Abstract
Context
Surface electromyography (SEMG) may be a sensitive marker for distinguishing the activity of trunk muscles, which are critical to functional mobility recovery in patients with spinal cord injury (SCI).
Objectives
This manuscript presents a systematic review and meta-analysis of the published literature on the effect of SEMG as a measure of trunk muscle activity in patients with SCI.
Methods
A comprehensive search of the research literature included Pubmed, Medline, CNKI, WANFANG DATA, Web of Science, Elsevier, Wiley-Blackwell, Karger, OVID, and a review of reference lists within found articles. Case-control, cohort, and cross-sectional studies were included in the review.
Results
Eleven studies were included in this meta-analysis. Trunk muscle activities for the sitting condition were greater in patients with SCI than normal subjects. SEMG activity of trunk muscles for the sitting condition and posterior transfer was greater in patients with high level (HL)-SCI compared to those with low level (LL)-SCI. In addition, across studies, the level of trunk muscle activity for various difficulty settings was different for a given SCI group.
Conclusion
This systematic review evaluated the value of trunk muscles for patients with SCI. We recommend use of SEMG as an assessment tool for improving the comparability and interpretability of trunk muscle activity of SCI therapeutic strategies.
Keywords: Surface electromyography, Spinal cord injury, Trunk muscle
Introduction
Each year there are approximately 12,000 new cases of spinal cord injury (SCI) reported in the United States.1 When the neural elements of a spinal cord are injured during SCI, muscular dysfunction and/or paralysis occur below the injury site.2 Since integrity of the motor neurological system is impaired in most individuals with SCI, rehabilitation of patients with paraplegia or tetraplegia should revolve around the development of their surviving motor function.3
Restoration of trunk function is crucial for developing patient independence and improving quality of life. A large part of rehabilitation after SCI consists of task directed training via the performance of many repetitions of movements relevant for activities of daily living (ADLs). In this context, by understanding how the central nervous system (CNS) uses motor control strategies to govern trunk movements in patients with SCI, we can better develop rehabilitation processes for patients with SCI and ensure that the exercises are performed effectively to restore functional ability.4 The latissimus dorsi (LAT), trapezius, pectoralis (PECT) major, and serratus anterior (SERR) muscles contribute to the maintenance and restoration of sitting balance in patients with SCI.5 For example, paraplegic subjects use both latissimus dorsi and trapezius to stabilize their sitting posture, in contrast to nondisabled persons.6
A series of research studies have provided electrophysiological data related to trunk muscles as an aid to plan efficient rehabilitation interventions for functional mobility recovery in individuals with SCI. These studies often employ SEMG to measure action potentials in muscles. There is mounting evidence suggesting that there are obvious differences in the activity of trunk muscles between patients with SCI and healthy controls and between patients with high level (HL)-SCI and low level (LL)-SCI. Use of SEMG can accurately differentiate distinct electrical activity in trunk muscles during the performance of tasks of varying difficulty. Here, we provide a systematic review and meta-analysis of the published literature regarding the differences in SEMG between the aforementioned patient groups and determine the effect size associated with specific SEMG measures. Based on our findings, we provide recommendations on the utility of SEMG as an objective marker for the activity of trunk muscles in patients with SCI.
Materials and methods
Study selection
This study is a systematic literature review conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol. Two independent raters performed all stages, and both reached a consensus. If a consensus could not be reached, a third rater was recruited to resolve the disagreement.6 First, systematic literature searches of the following databases were performed to identify relevant studies: Pubmed, Medline, CNKI, WANFANG DATA, Web of Science, Elsevier, Wiley-Blackwell, Karger, and OVID. Keyword search terms included surface electromyography, SEMG, spinal cord injury, SCI, paraplegia, tetraplegia, quadriplegia, and trunk. Studies were included in the meta-analysis if SEMG was used clinically to evaluate trunk function of subjects with SCI. There was no language restriction, and they were published from the earliest records to January 2014.
Eligibility criteria
Case-control, cohort, and cross-sectional studies were included in the review, and case reports or case series were excluded. Only studies where clinical intervention was limited to administration of SEMG to adult participants were eligible for enrollment in the review.
