Abstract
Amblyopia is a neurodevelopmental disorder of vision that occurs when the visual cortex receives decorrelated inputs from the two eyes during an early critical period of development. Amblyopic eyes are subject to suppression from the fellow eye, generate weaker visual evoked potentials (VEPs) than fellow eyes and have multiple visual deficits including impairments in visual acuity and contrast sensitivity. Primate models and human psychophysics indicate that stronger suppression is associated with greater deficits in amblyopic eye contrast sensitivity and visual acuity. We tested whether transcranial direct current stimulation (tDCS) of the visual cortex would modulate VEP amplitude and contrast sensitivity in adults with amblyopia. tDCS can transiently alter cortical excitability and may influence suppressive neural interactions. Twenty-one patients with amblyopia and twenty-seven controls completed separate sessions of anodal (a-), cathodal (c-) and sham (s-) visual cortex tDCS. A-tDCS transiently and significantly increased VEP amplitudes for amblyopic, fellow and control eyes and contrast sensitivity for amblyopic and control eyes. C-tDCS decreased VEP amplitude and contrast sensitivity and s-tDCS had no effect. These results suggest that tDCS can modulate visual cortex responses to information from adult amblyopic eyes and provide a foundation for future clinical studies of tDCS in adults with amblyopia.
Abnormal binocular visual experience during early childhood can result in amblyopia, a neurodevelopmental disorder of the visual cortex1,2. Amblyopia impairs a wide range of visual functions including contrast sensitivity3, visual acuity2, stereopsis4,5, global motion perception6,7 and contour integration8 (see9,10 for reviews). Amblyopic eyes also exhibit high levels of crowding11,12 and abnormal, suppressive lateral spatial interactions that are related to the loss of visual acuity in the affected eye13,14,15. The neural basis of amblyopia is yet to be fully elucidated, however primate models of strabismic and anisometropic amblyopia have reported weaker responses and losses of spatial resolution within V1 when cells were driven by the amblyopic eye compared to the fellow eye16,17. However, these V1 deficits were not sufficient to explain the behavioral deficits in contrast sensitivity within the same animals and therefore it is likely that extrastriate areas are also affected16. Indeed, abnormal responses to inputs from the amblyopic eye have recently been reported in V217,18,19 and MT20 in primate models of strabismic and anisometropic amblyopia. Strong interocular suppression has also been observed within V1 and V2 in primate models of strabismic18 and anisometropic19 amblyopia. Importantly, in these studies, the magnitude of suppression was closely related to the behavioral loss of contrast sensitivity in the amblyopic eye suggesting that interocular suppression may play an important role in the monocular loss of contrast sensitivity that occurs in primate models of amblyopia18,19.
The results of investigations into the neural basis of amblyopia in humans are broadly consistent with the primate neurophysiology data. Visual evoked potential (VEP) measurements have shown that stimulation of the amblyopic eye evokes a weaker cortical response than stimulation of the fellow eye21. Studies utilizing functional magnetic imaging (fMRI) have extended these findings to reveal that attenuated and abnormal responses to the amblyopic eye occur in V122,23, the lateral geniculate nucleus24,25,26, and a range of extrastriate visual areas including V222,23 and MT27,28. In addition, psychophysical evidence indicates that stronger suppression of the amblyopic eye by the fellow eye is correlated with poorer amblyopic eye visual acuity in human patients29,30,31,32. Current evidence also suggests that dichoptic treatment interventions designed to promote binocular vision and reduce suppression can improve aspects of both binocular (stereopsis) and monocular (visual acuity and contrast sensitivity) vision in adult patients with amblyopia33,34,35,36,37 (see38 for a recent review). Reduced visual cortex excitability measured using phosphene thresholds for single pulses of transcranial magnetic stimulation (TMS) has also been observed in patients with amblyopia38,39, possibly reflecting abnormally high levels of cortical inhibition. Together, these findings are consistent with the idea that suppression is a key component of the amblyopia syndrome.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that can transiently alter the excitability of targeted brain areas in a polarity specific manner40. When applied to the motor cortex, anodal tDCS (a-tDCS) tends to increase cortical excitability, measured as an increase in the amplitude of motor evoked potentials elicited by single pulse TMS40. Cathodal tDCS (c-tDCS), on the other hand, tends to reduce motor cortex excitability40. Comparable polarity-specific effects of tDCS on excitability have been reported for the visual cortex41. For example, a-tDCS of the occipital poles transiently decreases TMS phosphene thresholds whereas c-tDCS has the opposite effect42,43.
One potential mechanism for the increase in cortical excitability following a-tDCS is a reduction in GABA-mediated inhibition within the stimulated region. Stagg et al.44 used magnetic resonance spectroscopy to assess the relative concentration of glutamate and GABA within the motor cortex before and after tDCS. A-tDCS reduced the concentration of GABA relative to glutamate whereas c-tDCS induced comparable reductions in both GABA and glutamate. The selective reduction of GABA concentration following motor cortex a-tDCS has subsequently been replicated45,46. GABA mediated inhibition has been linked to a number of suppressive neural interactions within the visual cortex such as those underlying surround suppression in humans and primates47,48,49, bistable perception in humans50 and interocular suppression in cats with experimentally induced strabismus51. Furthermore, GABA mediated inhibition has been identified as one of a number of mechanisms that regulate adult visual cortex plasticity in rodent models of deprivation amblyopia52. Together, these findings raise the possibility that a-tDCS may modulate visual cortex function in patients with amblyopia.
In an initial study, we found that a-tDCS of the occipital poles significantly reduced psychophysically measured surround suppression in adults with normal vision53. This result suggested that a-tDCS might act to transiently reduce GABA mediated inhibition within the visual cortex as had previously been reported for the motor cortex44. We subsequently observed that a-, but not c-tDCS, transiently improved amblyopic eye contrast sensitivity in 8 out of 13 adult patients with strabismic and/or anisometropic amblyopia54. A-tDCS had no effect on fellow eye contrast sensitivity. Functional MRI measurements in 5 of these patients indicated that a-, but not sham tDCS (s-tDCS) significantly reduced the response bias to inputs from the fellow eye vs. the amblyopic eye in V2 and V354. In other words, a-tDCS reduced the cortical response asymmetry to inputs from the fellow and amblyopic eye in these patients.
Building on this previous work, the aim of this study was to further investigate the effects of tDCS on visual cortex responses and contrast sensitivity in a larger group of adults with amblyopia and controls. In particular, we addressed the following questions that were not part of our previous studies: 1) Are changes in amblyopic eye contrast sensitivity induced by tDCS associated with changes in the cortical response to inputs from the amblyopic eye? Cortical responses were measured using monocular pattern reversal VEPs. 2) Do the effects of tDCS on contrast sensitivity and VEP amplitude differ between patients with amblyopia and controls? 3) Do the effects of tDCS on contrast sensitivity and VEP amplitude differ significantly from a sham stimulation control condition?
