of genes encoding enzymes involved in glycolysis, lipogenesis, triglyceride biosynthesis and lipid oxidation in caChREBP cells (A) and dnChREBP cells (B). *p< 0.05 compared with eYFPnuc cells. (C-D) Expression of metabolic genes, including Rgs16 and Mid1ip1, Srebp1c and its target Irs2, and genes involved in cell proliferation, Hbegf and Myc, in caChREBP cells (C) and dnChREBP cells (D). *p< 0.05 compared with eYFPnuc cells.
We next examined the expression level of these genes in dnChREBP cells. Our dnChREBP, a mouse ChREBP mutant lacking of transactivation domain, occupies ChoRE without transcriptional induction of target genes (data not shown). We found that levels of gene expression of Eno1, Pklr, Mdh1, Me1, Pdha1, Acly, Acaca, Fasn, Elovl6, and Gpd1were downregulated after induction of dnChREBP expression for 36–72 hours (Fig 2B). Expression of Pgk1, Dgat2 and Cpt1a was increased significantly.
We also evaluated the expression of other lipogenic genes, including Rgs16, MID1 interacting protein 1 (Mid1ip1), and Txnip [37–39], and found that these genes were induced in caChREBP cells (Fig 2C) and were reduced in dnChREBP cells (Fig 2D). Sterol regulatory element-binding protein-1c (SREBP-1c) is a key transcription factor that shares a part of downstream target genes with ChREBP, e.g. Pklr, Fasn and Acaca, and controls hepatic fatty acid synthesis [40]. It has been reported that ChREBP binds to human SREBP-1c gene and conveys glucose action on SREBP-1c gene expression in hepatocytes [41]. In INS-1 β-cell line, overexpression of nuclear active form of SREBP-1c led to marked accumulation of lipid droplets [42]. To exclude the possibility that ChREBP indirectly affected expression of lipogenic genes via SREBP-1c, we examined the expression of Srebp1c using variant-specific primers and found that Srebp1c gene expression was not altered in caChREBP or dnChREBP cells (Fig 2C and 2D). In addition, expression of insulin receptor substrate 2 (Irs2), a Srebp1c target in β-cells [43], did not change in either type of cells. These findings suggest that ChREBP controls downstream glycolytic and lipogenic genes independent of SREBP-1c.
Recently published article reports that ChREBP controls expression of heparin-binding EGF-like growth factor (Hbegf) and Myc genes which are involved in cellular proliferation [36]. In our study, we found that Hbegf expression is significantly increased in caChREBP cells and decreased in dnChREBP cells (Fig 2C and 2D), whereas the expression of Myc remained unchanged in the two types of cells.
Evolutionarily conserved Gpd1 ChoRE sequence does not respond to ChREBP in β-cells
Gpd1 is another gene whose expression was increased by caChREBP and decreased by dnChREBP in this study. In hepatocytes, Gpd1 was induced by glucose and this induction was repressed by dnMlx [20]. A potential ChoRE on was identified at position -1943 to -1927 of the rat Gpd1 promoter and binding of ChREBP to this ChoRE was confirmed by electrophoretic mobility shift assay [20]. However, glucose cannot activate either ChoRE-containing minimal Gpd1 promoter or minimal TATA-containing promoter with two copies of Gpd1 ChoRE [20].
We found that Gpd1 ChoRE is highly conserved not only between rodents and human, but also in several other species (Fig 3A). To evaluate whether this Gpd1 ChoRE can respond to ChREBP in β-cells, we cloned two copies of Gpd1 ChoRE upstream of the minimal TATA promoter in the reporter plasmid and performed luciferase assay in the existence of caChREBP or empty vector in 832/13 cells. We found that caChREBP had no impact at all on activity of this promoter (Fig 3B). Our finding supports a previous report that ChREBP does not regulate Gpd1 expression through this putative ChoRE [20].
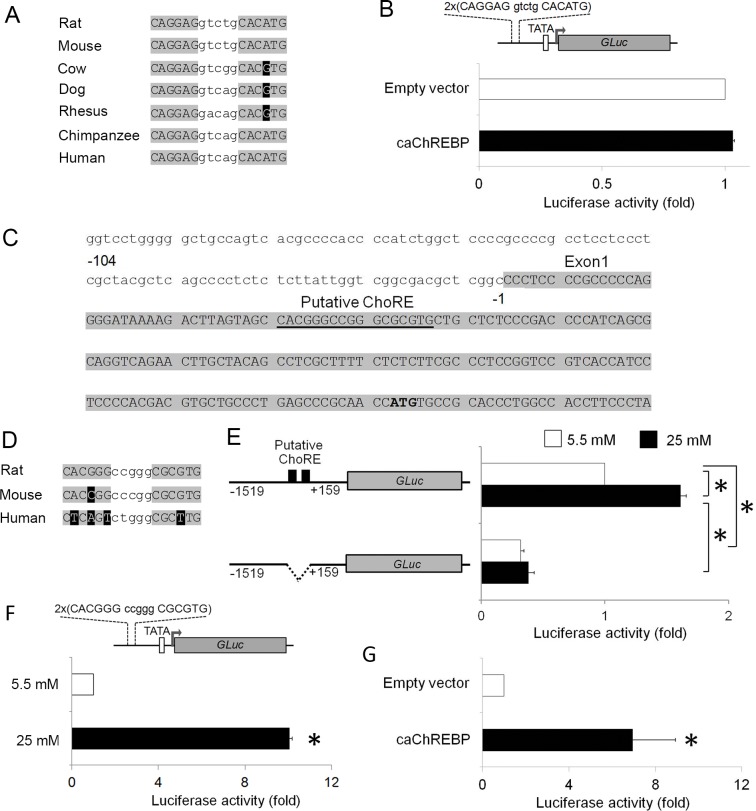
Fig 3. Effect of caChREBP on Gpd1 or Rgs16 ChoRE-containing promoters.
