Abstract
Changes in litterfall dynamics and soil properties due to anthropogenic or natural perturbations have important implications to soil carbon (C) and nutrient cycling via microbial pathway. Here we determine soil microbial responses to contrasting types of litter inputs (leaf vs. fine woody litter) and nitrogen (N) deposition by conducting a multi-year litter manipulation and N addition experiment in a mixed-wood forest. We found significantly higher soil organic C, total N, microbial biomass C (MBC) and N (MBN), microbial activity (MR), and activities of four soil extracellular enzymes, including β-glucosidase (BG), N-acetyl-β-glucosaminidase (NAG), phenol oxidase (PO), and peroxidase (PER), as well as greater total bacteria biomass and relative abundance of gram-negative bacteria (G-) community, in top soils of plots with presence of leaf litter than of those without litter or with presence of only fine woody litter. No apparent additive or interactive effects of N addition were observed in this study. The occurrence of more labile leaf litter stimulated G-, which may facilitate microbial community growth and soil C stabilization as inferred by findings in literature. A continued treatment with contrasting types of litter inputs is likely to result in divergence in soil microbial community structure and function.
Intensification of global change has profound impacts on the structure and function of forest ecosystems. Such events as windthrow and tree mortality as results of extreme climatic events, or anthropogenic N deposition and harvesting, can markedly modify the biotic and abiotic conditions on forest floor, thereby altering soil C and nutrient cycling via microbial pathway1,2. However, few studies have investigated how combined changes in forest floor litter dynamics and N deposition would affect soil processes. Forest floor litter consists of both nutrient-rich leaf litter and lignin-rich woody detritus. Shifts in the relative abundance of the two types of litter may lead to changes in soil microbial community structure, consequently affecting microbial regulation of soil C and nutrient processes through the linkage between microbial community structure and function3,4,5,6,7. While leaf litter is commonly found to be associated with abundance of diverse soil microbial communities8,9, the occurrence of woody litter tends to promote the abundance of saprophytic fungi specialized in lignin degradation10. N deposition, on the other hand, can either stimulate the growth of soil microbial community by ameliorating N-limitation and relieving C-limitation through enhancement of aboveground NPP and litter quality1,11, or impose negative impacts on soil microorganisms, especially the white-rot fungi, which is a major producer of soil oxidase, by increasing soil acidity or enriching toxic [NH4+]2,12,13. Moreover, excessive N leads to microbial C-limitation by increasing resistance to lignin degradation, decreasing plant belowground NPP or inhibiting soil microorganisms11,13. In labile substrate, N addition stimulates the growth of microbes and enzyme activities by alleviating microbial N-limitation; whereas in recalcitrant substrate, lignin-limitation caused by a reduction of N-suppressive phenol oxidase may restrain soil microbial community14,15,16,17,18,19.
Based on relevant findings in literature, we postulate that differential occurrence of leaf litter and woody litter on forest floor would lead to divergence in soil microbial community structure and function because of contrasting chemistry and recalcitrance, i.e. nutrient-rich vs. lignin-rich, with different levels of N deposition exacerbating the substrate-induced differences in soil microbial traits. To test this hypothesis, we conducted a multi-year litter manipulation and N addition experiment in a mixed pine (Pinus tabulaeformis Carr.) and oak (Quercus wutaishanica Mayr.) forest in temperate northern China. Replicated field plots were set up in 2009 with three rates of N addition (0, N0; 5 gN·m−2·a−1, N5; 10 g N·m−2·a−1, N10) and four types of litter placement (removal, Littnil; leaf litter, Littleaf; fine woody litter, Littwoody; mixture of leaf and fine woody litter, Littmix), and selective soil physiochemical properties and microbial traits were studied four years after the initial treatments.
Results
Both litter mass density and chemistry varied among plots of different types of litter placement. Litter mass density in the Littwoody plots was less than half of that in the Littleaf and Littmix plots. The litter samples from the Littwoody plots had significantly lower contents of N (%N) (F = 31.587, d.f. = 2, p < 0.001) and acid-unhydrolyzable residue (%AUR) (F = 14.882, d.f. = 2, p < 0.001), and significantly higher C:N ratio (F = 18.711, d.f. = 2, p < 0.001) and AUR:N ratio (F = 7.928, d.f. = 2, p = 0.001), than those from the Littleaf and Littmix plots (Table 1). The effects of the litter treatment were significant on both soil organic C (SOC) content (F = 11.182, d.f. = 3, p < 0.001) and total N (TN) content (F = 17.556, d.f. = 3, p < 0.001); values of SOC and TN were all higher in the Littmix and Littleaf plots than in the Littwoody and Littnil plots. TN was also significantly affected by the level of N addition (F = 3.051, d.f. = 2, p = 0.050), and was highest in the N5 treatment and lowest in the N0 treatment (Table 2). Soil C:N ratio and soil pH were not significantly affected by the litter treatment and the level of N addition (Table 2).
