Abstract
Context
Spinal cord injury (SCI) causes disruption of the efferent input to and afferent input from respiratory muscles, which impairs respiratory motor and sensory functions, respectively. This disturbs the injured individual's ability to respond to ventilatory loads and may alter the respiratory perceptual sensitivity of applied loads. Acute intermittent hypoxia with elevated CO2 (AIH treatment) has been shown to induce ventilatory long-term facilitation in individuals with chronic SCI. This study evaluated the effect of ten days of AIH treatment on ventilatory load compensation and respiratory perceptual sensitivity to inspiratory resistive loads (IRL), in an individual with chronic, incomplete cervical SCI.
Methods
Case report and literature review.
Findings
We report a case of a 55-year-old female with a C4 chronic, incomplete SCI (American Spinal Injury Association Impairment Scale D). The subject underwent evaluation at four time-points: Baseline, Post Sham, AIH Day 1 and AIH Day 10. Significant improvements in airflow generated in response to applied IRL were found after AIH treatment compared to Baseline. There were no significant changes in the respiratory perceptual sensitivity to applied IRL after AIH treatment.
Clinical relevance
Rehabilitative interventions after SCI demand restoration of the respiratory motor function. However, they must also ensure that the respiratory perceptual sensitivity of the injured individual does not hinder their capability to compensate to ventilatory challenges.
Keywords: Acute intermittent hypoxia, Inspiratory resistive loads, Load detection, Rehabilitation, Respiratory sensation, Spinal cord injury
Introduction
An estimated 12 000 new cases of spinal cord injury (SCI) arise every year in the United States1 and respiratory dysfunction is the leading cause of mortality in this population.2,3 A disruption of the neural efferent output to respiratory muscles due to SCI results in reduced motor function which, in turn, alters the individual's ability to breathe and compensate to increased respiratory loads.4,5 Also, depending on level and severity of injury, SCI interrupts the ascending afferent input from respiratory and accessory respiratory muscles innervated by the cervical, thoracic and lumbar spinal cord. This afferent feedback plays a key role in the modulation of breathing.6 Rehabilitation after SCI is primarily aimed at restoration of respiratory motor function. However, the affective response to an experienced and/or applied respiratory challenge plays a significant role in determining the motor output response.
Magnitude estimation (ME) is a technique used to cognitively scale the size of a given stimulus by assigning a proportional number to the perceived magnitude. The respiratory perceptual sensitivity follows Steven's law: Ψ = KΦn. The estimated magnitude, Ψ, is a power function of the load magnitude, Φ; n is the power exponent and K is a constant.7 The slope of the log-log plot of the reported ME and mouth pressure generated in response to an inspiratory resistive load (IRL) corresponds to “n”, which is the perceptual sensitivity for the given stimulus modality. Manning et al. have shown that healthy humans exposed to high and low levels of carbon dioxide (CO2)8 and changes in inspiratory flow rate9 do not show changes in the ME of tidal volume. However, in quadriplegics, with functionally complete low cervical transection, presented with IRL the ME of respiratory force is diminished,10 but that of inspired volume is unchanged.11 The procedure for ME of IRL has been previously used to demonstrate improvements after inspiratory muscle strength training12 and to investigate the impact of emotional state13 on the respiratory perceptual sensitivity in healthy subjects.
Exposure to acute intermittent hypoxia (AIH) has been shown to induce respiratory plasticity and improve ventilation in animal models of SCI14–17 by means of phrenic and respiratory long-term facilitation (LTF).18,19 Repetitive exposure to AIH while sustaining an elevated level of CO2 was also shown to induce ventilatory LTF during consciousness in neurologically-intact subjects20,21 and subjects with chronic, incomplete SCI.22 We hypothesized that ten days of AIH with elevated CO2 would improve ventilatory load compensation and increase the respiratory perceptual sensitivity to IRL in an individual with chronic incomplete cervical SCI.