(A) Alignment of ChoRE sequence presented on Gpd1 promoters among rat (at the position -1943 to -1927), mouse, cow, dog, rhesus, chimpanzee, and human. Sequence assemblies and coordinates are as follows: Rat: Mar.2012 ChrX(+): 115873245–11587361, Mouse: Dec.2011 Chr15(+): 99716109–99716125, Cow: Oct.2011 Chr5(-): 32732081–32732065, Dog: Sep.2011 Chr27(-): 4623314–4623298, Rhesus: Oct.2010 Chr11(+): 47359512–47359528, Chimpanzee: Feb.2011 Chr12(-): 39238594–39238578, and Human: Feb.2009 Chr12(+): 50496575–50496591. Color coding: light grey, identical residues; dark grey, unconserved residues. (B) caChREBP cannot activate Gpd1 ChoRE-containing promoter. We co-transfected luciferase reporter driven by two copies of rat Gpd1 ChoRE (position -1943 to -1927 relative to TSS) upstream of minimal TATA promoter with caChREBP in 832/13 cells in RPMI with 5.5 mmol/l D-glucose. Gaussia luciferase activity was measured at 48h and normalized to Cypridina luciferase, and expressed as fold activation over the activity seen in cells transfected with two copies of ChoRE with minimal TATA promoter and empty vector. *, p< 0.05. (C) Sequence of rat Rgs16 proximal promoter (-104 to +196, relative to TSS). Bold text, Start codon. Color coding: light grey, Exon 1. (D) Alignment of ChoRE sequence presented on Rgs16 promoter among rat (at the position +37 to +53), mouse, and human. Sequence assemblies and coordinates are as follows: Rat: Mar.2012 Chr13(+): 76145198–76145214, Mouse: Dec.2011 Chr1(+): 153740341–153740357, and Human: Dec.2013 Chr1(-): 182604401–182604385. Color coding: light grey, identical residues; dark grey, unconserved residues. (E) The effect of high glucose on the activity of natural Rgs16 promoter and mutated Rgs16 promoter. We transfected luciferase reporter driven by natural Rgs16 promoter (position -1519 to +159 relative to TSS) or ChoRE-deleted Rgs16 promoter in 832/13 cells cultured in RPMI with 5.5 mmol/l or 25 mmol/l D-glucose. Gaussia luciferase activity was measured at 48h and normalized to Cypridina luciferase, and expressed as fold activation over the activity seen in cells transfected with natural Rgs16 promoter and exposed to 5.5 mmol/l D-glucose. *, p< 0.05. (F-G) Glucose and caChREBP stimulates Rgs16 ChoRE-containing promoter. We transfected luciferase reporter driven by two copies of rat Rgs16 ChoRE (position +37 to +53 relative to TSS) upstream of minimal TATA promoter in 832/13 cells in RPMI with 5.5 or 25 mmol/l D-glucose (F) or co-transfected with caChREBP or empty vector in 832/13 cells in RPMI with 5.5 mmol/l D-glucose (G). Gaussia luciferase activity was measured at 48h and normalized to Cypridina luciferase, and expressed as fold activation over the activity seen in cells transfected with two copies of ChoRE with minimal TATA promoter and exposed to 5.5 mmol/l D-glucose (F) or co-transfected with empty vector (G). *, p< 0.05.
Proximal promoter of Rgs16 contains functional ChoRE sequence
Among the genes that are altered in both caChREBP and dnChREBP cells in the current study, Txnip is the most affected gene by ChREBP. Functional ChREBP binding sites on human and murine Txnip promoters were well described [30, 44]. Rgs16 is the second most upregulated gene by caChREBP (~9–fold increased). Few studies in hepatocytes have shown that ChREBP regulates Rgs16 expression [20, 39]. Iizuka et al have demonstrated that either high glucose condition or dominant active form of ChREBP is able to induce Rgs16 mRNA expression in 832/13 cells [45]. We have also confirmed that glucotoxic condition upregulates Rgs16 expression in these cells. Our RT-qPCR result showed that Rgs16 expression in 832/13 cells under 25 mmol/l D-glucose condition is ~8.9-fold higher than that in cells exposed to 5.5 mmol/l D-glucose (data not shown).