Table 1. Litter mass density and chemistry in different litter treatment plots in a mixed pine/oak forest in central North China.
| Litter treatment | Mass density (kg·m−2) | %C | %N | %AUR | Litter C:N | AUR:N |
|---|---|---|---|---|---|---|
| Littwoody | 1.04 ± 0.11 B | 48.8 ± 0.9 A | 0.37 ± 0.03 B | 40.3 ± 2.0 B | 142.0 ± 12.6 A | 115.6 ± 11.1 A |
| Littleaf | 2.35 ± 0.29 A | 48.7 ± 0.5 A | 0.61 ± 0.02 A | 49.6 ± 0.9 A | 82.1 ± 3.0 B | 83.7 ± 2.8 B |
| Littmix | 2.96 ± 0.23 A | 47.4 ± 1.0 A | 0.64 ± 0.03 A | 49.6 ± 1.0 A | 79.6 ± 4.5 B | 79.5 ± 1.8 B |
Treatments began in September 2009 and were applied annually; samples were taken in April 2015. Values presented are means ± standard errors (n = 15). Values designated by the same letters are not significantly different among different litter treatments (p < 0.05). Littnil, litter removal (no data presented as measurements are not applicable); Littwoody, fine woody litter; Littleaf, leaf litter; Littmix, mixed leaf and fine woody litter.
Table 2. Soil organic carbon (SOC), total nitrogen (TN) and C:N ratio under different treatments of litter and nitrogen in a mixed pine/oak forest in central North China.
| Treatment | SOC (g·kg−1) | TN (g·kg−1) | Soil C:N | Soil pH | |
|---|---|---|---|---|---|
| Litter | Littnil | 72.0 ± 3.1 C | 4.28 ± 0.13 C | 16.5 ± 0.5A | 6.61 ± 0.04 A |
| Littwoody | 85.8 ± 4.0 B | 4.96 ± 0.16 B | 17.2 ± 0.5A | 6.58 ± 0.05 A | |
| Littleaf | 104.1 ± 4.8 A | 5.73 ± 0.15 A | 17.4 ± 0.6A | 6.60 ± 0.04 A | |
| Littmix | 101.4 ± 5.5 A | 5.47 ± 0.17 A | 17.7 ± 0.6A | 6.59 ± 0.05 A | |
| N Addition | N0 | 89.2 ± 4.2 A | 4.83 ± 0.16 B | 17.4 ± 0.5A | 6.59 ± 0.04 A |
| N5 | 93.6 ± 4.5 A | 5.30 ± 0.15 A | 17.3 ± 0.4A | 6.62 ± 0.04 A | |
| N10 | 89.6 ± 4.0 A | 5.13 ± 0.14 AB | 16.9 ± 0.5A | 6.58 ± 0.03 A | |
Treatments began in September 2009 and were applied annually, and samples were collected in June, August and October 2014 and mixed within the same treatment plots. Data are presented as means ± standard errors (n = 5). N0, zero rate of nitrogen addition; N5, nitrogen addition at 5 g∙m−2∙a−1; N10, nitrogen addition at 10 g∙m−2∙a−1; Littnil, litter removal; Littwoody, fine woody litter; Littleaf, leaf litter; Littmix, mixed leaf and fine woody litter. Values designated by the same letters are not significantly different among nitrogen treatments or among different litter treatments (p < 0.05).
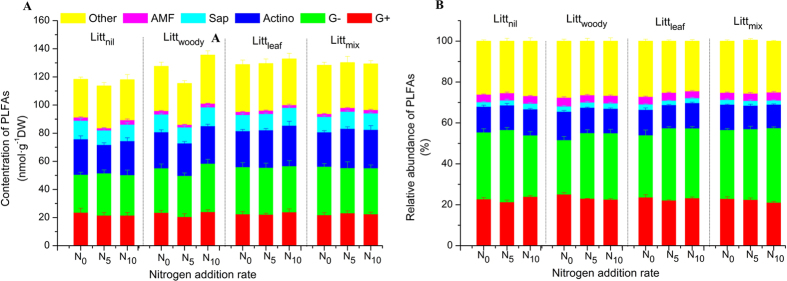
The soil microbial community composition in terms of PLFAs was significantly affected by the litter treatment (F = 3.274, d.f. = 1, p = 0.043) as tested by the PERMANOVA procedure, in particular the quantity of total bacteria (F = 2.934, d.f. = 3, p = 0.042) and the relative abundance of gram-negative bacteria, G− (F = 3.371, d.f. = 3, p = 0.025). The quantity of total bacteria and the relative abundance of G− were both higher in the Littmix and Littleaf plots than in the Littnil and Littwoody plots (Fig. 1). The relative abundance of G− was also significantly (F = 4.409, d.f. = 2, p = 0.023) affected by the level of N addition, and was higher in the N5 treatment than in the N0 treatment; whilst the effect of N addition on the concentration of total PLFAs was only marginal (F = 2.934, d.f. = 1, p = 0.051). The ratio of fungal to bacterial biomass (F:B ratio) was not significantly affected by either the litter treatment or the level of N addition, nor were there significant interactions between the litter treatment and the level of N addition in the effects on soil microbial community structure.