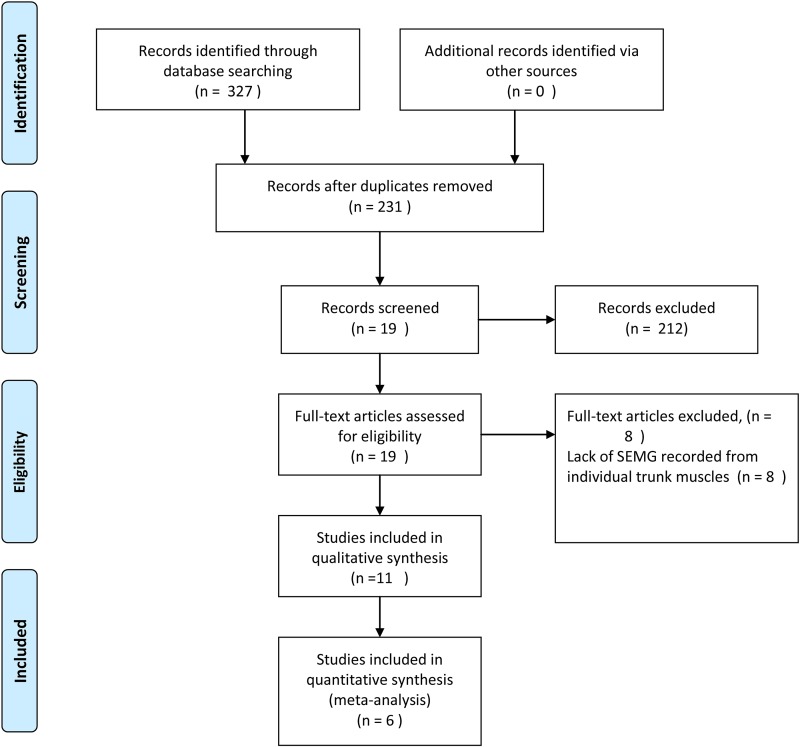
Data extraction and quality assessment
Two reviewers independently evaluated the titles and abstracts from all articles, and those studies that clearly did not meet the inclusion criteria were excluded. The remaining studies were read fully, and included or excluded based on the predetermined criteria. Subsequently, the references of each included article were searched for additional studies that may have been missed in the initial search.7,8 Thirty-two references were included, and 19 full-text articles were screened from 231 records according to eligibility criteria. Eight articles were excluded for lack of SEMG of individual trunk muscles. In total, we identified 11 clinical trials that examined SEMG and SCI, and these were included in qualitative synthesis. Only six of these studies were included in meta-analysis because five studies9–13 did not report mean and standard deviation (Fig. 1). SCI in all eligible studies was evaluated using the International Standards for Neurological and Functional Classification of Spinal Cord Injury.14
Figure 1 .
Flow diagram of the evaluation process for those included and excluded from studies.
A summary of the studies reviewed are presented in Tables 1 and 2. Table 1 presents the studies included in the meta-analysis, while Table 2 presents the remaining studies that did not report the necessary means and standard deviations.
Table 1 .
Summary of studies examined in meta-analysis
| Study | Subjects | Position/activity measured | Type SEMG | Findings |
|---|---|---|---|---|
| Lalumiere et al. (2013)25 | 15 SCI | Ascending curbs by wheelchair | MUR of PECT | MUR increased with the higher curb |
| Gagnon et al. (2003)3 | 6 HL-SCI 5LL-SCI |
Posterior transfer | MUR of the PECT, LAT, TRAP, and RECT | Muscular demands higher in HL-SCI than LL-SCI |
| Gagnon et al. (2005)24 | 10 SCI | Posterior transfers | MUR of the PECT, LAT, and TRAP | High muscular demand when hands positioned on the elevated surface |
| Terson de Paleville et al. (2013)23 | 8 SCI | Respiration | VRI of respiratory muscles activity | Respiratory muscles activity improved by the LT |
| Wang et al. (2010)20 | 30 SCI 10 healthy subjects |
Static sitting | MUR of EREC, LAT, RECT, and OBLI | Higher level SCI linked with lower MUR |
| Liu et al. (2008)21 | 15 SCI 15 health subjects |
Grasping cup, touching light switch, wheelchair ambulation, upper limb weight- bearing. | MUR of LAT, PECT, and SERR | SCI patients increased motor level of muscles to complete motive tasks. |
Table 2 .