To further investigate whether ChREBPβ mediates glucose-induced Rgs16 expression, we have constructed lentiviral vectors with Tet-inducibility to generate microRNA-adapted shRNA based on optimized miRNA mimic design (S1A Fig.) [33]. We transduced viral vectors in 832/13 cells to establish inducible cell lines harboring Chrebpβ shRNA targeting sequence (mirGE-β cells) and non-silencing sequence (mirGE-N cells). The expression of Chrebpβ in induced mirGE-β cells was ~46% as compared to expression in induced mirGE-N cells (S1B Fig.). However, Rgs16 expression was not different between these two cell lines.
The ChoRE on Rgs16 promoter has never been identified or tested. We analyzed rat Rgs16 promoter in silico and found a putative ChoRE sequence at position +37 to +53 from the transcription start site based on the Ensembl database (ENSRNOT00000032236) (Fig 3C). Sequence alignment of each ChoRE exhibited high conservation between rat and mouse, but not well-conserved in the human sequence (Fig 3D).
To dissect the role of a putative ChoRE in the glucose activation of the Rgs16 promoter, we used luciferase reporter constructs driven by the Rgs16 promoter (-1519 to +159) with or without this ChoRE (Fig 3E) to transfect into 832/13 cells under low (5.5 mmol/l) or high (25 mmol/l) glucose condition and performed luciferase assay. We found a small but significant increase (~1.6-fold) in the activity of natural Rgs16 promoter by glucose, whereas Rgs16 promoter without ChoRE exhibited a lower basal activity and did not respond to glucose (Fig 3E).
To test whether ChoRE sequence on the Rgs16 promoter is able to mediate glucose- and ChREBP-induced promoter activity, we performed luciferase assay in 832/13 cells using reporter construct driven by minimal TATA promoter and 2 copies of putative rat Rgs16 ChoRE in low glucose (5 mmol/l D-glucose) or high glucose (25 mmol/l D-glucose) condition (Fig 3F) and in the presence of caChREBP or empty vector construct (Fig 3G). We found that glucose and caChREBP induced activity of the promoter that contains Rgs16 ChoRE at position +37 to +53 by ~10-fold and 6.9–fold, respectively (Fig 3F and 3G). Our result indicates that this sequence is both glucose- and ChREBP-responsive in pancreatic β-cells.
Increased Rgs16 enhances accumulation of neutral lipid in β-cells
Transgenic mice overexpressing liver-specific Rgs16 protein had reduced hepatic fatty acid oxidation and developed hepatosteatosis [39]. To investigate whether Rgs16 plays a similar role in β-cells, we overexpressed rat Rgs16 using Tet-on inducible system in 832/13 cells (Fig 4A). Exogenous Rgs16 was successfully induced after addition of doxycycline to culture medium for 72 hour (data not shown). We then performed Oil Red O staining of Rgs16-overexpressing cells (Rgs16 cells), caChREBP cells and eYFPnuc cells to visualize intracellular neutral lipids (Fig 4B). Quantitative analysis showed that Rgs16 cells exhibited ~3.3–fold increased cytoplasmic lipids as compared to eYFPnuc cells (Fig 4C). As we expected, caChREBP cells displayed even higher intracellular lipids, ~8.3–fold compared with eYFPnuc cells.
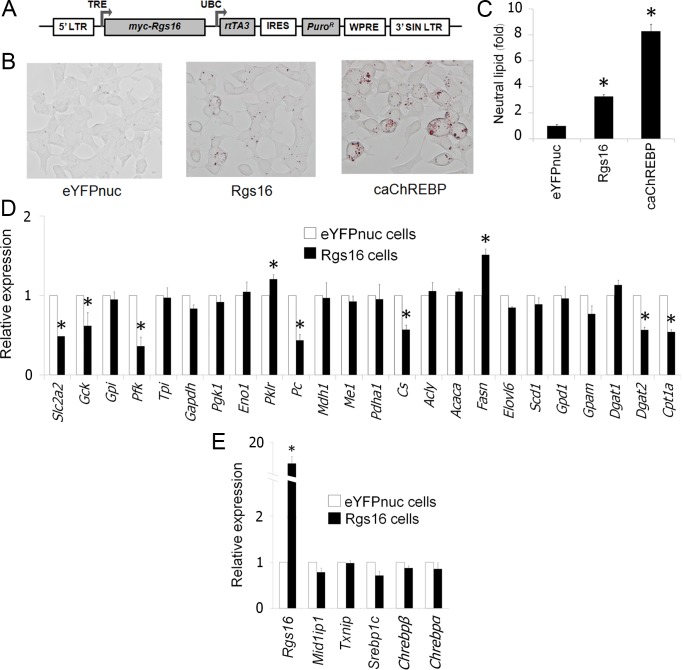
Fig 4. Rgs16 enhances accumulation of neutral lipid in 832/13 β-cells.
