Abstract
Background
Necroptosis is an emerging programmed necrosis other than traditional necrosis and apoptosis. Until recently, there have not been studies that have investigated a relationship between necroptosis and pathogenesis of cell death after spinal cord injury (SCI).
Objective
To investigate whether necroptosis takes part in the early pathophysiological processes of traumatic SCI in mice.
Methods
Female ICR mice were randomized equally into three groups: the sham, the vehicle-treated + SCI group, and the Nec-1-treated + SCI group. To induce SCI, the mice were subjected to a laminectomy at T9 and compression with a vascular clip. After mice were sacrificed 24 hours post-SCI, propidium iodide (PI)-positive cells were detected using in vivo PI labeling. Morphological analyses were performed by hematoxylin and eosin staining and Nissl staining. The samples were evaluated for apoptosis by the in situ TUNEL assay. The expression of caspase-3 was assessed by western blot. Locomotor behavior of hindlimb was evaluated by BMS (Basso mouse scale) score at 1, 3, 5, 7, and 14 days post-injury.
Results
Compared with dimethyl sulfoxide -treated mice, necrostatin-1-treated mice showed decreased PI-positive cells (P < 0.05), alleviated tissue damage, more surviving neuron at 24 hours after SCI (P < 0.05), and improved functional recovery from days 7 to 14 (P < 0.05). Necrostatin-1 did not reduce the expression of caspase-3 and the number of TUNEL-positive cells at 24 hours after SCI (P > 0.05).
Conclusions
Necroptosis contributes to necroptotic cell death and influences functional outcome after SCI in adult mice. The inhibition of necroptosis by necrostatin-1 may have therapeutic potential for patients with SCI.
Keywords: Functional outcome, Necroptosis, Necrostatin-1, Spinal cord injury
Introduction
The annual incidence of spinal cord injury (SCI), excluding mortalities at the scene of the accident, is ∼40 cases per million population in the USA or ∼12 000 new cases each year.1 Individuals paralyzed from SCI experience one of the most physically disabling and psychologically devastating conditions known to humans.2 Unfortunately, although great efforts have been made to improve the outcome of patients with SCI, at present there is little effective therapy in clinic except high-dose methylprednisolone, which does not significantly improve functional recovery and has serious adverse effects.3 Nowadays, traumatic SCI and its devastating consequences represent one of the greatest challenges for clinicians. So, it is crucial and significant to find new and promising therapeutic approaches.
The pathophysiological processes of traumatic SCI generally include two phases: the primary injury, characterized by hemorrhage and cell necrosis (neurons, oligodendrocytes, and endothelial cells, etc.) in the epicenter of the lesion, which is an irreversible, mechanical insult and a subsequent secondary insult, characterized by excitotoxicity, oxidative stress, ischemia, inflammation, ionic homeostasis, and necrotic and apoptotic cell death, all of which lead to neuronal and supporting cell death.2 Cell death is a fundamental and core issue following spinal cord damage. The extent of tissue damage and demyelination can be alleviated through mechanisms that attenuate neuronal and/or oligodendrocytic death. Generally, cell death includes apoptosis, necrosis, and autophagy.4,5 Apoptosis is often characterized by cellular shrinkage, plasma membrane blebbing, chromatin condensation, appearance of apoptotic body, and nuclear degradation.6 Necrosis is morphologically identified by increased cell volume, swelling of organelles, and early ruptures of plasma membrane. Autophagy is characterized by lack of chromatin condensation, redistribution of light chain 3 into autophagosomes membrane, and accumulation of double-membrane covered vacuoles containing cytoplasmic organelles or cytosol.7 Most prior studies about SCI focused on apoptosis and autophagy because necrosis traditionally was considered to be irreversible.8–10 While necrosis is a complex traumatic effect to cells, therapeutic attempts are currently being undertaken in the hope of functional recovery or alleviation of tissue damage. One such approach is through the use of a relatively new compound: necrostatin-1. Necrosis is often viewed as an accidental and unregulated cellular event. However, accumulating evidence suggests that necrosis, like apoptosis, can partly be executed by regulated mechanisms. In 2005, necrostatin-1 was initially synthesized by Degterev as a specific tool drug to distinguish necroptosis from other cell death processes.11 Since, necroptosis has been described as a receptor-induced, caspase-independent, highly regulated type of programmed cell death process with morphological resemblance of necrosis.7
More recently, necroptosis has been reported to contribute to the attenuation of pathological injury and to improve functional outcome in animal models of cerebral ischemia, traumatic brain injury, and myocardial infarction, etc.12–14 There has been a paucity of studies on necroptosis in SCI. In this present study, we tested the hypothesis that in a mouse model of SCI, necroptosis contributes to neural cell death and that treatment with necrostatin-1 reduces histopathological and functional deficits. Until recently, none of research about necroptosis following SCI has been reported. So in this study, we tested the hypothesis that necroptosis contributes to neural cell death after SCI in mice, and that treatment with necrostatin-1 would reduce histopathological and functional deficits.