Summary of studies reviewed but not included in meta-analysis
| Study | Subjects | Position/activity measured | Type SEMG | Findings |
|---|---|---|---|---|
| Desroches et al. (2013)12 | 32 with SCI | Three types of sitting pivot transfers | MUR of PECT, TRAP, and LAT | Greater muscle activity of PECT at the forward trunk flexion Upright trunk strategy yielded greater muscle activity of LAT |
| Louis et al. (2010)13 | 10 with SCI 10 healthy subjects |
Propelled wheelchair | Integrated electromyography of PECT, LAT, and TRAP. | Subjects with SCI had a higher muscle activation during propulsion and higher muscle activation for TRAP during recovery |
| Chow et al. (2009)14 | 10 with SCI | Wheelchair propulsion over ramps of different slopes. | MUR of PECT and LAT | The trunk becomes more active with increasing slope. |
| McKay et al. (2011)15 | 11 with SCI 5 healthy subjects |
Expanded brain motor control assessment protocol | Magnitude and SI of TRAP and RECT | Overall, SEMG amplitudes were lower after SCI. Recovery showed an increase in SEMG amplitudes (in 9 of 11) |
| Lin et al. (2008)16 | 11 with SCI | Subjects with KAFOs maintain self-supported standing until falling with grasping. | MUR of the OBLI | Abdominal muscle activity increased after the reach-and-grasp reaction |
Information gathered from the selected trials included study design, number of patients, position/activity measured, type of SEMG, and outcome measurements. In five articles, SEMG analysis was conducted with the muscular utilization ratio (MUR), and in one article, analysis was conducted with the voluntary response index (VRI). The MUR is calculated by dividing the EMG recorded at any given time during an activity by the maximal voluntary isometric contractions (MVIC) obtained from the previous maximal effort. The result is then multiplied by 100 to provide a percentage. Recently, a new method independent of maximal effort for analyzing SEMG data was reported by Lee et al.15 This vector-based analytical tool derived from SEMG signals is termed the VRI. It is sensitive enough to detect motor control differences between healthy subjects and patients with SCI, across a group of subjects with SCI, and within individual subjects with SCI across time and following intervention.16,17 The VRI consists of two numeric values, one from the total muscle activity recorded for a voluntary motor task (magnitude) and the other from the SEMG distribution across the recorded muscles (similarity index).15
The non-randomized studies in this meta-analysis were evaluated using the Newcastle-Ottawa scale to determine the qualities of selection, comparability, and exposure.18 A case-control study can be awarded a maximum of one star for each numbered item within the selection and exposure categories, and a maximum of two stars can be given for comparability. The goal of this scale is to identify a threshold score to distinguish between good and poor quality studies. The assessment of the quality of the studies included in the meta-analysis are presented in Tables 3.
Table 3 .
Assessment of the quality of case-control studies
Since the methodology and conditions for studying SEMG varied greatly across studies, the effect sizes from all studies could not be summarized together in a meaningful way. For ease of presentation, the trunk SEMG effect sizes were grouped as follows: (1) healthy, normal controls versus patients with SCI; (2) HL-SCI versus LL-SCI; (3) high difficult movement (HDM)-SCI versus low difficult movement (LDM)-SCI. The effect sizes reported, however, were not entirely independent of each other. If a study reported a difference in SEMG between SCI groups, the comparisons made between the control and patient groups were calculated as separate effect sizes, and the data for the control group were the same. In addition, some subjects with SCI underwent multiple conditions in a given experiment and separate effect sizes had to be calculated.
The meta-analysis examined the effect sizes associated with various SEMG measures. Effect sizes for each study (standardized mean difference, d) were computed by subtracting the mean of the normal group from the mean of the SCI group, subtracting the mean of the LL-SCI group from the mean of the HL-SCI group, and subtracting the mean of the LDM-SCI group from the HDM-SCI group, and this difference was divided by the pooled standard deviation.19 A positive effect size indicates that subjects with SCI/ HL-SCI group/ HDM-SCI group had a higher SEMG than normal control group/ LL-SCI group/ LDM-SCI group, whereas a negative effect size denoted the opposite. Since treating non-independent effects as independent can lead to statistical errors,20 we summarized the effect sizes qualitatively based on the guidelines established by Cohen.19 According to Cohen, an effect size of 0.2 is small, 0.5 is a moderate effect size, and 0.8 is a large effect size. Because some effect sizes were based on a small number of subjects, a weighted effect size was calculated using the following formula: d = J(m)g, where g is the uncorrected effect size and J(m) = 1– (3/(4 × df) – 1).21,22 Based on the data, there was a small difference between the actual and adjusted effect sizes. Therefore, we used the adjusted effect sizes throughout to discuss and interpret the data, although both actual and adjusted effect sizes are presented in the tables.