(A) Schematic diagram of tetracycline-inducible vectors for overexpression of Rgs16. LTR, long terminal repeat; TRE, tetracycline-responsive promoter element; UBC, human ubiquitin C promoter; rtTA3, reverse tetracycline-transactivator 3; IRES, internal ribosomal entry site; PuroR, puromycin resistance gene; WPRE, Woodchuck hepatitis posttranscriptional regulatory element; SIN LTR, self-inactivating long terminal repeat. (B-C) Overexpression of Rgs16 triggers lipid accumulation in 832/13 cells. We incubated eYFPnuc cells, Rgs16 cells, and caChREBP cells for 72h in RPMI with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. We stained these cells for neutral lipid by Oil red O (B). Histograms (C) represent the amount of stained intracellular lipid compared with eYFPnuc cells. *, p< 0.05 compared with eYFPnuc cells. (D-E) Effects of Rgs16 overexpression on genes encoding metabolic enzymes (D) and related metabolic genes (E). We incubated Rgs16 cells for 72h in RPMI with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. We isolated the RNA and performed RT-qPCR using gene-specific primers. The histograms are the means of relative RNA levels normalized to Eef1g and Hprt1 and expressed as fold activation over the activity seen in eYFPnuc cells preincubated with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. *, p< 0.05 compared with eYFPnuc cells.
We next measured gene expression in Rgs16 cells by RT-qPCR (Fig 4D and 4E). Total Rgs16 transcripts were increased ~17.0–fold (Fig 4E). Concomitantly, Pklr and Fasn were slightly upregulated, while Cpt1a expression was reduced to half (Fig 4D). Expression of Slc2a2, Gck, Pfk, Pc, Cs, and Dgat2, was significantly downregulated. Chrebpα and Chrebpβ expression did not change significantly in Rgs16 cells (Fig 4E).
Discussion
ChREBP is a glucose-responsive transcription factor that was shown to play an important role in β-cell glucotoxicity [30]. Although we have shown that accumulation of lipid droplets, a major phenotypic characteristic in glucotoxic β-cells, appears to be mediated by ChREBP expression, the mechanisms underlying this phenomenon are only partly understood. Alteration in expression of metabolic genes may partially explain ChREBP-induced lipid accumulation. A few metabolic genes have been well-characterized as direct downstream targets of ChREBP in pancreatic β-cells, including Txnip, Fasn, and Pklr. ChREBP binds to their respective promoters, regulates promoter activities as well as expression of these genes [30, 31, 44, 45]. Gpd1 and Rgs16 expression was shown to be activated by a dominant active mutant of ChREBP in INS-1E β-cell line [45]. It is therefore not surprising to see upregulation of these genes by caChREBP and downregulation by dnChREBP in our experiments. Several other metabolic genes exhibited the same expression pattern, i.e. Eno1, Mdh1, Me1, Pdha1, Acly, Acaca, Elovl6, Mid1ip1 and Chrebpβ, while a gene encoding the lipid oxidation enzyme, Cpt1a, manifested the opposite expression pattern. Our results suggest that ChREBP is sufficient and necessary for regulation of these genes. Among this list, Acaca, Mid1ip1 and Chrebpβ genes are ChREBP targets with ChoRE on their promoters [4, 15, 46]. Although Gapdh and Pc are also ChREBP target genes which ChoREs have been identified [22–24], their expressions were controlled by caChREBP, but not dnChREBP, in our experiments. A similar pattern was found in expression of Slc2a2, Gpi, Pfk, Cs, Tpi, and Scd1. These findings indicate that ChREBP is sufficient but not necessary for regulation of these genes in β-cell line. Pgk1 and Dgat2 were upregulated by both caChREBP and dnChREBP. There may be other factors that modulate the effect of ChREBP on these genes. Despite the fact that Srebp1c is a candidate ChREBP target gene [41], we clearly showed that ChREBP has no influence on the expression of Srebp1c and its target gene Irs2 in 832/13 β-cells, suggesting that ChREBP regulates expression of several metabolic genes independent of SREBP-1c.
Previous studies have used different approaches to demonstrate functional ChoREs in β-cells. Txnip and Pklr ChoREs confer ChREBP activation of the respective promoters in β-cells [45]. G6pc and Fasn ChoREs have been functionally analyzed in term of responsiveness to high glucose, but not ChREBP [21, 31]. In the current study, we describe two new functional ChoRE sequences on the promoters of Chrebpβ and Rgs16. In agreement with a recent study by Zhang et al. [36] that was published while our manuscript was under review, we found that the same Chrebpβ ChoRE sequence played a major role on the Chrebpβ promoter in response to glucose. The Chrebpβ ChoRE sequence is highly conserved. Our results further demonstrate that the caChREBP, which has similar amino acid sequence domains as Chrebpβ, was able to activate artificial promoters which contain 2 copies of these ChoREs. Our experiments suggest the possibility that Chrebpβ auto-regulates its own expression, but not Chrebpα expression, perhaps through ChoRE at the position -109 to -93 of rat Chrebpβ, that is absent in Chrebpα. It is noteworthy that nucleotide at the third position of this rat Chrebpβ sequence is guanine (G), which is inconsistent with a previously articulated hypothesis on functional ChoRE sequence used to explain the absence of Gpd1 ChoRE responsiveness to high glucose [20]. On the other hand, our result is supported by previous reports on ChoREs of Krüppel-like factor-10 (Klf10) and Fgf21 which can be activated by ChREBP and high glucose [25, 47]. It is likely that the sequence context surrounding the third nucleotide on functional ChoRE contributes to the response.