Figure 1. Soil microbial community structure based on PLFAs under different treatments of litter and nitrogen in a mixed pine/oak forest in central North China:
(A) Concentrations; (B) Relative abundance. Treatments began in September 2009 and were applied annually; samples were collected in August 2014. Vertical bars indicate one standard error of means (n = 5). N0, zero rate of nitrogen addition; N5, nitrogen addition at 5 g∙m−2∙a−1; N10, nitrogen addition at 10 g∙m−2∙a−1; Littnil, litter removal; Littwoody, fine woody litter; Littleaf, leaf litter; Littmix, mixed leaf and fine woody litter.
The activities of four extracellular enzymes studied, including BG, NAG, PO, and PER, were all significantly affected by the litter treatment as tested by repeated measures ANOVA acoss sampling times (BG: F = 4.208, p = 0.010; NAG: F = 15.90, p < 0.001; PO: F = 5.727, p = 0.002; PER: F = 3.270, p = 0.030; d.f. = 3 for all) (Table 3); they were mostly higher in the Littmix and Littleaf plots than in the Littwoody and Littnil plots (Fig. 2). MBC, MBN and MR were also significantly affected by the litter treatment (MBC: F = 6.349, p = 0.001; MBN: F = 3.506, p = 0.022; MR: F = 9.155, p < 0.001; d.f. = 3 for all) (Table 3); they were mostly higher in the Littmix and Littleaf plots than in the Littwoody and Littnil plots (Fig. 3). There was a significant interactive effect on the NAG activity between the litter treatment and the level of N addition when sampled in October 2014 (F = 2.306, d.f. = 6, p = 0.049) (Table 3); the NAG activity in the Littleaf and Littmix plots of the N5 treatment was markedly greater than in other treatments (Fig. 4).
Table 3. F-values and p-values (in parentheses) for significance tests of the effects of nitrogen (N) and litter treatments on soil extracellular enzyme activities and microbial variables in a mixed pine/oak forest in central North China.
| Variable | Factor | Time of sampling |
repeated measurements | ||
|---|---|---|---|---|---|
| Jun. 2014 | Aug. 2014 | Oct. 2014 | |||
| BG | N | 0.024 (0.977) | 0.092 (0.912) | 2.439 (0.096) | 0.398 (0.674) |
| Litter | 3.335 (0.026)* | 8.841 (0.000)*** | 1.467 (0.233) | 4.208 (0.010)* | |
| N × Litter | 0.609 (0.722) | 0.775 (0.593) | 0.305 (0.931) | 0.408 (0.870) | |
| NAG | N | 0.214 (0.808) | 0.430 (0.652) | 6.217 (0.045)* | 1.440 (0.247) |
| Litter | 22.24 (0.000)*** | 28.07 (0.000)*** | 5.424 (0.002)** | 15.90 (0.000)*** | |
| N × Litter | 0.972 (0.455) | 0.244 (0.960) | 2.306 (0.049)* | 1.075 (0.391) | |
| PO | N | 0.714 (0.494) | 0.011 (0.989) | 0.203 (0.817) | 0.225 (0.800) |
| Litter | 15.21 (0.002)** | 8.688 (0.000)*** | 8.712 (0.033)* | 5.727 (0.002)** | |
| N × Litter | 0.708 (0.645) | 0.720 (0.636) | 0.137 (0.991) | 0.261 (0.952) | |
| PER | N | 1.895 (0.160) | 0.920 (0.404) | 0.587 (0.559) | 0.251 (0.779) |
| Litter | 3.108 (0.375) | 3.254 (0.028)* | 6.156 (0.104) | 3.270 (0.030)* | |
| N × Litter | 0.199 (0.976) | 0.419 (0.863) | 0.270 (0.948) | 0.189 (0.978) | |
| MBC | N | 0.086 (0.918) | 0.084 (0.919) | 2.422 (0.098) | 0.569 (0.570) |
| Litter | 2.561 (0.064) | 2.764 (0.050)* | 0.507 (0.679) | 6.349 (0.001)** | |
| N × Litter | 0.662 (0.681) | 0.369 (0.895) | 0.535 (0.779) | 0.793 (0.580) | |
| MBN | N | 0.232 (0.793) | 0.144 (0.866) | 2.878 (0.064) | 0.752 (0.477) |
| Litter | 3.131 (0.033)* | 3.946 (0.013)* | 0.587 (0.626) | 3.506 (0.022)* | |
| N × Litter | 1.001 (0.436) | 0.333 (0.916) | 0.303 (0.933) | 0.464 (0.832) | |
| MR | N | 0.115 (0.891) | 0.615 (0.554) | 0.073 (0.929) | 0.044 (0.957) |
| Litter | 6.097 (0.001)** | 9.435 (0.000)*** | 8.182 (0.000)*** | 9.155 (0.000)*** | |
| N × Litter | 0.546 (0.771) | 0.555 (0.764) | 0.908 (0.498) | 0.542 (0.774) | |
Treatments began in September 2009 and were applied annually. BG, β-glucosidase; NAG, N-acetyl-β-glucosaminidase; PO, phenol oxidase; PER, peroxidase; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; MR, microbial activity. *p < 0.05; **p < 0.01; ***p < 0.001; values in italic are results based on non-parametric tests.