Results
Comparison of SEMG activity of trunk muscles in people with and without SCI
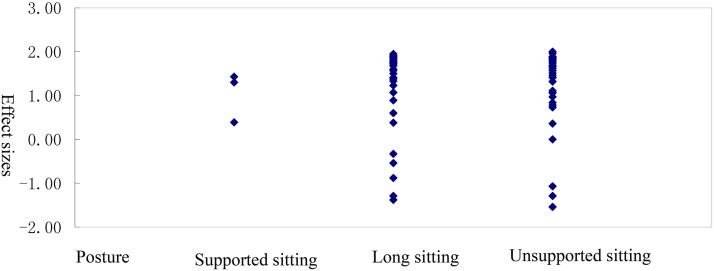
Overall, we identified two studies23,24 that reported significant differences in SEMG of trunk muscles in normal, healthy control and patients with SCI during sitting. A total of 75 effect sizes for static postures were extracted, and they are presented in Table 4. In one of those studies, SEMG was measured in trunk muscles during supported sitting, an upper limb weight-bearing posture.24 Liu et al,24 observed that subjects with SCI had significantly higher relative EMG intensities of upper PECT and SERR compared to controls during upper limb supported sitting. In Wang et al.,23 subjects with SCI displayed the greatest change in SEMG levels in erector spinae (EREC), LAT, rectus abdominus (RECT), and obliquus externus abdominis (OBLI) compared to normal controls during unsupported sitting. We found that the largest effect size was for the orthopnea position (n = 36, d = 1.50). The effect sizes for long sitting (n = 36, d = 1.46) and upper limb weight - bearing (n = 3, d = 1.30) were also large.
Table 4 .
Effect sizes for the examination of SEMG activity in trunk muscles between SCI patients and healthy controls
| Posture | Number of effect sizes | Mean effect size (SD) | Range | Mean weighted effect size (SD) |
|---|---|---|---|---|
| All postures | 75 | 1.19 (0.90) | –1.54 to 2.00 | 1.48 (1.13) |
| Supported sitting | 3 | 1.04 (0.57) | 0.39 to 1.43 | 1.30 (0.71) |
| Long sitting | 36 | 1.19 (0.95) | –1.38 to 1.95 | 1.46 (1.21) |
| Unsupported sitting | 36 | 1.20 (0.90) | –1.54 to 2.00 | 1.50 (1.12) |
Additional findings from studies from which effect sizes could not be calculated are summarized in Table 3. Louis et al.10 recorded TRAP, PECT major, and LAT recruitment patterns using SEMG during wheelchair propulsion in paraplegic (n = 10) and able-bodied subjects (n = 10). They found that the SEMG was higher in patients with SCI than healthy controls in the early push phase for TRAP, PECT, and LAT, higher in the late push phase for LAT, and higher during recovery for TRAP and LAT. Using an expanded brain motor control assessment protocol,25 McKay et al.12 assessed bilateral upper TRAP and RECT during upper-limb tasks and published lower-limb tasks in subjects with SCI and controls. They observed in initial recordings that the mean similarity index (SI) and magnitude values were lower in patients with SCI than healthy controls.
Comparison of SEMG activity of trunk muscles in patients with HL-SCI and LL-SCI
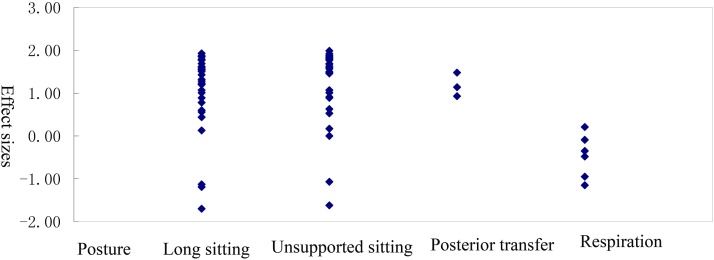
In three studies,3,23,26 SEMG was used to compare the muscular demand of trunk muscles during a given maneuver between patients with SCI with different levels of trunk musculature impairment.3 Eighty-one SEMG effect sizes of trunk muscles for patients with HL-SCI and LL-SCI were calculated, and they are summarized in Table 5.
Table 5 .