Materials and methods
Animal preparation
Adult female ICR mice (25–30 g) were purchased from Jinling Hospital Laboratory Animal Center. The mice were raised on a 12-hour dark–light cycle with free access to food and water. All experiments were approved by the Animal Care and Use Committee of Southern Medical University (China) and accorded to Guides for the Care and Use of Laboratory Animals by National Institutes of Health.
Seventy-eight mice were randomly assigned to three main groups: (a) the sham group that underwent sham surgery; (b) the vehicle-treated group that underwent surgery for SCI induction and was given dimethyl sulfoxide (DMSO) by intrathecal injection; (c) the Nec-1-treated group that underwent surgery for SCI induction and was given necrostatin-1 by intrathecal injection. Each group was randomly divided into four subgroups. The first subgroup (n = 8) was used to detect propidium iodide (PI)-positive cells; the second subgroup (n = 6) was used for histopathological evaluation and TUNEL staining; the third subgroup (n = 6) was used for western blot analysis; while locomotor assessment was performed in the last subgroup (n = 6).
Induction of experimental SCI and administration of necrostatin-1
The model of SCI in mice was produced according to a previous report with minor modifications.15 After intraperitoneal anesthesia with pentobarbital sodium (50 mg/kg) (Sigma, St Louis, MO, USA), 0.1 ml of 2% lidocaine was injected around the incision site. A 2.5 cm skin incision along the midline of the back was made in each mouse. The subsequent operations were performed with an operating microscope (M500-N; Leica, Heerbrugg, Switzerland). The paravertebral muscles of the thoracic level (T8–T10) vertebrae were dissected out. Laminectomy was performed with mouse laminectomy forceps at the T9 level. Extradural compression with a vascular clip (10 g force, Kent Scientific Corporation, INS 15911, Torrington, CT, USA) was performed for 1 minute around the exposed spinal cord. Intrathecal injections were performed with 4 µl of 4 mM necrostatin-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or DMSO at 5 minutes after clipped injury by inserting a 33-gauge needle connected to a 5 µl Hamilton syringe (Hamilton, Reno, NV, USA) with each injection lasting for 5 minutes. After injection, muscles and skin were sutured in layers, and an antibacterial spray was applied topically. The mice were given a subcutaneous saline injection (1–2 ml). Mice were allowed to recover on a warm pad until thermoregulation and an alert state was reestablished. Mice were then returned to their home cages and raised at 23 ± 1°C.
Locomotor assessment
The Basso mouse scale (BMS) score, a sensitive and reliable rating system to measure hindlimb recovery in mice following SCI,16 was used to evaluate the locomotor function of mice at 1, 3, 5, 7, and 14 days after SCI. According to the BMS scale, the mice were allowed to move in an open field for 5 minutes. If the scores differed between the individuals, the lower score was taken. After a brief observation, it was initially judged whether it was plantar stepping after a mouse was placed in the open field. If there was plantar stepping, then the frequency of stepping and coordination was evaluated. If not, then ankle movement of dorsal stepping was evaluated and appropriately scored.17 The hindlimb movements were observed and scored by two independent observers, who were blinded to the experiment.
Administration of PI and detection of PI-positive cells
PI (10 mg/ml, Sigma) was diluted in 0.9% NaCl and 1 mg/kg was administered 1 hour prior to sacrifice by intraperitoneal injection. Mice were sacrificed at 24 hours after SCI, the samples of spinal cord were harvested and frozen in nitrogen vapor, and cryostat spine sections (8 µm) were cut at 150–200 µm intervals. Cryostat sections were placed on poly-l-lysine slides and were fixed in 100% ethanol for 10 minutes at room temperature. For detection of PI-labeled cells, the slides were washed with phosphate-buffered saline (PBS) again three times for 45 minutes. After the three washes, the slides were covered by microscopic glass with anti-fade mounting medium. The sections were photographed using a Nikon Eclipse T300 fluorescence microscope (Tokyo, Japan) fitted with excitation/emission filters 568/585 for PI.
Perfusion-fixation and tissue preparation
Mice were deeply anesthetized, and perfused through the left cardiac ventricle with ice-cold 0.9% NaCl solutions until the effluent from the right atrium was clear. The spinal cord within 15 mm rostral, epicenter, and caudal regions was harvested on ice and stored at −80°C for western blot analysis. For hematoxylin and eosin (HE) staining and Nissl staining, the mice were perfused with 0.9% NaCl solutions followed by 4% buffered paraformaldehyde, then the spinal cord within 15 mm rostral, epicenter, and caudal regions was removed and immersed in 4% buffered paraformaldehyde overnight and then embedded in paraffin for further study.
Measurement of lesion size
The longitudinal sections of spinal cord were stained with HE, and the area of the lesion was quantitated using image analysis software (Image Pro Plus 6.0, Media Cybernetics, Inc., Rockville, MD, USA) by two investigators blinded to the grouping, and the estimated lesion area was calculated by the average of the injury areas. Lesion size was expressed in mm2.