Case report
Institutional and governmental regulations concerning the ethical use of human research were followed. Protocols were approved by the University of Florida and North Florida/South Georgia Veterans Affairs Medical Center. Informed consent was obtained from the participant. The study participant was a 55-year-old female with a C4 chronic (4.7 years), incomplete SCI classified as Brown Sequard Syndrome (American Spinal Injury Association Impairment Scale D). Upper motor neuron signs were present. The participant was without tracheostomy or assisted ventilation. A study physician and therapist confirmed the absence of heart and lung complications after obtaining medical authorization from the subject's personal physician. The subject was sequentially exposed to 2 sham sessions and 10 days of AIH, and was assessed at four time-points in the following order:
-
•
Baseline – Naïve to any testing
-
•
Post Sham—after two days of exposure to elevated CO2, alone
-
•
AIH Day 1—after exposure to AIH treatment on day one
-
•
AIH Day 10—after exposure to ten days of AIH treatment.
Tests to assess pulmonary function and respiratory muscle pressure generating capacity were conducted after the IRL procedure at each time-point. No prior history of smoking was reported.
Pulmonary function and respiratory muscle pressure generating capacity tests
A digital spirometer (Futuremed, Granada Hills, CA, USA) and a respiratory pressure meter (Micro Direct, Inc., Lewiston, ME, USA) were used to assess pulmonary function and respiratory muscle pressure generating capacity. The subject was given standard directions for task performance and measurements were conducted three times each. Mean and standard deviation values for the outcomes were calculated. Statistical analyses using RMANOVA and Holm-Sidak test for multiple comparisons were used and the significance value was set at P ≤ 0.05 (SigmaPlot 12.5, Systat Software, Inc., San Jose, CA, USA).
Sham sessions and acute intermittent hypoxia treatment
Sham and AIH treatment trials were delivered via a non-rebreathing system and a facemask during wakefulness. The subject maintained a supine position throughout all treatment sessions. Partial pressures of CO2 (PETCO2) and oxygen (PETO2) were sampled from a port in the facemask (models 17515 and 17518, respectively, VacuMed, Ventura, CA, USA) in real time. Heart rate and O2 saturation were continuously monitored using an electrocardiogram (model 17032, VacuMed, Ventura, CA, USA) and pulse oximetry (Biox 3740, Ohmeda, Boulder, CO, USA), respectively. The subject was blinded to the two sham sessions for 2 days before the 10 days of AIH treatment. During each sham and AIH treatment session the subject breathed room air for 10 minutes to establish eupneic baseline (Eupnea) values. Supplemental CO2 was added to the inspirate and PETCO2 was elevated and maintained for the remainder of the session. After a 10-minute period with elevated CO2 levels (average = 3.8 ± 0.09 mmHg above Eupnea), eight 2-minute episodes of 8% O2/balance N2 were administered while maintaining PETO2 at ≥50 mmHg using supplemental 100% O2 during AIH sessions. On days when subject underwent sham sessions, room air was used instead of 8% O2/balance N2. Every hypoxic/sham episode was terminated abruptly by administering one breath of 100% O2 to rapidly normalize PETO2. This was followed by a 2-minute period, where the subject breathed room air. After the eighth hypoxic/sham episode the subject was monitored for an additional 30-minute Recovery Period during which average increases in PETCO2 were 4.3 ± 0.19 mmHg above Eupnea. The PETCO2 during Eupnea, the 10-minute period where CO2 was elevated, and the 30-minute Recovery Period was 36.3 ± 0.5, 40.1 ± 0.5, and 40.6 ± 0.4 mmHg, respectively. Oxygen saturation values during Eupnea, the 10-minute period where CO2 was elevated, and the 30-minute Recovery Period was 97.2 ± 0.3, 98.0 ± 0.3, and 97.5 ± 0.2%, respectively. Average values reported were across the two sham and ten AIH sessions.