Figure 2. Differences in extracellular enzyme activities among treatments of litter and nitrogen in a mixed pine/oak forest in central North China.
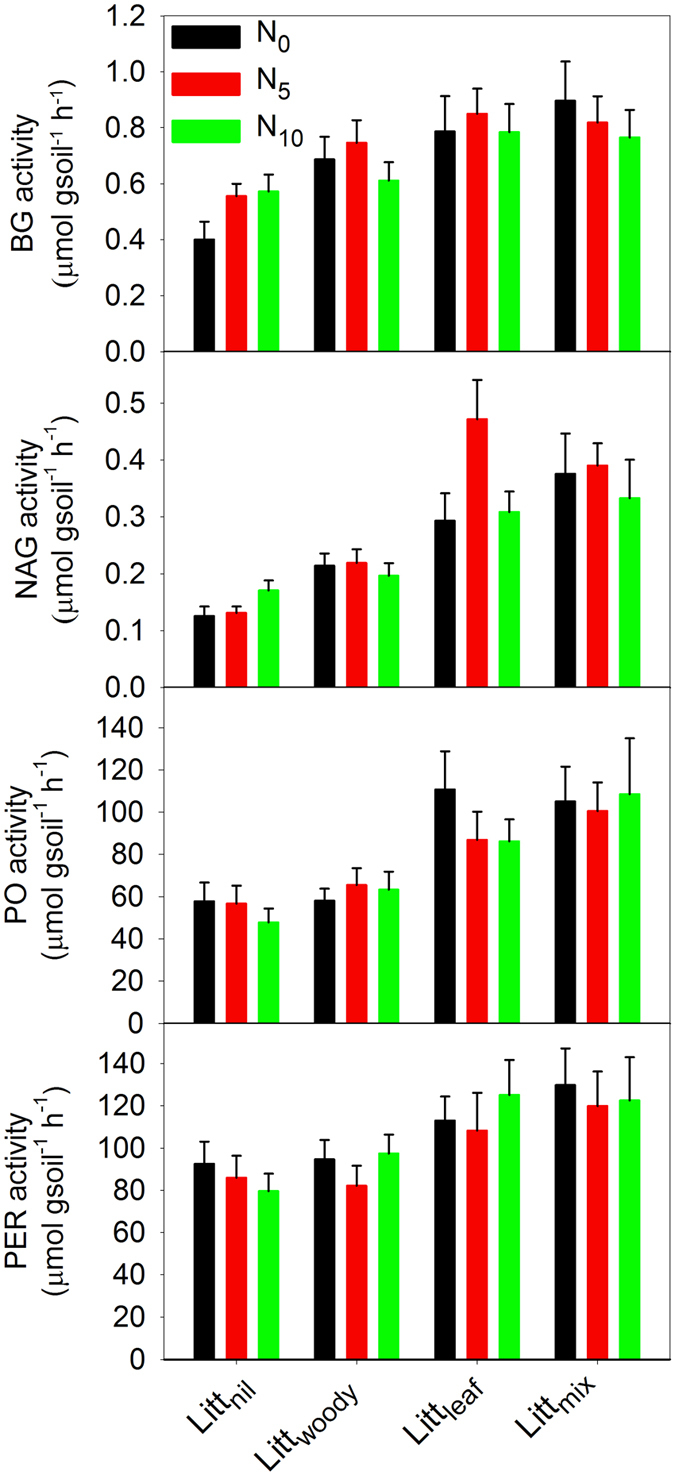
Treatments began in September 2009 and were applied annually. Vertical bars indicate one standard error of means (n = 5). BG, β-glucosidase; NAG, N-acetyl-β-glucosaminidase; PO, phenol oxidase; PER, peroxidase; N0, zero rate of nitrogen addition; N5, nitrogen addition at 5 g∙m−2∙a−1; N10, nitrogen addition at 10 g∙m−2∙a−1; Littnil, litter removal; Littwoody, fine woody litter; Littleaf, leaf litter; Littmix, mixed leaf and fine woody litter.
Figure 3. Differences in microbial biomass carbon (MBC), nitrogen (MBN) and microbial activity (MR) among treatments of litter and nitrogen in a mixed pine/oak forest in central North China.