Nissl staining
After deparaffinization and rehydration, all sections were dyed in 1% toluidine blue for 4 minutes at 37°C. Then, the sections were dehydrated in increasing concentrations of ethanol and mounted with Permount. Five Nissl-stained sections from each animal were randomly selected that were ∼200 µm apart from each other and running across the gray matter. All neurons within 200 µm rostral to the lesion edge in each section were counted, with counting restricted to neurons having a well-defined nucleolus and a soma rich in Nissl bodies. All tissue processing was conducted by two investigators blinded to the grouping.
TUNEL staining
Mice were deeply anesthetized as before in all groups (n = 6 for each group) at 24 hours after SCI. Apoptotic cells were analyzed using an In Situ Cell Death Detection kit (Boehringer Mannheim, Mannheim, Germany). According to the kit's protocol and the previous study,18 after deparaffinization and rehydration, the tissue was washed with PBS, and then was digested for 15 minutes in proteinase K (20 µg/ml; Sigma). The reaction was terminated with tap water, and the tissue was washed with PBS for 10 minutes. Sections were incubated at 37°C with labeling solution containing TUNEL reaction fluid for 60 minutes. After washing with PBS again, the sections were blocked with 10% goat serum in 0.1 M Tris for 15 minutes. DNA was visualized by treating the tissue with a 1:40 dilution of streptavidin peroxidase (horseradish peroxidase (HRP)) and staining with DAB as chromogen. Five sections from each spinal cord 200 µm apart from each other were randomly chosen. The TUNEL-positive cells of six microscopic fields at a magnification of ×400 in each section were identified, counted, and analyzed under the light microscope by two investigators blinded to the grouping.
Western blot analysis
Spinal tissues harvested as before were homogenized and centrifuged at 14 000 g for 15 minutes at 4°C. After adding sodium dodecyl sulfate (SDS) sample buffer, the supernatant were boiled for 5 minutes at 100°C. Protein concentrations in the samples were determined using the Bradford method. Then, appropriate amounts of proteins were loaded onto 10% SDS–polyacrylamide gel electrophoresis and electrotransferred it onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% skimmed milk for 2 hours at room temperature, then incubated overnight at 4°C with primary antibodies anti-caspase-3 antibody (diluted 1:1000, Cell Signaling Technology, Danvers, MA, USA) and β-actin (diluted 1:3000, Bioworld Technology, Inc., St Louis Park, MN, USA). After the membrane was washed for 15 minutes three times in TBST, the membranes were incubated with the appropriate