Inspiratory resistive load and magnitude estimation procedures
The subject reclined comfortably on a flat bench, with her back supported. With nose-clips on, she was asked to breathe through a mouthpiece, which was attached to the non-rebreathing valve. No-load (0 cm H2O/l/s) and four magnitudes of IRL (5, 15, 30 and 50 cm H2O/l/s) were applied five times each (seven times each during baseline), in a randomized block design.13 During baseline, the first two blocks of IRL were presented to familiarize the subject to the respiratory load sensation, testing device and protocol. The data from these presentations were not used for analysis. The IRL manifold was placed at the end of the experimental set-up and was attached to the inspiratory side of the non-rebreathing valve via a reinforced flexible plastic tube (∼30 cm long). The manifold was out of view from the participant and she was not aware of the order in which the IRLs were presented. A light cued the subject that her next inspiratory effort may (randomized graded IRL) or may not (no-load) have an IRL. When the light cue was turned off, the subject was asked to rate her perceived magnitude of breathing effort for the light-cued breath using a modified Borg scale23 ranging from 0 (nothing at all) to 10 (very, very severe). The subject indicated by hand gesture the ME rating for the IRL. One of the investigators recorded these ME values after repeating the subject's rating aloud to ensure that it was communicated properly. The subject and the investigator recording the subject reported ME were blinded to the sequence and the magnitude of IRL being applied. Each IRL was presented for a single inspiration only. Mouth pressure (P) was measured from a port in the center of the non-rebreathing valve and airflow (AF) was measured with a spirometer (Vernier Software & Technology, Beaverton, OR, USA) attached to the inspiratory port of the non-rebreathing valve. The data were recorded with a portable signal processor and transferred to a computer and stored for subsequent data analysis.
Data analysis
The data collection software (Vernier Software & Technology, Beaverton, OR, USA) recorded numerical P (mbar), AF (l/s) and time. The experimenter marked each single presentations of IRL during the recording session. P mbar were converted to P cmH2O values, which were used for all further P analyses. P and AF values and log transformed P and ME values were used for statistical analysis. Mean slopes for P vs. resistance, AF vs. resistance and Log ME vs. Log P were calculated. All data were analyzed by RMANOVA and Student–Neuman–Keuls test for multiple comparisons (SigmaPlot 12.5, Systat Software, Inc., San Jose, CA, USA). Data are presented as mean ± standard error of mean and significance was set for P ≤ 0.05.
Results
Pulmonary function and respiratory muscle pressure generating capacity tests
Table 1 summarizes subject data from pulmonary function, and maximal inspiratory (MIP) and maximal expiratory (MEP) pressure tests. Values for percent-predicted forced vital capacity (FVC) increased significantly (Table 1) on Post Sham (74.66 ± 3.05%, P ≤ 0.01), and AIH Days 1 (76.33 ± 0.58%, P ≤ 0.01) and 10 (80.00 ± 2.00%, P ≤ 0.001) compared to Baseline (67.33 ± 4.04%). Forced expiratory volume in one second (FEV1) also increased significantly (Table 1) on Post Sham (63.66 ± 2.51%, P ≤ 0.05), and AIH Days 1 (60.00 ± 2.00%, P ≤ 0.05) and 10 (67.00 ± 2.65%, P ≤ 0.05) compared to Baseline (46.33 ± 12.66%). The MIP and MEP were not significantly different from Baseline values (Table 1).
Table 1 .
Summary of results (Mean ± standard deviation) from pulmonary function and respiratory muscle pressure generating capacity tests
| Functional measure | Baseline | Post Sham | AIH Day 1 | AIH Day 10 |
|---|---|---|---|---|
| Forced vital capacity (FVC, %) | 67.33 ± 4.04 | 74.66 ± 3.05** | 76.33 ± 0.58** | 80.00 ± 2.00*** |
| Forced expiratory volume (FEV1, %) | 46.33 ± 12.66 | 63.66 ± 2.51* | 60.00 ± 2.00* | 67.00 ± 2.65* |
| Maximal inspiratory pressure (MIP, cm H2O) | 56.00 ± 3.61 | 57.33 ± 1.53 | 57.00 ± 6.56 | 59.33 ± 3.21 |
| Maximal expiratory pressure (MEP, cm H2O) | 63.00 ± 8.88 | 65.66 ± 3.05 | 73.00 ± 3.46 | 75.00 ± 4.00 |
Values for percent predicted forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) showed significant improvements on Post Sham and AIH treatment Days 1 and 10, ***, P ≤ 0.001, **P ≤ 0.01 and *P ≤ 0.05 compared to Baseline. No significant difference was observed in maximal inspiratory (MIP) and expiratory (MEP) pressure values after AIH treatment.