Effect sizes for the examination of SEMG activity in trunk muscles between patients with HL-SCI and LL-SCI
| Posture | Number of effect sizes | Mean effect size (SD) | Range | Mean weighted effect size (SD) |
|---|---|---|---|---|
| All postures | 81 | 1.04 (0.94) | –1.70 to 1.99 | 1.31 (1.19) |
| Static | ||||
| Long sitting | 36 | 1.10 (0.87) | –1.70 to 1.93 | 1.39 (1.10) |
| Unsupported sitting | 36 | 1.22 (0.90) | –1.62 to 1.99 | 1.57 (1.11) |
| Dynamic | ||||
| Posterior transfer | 3 | 1.18 (0.28) | 0.93 to 1.48 | 1.48 (0.35) |
| Respiration | 6 | –0.47 (0.51) | –1.15 to 0.21 | –0.59 (0.64) |
Studies can be divided into two groups: static and dynamics postures. Static postures include long sitting and unsupported sittings, whereas dynamic postures include posterior transfer and respiration. Regarding effect sizes, the largest was for unsupported sitting (n = 36, d = 1.57),23 followed by posterior transfer (n = 3, d = 1.48),3 long sitting, and respiration.23,26
In SEMG studies, trunk muscles of patients with SCI (n = 30) displayed different activity on SEMG than healthy controls (n = 10). During a laboratory simulation of posterior transfer maneuvers on a level surface, Gagnon et al.3 compared SEMG data from patients with SCI with either high or low level neurological lesions. For patients with HL-SCI, higher muscular demands were calculated for all muscles recorded during transfer relative to patients with LL-SCI. During the lift phase, however, only the PECT major was statistically different between the groups.3 Terson de Paleville et al. calculated a vector-based VRI of respiratory muscle (PECT, RECT, and OBLI) activity during respiratory tasks from individuals with chronic C3–T12 SCI (n = 8).26
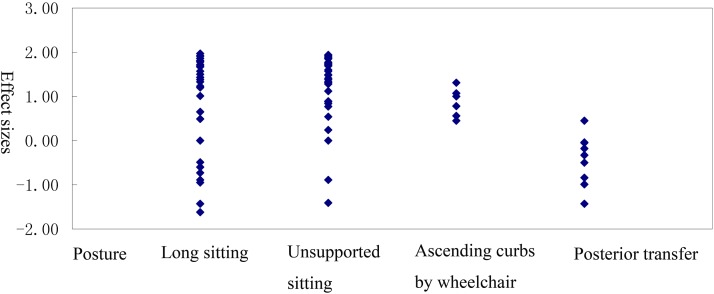
Comparison of SEMG activity in trunk muscles in self-control study of HDM-SCI and LDM-SCI
We identified three studies where trunk muscle activity was compared in HDM-SCI and LDM-SCI using SEMG. Almost all effect sizes were large, except for a moderate to large \ the dominant upper extremity during posterior transfers (Table 6).27 Lalumiere et al.28 compared muscular demand among manual wheelchair users (MWUs) with SCI (n = 15) when performing a curb ascent task at different heights. It was found that the MUR of the main muscles increased as the height of the curb increased. Wang et al.23 collected SEMG signal of EREC, LAT, RECT, and OBLI of patients with spinal cord injury (N = 30) when they pulled themselves into the wheelchair with different load levels. The rate of increase of the LAT muscle was higher than the EREC in patients with SCI with injury level higher than L1. In contrast, patients with injury at L1 and lower with the same increase in load exhibited an EREC rate higher than the LAT muscle.
Table 6 .
Effect sizes for examination of SEMG activity of trunk muscles in self-controlled study (HDM-SCI and LDM-SCI)
| Posture | Number of effect sizes | Mean effect size (SD) | Range | Mean weighted effect size (SD) |
|---|---|---|---|---|
| All postures | 87 | 0.86 (1.00) | –1.62 to1.97 | 1.08 (1.25) |
| Static | ||||
| Long sitting | 36 | 0.89 (1.11) | –1.62 to 1.97 | 1.11 (1.38) |
| Unsupported sitting | 36 | 1.16 (0.80) | –1.41 to1.94 | 1.45 (1.00) |
| Dynamic | ||||
| Ascending curbs by wheelchair | 6 | 0.86 (0.33) | 0.45 to 1.31 | 1.08 (0.41) |
| Posterior transfer | 9 | –0.43 (0.57) | –1.43 to 0.45 | –0.54 (0.72) |
In one study the effect sizes could not be determined. Desroches et al.9 recorded bilateral SEMG of PECT, TRAP, and LAT of subjects with SCI during the performance of three types of sitting pivot transfers: natural technique, exaggerated forward trunk flexion, and upright trunk position. In the exaggerated forward trunk flexion condition, activity in PECT was greatly increased over natural strategy, whereas activity of LAT was increased during the upright trunk strategy condition compared to the natural strategy. Chow et al.11 found in young men (N = 10) with paraplegia that PECT and LAT trunk muscle activity significantly changes with various speed and slope conditions. During the mid-portion of the push phase, the PECT major was most active, whereas the LAT was most active at the end of the push phase and at the start of the recovery phase.