HRP-conjugated secondary antibody (diluted 1:5000 in TBST) for 2 hours at room temperature. Blotted protein bands were visualized by enhanced chemiluminescence (Thermo Fisher Scientific Inc., Waltham, MA, USA) and were exposed to X-ray film. Relative changes in protein expression were estimated from the mean pixel density using UN-Scan-It 6.1 software (Silk Scientific Inc., Orem, UT, USA), normalized to β-actin, and calculated as target protein expression/β-actin expression ratios.
Statistical analysis
All data were presented as mean ± SEM. SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the data. The measurements were subjected to two-tailed Student's t-test. A value of P < 0.05 was considered statistically significant.
Results
Necrostatin-1 reduces tissue damage and neuronal cell death after SCI
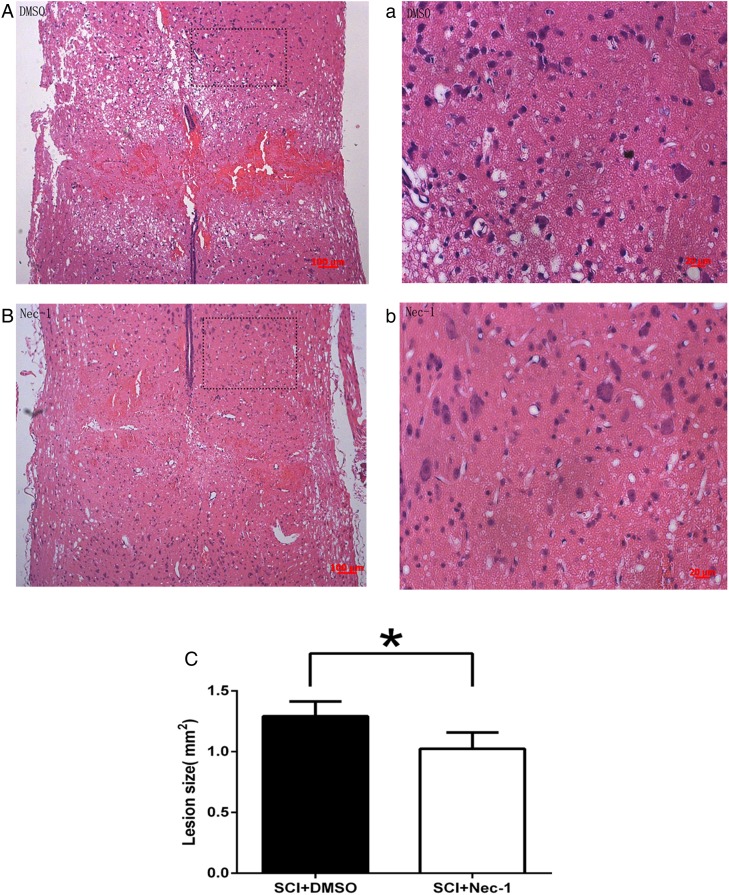
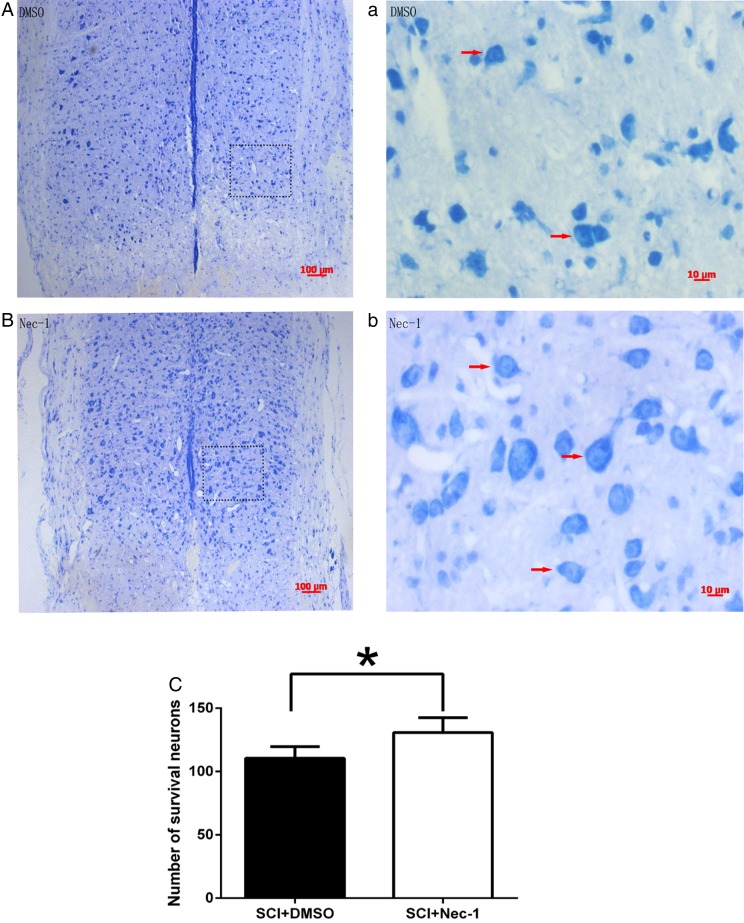
HE staining showed that the injured area of spinal cord formed cavities characterized by the typically “spongy” appearance at 24 hours after SCI, more severe cavitation can be observed in the vehicle group compared with the necrostatin-1 group (Fig. 1A, a and B, b). The lesion size as defined by HE staining was significantly reduced by administration of necrostatin-1 24 hours after SCI (Fig. 1C). Surrounding the lesion, fewer neurons survived in the vehicle group compared with the necrostatin-1 group (Fig. 2A and B). Nissl staining demonstrated that the number of surviving neurons in the necrostatin-1 group was significantly more than in the vehicle group (Fig. 2C). All of the above suggest that necrostatin-1 can reduce tissue damage and neuronal cells death 24 hours after SCI.
Figure 1 .
Longitudinal sections of spinal cord stained with HE. (A) and (a) are from a representative section of the DMSO-treated group; (B) and (b) show a representative section of the necrostatin-1-treated group. (C) The lesion sizes of spinal cord were significantly smaller in the necrostatin-1 group than that of the DMSO group at 24 hours post-SCI. *P < 0.05 vs. the DMSO-treated group (n = 6 per group). Scale bars: (A) and (B), 100 µm; (a) and (b), 20 µm.
Figure 2 .
Survival of neurons in the peripheral region of the lesion was shown using Nissl staining at 24 hours after SCI. Necrostatin-1 markedly decreases neurons death at 24 hours after SCI in injured spinal cord. (A, a and B, b) Representative photomicrographs showing neurons in surrounding injured regions after SCI in necrostatin-1 and DMSO-treated mice. Arrows indicate Nissl-positive neurons. (C) Quantitation of neurons in injured region. *P < 0.05 vs. the DMSO-treated group (n = 6 per group). Scale bars: (A) and (B), 100 µm; (a) and (b), 10 µm.