Ventilatory load compensation
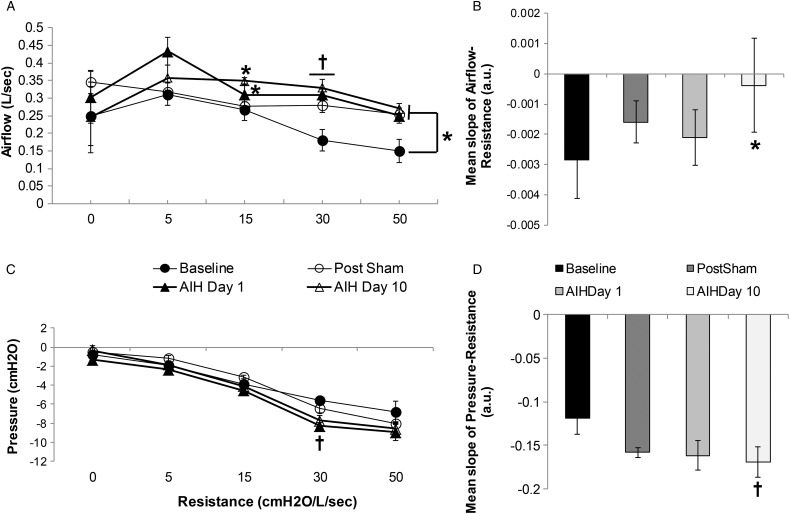
The results for AF and P in response to IRL indicate an improvement in ventilatory load compensation ability after repeated exposure to AIH treatment for ten days (Fig. 1). The mean slope for the AF vs. resistance was significantly greater on AIH Day 10 (–0.0004 ± 0.002, P ≤ 0.05) compared to Baseline (–0.003 ± 0.001) (Fig. 1B). No significant difference was found for mean slope at Post Sham (P = 0.06) and AIH Day 1 (P = 0.65) compared to Baseline. The subject produced a significantly larger AF (Fig. 1A) in response to a 15-cm H2O/l/s IRL on AIH Day 1 (0.31 ± 0.03 l/s, P ≤ 0.05) and AIH Day 10 (0.35 ± 0.01 l/s, P ≤ 0.05) compared to Baseline (0.27 ± 0.03 l/s); a 30-cm H2O/l/s IRL on Post Sham (0.28 ± 0.02 l/s, P ≤ 0.01), AIH Day 1 (0.31 ± 0.02 l/s, P ≤ 0.01) and AIH Day 10 (0.33 ± 0.02 l/s, P ≤ 0.01) compared to Baseline (0.18 ± 0.03 l/s) and a 50-cm H2O/l/s IRL on Post Sham (0.25 ± 0.003 l/s, P ≤ 0.05), AIH Day 1 (0.25 ± 0.02 l/s, P ≤ 0.05) and AIH Day 10 (0.27 ± 0.02 l/s, P ≤ 0.05) compared to Baseline (0.15 ± 0.03 l/s) (Fig. 1A). The results for AF were not significantly different for 0 and 5-cm H2O/l/s IRL during any of the time-points studied or 15-cm H2O/l/s at Post Sham.
Figure 1 .
Ventilatory load compensation, airflow (A and B) and pressure (C and D). (A) Airflow (l/s) vs. resistance (cm H2O/l/s) for Baseline, Post Sham, AIH Day 1 and AIH Day 10. The subject generated significantly greater airflow when presented with the following IRLs; 15-cm H2O/l/s on AIH Days 1 and 10 (*P ≤ 0.05); 30-cm H2O/l/s on Post Sham, AIH Day 1 and AIH Day 10 (†P ≤ 0.01) and 50-cm H2O/l/s on Post Sham, AIH Day 1 and 10 (*P ≤ 0.05). (B) Mean slope of airflow vs. resistance. Mean slope of this plot on AIH Day 10 was significantly greater than that at Baseline (*P ≤ 0.05). (C) Pressure (cm H2O) vs. resistance (cm H2O/l/s) for Baseline, Post Sham, AIH Day 1 and AIH Day 10. The subject generated significantly greater pressure on AIH Day 1 (†P ≤ 0.01) when presented with the 30 cm H2O/l/s IRL. (D) Mean slope of pressure vs. resistance. Mean slope of this plot on AIH Day 10 was significantly smaller than that at Baseline (†P ≤ 0.01).