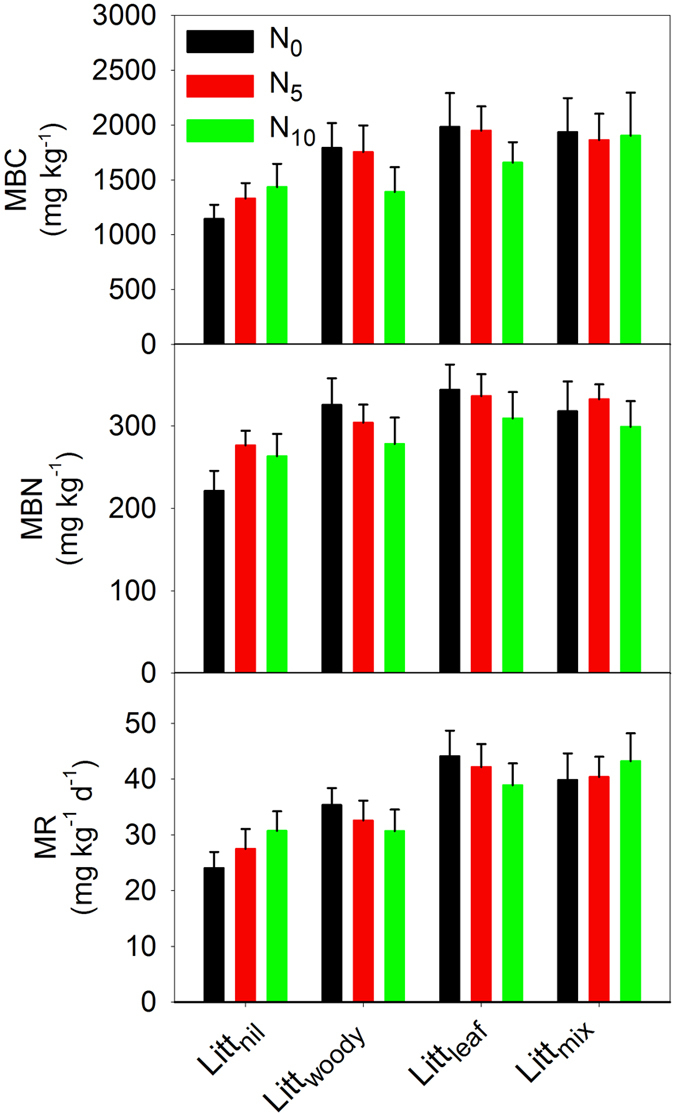
Treatments began in September 2009 and were applied annually. Vertical bars indicate one standard error of means (n = 5). N0, zero rate of nitrogen addition; N5, nitrogen addition at 5 g∙m−2∙a−1; N10, nitrogen addition at 10 g∙m−2∙a−1; Littnil, litter removal; Littwoody, fine woody litter; Littleaf, leaf litter; Littmix, mixed leaf and fine woody litter.
Figure 4. Differences in activity of N-acetyl-β-glucosaminidase (NAG) among treatments of litter and nitrogen sampled in October 2014 in a mixed pine/oak forest in central North China.
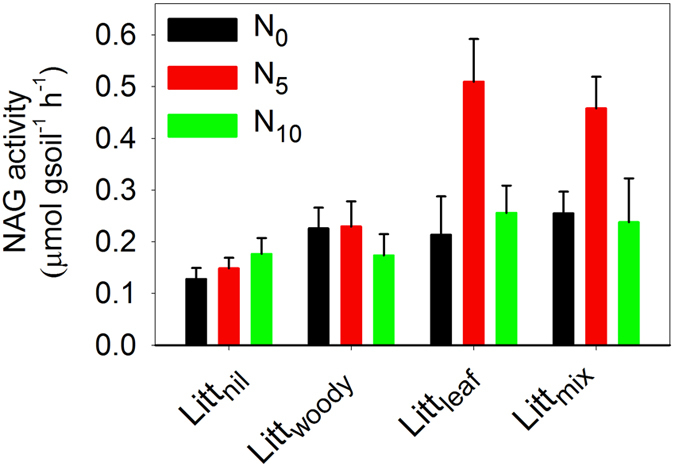
Treatments began in September 2009 and were applied annually. Vertical bars indicate one standard error of means (n = 5). N0, zero rate of nitrogen addition; N5, nitrogen addition at 5 g∙m−2∙a−1; N10, nitrogen addition at 10 g∙m−2∙a−1; Littnil, litter removal; Littwoody, fine woody litter; Littleaf, leaf litter; Littmix, mixed leaf and fine woody litter.
Discussion
In this study, we found that the microbial traits in the top soils of a mixed-wood forest in temperate northern China are mainly differentiated by the occurrence of leaf litter, without apparent additive or interactive effects of N addition. In the experimental plots where leaf litter are present (i.e. in Littleaf and Littmix plots), the top soils had greater activities of BG, NAG, PO, and PER, which are soil extracellular enzymes directly involved in C metabolisms, and greater microbial biomass C, microbial biomass N and microbial activity, corresponding to greater litter mass density and lower values of C:N and AUR:N ratios, than in the plots where leaf litter are removed (i.e. in Littnil and Littwoody plots). This is because that nutrient-rich leaf litter is more readily degradable than lignin-rich woody litter14, and that exclusion of leaf litter would constrain soil microbial growth and function by imposing energy limitation. Varying findings have been reported regarding the differential effects of leaf litter and woody litter on soil microbial characteristics20,21,22. Changes in soil microbial traits in response to contrasting types of litter inputs were also found to be associated with significant changes in soil organic C and total N, signifying the importance of leaf litter as a source of soil organic matter in top soils and the underlying controls of microbial communities in soil C and N processes.