Necrostatin-1 reduces acute plasmalemma permeability in neural cells after SCI
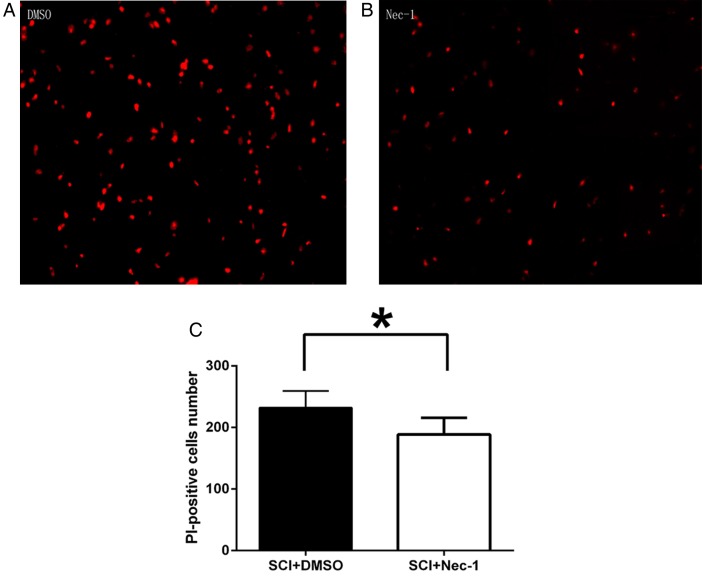
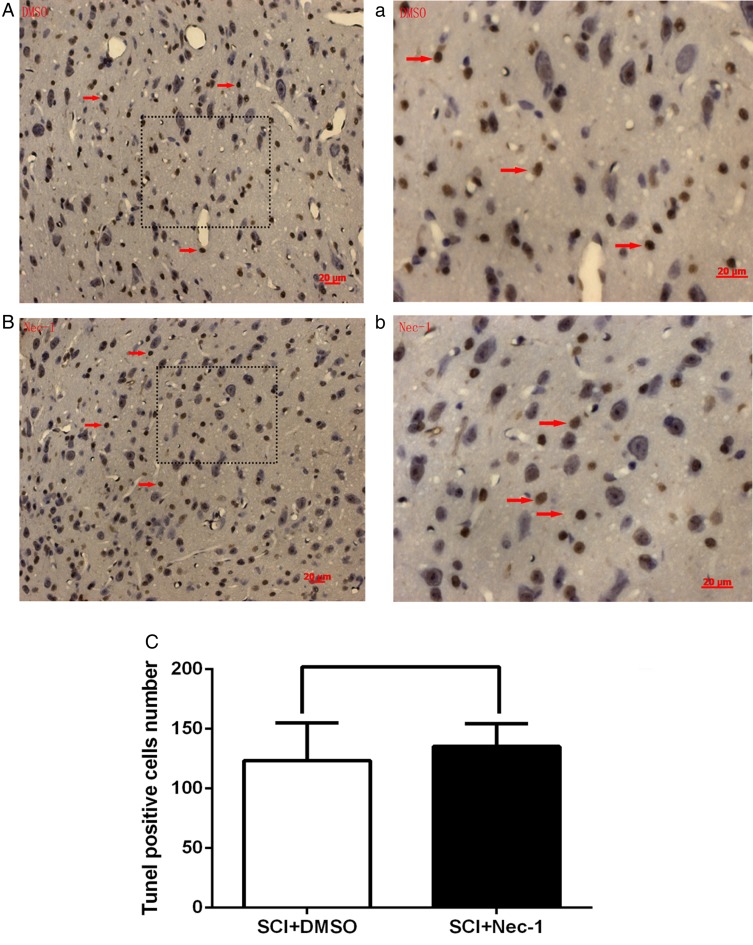
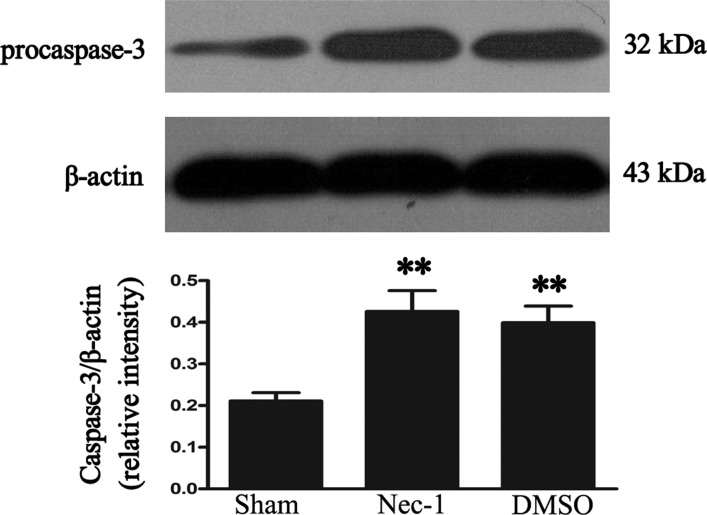
Plasmalemma permeability is a hallmark of necroptosis distinguished from other programmed cell death, like apoptosis. The effect of necrostatin-1 on loss of plasma membrane integrity was evaluated using in vivo PI labeling. Compared with the vehicle group, necrostatin-1 reduced significantly the number of PI-positive cells at 24 hours after SCI (P < 0.05; Fig. 3). Because plasma membrane permeability may also take place late in apoptosis, we next assessed the effect of necrostatin-1 on apoptosis using two classic markers of apoptosis at 24 hours after SCI: caspase-3 expression and TUNEL staining. TUNEL staining illustrated that necrostatin-1 did not significantly vary the number of TUNEL-positive cells at 24 hours in areas surrounding the lesion compared with the vehicle-treated group (P > 0.05; Fig. 4). The level of caspase-3 was not significantly different between necrostatin-1-treated and vehicle-treated mice (P > 0.05; Fig. 5). These results suggest that necrostatin-1 does not influence the apoptosis of neurons and/or neuroglia cells after SCI, further supporting its specific role in reduction of necrosis-like cellular injury.