Materials and Methods
Participants
Twenty-one adult participants with unilateral amblyopia (Table 1) and twenty-seven adults with normal vision (mean age 23.0 years, SD 2.3, range 19–30 years; 21 females) participated in this study. The Zhongshan Ophthalmic Center ethics committee approved the study and all study protocols were in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants prior to data collection. Participants with amblyopia had an intraocular acuity difference of at least 0.2 LogMAR with no organic cause and 0.1 LogMAR visual acuity or better in the fellow eye. Participants with amblyopia were classified as having strabismic, anisometropic or mixed mechanism amblyopia (both strabismus and anisometropia). Anisometropia was defined as a spherical equivalent difference of 1 dioptre or more between the two eyes. Participants with normal vision had 0.1 LogMAR acuity or better in each eye and no history of visual disorders. Best refractive correction was worn during testing for all experimental sessions. No participants had a history of neurological or psychiatric disorder, any implanted medical devices or were currently taking medication.
Table 1. Clinical details of the participants with amblyopia.
| ID | Current Age [Age of First Detection]/Gender | History of Previous Treatment | Type of Amblyopia | Visual Acuity [log MAR] | Current Refractive Error |
|---|---|---|---|---|---|
| 01 | 1715/F | None | RE Aniso | 0.30 | +3.00 + 0.50 × 090 |
| LE | −0.18 | +0.25 + 0.50 × 080 | |||
| 02 | 175/M | None | RE Strab (26ΔET) | 0.24 | +0.25 + 0.75 × 095 |
| LE | 0.04 | Plano + 1.00 × 080 | |||
| 03 | 178/M | None | RE | −0.08 | +0.25–0.50 × 175 |
| LE Mixed (9~17ΔET) | 0.70 | +5.00 + 2.50 × 105 | |||
| 04 | 1914/M | None | RE | 0.26 | +6.25 + 0.50 × 090 |
| LE | 0.08 | +6.75 DS | |||
| 05 | 17 [unknown]/ F | None | RE | −0.08 | −0.25 DS |
| LE Aniso | 1.00 | +5.00 + 1.50 × 115 | |||
| 06 | 215/ M | Patching | RE Aniso | 1.00 | +5.25 + 0.75 × 025 |
| LE | 0.00 | +2.75 DS | |||
| 07 | 17 [unknown]/F | None | RE | −0.08 | +0.75 DS |
| LE Aniso | 0.40 | +4.00 + 0.75 × 085 | |||
| 08 | 1614/F | None | RE Aniso | 0.52 | +4.50 + 1.75 × 070 |
| LE | 0.00 | −1.00 DS | |||
| 09 | 16 [unknown]/M | None | RE Aniso | 1.30 | +6.25 DS |
| LE | 0.10 | +0.25 DS | |||
| 10 | 1916/M | None | RE Aniso | 0.50 | +1.75 + 2.50 × 090 |
| LE | −0.08 | +1.00 + 2.00 × 088 | |||
| 11 | 18 [unknown]/ M | None | RE Aniso | 0.30 | +1.50–4.25 × 180 |
| LE | 0.00 | −1.50 DS | |||
| 12 | 20 [unknown]/F | None | RE | 0.00 | +1.00 + 0.75 × 85 |
| LE Aniso | 0.52 | +3.25 + 0.75 × 60 | |||
| 13 | 2219/ F | None | RE | 0.14 | +1.00–1.75 × 015 |
| LE Aniso | 0.40 | +3.50–4.25 × 170 | |||
| 14 | 2323/F | None | RE | −0.1 | +1.25 + 0.50 × 105 |
| LE Aniso | 0.10 | +4.50 + 1.75 × 115 | |||
| 15 | 256/F | Patching | RE | −0.08 | +4.25 DS |
| LE Aniso | 0.84 | +5.75 + 0.50 × 105 | |||
| 16 | 216/ M | Patching | RE | −0.08 | +6.00 DS |
| LE Strab (17ΔET) | 0.56 | +5.75 + 0.25 × 160 | |||
| 17 | 177/ F | Patching and Surgery | RE | 0.00 | Plano + 0.50 × 180 |
| LE Aniso | 1.00 | +6.50 + 1.75 × 105 | |||
| 18 | 1919/M | None | RE | −0.02 | −3.25 DS |
| LE Aniso | 0.54 | +4.50 + 1.50 × 100 | |||
| 19 | 237/M | None | RE Aniso | 0.30 | +4.50 DS |
| LE | 0.00 | Plano + 0.50 × 100 | |||
| 20 | 2113/M | Patching | RE | 0.00 | +0.25 + 0.25 × 090 |
| LE Aniso | 0.72 | +3.50 + 2.00 × 088 | |||
| 21 | 265/F | None | RE | 0.00 | +5.00 DS |
| LE Mixed (17ΔET) | 0.72 | +6.00 DS |
M, male, F, female, RE, right eye, LE, left eye, Aniso, anisometropic amblyopia, Strab, strabismic amblyopia, DS, diopter sphere.
Each participant took part in 7 experimental sessions; three VEP sessions, one each for anodal tDCS (a-tDCS), cathodal tDCS (c-tDCS) and sham tDCS (s-tDCS), and four contrast sensitivity sessions (familiarization, a-, c-, and s-tDCS). For control participants only one eye, selected at random, was tested.
Transcranial Direct Current Stimulation
tDCS was delivered by a battery-driven constant-current stimulator (Chattanooga Ionto, USA) using a pair of conductive rubber electrodes (4 mm × 6 mm stimulating electrode, 5 mm × 7 mm reference electrode) housed in saline-soaked synthetic sponges. A-tDCS, c-tDCS or s-tDCS was applied with the stimulating electrode placed over Oz and the reference over Cz. Electrode size and placement was adopted from previous studies of visual cortex stimulation53,54. All participants with amblyopia and 12 control participants received 20 minutes of tDCS. A separate group of 15 control participants received 10 minutes of tDCS to provide initial dose-response data for visual cortex tDCS. A- and c-tDCS were delivered at 2 mA. Sham stimulation involved a 30 second ramp up of anodal stimulation after which the stimulator was shut off out of sight of the participant. Stimulation sessions were separated by at least 48 hours and a-, c- and s- tDCS were delivered in a random sequence. For the VEP experiment, VEPs were recorded immediately before (baseline), after and 30 minutes after stimulation. For the contrast sensitivity experiment, measurements were made immediately before (baseline), during, after and 30 minutes after stimulation.
Contrast sensitivity measurements
Contrast sensitivity was measured using a 10 cpd Gabor patch (radius 1.3°, sigma 1°), presented on a uniform grey background (50 cd/m2) for 500 ms within a Gaussian temporal envelope (100 ms ramp up and 100 ms ramp down). A relatively high spatial frequency was chosen, as the effects of amblyopia on contrast sensitivity are most pronounced at high spatial frequencies3. On each trial, participants judged the orientation of the patch (vertical vs. horizontal). Stimuli were generated using Psykinematix software, which allows for 10.8 bits of contrast resolution, and presented on an Eizo CRT monitor (1024 × 768 resolution, 120 Hz refresh rate). The viewing distance was 200 cm and a tight fitting opaque patch was worn over the non-viewing eye.