Examination of the microbial community structure using PLFAs revealed that the total biomass of bacteria was higher in soils with presence of leaf litter than in soils with presence of woody litter, in agreement with findings from other studies that bacteria are adapted to utilize easily decomposed C sources (more cellulose, less lignin)7,13,23. Moreover, the relative abundance of gram-negative bacteria was significantly higher with presence of leaf litter, indicating that the occurrence of more labile leaf litter stimulated gram-negative bacteria, which is a specific group of bacteria often classified to the copiotrophic community and is activated by high C and nutrient availability3,4. It is known that the extracellular enzymes such as BG are produced by gram-negative bacteria and are cell-bounded, giving them advantages in avoiding diffusive loss and enhancing resources utilization4,24. Our results suggest that the presence of leaf litter increases production of cellulosic hydrolases and lignin oxidase as compared with occurrence of woody litter, facilitating microbial community growth and soil C stabilization as inferred by findings in literature6. It can be predicted that a continued treatment with contrasting types of litter inputs would result in divergence in soil microbial community structure and function.
Many previous studies have established that, in labile substrate, N addition stimulates the growth of microbes and enzyme activities by alleviating microbial N-limitation14,15,16,17,18,19. In this study, we found that a moderate N addition at 5 gN·m−2·a−1 markedly increased the activity of N-acetyl-β-glucosaminidase.
Methods
Study site
The study is located in Ling Kong Mountain (latitude 36°38.736′N and longitude 112°06.967′E) of Shanxi province, northern China. The site is under the influence of a warm temperate and continental monsoon climate, with cinnamon and brown forest soils developed on limestone. Annual average temperature is 8 °C, with mean monthly minimum temperature of −5 °C in January and mean monthly maximum temperature of 21.5 °C in July25. The experimental plots were set up in a mixed P. tabulaeformis and Q. wutaishanica forest stand, which is a typical zonal vegetation type of the region.
Experimental design and treatments
The experiment was initiated in September 2009, setting up as a randomized block design with four types of litter placement and three rates of N addition. There were five blocks laid out along the contour of the site and kept at least 1 m apart; within each block, combinations of litter treatment and N addition treatment were randomly assigned to 12 2 × 2 m plots at a minimum of 0.5 m apart26. Litter treatments included complete removal of all litter (Littnil), placement of fine woody litter (Littwoody), placement of leaf litter (Littleaf), and placement of mixed leaf and fine woody litter (Littmix); N was applied as urea at rates of 0 (N0), 5 (N5) and 10 gN∙m−2∙a−1 (N10). Each year, all litterfall on Littnil plots were collected and transferred to Littmix plots, whilst leaf litter and fine woody litter were sorted and exchanged between Littwoody and Littleaf plots. N applications were implemented four times per annum, in April, June, August and October, respectively. Urea was used in this study as the form of N addition instead of NH4NO3 because the latter is not easily obtainable in sufficient quantity in China for security concerns. Previous studies suggested that the form of added N is not of major importance on microbial activity and decomposition27,28.
Sample collections and processing
Soil samples were collected in June, August and October of 2014, each to a depth of 0–5 cm on each plot at five random points using a stainless soil corer (3 cm inner diameter and 20 cm long), which were then mixed to form a composite sample. All soil samples were kept in sealed bags before being taken back to laboratory within 2 h, and gravels, roots, and large organic residues were manually removed before passing a 2 mm sieve. Each sample was separated into two parts: one stored at −20 °C for analyses of soil enzyme activities and microbial properties, and the other air-dried and passed through a 0.5-mm sieve for soil physicochemical analyses. Litter samples were collected from forest floor on each plot in April 2015 and oven-dried at 65 °C to constant weight before grounding for determination of mass density and chemical analyses.
Litter and soil analyses
C concentration in litter samples was measured using the K2Cr2O7-H2SO4 calefaction method. Litter N concentration was analyzed following the Kjeldahl digestion procedure. Litter AUR was determined as the percentage of residue over litter sample after acidolysis at 105 °C for 2 h by 75% sulfuric acid. SOC was analyzed by K2Cr2O7-H2SO4 calefaction method. Soil TN was analyzed using the Kjeldahl digestion procedure. Soil MBC and MBN were measured by fumigation-extraction method, using 0.5 M K2SO4 as extracting agent29. Dichromate oxidation method and semi-micro Kjeldahl method were used to determine C and N in the extracts, respectively. Soil microbial activity was determined as the basal rate of microbial respiration (MR), estimated as CO2 evolution over a 12-day period of incubation at 25 °C in dark. Soil pH value was measured by mixing the soil sample with deionized water at 1:2.5 ratio (w/v), and the supernatants were measured using a pH meter (HI-9125, Hanna Instruments Inc, Woonsocket, RI).