It has been shown that previously identified Chrebpβ ChoRE, at the position +158 to +174, mediates the activation of mouse Chrebpβ promoter by Chrebpα [15]. In our study, activity of the promoter with two copies of its conserved ChoRE sequence at position +160 to +176 of rat Chrebpβ did not alter. These findings cannot be directly compared to each other because there are at least four important differences in experimental design/condition: 1) our study focused on function of ChoRE independent of other response elements on Chrebpβ promoter. 2) unconserved fourth nucleotide of these ChoREs (C versus T nucleotide), 3) distinction between response to activation by caChREBP and Chrebpα/Mlx with glucose, and 4) difference in cell types between experiments (832/13 β-cells versus HEK293T cells).
While this manuscript was under review, Zhang et al. reported that ChREBP differentially binds to Chrebpβ ChoREs in a tissue-specific manner [36]. It is noteworthy that our recently published high through-put DNA sequencing (ChIP-seq) data [11] also showed that ChREBP binds to the Chrebpβ promoter in both mouse liver and white adipose tissue with a higher preference for the liver. Interestingly, the ChoRE identified in the current study is located at the summit of the ChIP-seq peaks in both tissues (S2 Fig); there is no evidence of ChREBP binding occurs at the position that had previously been identified as ChoRE [15]. Additional studies will be needed to further dissect the tissue specificity of ChREBP binding on two Chrebpβ ChoREs.
Rgs16 is one of the proteins that regulate G protein-coupled receptor signaling. It has been reported that expression of Rgs16 is quiescent in adult pancreatic islets and chronic hyperglycemia reactivates Rgs16 re-expression [48]. In this study, we have tried to knockdown Chrebpβ to determine if Chrebpβ mediates high glucose-induced Rgs16 expression in β-cells. We found that ~54% Chrebpβ silencing has no effect on Rgs16 expression. This diminished Chrebpβ expression level may not be sufficient to affect target gene expression and the existing Chrebpα protein could activate its target genes in high glucose condition. A similar phenomenon was recently reported that ~50% Chrebpβ silencing could not alter Txnip expression in 832/13 cells [36]. Therefore it is still unclear whether Chrebpβ plays a major role on Rgs16 expression in β-cells under high glucose condition.
Our study demonstrates that functional ChoRE sequence of rat Rgs16 is located at the position +37 to +53 relative to its transcription start site. Induced expression of caChREBP increased expression of Rgs16 in β-cells, while dnChREBP had an opposite effect. Overexpression of rat Rgs16 led to an increase in lipid accumulation in 832/13 cells in a way analogous to that found in liver-specific Rgs16 overexpression in mice [39]. Gene expression analysis reveals effects of Rgs16 on expression of three genes encoding key enzymes that may mediate the accumulation of lipid droplets. Decreased expression of Cpt1a is similar to that found in the liver of Rgs16 transgenic mice. It was proposed that Rgs16 does not promote genes involved in glycolysis or fatty acid synthesis, however, expression of Pklr and Fasn was mildly increased in our Rgs16-overexpressing 832/13 cells. Interestingly, we observed reduced expression of Slc2a2, Pfk, Pc, Cs and Dgat2, opposites to what we have seen in caChREBP cells. These effects of increased Rgs16 expression may somehow interfere with impact of caChREBP on expression of these genes in caChREBP-overexpressing 832/13 cells. Neither Chrebpα nor Chrebpβ expression was influenced by induction of Rgs16, indicating that no feedback loop existed.
In this work we have for the first time provided evidence for possible auto-regulation of Chrebpβ through newly identified ChoRE on its proximal promoter and broad consequences of ChREBP on metabolic gene expression (Fig 5). We also described the molecular mechanisms underlying ChREBP-mediated lipid accumulation a phenotype that occurs commonly in pancreatic beta-cells glucotoxicity. We further demonstrated that Rgs16 is a ChREBP target gene in β-cells with functional Rgs16 ChoRE sequence, whose activation contributes in part to the lipid accumulation in glucotoxicity.
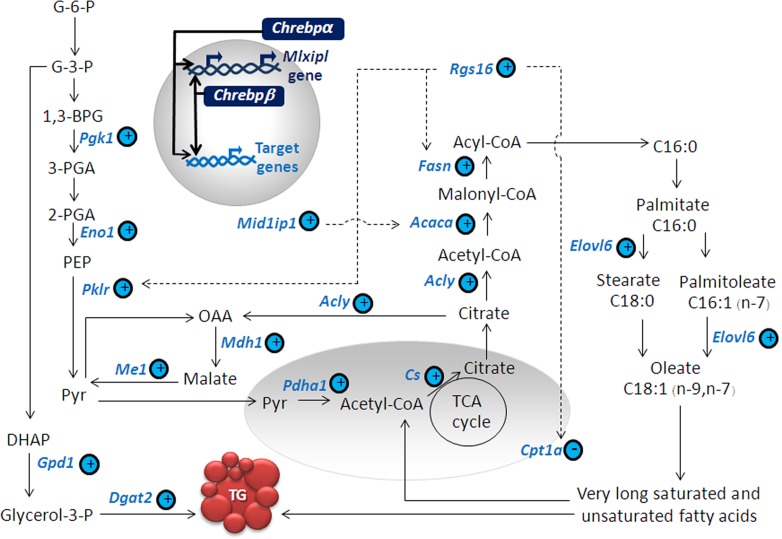
Fig 5. Model of ChREBP-induced lipid accumulation in β-cells.