Discussion
Motor function following SCI can be assessed in several different ways.29 To date, the primary method for evaluating motor function for SCI is the American Spinal Injury Association Impairment Scale (AIS), which tests manual muscle strength in five key muscles in each limb and examines sensory function.30 However, this approach is highly subjective, making it difficult to monitor appropriately patterned muscle activation, and it does not measure activities of trunk muscles.27,29 The primary goal of rehabilitation is to restore as much function as possible and to enable the performance of everyday life tasks from a seated position. In this context, the trunk muscles in particular become critical, since the ability to control the trunk is essential for performance of these everyday tasks. Proper rehabilitation is of key importance, as proficiency of these tasks is usually maintained, or even improved, after discharge from the hospital.3 To further aid in this recovery, there needs to be available better and more objective functional assessment tools for the clinical evaluation of residual trunk motor control. Trunk muscles, however, are not routinely assessed to classify motor function in patients with SCI. Surface electromyography (SEMG) is a non-invasive method to assess motor function that is already used clinically to evaluate neuromuscular pathology in patients with SCI. SEMG records electrical potentials from muscles and can be used to analyze trunk muscular activities and to detect appropriately patterned muscle activation.29 One advantage of SEMG is that the appropriateness of muscle activation during voluntary attempts of movement can be determined.9,27 Due to the integrity of the motor and sensory neurological systems is impaired, trunk demand increases in individuals with SCI. Understanding the pattern of trunk muscle activity and movement will likely translate into the refinement of trunk skill training programs offered by physical and occupational therapists.27
Two studies23,24 that examined SEMG activity in static trunk muscles between people with and without SCI found higher activity levels in patients with SCI relative to healthy controls during some tasks The largest effect size observed was for the seated position. The SEMG effect size between HL-SCI and LL-SCI tended to be less and inconsistent relative to effect size between people with and without SCI. Studies examining SEMG while patients with SCI were posterior transferring or respirating produced mixed results. One study reported large, positive effect size in SEMG activity between patients with HL-SCI and LL-SCI in posterior transfer,3 and another study found negative, moderate to large effect size in SEMG activity between patients with HL-SCI and LL-SCI.26 Regarding static SEMG and dynamic SEMG during the performance of ascending curbs, both studies found a large, positive effect in SEMG activity between patients with HL-SCI and LL-SCI. For dynamic SEMG during posterior transfer, the effect size was negative and moderate to large between the HDM-SCI and LDM-SCI groups. Given the inconsistency of these findings, further examination is required of SEMG effect sizes of trunk muscles during dynamic activities.
As shown in Figs. 2–4, the MUR of trunk muscles during sitting in patients with SCI was greater than normal controls. For static sitting, posterior transfer, or respiration, the activity level of trunk muscles in HL-SCI was different from LL-SCI. With the exception of respiration, the higher the level of SCI was linked with greater trunk muscle activity. The decrease in trunk muscle activity during respiration in patients with HL-SCI was likely associated with the differences in motion patterns of trunk musculature during performance of this task. Performance of the high difficult sitting and ascending curbs by wheelchair generated higher MUR of trunk muscle in patients with SCI than controls. Surprisingly, high difficult posterior transfer did not require greater muscular demand than low difficult posterior transfer. These results suggested that the performance of high difficult posterior transfer required high upper limb muscular demand and different, but not greater, trunk motor patterns.
Figure 2 .
Distribution of effect sizes for the trunk muscles between patients with SCI and healthy controls
Figure 3 .
Distribution of effect sizes for the trunk muscles between between patients with HL-SCI and LL-SCI.
Figure 4 .