The mean slopes for the P vs. resistance plot from baseline to AIH Day 10 demonstrated an increased negative slope (Fig. 1D), the result of more negative P for the same magnitude of IRL. The mean slope for AIH Day 10 (–0.17 ± 0.02 a.u.) was significantly more negative (P ≤ 0.01, Fig. 1D) when compared to mean slope at Baseline (−0.12 ± 0.02 a.u.). Mean slope values for Post Sham (P = 0.075) and AIH Day 1 (P = 0.09) were not statistically significant. At the low load magnitudes (0–15 cm H2O/l/s) there was no significant difference in P Post Sham (P = 0.19), AIH Day 1 (P = 0.06) and AIH Day 10 (P = 0.28) compared to Baseline values. The subject generated a significantly more negative P at the 30-cm H2O/l/s load on AIH Day 1 (–8.28 ± 0.36 cm H2O, P ≤ 0.01) compared to Baseline (–5.61 ± 0.36 cm H2O) (Fig. 1C). At the highest load magnitude, 50-cm H2O/l/s, the P was not significantly different when compared across Baseline, Post Sham (P = 0.36), AIH Day 1(P = 0.15) and AIH Day 10 (P = 0.11).
Magnitude estimation
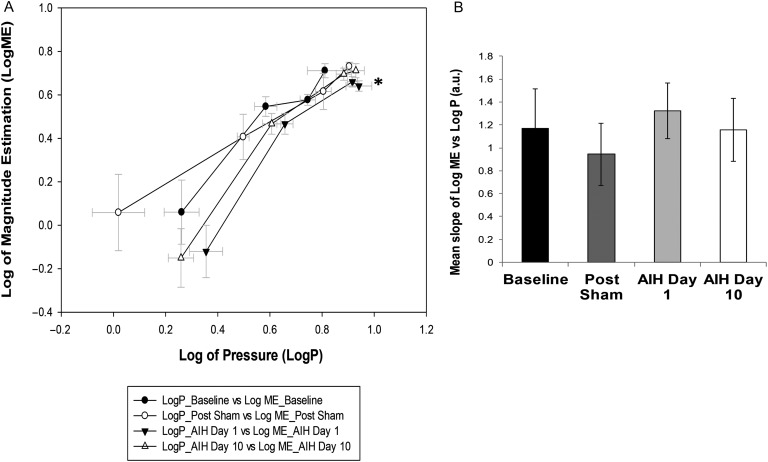
The ME for each IRL was log-transformed (Log ME) and plotted against log-transformed values of P generated against each load magnitude (Log P). The Log ME at the 50-cm H2O/l/s load on AIH Day 1 (0.64 ± 0.02 a.u., P ≤ 0.05, Fig. 2A) was significantly lower than on Post Sham (0.73 ± 0.02 a.u.). No significant differences were found in Log ME on AIH Day 10. The mean slopes of Log ME vs. Log P (Fig. 2B) were not significantly different between any of the time-points measured in this study.
Figure 2 .
Magnitude estimation of IRL (A) Log-log plot for magnitude estimation (ME) vs. P. Log ME was significantly lower on AIH Day 1 in response to a 50-cm H2O/l/s load compared to Post Sham (*P ≤ 0.05). (B) Mean slope of the log-log plot of ME vs. P. No significant differences were found in the perceptual sensitivity to IRL after AIH treatment in the study subject.
Discussion
The key result from this study is an improvement in ventilatory load compensation and pulmonary function after ten days of exposure to AIH treatment, in an individual with chronic, incomplete cervical SCI. However, this paradigm did not demonstrate an AIH effect on the ME of IRL, and therefore the perceptual sensitivity to IRL, in the study subject.