Plus symbol, upregulation; Minus symbol, downregulation; Pgk1, phosphoglycerate kinase1; Eno1, enolase1; Pklr, L-type pyruvate kinase; Mdh1, malate dehydrogenase 1; Me1, malic enzyme1; Pdha1, pyruvate dehydrogenase (lipoamide) alpha 1; Cs, citrate synthase; Acly, ATP citrate lyase; Mid1ip1, MID1 interacting protein 1; Acaca, acetyl-CoA carboxylase alpha; Fasn, fatty acid synthase; Elovl6, ELOVL fatty acid elongase 6; Rgs16, regulator of G-protein signaling 16; Cpt1a, carnitine palmitoyltransferase 1; G-6-P, glucose-6-phosphate; G-3-P, glyceraldehydes-3-phosphate; 1,3-BPG, 1,3-bisphosphoglycerate; 3-PGA, 3-phosphoglycerate; 2-PGA, 2-phosphoglycerate; PEP, phosphoenolpyruvate; Pyr, pyruvate; DHAP, dihydroxyacetone phosphate; Glycerol-3-P, glycerol;-3-phosphate; OAA, oxaloacetate.
Supporting Information
(A) Schematic diagram of tetracycline-inducible lentiviral vectors for expression of microRNA-adapted short hairpin RNA to target Chrebpβ (mirGE-β) or non-silencing (mirGE-N) sequences. (B) Effect of Chrebpβ shRNA on the expression of Chrebpβ and Rgs16 in 832/13 cells. We pre-incubated mirGE-β cells and mirGE-N cells for 24h in RPMI with 5.5 mmol/l D-glucose in the presence of doxycycline 1 μg/mL, and switched to RPMI with 25 mmol/l D-glucose in the presence of doxycycline 1 μg/mL for 48h. The histograms are the means of relative RNA levels normalized to Ywhaz and Hprt1 and expressed as fold activation over the activity seen in mirGE-N cells. *, p< 0.05.
(TIF)
We explored the anti-ChREBP ChIP-seq data using the Integrated Genome Browser and demonstrated the presence of ChoRE sequence identified in this study at the summit of ChIP-seq peaks in mouse liver and white adipose tissue. Gray vertical line indicates the position where previously identified ChoRE is located.
(TIF)
Acknowledgments
We thank Dr.Christopher Newgard for providing 832/13 cells and Priyakorn Chinnasang for technical assistance. A part of this work was presented at the Seoul International Congress of Endocrinology and Metabolism, held at Seoul, Korea, on May 3, 2013.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Siriraj research fund (R015433020) from Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand (NP) and Discovery-based Development Grant (DD Grant) from National Science and Technology Development Agency (NSTDA), Thailand (NP). It was supported in part by National Institutes of Health grants HL051586/DK105527 and P30DK079638 and the Rutherford Chair in Diabetes research to LC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Decaux JF, Antoine B, Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem. 1989. July 15;264(20):11584–90. [PubMed] [Google Scholar]
- 2.Roche E, Farfari S, Witters LA, Assimacopoulos-Jeannet F, Thumelin S, Brun T, et al. Long-term exposure of beta-INS cells to high glucose concentrations increases anaplerosis, lipogenesis, and lipogenic gene expression. Diabetes. 1998. July;47(7):1086–94. [DOI] [PubMed] [Google Scholar]
- 3.Prip-Buus C, Perdereau D, Foufelle F, Maury J, Ferre P, Girard J. Induction of fatty-acid-synthase gene expression by glucose in primary culture of rat hepatocytes. Dependency upon glucokinase activity. Eur J Biochem. 1995. May 15;230(1):309–15. [DOI] [PubMed] [Google Scholar]
- 4.O'Callaghan BL, Koo SH, Wu Y, Freake HC, Towle HC. Glucose regulation of the acetyl-CoA carboxylase promoter PI in rat hepatocytes. J Biol Chem. 2001. May 11;276(19):16033–9. [DOI] [PubMed] [Google Scholar]
- 5.Mourrieras F, Foufelle F, Foretz M, Morin J, Bouche S, Ferre P. Induction of fatty acid synthase and S14 gene expression by glucose, xylitol and dihydroxyacetone in cultured rat hepatocytes is closely correlated with glucose 6-phosphate concentrations. Biochem J. 1997. September 1;326 (Pt 2):345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rufo C, Teran-Garcia M, Nakamura MT, Koo SH, Towle HC, Clarke SD. Involvement of a unique carbohydrate-responsive factor in the glucose regulation of rat liver fatty-acid synthase gene transcription. J Biol Chem. 2001. June 15;276(24):21969–75. [DOI] [PubMed] [Google Scholar]
- 7.Bergot MO, Diaz-Guerra MJ, Puzenat N, Raymondjean M, Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 1992. April 25;20(8):1871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Thompson KS, Towle HC. Carbohydrate regulation of the rat L-type pyruvate kinase gene requires two nuclear factors: LF-A1 and a member of the c-myc family. J Biol Chem. 1993. June 15;268(17):12787–95. [PubMed] [Google Scholar]
- 9.Koo SH, Towle HC. Glucose regulation of mouse S(14) gene expression in hepatocytes. Involvement of a novel transcription factor complex. J Biol Chem. 2000. February 18;275(7):5200–7. [DOI] [PubMed] [Google Scholar]