A two-alternative forced choice (2AFC) paradigm and a 2-down-1-up adaptive staircase procedure (proportional step size of 25% before the first reversal and 7.5% increments and 15% decrements after the first reversal) was used to measure detection thresholds. Thresholds were calculated as the mean of the last four reversals out of a total of six reversals. Participants completed at least 1 hour of task familiarization in a separate session prior to the tDCS sessions. The order of the a-, c- and s-tDCS sessions was randomized across participants. Contrast sensitivity was measured before, during, after and 30 minutes after tDCS. Only a single spatial frequency was measured because tDCS can only be administered for a finite period of time and the after-effects can decay rapidly. Therefore we did not anticipate that there would be time to measure a full contrast sensitivity function.
Pattern visual evoked potentials
The VEP stimuli were standard black (5 cd/m2) and white (80 cd/m2) pattern-reversal checkerboards generated with a Roland Consult clinical electrophysiology system viewed from 100 cm with a temporal frequency of 2 Hz. The stimuli were presented with a resolution of 1280 × 1024 at two contrasts (100% and 50% contrast) and two check sizes (15′ and 60′, equating to a fundamental spatial frequency of 2 cpd and 0.5 cpd respectively). VEPs for each of the 4 stimuli were collected at each time point (pre, post and 30 minutes post tDCS) in a random sequence. For participants with amblyopia, the fellow eye was always tested first. Measurements conformed to the ISCEV standards for clinical VEP recording. The non-viewing eye was occluded with a tight fitting opaque patch.
Data analysis
Contrast detection thresholds were converted to log contrast sensitivity. VEP amplitude was defined as the difference between the N75 negative peak and the P100 positive peak amplitudes in μV. Using this difference rather than absolute values facilitated the comparison of VEP data across separate sessions. The N75 peak was defined as a negative peak 60 to 110 ms after the pattern reversal. The peak of the first positive wave after the N75 peak was defined as the P100. Latencies for both the N75 and P100 were also calculated. Changes from pre-stimulation baseline within each session were calculated by subtraction of the baseline value from the subsequent values within the session.
Four ANOVAs were conducted on the change from baseline data for contrast sensitivity to compare the effects of a-, c- and s-tDCS on the amblyopic, fellow fixing and control eyes and to compare the effects of 10 and 20 minutes of tDCS in control participants. 1) An ANOVA with factors of Eye (amblyopic vs. fellow fixing eye), Stimulation (anodal vs. cathodal vs. sham) and Time (during vs. post vs. post 30 minutes) was conducted on the results from the observers with amblyopia. 2) A mixed ANOVA with within-subject factors of Stimulation (anodal vs. cathodal vs. sham) and Time (during vs. post vs. post 30 minutes) and a between-subjects factor of Eye (amblyopic vs. control) was conducted on the data from participants with amblyopia and controls who received 20 minutes of tDCS. 3) A mixed ANOVA with within-subject factors of Stimulation (anodal vs. cathodal vs. sham) and Time (during vs. post vs. post 30 minutes) and a between-subjects factor of Eye (fellow fixing eye vs. control) was conducted on the data from participants with amblyopia and controls who received 20 minutes of tDCS. 4) A mixed ANOVA with within-subject factors of Stimulation (anodal vs. cathodal vs. sham) and Time (during vs. post vs. post 30 minutes) and a between-subjects factor of tDCS Duration (20 minutes vs. 10 minutes) was conducted on the data from controls who received either 20 or 10 minutes of tDCS.
Post-hoc paired samples t-tests were used to compare contrast sensitivity changes induced by c- or a-tDCS to the within session baseline and s-tDCS. Pearson’s correlation was employed to evaluate the relationship between the mean contrast sensitivity change (the mean of the during, post and post 30 minutes changes) and the severity of amblyopia.
The same analysis approach was applied to the VEP amplitude and latency data. Additional within-subjects factors of Spatial frequency (15′ vs 60′) and Contrast (50% vs. 100%) were added to the four ANOVAs and the ‘during stimulation’ timepoint was removed from the analysis as this was not collected for the VEP experiments due to technical constraints. Pearson’s correlation was employed to evaluate the relationship between the mean VEP change (the mean of post and post 30 minutes changes) and severity of amblyopia.
Finally Pearson’s correlations between the average changes in contrast sensitivity and the average changes in the VEP amplitude following anodal tDCS were conducted for four data sets; amblyopic eyes, fellow fixing eyes, control eyes (20 min tDCS) and control eyes (10 min tDCS).
Results
We first assessed the effect of a-tDCS on pattern reversal VEPs (statistical results are provided in Tables 2 and 3). VEP amplitude was significantly weaker at baseline for amblyopic eyes than fellow eyes for each session. The mean baseline VEP amplitude for amblyopic eyes collapsed across stimulus contrast, spatial frequency and session was 6.95 ± 3.44 uV compared to 10.84 ± 5.09 uV for fellow eyes (t20 = 4.316, p < 0.001).
Table 2. The results of ANOVAs testing the effect of tDCS on VEP amplitudes.
| ANOVA factor(s) | AME vs. FEE | AME vs. Control | FFE vs. Control | Control 10 vs. 20 min |
|---|---|---|---|---|
| Stimulation (a-tDCS vs. c-tDCS vs. s-tDCS) | F2, 40 = 57.840, p < 0.001 | F2, 62 = 36.227, p < 0.001 | F2, 62 = 30.622, p < 0.001 | F2, 50 = 21.441, p < 0.0001 |
| Time (pre vs. post vs. 30 min post) | F1, 20 = 0.088, p = 0.769 | F1, 31 = 4.462, p = 0.043 | F1, 31 = 0.574, p = 0.454 | F1, 25 = 17.490, p < 0.0001 |
| Eye (amblyopic vs. fellow) | F1, 20 = 9.723, p = 0.005 | |||
| SF (low vs. high) | F1, 20 = 0.483, p = 0.495 | F1, 31 = 2.415, p = 0.13 | F1, 31 = 0.146, p = 0.705 | F1, 25 = 0.005, p = 0.946 |
| Contrast (low vs. high) | F1, 20 = 2.794, p = 0.609 | F1, 31 = 2.093, p = 0.158 | F1, 31 = 0.335, p = 0.567 | F1, 25 = 11.092, p = 0.003 |
| Stimulation*Time | F2, 40 = 0.804, p = 0.455 | F2, 62 = 0.509, p = 604 | F2, 62 = 1.643, p = 0.202 | F2, 50 = 2.452, p = 0.98 |
| Stimulation*Eye | F2, 40 = 3.985, p = 0.026 | |||
| Stimulation*SF | F2, 40 = 0.21, p = 0.811 | F2, 62 = 0.672, p = 0.514 | F2, 62 = 0.457, p = 0.635 | F2, 50 = 0.176, p = 0.838 |
| Stimulation*Contrast | F2, 40 = 0.765, p = 0.472 | F2, 62 = 0.175, p = 0.84 | F2, 62 = 0.531, p = 0.591 | F2, 50 = 0.196, p = 0.818 |
| Stimulation*Time*Eye | F2, 40 = 0.804, p = 0.454 | |||
| Stimulation*Eye (between-subjects factor) | F2, 62 = 1.476, p = 0.236 | F2, 62 = 4.708, p = 0.012 | ||
| Stimulation*tDCS Duration (between-subjects factor) | F2, 50 = 2.767, p = 0.073 |
AME, amblyopic eye, FFE, fellow fixing eye.