Figure 3 .
Necrostatin-1 dramatically reduces PI-positive cells at 24 hours after SCI. (A and B) Representative photomicrographs showing PI-positive cells in surrounding injured regions after SCI in necrostatin-1-treated and DMSO-treated mice. Magnification ×200. (C) The bar graph demonstrating quantitation PI-positive cells in surrounding injured regions of DMSO and necrostatin-1 administrated mice (the Nec-1-treated group vs. the DMSO-treated group. *P < 0.05, n = 6 per group).
Figure 4 .
TUNEL-positive cells at 24 hours after SCI were shown by TUNEL staining. (A) and (a): the DMSO-treated group; (B) and (b): the necrostatin-1-treated group. No difference in numbers of TUNEL-positive cells was observed between the necrostatin-1 and the DMSO-treated groups. Arrows indicate TUNEL-positive cells. Statistical results are shown in (C). P > 0.05 vs. the DMSO-treated group, n = 6 per group. Scale bars: 20 µm.
Figure 5 .
Representative autoradiogram of caspase-3 expression in injured spinal cord tissue. It shows that no difference can be found in the expression of caspase-3 protein between the necrostatin-1 and the DMSO-treated groups at 24 hours. **P < 0.05, the Nec-1-treated group vs. the DMSO-treated group. n = 6 per group.
Effects of necrostatin-1 on functional recovery after SCI
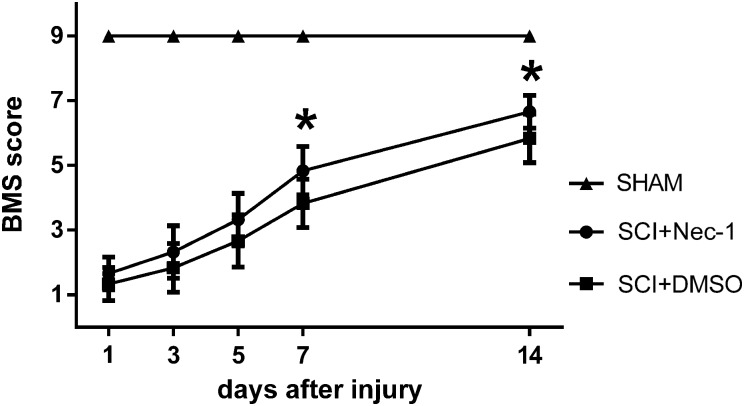
All mice used in this study showed a normal BMS score (score 9) during assessment before the injury. The hindlimbs of each mouse were totally paralyzed immediately after SCI, corresponding to a BMS score of 0–1. The BMS scores of laminectomy control groups throughout the extent of experiments remained at normal scores of 9. And a gradual recovery of hindlimbs locomotion was observed in all injured animals regardless of treatment. The statistical analysis showed a significant improvement in the BMS score from days 7 to 14 in the necrostatin-1 group than in the SCI group, indicating that necrostatin-1 could promote the functional recovery of mice during early stages of recovery after SCI (Fig. 6).
Figure 6 .
Effects of necrostatin-1 on functional recovery of mice after SCI. The function of hindlimb recovery was evaluated from days 1 to 14 after SCI by BMS scores, indicating that necrostatin-1 significantly improved functional recovery beginning on day 7 after SCI. *P < 0.05 vs. DMSO, n = 6.
Discussion
Traumatic SCI initiates a complex series of cellular and molecular events that induce massive cell death of neurons and glial cells, extensive demyelination, and axonal destruction, leading to permanent neurologic deficits.19 Some of the previous literature suggests that apoptosis plays an important role in cell death resulting from SCI, but none of them targets necroptosis.19–21 In this study, we demonstrated for the first time the existence of necroptosis, a novel type of caspase-independent programmed necrosis, involving in the cell death after experimental SCI in mice. Our data demonstrated that necrotatin-1 could attenuate spinal cord tissue damage, rescue neurons, and improve functional recovery after experimental SCI in mice.