- 10.Shih HM, Liu Z, Towle HC. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J Biol Chem. 1995. September 15;270(37):21991–7. [DOI] [PubMed] [Google Scholar]
- 11.Poungvarin N, Chang B, Imamura M, Chen J, Moolsuwan K, Sae-Lee C, et al. Genome-Wide Analysis of ChREBP Binding Sites on Male Mouse Liver and White Adipose Chromatin. Endocrinology. 2015. June;156(6):1982–94. 10.1210/en.2014-1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih H, Towle HC. Definition of the carbohydrate response element of the rat S14 gene. Context of the CACGTG motif determines the specificity of carbohydrate regulation. J Biol Chem. 1994. March 25;269(12):9380–7. [PubMed] [Google Scholar]
- 13.Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001. July 31;98(16):9116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metukuri MR, Zhang P, Basantani MK, Chin C, Stamateris RE, Alonso LC, et al. ChREBP mediates glucose-stimulated pancreatic beta-cell proliferation. Diabetes. 2012. August;61(8):2004–15. 10.2337/db11-0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012. April 19;484(7394):333–8. 10.1038/nature10986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A. 2001. November 20;98(24):13710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsatsos NG, Towle HC. Glucose activation of ChREBP in hepatocytes occurs via a two-step mechanism. Biochem Biophys Res Commun. 2006. February 10;340(2):449–56. [DOI] [PubMed] [Google Scholar]
- 18.Li MV, Chang B, Imamura M, Poungvarin N, Chan L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes. 2006. May;55(5):1179–89. [DOI] [PubMed] [Google Scholar]
- 19.Li MV, Chen W, Poungvarin N, Imamura M, Chan L. Glucose-mediated transactivation of carbohydrate response element-binding protein requires cooperative actions from Mondo conserved regions and essential trans-acting factor 14-3-3. Mol Endocrinol. 2008. July;22(7):1658–72. 10.1210/me.2007-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006. September 29;281(39):28721–30. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen KB, Zhang P, Doumen C, Charbonnet M, Lu D, Newgard CB, et al. The promoter for the gene encoding the catalytic subunit of rat glucose-6-phosphatase contains two distinct glucose-responsive regions. Am J Physiol Endocrinol Metab. 2007. March;292(3):E788–801. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen KB, Buckley RS, Scioneaux R. Glucose induces expression of rat pyruvate carboxylase through a carbohydrate response element in the distal gene promoter. Biochem J. 2010. March 1;426(2):159–70. 10.1042/BJ20091266 [DOI] [PubMed] [Google Scholar]
- 23.Wutthisathapornchai A, Vongpipatana T, Muangsawat S, Boonsaen T, MacDonald MJ, Jitrapakdee S. Multiple e-boxes in the distal promoter of the rat pyruvate carboxylase gene function as a glucose-responsive element. PLoS One. 2014;9(7):e102730 10.1371/journal.pone.0102730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanke N, Scheibe RJ, Manukjan G, Ewers D, Umeda PK, Chang KC, et al. Gene regulation mediating fiber-type transformation in skeletal muscle cells is partly glucose- and ChREBP-dependent. Biochim Biophys Acta. 2011. March;1813(3):377–89. 10.1016/j.bbamcr.2010.12.021 [DOI] [PubMed] [Google Scholar]
- 25.Iizuka K, Takeda J, Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009. September 3;583(17):2882–6. 10.1016/j.febslet.2009.07.053 [DOI] [PubMed] [Google Scholar]
- 26.Iizuka K, Horikawa Y. Regulation of lipogenesis via BHLHB2/DEC1 and ChREBP feedback looping. Biochem Biophys Res Commun. 2008. September 12;374(1):95–100. 10.1016/j.bbrc.2008.06.101 [DOI] [PubMed] [Google Scholar]
- 27.Benhamed F, Denechaud PD, Lemoine M, Robichon C, Moldes M, Bertrand-Michel J, et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012. June 1;122(6):2176–94. 10.1172/JCI41636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004. May 11;101(19):7281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iizuka K, Takeda J, Horikawa Y. Hepatic overexpression of dominant negative Mlx improves metabolic profile in diabetes-prone C57BL/6J mice. Biochem Biophys Res Commun. 2009. February 6;379(2):499–504. 10.1016/j.bbrc.2008.12.100 [DOI] [PubMed] [Google Scholar]
- 30.Poungvarin N, Lee JK, Yechoor VK, Li MV, Assavapokee T, Suksaranjit P, et al. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in beta cell glucotoxicity. Diabetologia. 2012. June;55(6):1783–96. 10.1007/s00125-012-2506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva Xavier G, Rutter GA, Diraison F, Andreolas C, Leclerc I. ChREBP binding to fatty acid synthase and L-type pyruvate kinase genes is stimulated by glucose in pancreatic beta-cells. J Lipid Res. 2006. November;47(11):2482–91. [DOI] [PubMed] [Google Scholar]
- 32.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000. March;49(3):424–30. [DOI] [PubMed] [Google Scholar]