Table 3. The results of within subjects t-tests comparing the effects of tDCS on VEP amplitudes to within session baselines and the sham condition.
| VEP data t-tests | Post | Post30 | |
|---|---|---|---|
| AME | Anodal | t20 = 6.256, p < 0.001 | t20 = 6.351, p < 0.001 |
| Cathodal | t20 = −5.276, p < 0.001 | t20 = −3.225, p = 0.004 | |
| Sham | t20 = −0.44, p = 0.662 | t20 = 1.724, p = 0.1 | |
| Anodal vs. Sham | t20 = 6.129, p < 0.001 | t20 = 4.293, p < 0.001 | |
| Cathodal vs. Sham | t20 = −4.107, p = 0.001 | t20 = −4.188, p < 0.001 | |
| FEE | Anodal | t20 = 3.517, p = 0.002 | t20 = 3.267, p = 0.004 |
| Cathodal | t20 = −4.912, p < 0.001 | t20 = −8.596, p < 0.001 | |
| Sham | t20 = −1.359, p = 0.189 | t20 = −0.751, p = 0.461 | |
| Anodal vs. Sham | t20 = 3.483, p = 0.002 | t20 = 3.148, p = 0.005 | |
| Cathodal vs. Sham | t20 = −4.852, p < 0.001 | t20 = −5.263, p < 0.001 | |
| Control 20 min | Anodal | t11 = 2.792, p = 0.018 | t11 = 5.190, p < 0.001 |
| Cathodal | t11 = −1.020, p = 0.33 | t11 = −1.27, p = 0.23 | |
| Sham | t11 = −0.868, p = 0.404 | t11 = 0.018, p = 0.986 | |
| Anodal vs. Sham | t11 = 2.781, p = 0.018 | t11 = 5.067, p < 0.001 | |
| Cathodal vs. Sham | t11 = −0.795, p = 0.443 | t11 = −1.344, p = 0.206 | |
| Control 10 min | Anodal | t14 = 1.864, p=0.082 | t14 = 3.296, p = 0.005 |
| Cathodal | t14 = −4.598, p < 0.0001 | t14 = −5.297, p < 0.0001 | |
| Sham | t14 = 0.76, p = 0.486 | t14 = 2.091, p = 0.055 | |
| Anodal vs. Sham | t14 = 0.961, p = 0.353 | t14 = 1.363, p = 0.194 | |
| Cathodal vs. Sham | t14 = −4.032, p = 0.001 | t14 = −5.228, p < 0.001 | |
AME, amblyopic eye, FFE, fellow fixing eye. Unless indicated with “vs. Sham” the post hoc t-tests compare each condition to the within session baseline. Positive t values indicate an increase in VEP amplitude and negative values indicate a decrease.
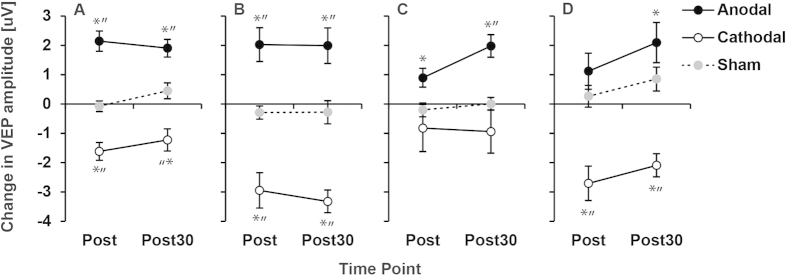
A-tDCS increased the amplitude of the pattern reversal VEP for amblyopic, non-amblyopic and control eyes directly after and 30 minutes after stimulation (Fig. 1). This effect was significantly different from s-tDCS and c-tDCS and was not dependent on the spatial frequency or contrast of the standard checkerboard VEP stimulus. Conversely, c-tDCS reduced the amplitude of the VEP and sham had no effect. VEP latencies were unaffected by tDCS suggesting that the effect was restricted to the response of cortical neurons and did not influence conduction time from retina to cortex. For controls, reducing the stimulation duration to ten minutes did not significantly alter the effects, although the c-tDCS induced decreases in VEP amplitude were more pronounced. Representative VEP waveforms are shown in Fig. 2.
Figure 1. A-tDCS increased the amplitude of the pattern reversal VEP.
The change in VEP amplitude from baseline after 20 minutes of anodal, cathodal or sham tDCS is shown for amblyopic (A; n = 21), non-amblyopic (B; n = 21) and control (C; n = 12) eyes. Positive values indicate an improvement. Measurements were made directly after (Post) and 30 minutes after (Post30) simulation. For controls, reducing the simulation duration to 10 minutes did not change the pattern of tDCS effects (D; n = 15). * = significant change from baseline, “ = significant difference from sham (paired t-test, p < 0.05).
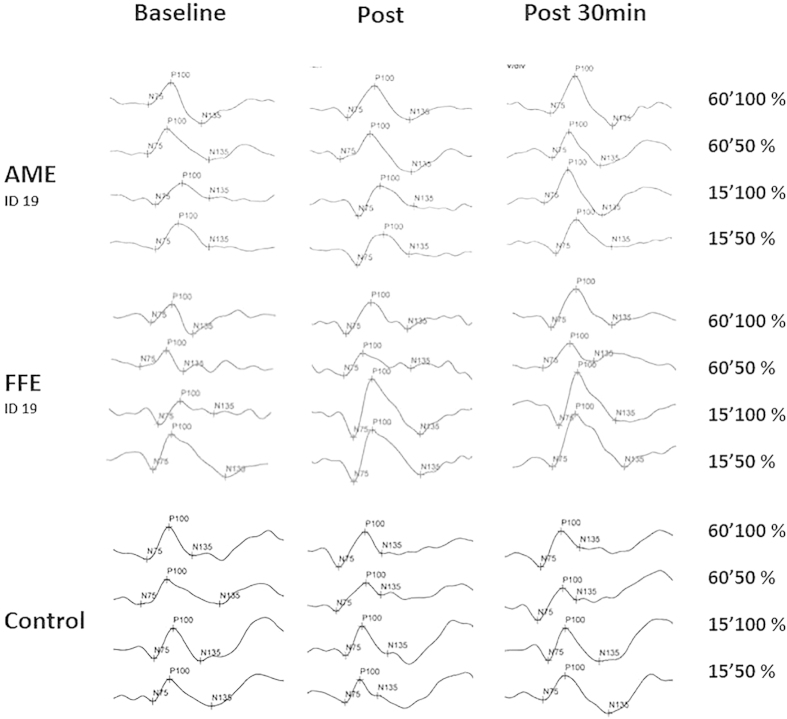
Figure 2. Example VEP Reponses for an amblyopic eye (Patient 19), a fellow eye (Patient 19) and a control eye before (baseline), after and 30 minutes after a-tDCS.
The right column identifies the size of the check in the VEP stimulus (either 60′ or 15′) and the contrast (either 50% or 100%). Each waveform is the average of 64 repetitions.