Distribution of effect sizes for the trunk muscles in self-controlled study (HDM-SCI and LDM-SCI)
Even though SEMG assessment is noninvasive, concerns have been raised about its use in SCI populations. Based on our analysis, at least two factors appear to influence the outcomes of studies examining SEMG and SCI. One factor is duration of rehabilitation. Patients with SCI who have only undergone a short-term rehabilitation program often have weaker trunk muscle strength than those who had a long-term rehabilitation program. Such experiences could introduce bias into the data. Many studies that evaluate SEMG in patients with SCI do within patient controls, normalizing maximal effort to no effort to reduce artifacts. Also, SEMG evaluation methods that do not rely on assessment of maximal effort have been shown to be less subject to bias.31
Another factor that likely influences SEMG assessment is population heterogeneity. Patients with various levels of SCI present with a number of different body function and psychosocial conditions. For example, participants with HL-SCI present with different movement characteristics and muscular demands during some tasks than patients with LL-SCI. In this review, we found that patients with HL-SCI typically perform poorer on respiration tasks than patients with LL-SCI, perhaps due to weakness. Therefore, when selecting subjects for data analysis related to SEMG and SCI, the level of the nerve lesion should be taken into consideration. Further research is needed to determine the extent by which SEMG measures are influenced by factors such as sincerity of effort. Studies should be designed to limit the effect such confounds could have on experimental outcomes.
One potential limitation of this review is that subgrouping yielded small sample sizes. This was a problem particularly for measuring effect size when using weighted measures, calculated to correct for sample size, differed significantly from the unweighted values. In the future, performing additional experiments with large sample sizes will address this limitation.
In summary, we identified SEMG measures as objective markers of SCI. Patients with SCI or patients with HL-SCI needed to generate higher trunk muscle strength to control the trunk during both sitting and posterior transfer. We have also shown that the activity of trunk muscles were not the same during different tasks. Use of SEMG not only makes it possible to compare trunk muscle activity of subjects with or without SCI, patients with different lesion levels, and patients performing different difficult tasks; but also allows for understanding the various activity patterns of trunk muscle by comparing muscle activity levels. These findings should prompt further investigation into trunk muscle function in SCI and highlight the importance of including SEMG tests for trunk muscles in the rehabilitation of people with SCI. Future studies are needed to identify more SEMG protocols to measure the variation in trunk muscle activity in different SCI patient populations.
Acknowledgments
This study was supported by China Rehabilitation Research Center (2014CZ-17).
Disclaimer statements
Contributors Y-JW participated in writing the article in whole. J-JL and H-JZ carried out revising the article. G-LL and YZ participated in interpreting the data. BW, YZ and C-XH participated in the analysing the data. H-QK, YY and L-JG carried out collecting the data. All authors read and approved the final manuscript.
Funding None.
Conflicts of interest The authors declare no conflict of interest.
Ethics approval None.
References
- 1.DeVivo M. Epidemiology of spinal cord injury. In: Lin VW, (ed.) Spinal cord medicine principles and practice. New York: Demos Medical Publishing; 2010. p. 78–84. [Google Scholar]
- 2.Thomas CK, Zaidner EY, Calancie B, Broton JG, Bigland-Ritchie BR. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp Neurol 1997;148(2):414–23. [DOI] [PubMed] [Google Scholar]
- 3.Gagnon D, Nadeau S, Gravel D, Noreau L, Lariviere C, Gagnon D. Biomechanical analysis of a posterior transfer maneuver on a level surface in individuals with high and low-level spinal cord injuries. Clin Biomech (Bristol, Avon) 2003;18(4):319–31. [DOI] [PubMed] [Google Scholar]
- 4.Zariffa J, Steeves J, Pai DK. Changes in hand muscle synergies in subjects with spinal cord injury: characterization and functional implications. J Spinal Cord Med 2012;35(5):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seelen HA, Potten YJ, Huson A, Spaans F, Reulen JP. Impaired balance control in paraplegic subjects. J Electromyogr Kinesiol 1997;7(2):149–60. [DOI] [PubMed] [Google Scholar]
- 6.Silva PF, Quintino LF, Franco J, Faria CD. Measurement properties and feasibility of clinical tests to assess sit-to-stand/stand-to-sit tasks in subjects with neurological disease: a systematic review. Braz J Phys Ther 2014;18(2):99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Int Med 2009;151(4):W-65–W-94. [DOI] [PubMed] [Google Scholar]