Multiple lines of evidence indicate that necroptosis is a significant cell death mode after SCI in mice, according to the characteristic of it described in previous studies.11,12,22 Firstly, necroptosis is a pathway of regulated necrotic cell death triggered by death receptor ligands in the presence of broad caspase inhibition.7,11 Accordingly, several studies have demonstrated an upregulation of death receptor ligands, including tumor necrosis factor-α (TNF-α) within hours after SCI.23,24 Therefore, it prompted us to directly examine whether necroptosis may contribute to SCI.
Secondly, the loss of plasma membrane integrity at an early point in time after SCI argues for a mechanism involving necrosis. On the one hand, although necroptosis shares some morphological features of necrosis (the loss of plasma membrane integrity, it is different from the latter, in that it is triggered by receptor signaling rather than a non-specific cellular injury. Furthermore, necroptosis can be inhibited by necrostatin-1, but necrosis cannot.11 On the other hand, despite the fact that the same death receptor agonist may trigger necroptosis and apoptosis, necroptosis can be distinguishable from apoptosis both morphologically and functionally. Apoptosis is often characterized by cellular shrinkage, plasma membrane blebbing, chromatin condensation, appearance of apoptotic body, and nuclear degradation, rather than the loss of plasma membrane integrity. Moreover, the activation of caspase-3 and TUNEL staining are also markers of apoptosis.6 In our study, necrostatin-1 reduced the number of cells with loss of plasma membrane integrity (PI staining positive), a hallmark of necrosis, at 24 hours after SCI. Moreover, compared with DMSO-treated animals, more survival neurons can be observed in the lesion periphery of injury in necrostatin-1-treated ones. These findings indicated that a portion of neurons and perhaps other cell deaths can be relieved by necrostatin-1, a specific inhibitor of necropotsis.
Finally, consistent with previous research, necrostatin-1 does not alleviate the expression of caspase-3 or TUNEL staining at 24 hours.11 It proves that necrostatin-1 does not decrease cells death through apoptotic or caspase-mediated pathways after SCI. However, it should be acknowledged that caspase and TUNEL are not absolute markers of apoptosis. Interestingly, we found an increase in the number of cells with an apoptotic profile in necrostatin-1-treated mice; however, the magnitude was not significant. Previous studies show that apoptosis induced by death receptor ligation could be switched to necroptosis when apoptosis signaling was blocked because apoptotic and necrotic cell death mechanisms share the same pathway.25 Therefore, we inferred that necroptosis may be partly diverted to apoptosis in the presence of necrostatin-1 in SCI model. Above all, a part of necrotic cell death following experimental SCI in mice can be suppressed by necrostatin-1, which means that necroptosis is a mode of cell death after SCI in adult mice.
Although extensive work has been done to characterize the mechanisms of necroptosis, the signaling pathways underlying necroptosis are still poorly understood. Until recently, the most extensively studied pathway leading to necroptosis was by inducing binding of TNFα to TNF-R1 with the involvement of kinase receptor interacting protein 1/3 (RIP1/3) when the apoptotic pathway is blocked.26–28 Because the primary intent of this study is to prove the existence of necroptosis in the pathological process of SCI, we did not undertake any further investigations of a mechanistic basis of necroptosis. But studies demonstrating the concrete mechanism of necroptosis post-SCI in vivo are currently underway in our laboratory.
Based on previous studies showing a beneficial effect of necrostatin-1 in ischemic brain injury and traumatic brain injury, we anticipated that necrostatin-1 would reduce lesion size and improve functional recovery after SCI.11,12 Fortunately, we showed that administration of necrostatin-1 at 5 minutes post-SCI can promote the recovery of motor functions in mice. But administration of necrostatin-1 at 5 minutes after controlled cortical impact (CCI) was not effective in reducing post-injury motor deficits in CCI model in mice. Necrostatin-1 significantly reduced lesion size only when it was given at 15–30 minutes post-injury. We suggested that a large proportion of necroptotic cell death possibly has no intrinsic relation with motor function in CCI model. So, in order to have a significant impact on motor deficits, it should rescue a great number of neurons. In contrast, a small amount of neural cell death, especially neurons and oligodendrocytes, are most likely to disrupt and ruin motor neurons or axon–myelin structural unit after SCI and hence destroy the motor conduction pathway, eventually resulting in functional deficits. So, even small gains in neural cell survival might significantly affect functionally relevant neurologic recovery in SCI.29 In contrast, we concluded that a smaller amount of neural cell survival have not a significant impact on motor deficits recovery in CCI. Therefore, we suggested that the relationship between cell residual and function recovery is tighter in SCI than in CCI models.
In prior studies, the effective manner of necrostatin-1 administration is intracerebroventricular injection in nervous system diseases.11,12,25 Instead, intrathecal injection was chosen as the method of administering necrostatin-1 in the study, because intrathecal injection is a more convenient and has a greater prospect of clinical application, especially in SCI model, compared with intracerebroventricular injection. Finally, we show, for the first time, that necrostatin-1 can also be effectively administered by intrathecal injection in SCI model. Further investigation is needed to identify necroptosis signaling pathways after SCI.