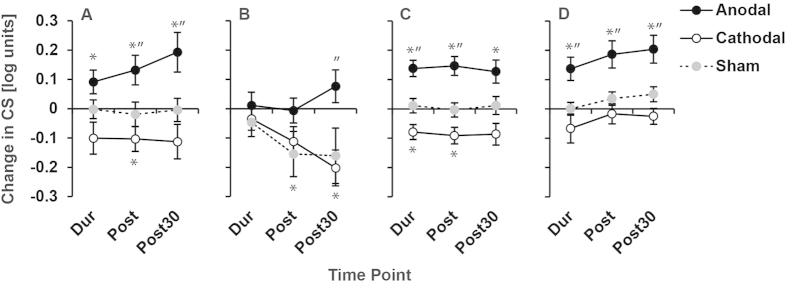
The results for contrast sensitivity were consistent with the VEP measurements. Amblyopic eye contrast sensitivity was significantly lower than that of the fellow eye for all baseline sessions (mean log sensitivity collapsed across sessions; amblyopic eye = 0.70 ± 0.52, fellow eye = 1.66 ± 0.34; t20 = 7.34, p < 0.001). A-tDCS significantly improved contrast sensitivity whereas c-tDCS had the opposite effect (Fig. 3; Tables 4 and 5). Contrast sensitivity measurements for two participants showed a large reduction in sensitivity following sham stimulation for the non-amblyopic eye condition only. This effect did not occur for other participants and is reflected in the standard error of the sham data in Fig. 3B.
Figure 3. A-tDCS enhanced contrast sensitivity.
The change in log contrast sensitivity relative to baseline after 20 minutes of anodal, cathodal or sham tDCS is shown for amblyopic (A), non-amblyopic (B) and control (C) eyes. Positive values indicate an improvement. Measurements were made during (Dur), directly after (Post) and 30 minutes after (Post30) simulation. For controls, reducing the stimulation duration to 10 minutes did not change the pattern of results (D). * = Significant change from baseline, “ = significant difference from sham (paired t-test, p < 0.05).
Table 4. The results of ANVOAs testing the effect of tDCS on log contrast sensitivity.
| ANOVA factor(s) | AME vs. FEE | AME vs. Control | FFE vs. Control | Control 10 vs. 20 min |
|---|---|---|---|---|
| Stimulation | F2, 40 = 12.364, p < 0.001 | F2, 72 = 24.798, p < 0.001 | F2, 72 = 11.069, p < 0.001 | F2, 58 = 26.909, p < 0.0001 |
| Time | F2, 40 = 0.997, p = 0.378 | F2, 72 = 0.326, p = 0.723 | F2, 72 = 2.067, p = 0.134 | F2, 58 = 2.174, p = 0.128 |
| Eye | F1, 20 = 4.381, p = 0.049 | |||
| Stimulation*Time | F4, 80 = 3.516, p = 0.011 | F4, 144 = 0.473, p = 0.755 | F4, 144 = 1.657, p = 0.163 | F2, 116 = 0.156, p = 0.960 |
| Stimulation*Eye | F2, 40 = 0.936, p = 0.4 | |||
| Stimulation*Time*Eye | F4, 80 = 0.321, p = 0.863 | |||
| Stimulation*Eye (between-subjects factor) | F2, 72 = 0.056, 0.946 | F2, 72 = 0.796, p = 0.455 | ||
| Stimulation*tDCS Duration (between-subjects factor) | F2, 58 = 0.102, p = 0.903 |
Data reported as in Table 2.
Table 5. The results of t-tests comparing the effects of tDCS on log contrast sensitivity to the within session baseline and the sham condition.
| VEP data t-tests | Dur | Post | Post30 | |
|---|---|---|---|---|
| AME | Anodal | t20 = 2.262, p = 0.035 | t20 = 2.623, p = 0.016 | t20 = 2.855, p = 0.01 |
| Cathodal | t20 = −1.841, p = 0.08 | t20 = −2.453, p = 0.023 | t20 = −1.903, p = 0.072 | |
| Sham | t20 = −0.66, p = 0.948 | t20 = −0.463, p = 0.648 | t20 = −0.107, p = 0.916 | |
| Anodal vs. Sham | t20 = 1.593, p = 0.127 | t20 = 2.429, p = 0.025 | t20 = 2.362, p = 0.028 | |
| Cathodal vs. Sham | t20 = −1.375, p = 0.184 | t20 = −1.729, p = 0.99 | t20 = −1.451, p = 0.162 | |
| FEE | Anodal | t20 = 0.257, p = 0.8 | t20 = −0.146, p = 0.885 | t20 = 1.367, p = 0.187 |
| Cathodal | t20 = 0.892, p = 0.383 | t20 = −2.225, p = 0.038 | t20 = −3.294, p = 0.004 | |
| Sham | t20 = −0.963, p = 0.347 | t20 = −2.007, p = 0.058 | t20 = −1.696, p = 0.105 | |
| Anodal vs. Sham | t20 = 0.97, p = 0.343 | t20 = 1.845, p = 0.08 | t20 = 2.372, p = 0.028 | |
| Cathodal vs. Sham | t20 = 0.229, p = 0.821 | t20 = 0.411, p = 0.685 | t20 = −0.382, p = 0.706 | |
| Control 20 min | Anodal | t16 = 4.945, p < 0.001 | t16 = 4.550, p < 0.001 | t16 = 3.208, p = 0.005 |
| Cathodal | t16 = −3.064, p = 0.007 | t16 = −3.240, p = 0.005 | t16 = −2.351, p = 0.032 | |
| Sham | t16 = 0.437, p = 0.668 | t16 = −0.149, p = 0.883 | t16 = 0.369, p = 0.717 | |
| Anodal vs. Sham | t16 = 4.095, p = 0.001 | t16 = 3.244, p = 0.005 | t16 6 = 2.165, p = 0.046 | |
| Cathodal vs. Sham | t16 = −2.456, p = 0.026 | t16 = −2.358, p = 0.031 | t16 = −1.866, p = 0.081 | |
| Control 10 min | Anodal | t13 = 3.449, t = 0.004 | t13 = 4.007, p = 0.001 | t13 = 4.282, p = 0.001 |
| Cathodal | t13 = −1.346, p = 0.201 | t13 = −0.489, p = 0.633 | t13 = −0.938, p = 0.365 | |
| Sham | t13 = 0.017, p = 0.986 | t13 = 1.539, p = 0.148 | t13 = 1.961, p = 0.072 | |
| Anodal vs. Sham | t13 = 2.874, p = 0.013 | t13 = 2.833, p = 0.014 | t13 = 2.860, p = 0.013 | |
| Cathodal vs. Sham | t13 = −1.368, p = 0.194 | t13 = −1.168, p = 0.264 | t13 = −2.007, p = 0.066 | |
Data reported as in Table 3.