Exposure to AIH with elevated levels of CO2 has been shown to induce ventilatory LTF in animal models of SCI,18,19 neurologically-intact humans20,21 and humans with chronic SCI.22 Based on our results, a decrease (more negative) in mean slope for P vs. resistance (Fig. 1D) and a corresponding increase (more positive) in mean slope for AF vs. resistance, on AIH Day 10 demonstrate that the subject in our study improved her ventilatory compensation capacity to IRL. During baseline testing the first two blocks of IRL were presented to familiarize the subject to the testing device and protocol. The data from these presentations were not used for analysis in this report. The possibility that the reported improvements in P and AF may be due to the combined influence of AIH treatment and a learning effect of repeated exposure to the IRL protocol cannot be ruled out. However, we also observed significant improvements in %FVC and %FEV1 values, thereby suggesting the study paradigm may have a beneficial effect on the subject's pulmonary function and associated lung mechanics. Since improvements occurred Post Sham, and further improvements were not evident Post AIH, the influence of CO2-alone must be considered. The subject reported no changes in perceptual sensitivity. This would suggest that our study paradigm might affect the discriminative but not affective processing of IRL.24 Thus, even if the subject learned to differentiate between loads (vs. Baseline) and generate greater negative pressure in the ventilatory load compensation response, the affective response to each load may remain the same over the treatment time. This is similar to findings from a previous study demonstrating that respiratory learning is not influenced by the elicited physiological responses.25 No improvements in respiratory muscle pressure generating capacity were observed. Values for MIP and MEP were not significantly different at any of the time-points measured in our study. This suggests that the increases in FVC%, FEV1%, AF and P are not due to increased respiratory pump function and may be due to increased central respiratory drive. However, it is interesting to note that despite non-significant differences, MEP appeared increased on AIH Days 1 and 10 compared to Post Sham. It is expected that exposure to elevated levels of CO2 increases central respiratory drive. However, from our22 and other studies20,21 it is recognized that elevated level of CO2 along with intermittent bouts of hypoxia (AIH) is required to elicit ventilatory long term-facilitation. To better understand the differential effects of AIH and CO2 alone on inspiratory loading and respiratory pressure generating capacity future studies with larger sample sizes, altered AIH treatment protocols and in subjects with different injury severities are warranted.
In patients with sleep apnea, the ventilatory response to AIH and the resulting LTF is dependent on time of day when the subject was exposed to AIH treatment.26 However, repeated exposure to AIH with elevated CO2 levels has been shown to augment the hypoxic ventilatory response, irrespective of time of day.26 To our knowledge there is no study determining the optimal time of day for conducting the ME of IRL protocol. We controlled for this effect and maintained a consistent time of the day when the patient underwent respiratory testing and AIH.
Posture significantly affects the ventilatory response to added loads in both individuals with and without SCI.27 Supine posture has been shown to have a mechanical advantage over upright sitting in tetraplegics.28 The subject in our study was supine during all AIH treatment trials and reclined comfortably with her back supported and legs straight on a flat bench. It is likely that the P and AF responses to lower intensities of IRL (0, 5-cm H2O/l/s) were influenced by posture. However, consistent increases in AF at the higher intensities of IRL (30, 50-cm H2O/l/s) with the same posture suggest the increased ventilatory load compensation response was not the result of the respiratory mechanical effects due to posture. Improvements in pulmonary function tests and ventilatory load compensation response suggest that this paradigm may be a potentially beneficial rehabilitation intervention for individuals with chronic incomplete c-SCI.
The baseline perceptual sensitivity of our study subject was slightly lower but comparable to that of healthy conscious adults, previously reported by our lab.13 Previous studies with quadriplegics have reported a much greater reduction in the value of the respiratory load sensitivity exponent.10 The perceptual sensitivity is dependent on the respiratory drive, breathing pattern and respiratory muscle fatigue.29 In our case, spinal injury severity also plays a key role in determining the perceptual sensitivity to IRL. The subject in our study has a chronic and less severe form of cervical SCI that may have spared some afferent and efferent pathways from the thoracic muscle receptors that modulate respiratory effort responses.6,10 The presentation of a single breath IRL does not cause respiratory muscle fatigue.30 In addition, diaphragm muscle fatigue does not influence the respiratory effort perceptual sensitivity of uninjured subjects.31 Thus, the perceptual response of this SCI patient is likely the result of spared neuromuscular function and not respiratory muscle fatigue.