Conclusion
In conclusion, necroptosis may be an important, emerging mode of cell death after experimental SCI in mice. The inhibition of necroptosis by necrostatin-1 may be a new and promising strategy for patients with SCI.
Disclaimer statements
Contributors All authors are in agreement with the content and have no conflict of interest to declare.
Funding This work was funded in part by grants from the National Natural Science Foundation, China (No. 81171170, 81371294) and Nature Science Foundation of Jiangsu Province, China (BK2010459) and Scientific Research and Innovation Foundation of Universities in Jiangsu Province, China (CXZZ12_0067).
Conflicts of interest None.
Ethics approval All experiments were approved by the Animal Care and Use Committee of Southern Medical University (China) and accorded to Guides for the Care and Use of Laboratory Animals by National Institutes of Health.
References
- 1.Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation & apoptosis in spinal cord injury. Indian J Med Res 2012;135:287–96. [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 2004;4(4):451–64. [DOI] [PubMed] [Google Scholar]
- 3.Braughler JM, Hall ED, Means ED, Waters TR, Anderson DK. Evaluation of an intensive methylprednisolone sodium succinate dosing regimen in experimental spinal cord injury. J Neurosurg 1987;67(1):102–5. [DOI] [PubMed] [Google Scholar]
- 4.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 2004;16(6):663–9. [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 2005;12Suppl 2:1463–7. [DOI] [PubMed] [Google Scholar]
- 6.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26(4):239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunai Z, Bauer PI, Mihalik R. Necroptosis: biochemical, physiological and pathological aspects. Pathol Oncol Res 2011;17(4):791–800. [DOI] [PubMed] [Google Scholar]
- 8.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 1997;3(1):73–6. [DOI] [PubMed] [Google Scholar]
- 9.Lou J, Lenke LG, Ludwig FJ, O'Brien MF. Apoptosis as a mechanism of neuronal cell death following acute experimental spinal cord injury. Spinal Cord 1998;36(10):683–90. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Zhang C, Hong Z, Chen H, Chen W, Chen G. C/EBP homologous protein (CHOP) mediates neuronal apoptosis in rats with spinal cord injury. Exp Ther Med 2013;5(1):107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005;1(2):112–9. [DOI] [PubMed] [Google Scholar]
- 12.You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab 2008;28(9):1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol 2012;107(4):270. [DOI] [PubMed] [Google Scholar]
- 14.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther 2007;21(4):227–33. [DOI] [PubMed] [Google Scholar]
- 15.Mao L, Wang HD, Wang XL, Tian L, Xu JY. Disruption of Nrf2 exacerbated the damage after spinal cord injury in mice. J Trauma Acute Care Surg 2012;72(1):189–98. [DOI] [PubMed] [Google Scholar]
- 16.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006;23(5):635–59. [DOI] [PubMed] [Google Scholar]
- 17.Tep C, Lim TH, Ko PO, Getahun S, Ryu JC, Goettl VM, et al. Oral administration of a small molecule targeted to block proNGF binding to p75 promotes myelin sparing and functional recovery after spinal cord injury. J Neurosci 2013;33(2):397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hang CH, Shi JX, Tian J, Li JS, Wu W, Yin HX. Effect of systemic LPS injection on cortical NF-kappaB activity and inflammatory response following traumatic brain injury in rats. Brain Res 2004;1026(1):23–32. [DOI] [PubMed] [Google Scholar]
- 19.Chen KB, Uchida K, Nakajima H, Yayama T, Hirai T, Watanabe S, et al. Tumor necrosis factor-alpha antagonist reduces apoptosis of neurons and oligodendroglia in rat spinal cord injury. Spine (Phila Pa 1976) 2011;36(17):1350–8. [DOI] [PubMed] [Google Scholar]
- 20.Wada S, Yone K, Ishidou Y, Nagamine T, Nakahara S, Niiyama T, et al. Apoptosis following spinal cord injury in rats and preventative effect of N-methyl-D-aspartate receptor antagonist. J Neurosurg 1999;911 Suppl:98–104. [DOI] [PubMed] [Google Scholar]
- 21.Yong C, Arnold PM, Zoubine MN, Citron BA, Watanabe I, Berman NE, et al. Apoptosis in cellular compartments of rat spinal cord after severe contusion injury. J Neurotrauma 1998;15(7):459–72. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res 2010;88(7):1569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal PM, Lemmens E, Avila A, Vangansewinkel T, Chalaris A, Rose-John S, et al. ADAM17 is a survival factor for microglial cells in vitro and in vivo after spinal cord injury in mice. Cell Death Dis 2013;4:e954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 2007;500(2):267–85. [DOI] [PubMed] [Google Scholar]
- 25.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab 2011;31(1):178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009;325(5938):332–6. [DOI] [PubMed] [Google Scholar]
- 27.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009;137(6):1100–11. [DOI] [PubMed] [Google Scholar]
- 28.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009;137(6):1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol 1995;132(2):220–8. [DOI] [PubMed] [Google Scholar]