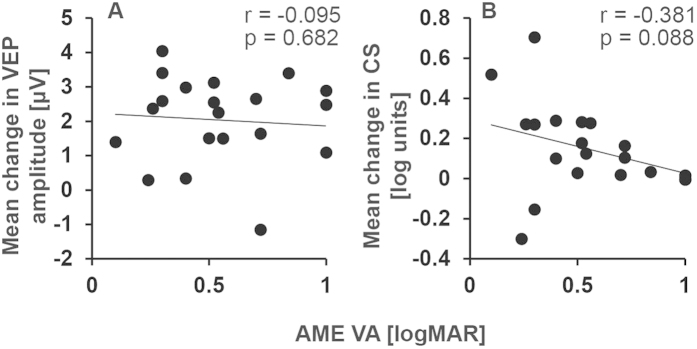
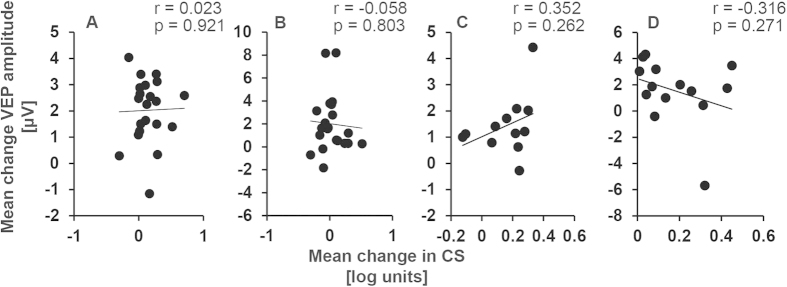
No significant relationships were found between amblyopic eye visual acuity at baseline and the a-tDCS induced changes in contrast sensitivity (Fig. 4A) or VEP amplitude (Fig. 4B) for amblyopic eyes. Data were collapsed across the ‘during’, ‘post’ and ‘post 30’ time points for correlation analyses. Changes in contrast sensitivity induced by a-tDCS and the changes in VEP amplitude induced by a-tDCS were also not significantly correlated (Fig. 5).
Figure 4.
Relationships between amblyopia severity and tDCS induced changes in VEP amplitude (A) and contrast sensitivity (B).
Figure 5.
Correlations between the effects of anodal tDCS on contrast sensitivity and VEP amplitude for amblyopic eyes (Panel A), fellow fixing eyes (Panel B), control eyes 10 min tDCS (Panel C) and control eyes 20 minutes tDCS (Panel D). The change in contrast sensitivity and VEP amplitude were collapsed across time points.
Given the significant increases in VEP amplitude and contrast sensitivity following a-tDCS, we investigated the duration of the effects. For the VEP measurements, fourteen participants with amblyopia completed a-tDCS as their first or second tDCS session and therefore completed a baseline measurement 48 hours later. There was a significant increase in VEP amplitude from the a-tDCS baseline to the baseline 48 hours later (t13 = 3.8, p = 0.002) suggesting an enduring effect of the a-tDCS. This did not occur for sham stimulation (n = 12, t11 = 1.5, p = 0.2). Post a-tDCS baselines did not differ significantly from pre a-tDCS baselines for amblyopic eye contrast sensitivity (n = 13, t12 = 2.0, p = 0.07).
Discussion
Our results demonstrate that a single session of a-tDCS can transiently increase VEP amplitude and contrast sensitivity in adult patients with amblyopia. Specifically, a-tDCS increased VEP amplitude and contrast sensitivity for amblyopic eyes. Similar effects were found for control eyes, however fellow eyes of patients with amblyopia did not show increased contrast sensitivity following a-tDCS. Importantly, increases did not occur following c- or s-tDCS, indicating that the effects were specific to a-tDCS and were not due to within session learning.
The results of this study contribute to a growing literature indicating that non-invasive brain stimulation can modulate contrast sensitivity in adults with amblyopia39,54,55. A possible mechanism for the a-tDCS effects we report is reduced inhibition within the visual cortex. Visual input from the amblyopic eye is subject to attenuation56 and suppression18,19,31 which may contribute to the weakened cortical response to information from the amblyopic eye22. The magnitude of suppression is positively correlated with the loss of visual acuity29,30,31,32 and contrast sensitivity18,19 in humans and primates with strabismic or anisometropic amblyopia, and reducing suppression may result in improvements in both visual acuity and stereopsis33,36,57. This suggests that suppression is an important contributor to the visual deficits associated with amblyopia. The inhibitory neurotransmitter GABA is involved in suppression of cortical inputs from one eye in cat models of strabismus51. GABA has also been implicated as one of a suite of mechanisms that gate recovery from amblyopia in adult rodents52. This role of GABA is directly relevant to a-tDCS effects because magnetic resonance spectroscopy measurements have shown that a-tDCS reduces the relative concentration of GABA when applied to the motor cortex44. Furthermore, a-tDCS induced reductions in motor cortex GABA have been associated with enhanced learning of a motor task, providing a link between changes in GABA and behavioral performance46. It is possible that a-tDCS reduces GABA within the visual cortex and therefore reduces chronic suppression of inputs from the amblyopic eye. This, combined with the excitatory effects of a-tDCS, may lead to a transient enhancement of the cortical response to amblyopic eye inputs in the form of an increased VEP amplitude and improved contrast sensitivity. Future work will test this hypothesis by assessing the effect of a-tDCS on measures of interocular suppression in patients with amblyopia.
We observed a dissociation between the a-tDCS induced increases in VEP amplitude that occurred for all eyes and the increases in contrast sensitivity that only occurred for amblyopic and control eyes. Fellow eyes did not show significant improvements in contrast sensitivity relative to baseline following a-tDCS. The increases in VEP amplitude are likely to reflect increased cortical excitability, a well-documented effect of a-tDCS within the motor cortex40. Therefore, our results suggest that the contrast sensitivity improvements induced by a-tDCS are not solely due to increased excitability. This finding raises the possibility that the improvements in contrast sensitivity may be more closely related to reduced inhibition/suppression within the visual cortex. While the control observers did not exhibit suppression under normal viewing conditions, a level of binocular competition or rivalry was introduced by the monocular viewing conditions used within the experiment (i.e. patching of the non-viewing eye). This may have been reduced by a-tDCS, leading to enhanced contrast sensitivity relative to baseline and sham. On the other hand, patching of the amblyopic eye would not induce rivalrous conditions for the fellow eye because suppressive interactions are biased in favor of the fellow eye18. Therefore no improvements in fellow eye contrast sensitivity would be expected following a-tDCS if reduced inhibition is involved.
An alternative explanation relates to the concept of homeostatic metaplasticity, whereby non-invasive brain stimulation acts to return a neural system to homeostasis58. Under this explanation, a-tDCS would have had a greater effect on neural populations preferring information from the amblyopic eye that have low levels of baseline activation. This explanation would also account for the trend towards more pronounced inhibitory effects of c-tDCS on fellow eye VEP amplitudes and contrast sensitivity. This is because fellow eye neural populations have a higher level of activation22 and are therefore likely to be more susceptible to the effects of inhibitory stimulation protocols. It is unclear how homeostatic metaplasticity relates to our control eye results. Based on the current data, we are not able to discriminate between these two explanations.