- 33.Myburgh R, Cherpin O, Schlaepfer E, Rehrauer H, Speck RF, Krause KH, et al. Optimization of Critical Hairpin Features Allows miRNA-based Gene Knockdown Upon Single-copy Transduction. Mol Ther Nucleic Acids. 2014;3:e207 10.1038/mtna.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002. June 18;3(7):RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das AT, Zhou X, Vink M, Klaver B, Verhoef K, Marzio G, et al. Viral evolution as a tool to improve the tetracycline-regulated gene expression system. J Biol Chem. 2004. April 30;279(18):18776–82. [DOI] [PubMed] [Google Scholar]
- 36.Zhang P, Kumar A, Katz LS, Li L, Paulynice M, Herman MA, et al. Induction of the ChREBPbeta isoform is essential for glucose-stimulated beta cell proliferation. Diabetes. 2015. September 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park MJ, Kim DI, Lim SK, Choi JH, Kim JC, Yoon KC, et al. Thioredoxin-interacting protein mediates hepatic lipogenesis and inflammation via PRMT1 and PGC-1alpha regulation in vitro and in vivo. Journal of hepatology. 2014. November;61(5):1151–7. 10.1016/j.jhep.2014.06.032 [DOI] [PubMed] [Google Scholar]
- 38.Aipoalani DL, O'Callaghan BL, Mashek DG, Mariash CN, Towle HC. Overlapping roles of the glucose-responsive genes, S14 and S14R, in hepatic lipogenesis. Endocrinology. [Research Support, N.I.H., Extramural]. 2010. May;151(5):2071–7. 10.1210/en.2009-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pashkov V, Huang J, Parameswara VK, Kedzierski W, Kurrasch DM, Tall GG, et al. Regulator of G protein signaling (RGS16) inhibits hepatic fatty acid oxidation in a carbohydrate response element-binding protein (ChREBP)-dependent manner. J Biol Chem. 2011. April 29;286(17):15116–25. 10.1074/jbc.M110.216234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dentin R, Pegorier JP, Benhamed F, Foufelle F, Ferre P, Fauveau V, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004. May 7;279(19):20314–26. [DOI] [PubMed] [Google Scholar]
- 41.Jeong YS, Kim D, Lee YS, Kim HJ, Han JY, Im SS, et al. Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression. PLoS One. 2011;6(7):e22544 10.1371/journal.pone.0022544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Maechler P, Antinozzi PA, Herrero L, Hagenfeldt-Johansson KA, Bjorklund A, et al. The transcription factor SREBP-1c is instrumental in the development of beta-cell dysfunction. J Biol Chem. 2003. May 9;278(19):16622–9. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005. September 1;118(Pt 17):3905–15. [DOI] [PubMed] [Google Scholar]
- 44.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005. May;146(5):2397–405. [DOI] [PubMed] [Google Scholar]
- 45.Iizuka K, Wu W, Horikawa Y, Takeda J. Role of glucose-6-phosphate and xylulose-5-phosphate in the regulation of glucose-stimulated gene expression in the pancreatic beta cell line, INS-1E. Endocr J. 2013;60(4):473–82. [PubMed] [Google Scholar]
- 46.Tsatsos NG, Augustin LB, Anderson GW, Towle HC, Mariash CN. Hepatic expression of the SPOT 14 (S14) paralog S14-related (Mid1 interacting protein) is regulated by dietary carbohydrate. Endocrinology. 2008. October;149(10):5155–61. 10.1210/en.2008-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iizuka K, Takeda J, Horikawa Y. Kruppel-like factor-10 is directly regulated by carbohydrate response element-binding protein in rat primary hepatocytes. Biochem Biophys Res Commun. 2011. September 9;412(4):638–43. 10.1016/j.bbrc.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 48.Villasenor A, Wang ZV, Rivera LB, Ocal O, Asterholm IW, Scherer PE, et al. Rgs16 and Rgs8 in embryonic endocrine pancreas and mouse models of diabetes. Dis Model Mech. 2010. Sep-Oct;3(9–10):567–80. 10.1242/dmm.003210 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schematic diagram of tetracycline-inducible lentiviral vectors for expression of microRNA-adapted short hairpin RNA to target Chrebpβ (mirGE-β) or non-silencing (mirGE-N) sequences. (B) Effect of Chrebpβ shRNA on the expression of Chrebpβ and Rgs16 in 832/13 cells. We pre-incubated mirGE-β cells and mirGE-N cells for 24h in RPMI with 5.5 mmol/l D-glucose in the presence of doxycycline 1 μg/mL, and switched to RPMI with 25 mmol/l D-glucose in the presence of doxycycline 1 μg/mL for 48h. The histograms are the means of relative RNA levels normalized to Ywhaz and Hprt1 and expressed as fold activation over the activity seen in mirGE-N cells. *, p< 0.05.
(TIF)
We explored the anti-ChREBP ChIP-seq data using the Integrated Genome Browser and demonstrated the presence of ChoRE sequence identified in this study at the summit of ChIP-seq peaks in mouse liver and white adipose tissue. Gray vertical line indicates the position where previously identified ChoRE is located.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.