Phospholipid fatty acids (PLFA) analysis
Microbial community structure was assessed by extractable ester-linked PLFAs composition analysis6,30. Concentrations of individual PLFAs were calculated based on 19:0 internal standard concentrations. The indicator PLFAs were used for classification of microbial community types. Bacterial community was represented by PLFAs i14:0, 15:0, i15:0, a15:0, i16:0, 16:1 × 7c, 17:0, a17:0, i17:0, cy17:0, 18:1 × 7c, and cy19:031,32. Gram-positive bacteria (G+) are composed of PLFAs i14:0, i15:0, a15:0, i16:0, a17:0, and i17:032,33; Gram-negative bacteria (G-) are composed of 16:1ω7c, cy17:0, 18:1ω7c, and cy19:034; actinomycete is composed of 10Me16:0, 10Me17:0, 10Me18:031,35,36; saprotrophic fungi are represented by 18:2ω6,9c33; and arbuscular mycorrhizal fungi (AMF) are represented by 16:1ω5c33,36,37. Other PLFAs such as 14:0, 16:0, 16:1 2OH, 16:1ω9c, 17:1ω8c, and 18:1ω9c were also used for analysis of the microbial composition32. The ratio of 18:2ω6,9c to total bacterial PLFAs was used to estimate the ratio of fungal to bacterial biomass (F:B) in soils31,32. The relative biomass of each microbial composition (their percentages) was calculated by dividing the concentration of each community type by the total amount of all types.
Enzyme assays
The activities of β-glucosidase (BG; EC: 3.2.1.21) and N-acetyl-β-glucosaminidase (NAG; EC: 3.2.1.30) were determined by p-nitrohpenol assays38,39; and the activities of phenol oxidase (PO) and peroxidase (PER) were measured using DOPA (3,4-Dihydroxy-L-phenylalamine) as their substrates6. For PO, the reaction mixture composed of 2 mL 5 mmol/L L-DOPA solution and soil slurry (1 g fresh soil with 1.5 mL 50 mmol/L sodium acetate buffer), and PER activity assays received 2 mL of 5 mmol/L-DOPA and soil slurry (1 g fresh soil with 1.5 mL 50 mmol/L sodium acetate buffer), plus 0.2 mL of 0.3% H2O2. All total enzyme activities were expressed as μmol g−1soil h−1. The results of all enzymatic assays were expressed on a dry-weight basis.
Statistical analyses
All variables were evaluated by analysis of variance (ANOVA) with litter treatment and N addition treatment as main factors for each sampling time. Non-parametric test was conducted if the variances were unequal. The values of BG, NAG, PO, PER, MBC, MBN and MR were also tested by repeated measures analysis of variance (RMANOVA) for the effects of litter treatment and N addition treatment across the three sampling times. Data on soil microbial community structure inferred by PLFAs were tested by permutational multivariate analysis of variance (PERMAVONA). The least significant difference (LSD) test was used to compare means of soil variables in absence of interactive effects between treatments. All data were analyzed using SPSS Version17.0 or R (Version 2.15.3), depending the statistical procedures, with p < 0.05 for testing the significance unless otherwise specified.
Additional Information
How to cite this article: Sun, X.-L. et al. Soil microbial responses to forest floor litter manipulation and nitrogen addition in a mixed-wood forest of northern China. Sci. Rep. 6, 19536; doi: 10.1038/srep19536 (2016).
Acknowledgments
This study was jointly supported by the National Basic Research Program of China (Grant No. 2011CB4032005) and the National Natural Science Foundation of China (Grant No. 31470623). We thank the Taiyueshan Long-Term Forest Ecosystem Research Station for access to the study sites and logistic assistance, and Yu Tu for help with setting up the initial field experiment.
Footnotes
The authors declare no competing financial interests.
Author Contributions X.L.S. and J.Z. jointly conducted the experiment, performed field sampling and data analyses, and wrote the manuscript. Y.M.Y. assisted with field sampling and data interpretations. O.J.S. conceived the study, designed the experiment, and wrote the manuscript.