In addition to its effects on GABA, tDCS may also enhance expression of brain derived neurotropic factor (BDNF)59 and increased BDNF levels have been linked with recovery from adult amblyopia in animal models60. Furthermore, the aftereffects of tDCS rely on the function of NMDA receptors61. This has led to the suggestion that a-tDCS has long term potential (LTP) - like effects. NMDA receptor dependent changes in synaptic strength have been linked to recovery of ocular dominance plasticity animal models of amblyopia62 and may underlie the increases in VEP amplitude and contrast sensitivity we report. We favor an explanation based on temporary changes in inhibition and excitation because LTP and BDNF effects are gradual, whereas the effects we observed were rapid. It is possible, however, that LTP-like changes underlie the longer-term effects of a-tDCS on VEP amplitude that we observed for amblyopic eyes 48 hours after stimulation.
Previous studies into the effects of tDCS on the healthy visual cortex have generated conflicting results. In terms of VEPs, a-tDCS has been reported to increase and c-tDCS decrease VEP amplitude63, however opposite results have also been observed64. Furthermore, tDCS has been reported to have no effect on flash VEPs65. The results from previous studies investigating tDCS induced changes in contrast sensitivity are similarly variable. Antal et al.66 found that c-tDCS decreased static and dynamic contrast sensitivity, whereas a-tDCS had no reliable effect. Using threshold perimetry, Kraft et al.67 found that a-tDCS enhanced contrast sensitivity within the central 2 degrees of the visual field whereas c-tDCS had no effect. More recently Costa et al.68 also found that a-tDCS increased contrast sensitivity on threshold perimetry, but only in the periphery. Finally, an absence of acute tDCS effects on contrast sensitivity have been reported53,69. These discrepancies could be due to differences in visual stimuli, viewing conditions, tDCS parameters and electrode placement. However, individual differences between the participants taking part in each of these studies may also contribute to these discrepancies as a number of factors such as BDNF polymorphisms and hormone levels have been found to alter the response to tDCS70. In the current study, electrophysiological and behavioral measures were made in the same participants and the polarity specific effects were consistent across both measures; a-tDCS tended to increase VEP amplitude and contrast sensitivity whereas c-tDCS had the opposite effect. These results are in a good agreement with the established polarity dependent effects of motor cortex tDCS40 and support the use of tDCS to modulate visual cortex function.
Reducing the duration of tDCS to 10 minutes did not change the pattern of results in control participants. Whether this is also the case for patients with amblyopia remains to be tested. The effect of repeated doses of a-tDCS also remains an open question and the exploration of ways to prolong the effects of a-tDCS is an important next step in this field.
Although the effects of tDCS were consistent between the VEP and contrast sensitivity measurements, we did not find a direct correlation between the changes in VEP and changes in contrast sensitivity. This may be due to the very different nature of the stimuli used to elicit VEPs and measure contrast sensitivity. The lack of a direct correlation may also reflect different neural mechanisms for the two effects, as described above.
These results indicate that a-tDCS can temporarily increase contrast sensitivity in adults with amblyopia in agreement with previous studies54. It has also previously been reported that a-tDCS can enhance the effect of dichoptic treatment on stereopsis in adults with amblyopia71. Further work using standard clinical outcome measures such as visual acuity is required to assess whether a-tDCS alone has any clinical relevance for the treatment of amblyopia in adulthood.
Additional Information
How to cite this article: Ding, Z. et al. The effect of transcranial direct current stimulation on contrast sensitivity and visual evoked potential amplitude in adults with amblyopia. Sci. Rep. 6, 19280; doi: 10.1038/srep19280 (2016).
Acknowledgments
This work was supported by grants from the University of Auckland, the Auckland Medical Research Foundation and The Health Research Council of New Zealand to BT and grants from the National Natural Science Foundation of China (81200715) and the Fundamental Research Funds of the State Key Laboratory of Ophthalmology to JL.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.L., D.P.S., Z.C., L.C. and B.T. wrote the manuscript. J.L., D.P.S., D.D., Z.C., L.C., B.T. and M.Y. designed the study. Z.D., J.L., D.D., Z.C., G.L. and J.Y. performed the data collection. M.Y. supervised the research. All authors reviewed the manuscript.
References
- Daw N. Visual development. (Springer, 2014). [Google Scholar]
- Holmes J. M. & Clarke M. P. Amblyopia. Lancet 367, 1343–1351 (2006). [DOI] [PubMed] [Google Scholar]
- Hess R. F. & Howell E. R. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Res. 17, 1049–1055 (1977). [DOI] [PubMed] [Google Scholar]
- Birch E. E. Amblyopia and binocular vision. Prog. Retin. Eye Res. 33, 67–84 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. S. & Giaschi D. E. Stereopsis-dependent deficits in maximum motion displacement in strabismic and anisometropic amblyopia. Vision Res. 47, 2778–2785 (2007). [DOI] [PubMed] [Google Scholar]
- Simmers A. J., Ledgeway T., Hess R. F. & McGraw P. V. Deficits to global motion processing in human amblyopia. Vision Res. 43, 729–738 (2003). [DOI] [PubMed] [Google Scholar]
- Simmers A. J., Ledgeway T., Mansouri B., Hutchinson C. V. & Hess R. F. The extent of the dorsal extra-striate deficit in amblyopia. Vision Res. 46, 2571–2580 (2006). [DOI] [PubMed] [Google Scholar]
- Chandna A., Pennefather P. M., Kovács I. & Norcia A. M. Contour integration deficits in anisometropic amblyopia. Invest. Ophthalmol. Vis. Sci. 42, 875–878 (2001). [PubMed] [Google Scholar]
- Asper L., Crewther D. & Crewther S. G. Strabismic amblyopia. Part 1. Psychophysics. Clin. Exp. Optom. 83, 49–58 (2000). [DOI] [PubMed] [Google Scholar]
- Hamm L. M., Black J., Dai S. & Thompson B. Global processing in amblyopia: a review. Front. Psychol. 5, 583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M., Hariharan S. & Klein S. A. Suppressive and facilitatory spatial interactions in amblyopic vision. Vision Res. 42, 1379–1394 (2002). [DOI] [PubMed] [Google Scholar]
- Levi D. M. & Klein S. A. Vernier acuity, crowding and amblyopia. Vision Res. 25, 979–991 (1985). [DOI] [PubMed] [Google Scholar]
- Levi D. M. & Polat U. Neural plasticity in adults with amblyopia. Proc. Natl. Acad. Sci. USA 93, 6830–6834 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U. Restoration of underdeveloped cortical functions: evidence from treatment of adult amblyopia. Restor. Neurol. Neurosci. 26, 413–424 (2008). [PubMed] [Google Scholar]
- Polat U., Sagi D. & Norcia A. M. Abnormal long-range spatial interactions in amblyopia. Vision Res. 37, 737–744 (1997). [DOI] [PubMed] [Google Scholar]