Ventilatory deficits in individuals with SCI vary depending on level and severity of injury, time after injury as well as age and gender of individual.1 The case presented is a subject with Brown Sequard Syndrome. Brown Sequard describes one specific type of SCI, and has a low incidence in the SCI population.32 This syndrome is commonly modeled in animals with C2 hemisections to study respiratory plasticity after SCI,33,34 making the parallel condition in a human an interesting case. Ventilatory deficits in pulmonary function and respiratory muscle pressure generating capacity were present in the subject at Baseline (Table 1); and the impact of the deficit magnitude on the subject's ability to respond to AIH treatment remains unknown. It is possible the lack of a progressive increase in ventilatory load compensation ability may be dependent on one or more of the following factors: the subject's injury severity, level of dysfunction, time since injury, and/or magnitude of ventilatory long-term facilitation. In addition, it is unclear whether altered AIH protocols (e.g. protocols with different levels of hypoxia or days of exposure) may influence outcomes. Therefore, further investigations using varied AIH protocols, in combination with parallel sham protocols, are necessary in a larger sample.
In summary, we conclude that repeated exposure to AIH with elevated CO2 levels improved pulmonary function without altering respiratory muscle pressure generating capacity in an individual with chronic incomplete c-SCI. The ventilatory load compensation response to added IRL increased, but the perceptual sensitivity to IRL was not affected after AIH treatment. Further studies are required to determine the applicability of AIH treatment as a rehabilitative intervention for SCI individuals.
Acknowledgments
The authors would like to acknowledge Dr Jason Fromm, Dr Martina Spiess, and Dr Jason Mateika for their assistance. This material is based upon work supported by the Office of Research and Development, Rehabilitation and Research Development Service, Department of Veterans Affairs (grants #B7182 W – NJT and #F2182C – Center Grant). The contents of this article do not represent the views of the Department of Veterans Affairs or the United States government.
Disclaimer statements
Contributors PBJ participated in: conceiving and designing the study, obtaining funding and ethics approval, collecting the data, analysing the data, interpreting the data, writing the article in whole or in part and revising the article. NJT participated in: conceiving and designing the study, obtaining funding and ethics approval, collecting the data, analysing the data, interpreting the data, writing the article in part and revising the article. PWD participated in: conceiving and designing the study, obtaining funding and ethics approval, collecting the data, interpreting the data, writing the article in part and revising the article.
Funding
This research was supported in part by the Office of Research and Development, Rehabilitation and Research Development Service, Department of Veterans Affairs, grant numbers #B7182 W - NJT and #F2182C - Center Grant. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States government.
Conflicts of interest None.
Ethics approval Institutional and governmental regulations concerning the ethical use of human research were followed.
References
- 1. 2013 Spinal Cord Injury Facts and Figures at a Glance, SCIMS/NDIRR [Internet] Available from: https://http://www.nscisc.uab.edu .