References
- Criquet S., Farnet A., Tagger S. & Le Petit J. Annual variations of phenoloxidase activities in an evergreen oak litter: influence of certain biotic and abiotic factors. Soil Biol. Biochem. 32, 1505–1513 (2000). [Google Scholar]
- DeForest J. L., Zak D. R., Pregitzer K. S. & Burton A. J. Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci. Soc. Am. J. 68, 132–138 (2004). [Google Scholar]
- Hu S., Van Bruggen A. & Grünwald N. Dynamics of bacterial populations in relation to carbon availability in a residue-amended soil. Appl. Soil Ecol. 13, 21–30 (1999). [Google Scholar]
- Waldrop M., Balser T. & Firestone M. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 32, 1837–1846 (2000). [Google Scholar]
- Romani A. M., Fischer H., Mille-lindblom C. & Tranvik L. J. Interactions of bacteria and fungi on decomposing litter: differential extracellular enzyme acticities. Ecology 87, 2559–2569 (2006). [DOI] [PubMed] [Google Scholar]
- You Y. et al. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 4, 633–647 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter M. & Dick R. Shifts in substrate utilization potential and structure of soil microbial communities in response to carbon substrates. Soil Biol. Biochem. 33, 1481–1491 (2001). [Google Scholar]
- Nottingham A. T., Griffiths H., Chamberlain P. M., Stott A. W. & Tanner E. V. J. Soil priming by sugar and leaf-litter substrates: a link to microbial groups. Appl Soil Ecol. 42, 183–190 (2009). [Google Scholar]
- Wang Q., Wang S., He T., Liu L. & Wu J. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol. Biochem. 71, 13–20 (2014). [Google Scholar]
- Brant J. B. et al. Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol. Biochem. 38, 2219–2232 (2006). [Google Scholar]
- Treseder K. K. Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol. Lett. 11, 1111–1120 (2008). [DOI] [PubMed] [Google Scholar]
- Waldrop M. P. & Zak D. R. Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 9, 921–933 (2006). [Google Scholar]
- Zhang N. et al. Impacts of urea N addition on soil microbial community in a semi-arid temperate steppe in northern China. Plant Soil 311, 19–28 (2008). [Google Scholar]
- Carreiro M. M., Sinsabaugh R.L., Repert D. A. & Parkhurst D. F. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81, 2359–2365 (2000). [Google Scholar]
- Saiya-Cork K. R., Sinsabaugh R. L. & Zak D. R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 34, 1309–1315 (2002). [Google Scholar]
- Waldrop M. P., Zak D. R., Sinsabaugh R. L., Gallo M. & Lauber C. Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol. Appl. 14, 1172–1177 (2004). [Google Scholar]
- Knorr M., Frey S. & Curtis P. Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86, 3252–3257 (2005). [Google Scholar]
- Sinsabaugh R. L., Gallo M. E., Lauber C., Waldrop M. P. & Zak D. R. Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75, 201–215 (2005). [Google Scholar]
- Ma Y. et al. The effects of simulated nitrogen deposition on extracellular enzyme activities of litter and soil among different-aged stands of larch. J. Plant Ecol. 7, 240–249 (2014). [Google Scholar]
- Fekete I., Varga C., Kotroczó Z., Tóth J. A. & Várbiró G. The relation between various detritus inputs and soil enzyme activities in a Central European deciduous forest. Geoderma 167, 15–21 (2011). [Google Scholar]
- Veres Z. et al. Soil extracellular enzyme activities are sensitive indicators of detrital inputs and carbon availability. Appl. Soil. Ecol. 92, 18–23 (2015). [Google Scholar]
- Brant J. B., Myrold D. D. & Sulzman E. W. Root controls on soil microbial community structure in forest soils. Oecologia 148, 650–659 (2006). [DOI] [PubMed] [Google Scholar]
- Hu Y. L., Wang S. L. & Zeng D. H. Effects of single Chinese fir and mixed leaf litters on soil chemical, microbial properties and soil enzyme activities. Plant Soil 282, 379–386 (2006). [Google Scholar]
- Burns R. G. et al. Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol. Biochem. 58, 216–234 (2013). [Google Scholar]
- Yu M. & Sun O. J. Effects of forest patch type and site on herb-layer vegetation in a temperate forest ecosystem. Forest Ecol. Manag. 300, 14–20 (2013). [Google Scholar]
- Tu Y., You Y. & Sun J. Effects of forest floor litter and nitrogen addition on soil microbial biomass C and N and microbial activity in a mixed Pinus tabulaeformis and Quercus liaotungensis forest stand in Shanxi Province of China. Chin. J. Appl. Ecol. 23, 2325–2331 (2012). [PubMed] [Google Scholar]
- Berg B. & Matzner E. Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ. Rev. 5, 1–25 (1997). [Google Scholar]
- Fog K. The effect of added nitrogen on the rate of decomposition or organic matter. Biol. Rev. 63, 433–462 (1988). [Google Scholar]
- Vance E. D., Brookes P. C. & Jenkinson D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987). [Google Scholar]
- Bossio D. & Scow K. Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 35, 265–278 (1998). [DOI] [PubMed] [Google Scholar]
- Frostegård Å. & Bååth E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fert. Soils 22, 59–65 (1996). [Google Scholar]
- Liu L., Gundersen P., Zhang T. & Mo J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 44, 31–38 (2012). [Google Scholar]
- Nie M. et al. Positive climate feedbacks of soil microbial communities in a semi‐arid grassland. Ecol. Lett. 16, 234–241 (2013). [DOI] [PubMed] [Google Scholar]
- Zelles L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol. Fert. Soils. 29, 111–129 (1999). [Google Scholar]
- Bell C. W. et al. Linking microbial community structure and function to seasonal differences in soil moisture and temperature in a Chihuahuan desert grassland. Microb. Ecol. 58, 827–842 (2009). [DOI] [PubMed] [Google Scholar]
- Brockett B. F., Prescott C. E. & Grayston S. J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 44, 9–20 (2012). [Google Scholar]
- Olsson P. A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 29, 303–310 (1999). [Google Scholar]
- Baldrian P. Microbial enzyme-catalyzed processes in soils and their analysis. Plant Soil Environ. 55, 370–378 (2009). [Google Scholar]
- Parham J. A. & Deng S. P. Detection, quantification and characterization of beta-glucosaminidase activity in soil. Soil Biol. Biochem. 32, 1183–1190 (2000). [Google Scholar]