- 9.Desroches G, Gagnon D, Nadeau S, Popovic M. Magnitude of forward trunk flexion influences upper limb muscular efforts and dynamic postural stability requirements during sitting pivot transfers in individuals with spinal cord injury. J Electromyogr Kinesiol 2013;23(6):1325–33. [DOI] [PubMed] [Google Scholar]
- 10.Louis N, Gorce P. Surface electromyography activity of upper limb muscle during wheelchair propulsion: influence of wheelchair configuration. Clin Biomech (Bristol, Avon) 2010;25(9):879–85. [DOI] [PubMed] [Google Scholar]
- 11.Chow JW, Millikan TA, Carlton LG, Chae WS, Lim YT, Morse MI. Kinematic and electromyographic analysis of wheelchair propulsion on ramps of different slopes for young men with paraplegia. Arch Phys Med Rehabil 2009;90(2):271–8. [DOI] [PubMed] [Google Scholar]
- 12.McKay WB, Ovechkin AV, Vitaz TW, Terson de Paleville DG, Harkema SJ. Neurophysiological characterization of motor recovery in acute spinal cord injury. Spinal Cord 2011;49(3):421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin KH, Lu TW, Hsu PP, Yu SM, Liao WS. Postural responses during falling with rapid reach-and-grasp balance reaction in patients with motor complete paraplegia. Spinal Cord 2008;46(3):204–9. [DOI] [PubMed] [Google Scholar]
- 14.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D, Lim H, McKay W, Priebe M, Holmes S, Sherwood A. Toward an objective interpretation of surface EMG patterns: a voluntary response index (VRI). J Electromyogr Kinesiol 2004;14(3):379–88. [DOI] [PubMed] [Google Scholar]
- 16.Lim HK, Lee DC, McKay WB, Protas EJ, Holmes SA, Priebe MM, et al. Analysis of sEMG during voluntary movement-part II: voluntary response index sensitivity. IEEE Trans Neural Syst Rehabil Eng 2004;12(4):416–21. [DOI] [PubMed] [Google Scholar]
- 17.Lim H, Lee D, McKay W, Priebe M, Holmes S, Sherwood A. Neurophysiological assessment of lower-limb voluntary control in incomplete spinal cord injury. Spinal Cord 2005;43(5):283–90. [DOI] [PubMed] [Google Scholar]
- 18.Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am 2012;94(4):298–307. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 20.Rosenthal R. Writing meta-analytic reviews. Psychol Bull 1995;118(2):183–92. [Google Scholar]
- 21.Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Behav Stat 1981;6(2):107–28. [Google Scholar]
- 22.LaFrance M, Hecht MA, Paluck EL. The contingent smile: a meta-analysis of sex differences in smiling. Psychol Bull 2003;129(2):305. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wang C. A study on the characteristics of surface electronic signals of some trunk muscles in SCI patients with hold static sitting balance. Master's thesis of Sun Yat-Sen University; 2010. [Google Scholar]
- 24.Liu Y, Li J, Hua G. A comparative study of electromyographic activities during four activities of daily living between normal subjects and patients with C5 and C6 quadriplegia. Chinese Journal of Rehabilitation Medicine 2008;23(4):317–9. [Google Scholar]
- 25.Sherwood AM, McKay WB, Dimitrijević MR. Motor control after spinal cord injury: assessment using surface EMG. Muscle Nerve 1996;19(8):966–79. [DOI] [PubMed] [Google Scholar]
- 26.Terson de Paleville D, McKay W, Aslan S, Folz R, Sayenko D, Ovechkin A. Locomotor step training with body weight support improves respiratory motor function in individuals with chronic spinal cord injury. Respir Physiol Neurobiol 2013;189(3):491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagnon D, Nadeau S, Gravel D, Noreau L, Lariviere C, McFadyen B. Movement patterns and muscular demands during posterior transfers toward an elevated surface in individuals with spinal cord injury. Spinal Cord 2005;43(2):74–84. [DOI] [PubMed] [Google Scholar]
- 28.Lalumiere M, Gagnon DH, Hassan J, Desroches G, Zory R, Pradon D. Ascending curbs of progressively higher height increases forward trunk flexion along with upper extremity mechanical and muscular demands in manual wheelchair users with a spinal cord injury. J Electromyogr Kinesiol 2013;23(6):1434–45. [DOI] [PubMed] [Google Scholar]
- 29.Nitzken M, Bajaj N, Aslan S, Gimel'farb G, El-Baz A, Ovechkin A. Local wavelet-based filtering of electromyographic signals to eliminate the electrocardiographic-induced artifacts in patients with spinal cord injury. J Biomed Sci Eng 2013;6(7B):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003;26(Suppl 1):S50–6. [DOI] [PubMed] [Google Scholar]
- 31.Geisser ME, Ranavaya M, Haig AJ, Roth RS, Zucker R, Ambroz C, et al. A meta-analytic review of surface electromyography among persons with low back pain and normal, healthy controls. J Pain 2005;6(11):711–26. [DOI] [PubMed] [Google Scholar]