- 2.Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care 2006;51(8):853–68; discussion 69–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 2003;82(10):803–14. [DOI] [PubMed] [Google Scholar]
- 4.Axen K. Ventilatory responses to mechanical loads in cervical cord-injured humans. J Appl Physiol 1982;52(3):748–56. [DOI] [PubMed] [Google Scholar]
- 5.Kelling JS, DiMarco AF, Gottfried SB, Altose MD. Respiratory responses to ventilatory loading following low cervical spinal cord injury. J Appl Physiol 1985;59(6):1752–6. [DOI] [PubMed] [Google Scholar]
- 6.von Euler C. The role of proprioceptive afferents in the control of respiratory muscles. Acta Neurobiol Exp (Wars) 1973;33(1):329–41. [PubMed] [Google Scholar]
- 7.Stevens SS. On the psychophysical law. Psychol Rev 1957;64(3):153–81. [DOI] [PubMed] [Google Scholar]
- 8.Manning HL, Slogic S, Leiter JC. Tidal volume perception in normal subjects: the effect of altered arterial PCO2. Respir Physiol 1994;96(1):99–110. [DOI] [PubMed] [Google Scholar]
- 9.Manning HL, Molinary EJ, Leiter JC. Effect of inspiratory flow rate on respiratory sensation and pattern of breathing. Am J Respir Crit Care Med 1995;151(3 Pt 1):751–7. [DOI] [PubMed] [Google Scholar]
- 10.Gottfried SB, Leech I, DiMarco AF, Zaccardelli W, Altose MD. Sensation of respiratory force following low cervical spinal cord transection. J Appl Physiol 1984;57(4):989–94. [DOI] [PubMed] [Google Scholar]
- 11.DiMarco AF, Wolfson DA, Gottfried SB, Altose MD. Sensation of inspired volume in normal subjects and quadriplegic patients. J Appl Physiol 1982;53(6):1481–6. [DOI] [PubMed] [Google Scholar]
- 12.Kellerman BA, Martin AD, Davenport PW. Inspiratory strengthening effect on resistive load detection and magnitude estimation. Med Sci Sports Exerc 2000;32(11):1859–67. [DOI] [PubMed] [Google Scholar]
- 13.Tsai HW, Chan PY, von Leupoldt A, Davenport PW. The impact of emotion on the perception of graded magnitudes of respiratory resistive loads. Biol Psychol 2013;93(1):220–4. [DOI] [PubMed] [Google Scholar]
- 14.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, et al. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 2012;32(11):3591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci 2008;28(11):2949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 2009;217(1):116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terada J, Mitchell GS. Diaphragm long-term facilitation following acute intermittent hypoxia during wakefulness and sleep. J Appl Physiol (1985) 2011;110(5):1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, et al. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci 2010;1198:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol. 2009;169(2):210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mateika JH, Sandhu KS. Experimental protocols and preparations to study respiratory long term facilitation. Respir Physiol Neurobiol 2011;176(1–2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 2006;291(4):R1111–9. [DOI] [PubMed] [Google Scholar]
- 22.Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med 2014;189(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14(5):377–81. [PubMed] [Google Scholar]
- 24.Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir Physiol Neurobiol 2009;167(1):72–86. [DOI] [PubMed] [Google Scholar]
- 25.Van den Bergh O, Stegen K, Van de Woestijne KP. Memory effects on symptom reporting in a respiratory learning paradigm. Health Psychol 1998;17(3):241–8. [DOI] [PubMed] [Google Scholar]
- 26.Gerst DG III, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, et al. The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J Appl Physiol 2011;110(1):15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loveridge B, Sanii R, Dubo HI. Breathing pattern adjustments during the first year following cervical spinal cord injury. Paraplegia 1992;30(7):479–88. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Dov I, Zlobinski R, Segel MJ, Gaides M, Shulimzon T, Zeilig G. Ventilatory response to hypercapnia in C(5-8) chronic tetraplegia: the effect of posture. Arch Phys Med Rehabil 2009;90(8):1414–7. [DOI] [PubMed] [Google Scholar]
- 29.Gandevia SC, Killian KJ, Campbell EJ. The effect of respiratory muscle fatigue on respiratory sensations. Clin Sci (Lond) 1981;60(4):463–6. [DOI] [PubMed] [Google Scholar]
- 30.von Leupoldt A, Vovk A, Bradley MM, Lang PJ, Davenport PW. Habituation in neural processing and subjective perception of respiratory sensations. Psychophysiology 2011;48(6):808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley TD, Chartrand DA, Fitting JW, Killian KJ, Grassino A. The relation of inspiratory effort sensation to fatiguing patterns of the diaphragm. Am Rev Respir Dis 1986;134(6):1119–24. [DOI] [PubMed] [Google Scholar]
- 32.McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med 2007;30(3):215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuller DD, Golder FJ, Olson EB Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol 2006;100(3):800–6. [DOI] [PubMed] [Google Scholar]
- 34.Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH, and respiratory rate in the adult rat. Exp Neurol 1986;93(2):440–5. [DOI] [PubMed] [Google Scholar]