- Kiorpes L., Kiper D. C., O’Keefe L. P., Cavanaugh J. R. & Movshon J. A. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J. Neurosci. 18, 6411–6424 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooner C. et al. Population representation of visual information in areas V1 and V2 of amblyopic macaques. Vision Res. 114, 56–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H. et al. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cereb. Cortex 21, 2033–2045 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X. et al. Early monocular defocus disrupts the normal development of receptive-field structure in V2 neurons of macaque monkeys. J. Neurosci. 34, 13840–13854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shamayleh Y., Kiorpes L., Kohn A. & Movshon J. A. Visual motion processing by neurons in area MT of macaque monkeys with experimental amblyopia. J. Neurosci. 30, 12198–12209 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M. & Harwerth R. S. Contrast evoked potentials in strabismic and anisometropic amblyopia. Invest. Ophthalmol. Vis. Sci. 17, 571–575 (1978). [PubMed] [Google Scholar]
- Barnes G. R., Hess R. F., Dumoulin S. O., Achtman R. L. & Pike G. B. The cortical deficit in humans with strabismic amblyopia. J. Physiol. 533, 281–297 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner I. P., Odom J. V., Schwartz T. L. & Mendola J. D. Monocular activation of V1 and V2 in amblyopic adults measured with functional magnetic resonance imaging. J. AAPOS 11, 341–350 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. F., Thompson B., Gole G. & Mullen K. T. Deficient responses from the lateral geniculate nucleus in humans with amblyopia. Eur. J. Neurosci. 29, 1064–1070 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. F., Thompson B., Gole G. A. & Mullen K. T. The amblyopic deficit and its relationship to geniculo-cortical processing streams. J. Neurophysiol. 104, 475–483 (2010). [DOI] [PubMed] [Google Scholar]
- Miki A., Liu G. T., Goldsmith Z. G., Liu C. S. & Haselgrove J. C. Decreased activation of the lateral geniculate nucleus in a patient with anisometropic amblyopia demonstrated by functional magnetic resonance imaging. Ophthalmologica 217, 365–369 (2003). [DOI] [PubMed] [Google Scholar]
- Bonhomme G. R. et al. Decreased cortical activation in response to a motion stimulus in anisometropic amblyopic eyes using functional magnetic resonance imaging. Journal of American Association for Pediatric Ophthalmology and Strabismus 10, 540–546 (2006). [DOI] [PubMed] [Google Scholar]
- Thompson B., Villeneuve M. Y., Casanova C. & Hess R. F. Abnormal cortical processing of pattern motion in amblyopia: Evidence from fMRI. Neuroimage 60, 1307–1315 (2012). [DOI] [PubMed] [Google Scholar]
- Li J. et al. How Best to Assess Suppression in Patients with High Anisometropia. Optom. Vis. Sci. 90, e47–52 (2013). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Quantitative Measurement of Interocular Suppression in Anisometropic Amblyopia: A Case-Control Study. Ophthalmology 120, 1672–1680 (2013). [DOI] [PubMed] [Google Scholar]
- Li J. et al. The role of suppression in amblyopia. Invest. Ophthalmol. Vis. Sci. 52, 4169–4176 (2011). [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Harrison E. R. & Giaschi D. E. Quantitative measurement of interocular suppression in children with amblyopia. Vision Res. 66, 1–10 (2012). [DOI] [PubMed] [Google Scholar]
- Hess R. F., Mansouri B. & Thompson B. A new binocular approach to the treatment of Amblyopia in adults well beyond the critical period of visual development. Restor. Neurol. Neurosci. 28, 1–10 (2010). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Dichoptic training improves contrast sensitivity in adults with amblyopia. Vision Res. 114, 161–172 (2015). [DOI] [PubMed] [Google Scholar]
- Ooi T. L., Su Y. R., Natale D. M. & He Z. J. A push-pull treatment for strengthening the ‘lazy eye’ in amblyopia. Curr. Biol. 23, R309–310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. F., Mansouri B. & Thompson B. A Binocular Approach to Treating Amblyopia: Anti-Suppression Therapy. Optom. Vis. Sci. 87, 697–704 (2010). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Dichoptic training enables the adult amblyopic brain to learn. Curr. Biol. 23, R308–309 (2013). [DOI] [PubMed] [Google Scholar]
- Hess R. F. & Thompson B. Amblyopia and the binocular approach to its therapy. Vision Res. 114, 4–16 (2015). [DOI] [PubMed] [Google Scholar]
- Thompson B., Mansouri B., Koski L. & Hess R. F. Brain plasticity in the adult: modulation of function in amblyopia with rTMS. Curr. Biol. 18, 1067–1071 (2008). [DOI] [PubMed] [Google Scholar]
- Nitsche M. A. & Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527 Pt 3, 633–639 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A., Nitsche M. A. & Paulus W. Transcranial direct current stimulation and the visual cortex. Brain Res. Bull. 68, 459–463 (2006). [DOI] [PubMed] [Google Scholar]
- Antal A., Kincses T. Z., Nitsche M. A. & Paulus W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp. Brain Res. 150, 375–378 (2003). [DOI] [PubMed] [Google Scholar]
- Lang N. et al. Bidirectional modulation of primary visual cortex excitability: a combined tDCS and rTMS study. Invest. Ophthalmol. Vis. Sci. 48, 5782–5787 (2007). [DOI] [PubMed] [Google Scholar]
- Stagg C. J. et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 29, 5202–5206 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Stephenson M. C., Morris P. G. & Jackson S. R. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: a 7 T magnetic resonance spectroscopy study. Neuroimage 99, 237–243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. J., Bachtiar V. & Johansen-Berg H. The role of GABA in human motor learning. Curr. Biol. 21, 480–484 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. et al. The effects of aging on the strength of surround suppression of receptive field of V1 cells in monkeys. Neuroscience 169, 874–881 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. H. et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J. Neurosci. 30, 3777–3781 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. H. et al. Diminished orientation-specific surround suppression of visual processing in schizophrenia. Schizophr. Bull. 35, 1078–1084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. M. et al. GABA shapes the dynamics of bistable perception. Curr. Biol. 23, 823–827 (2013). [DOI] [PubMed] [Google Scholar]
- Sengpiel F., Jirmann K.-U., Vorobyov V. & Eysel U. Strabismic suppression is mediated by interactions in the primary visual cortex. Cereb. Cortex 16, 1750–1758 (2006). [DOI] [PubMed] [Google Scholar]
- Sale A., Berardi N., Spolidoro M., Baroncelli L. & Maffei L. GABAergic inhibition in visual cortical plasticity. Front. Cell. Neurosci. 4, 10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D. P., Hansen B. C., Byblow W. D. & Thompson B. Anodal transcranial direct current stimulation reduces psychophysically measured surround suppression in the human visual cortex. PLoS One 7, e36220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D. P., Byblow W. D., Hess R. F. & Thompson B. Anodal transcranial direct current stimulation transiently improves contrast sensitivity and normalizes visual cortex activation in individuals with amblyopia. Neurorehabil. Neural Repair 27, 760–769 (2013). [DOI] [PubMed] [Google Scholar]
- Clavagnier S., Thompson B. & Hess R. F. Long lasting effects of daily theta burst rTMS sessions in the human amblyopic cortex. Brain Stimul 6, 860–867 (2013). [DOI] [PubMed] [Google Scholar]
- Baker D. H., Meese T. S. & Hess R. F. Contrast masking in strabismic amblyopia: attenuation, noise, interocular suppression and binocular summation. Vision Res. 48, 1625–1640 (2008). [DOI] [PubMed] [Google Scholar]
- To L. et al. A game platform for treatment of amblyopia. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 280–289 (2011). [DOI] [PubMed] [Google Scholar]
- Silvanto J., Muggleton N. & Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 12, 447–454 (2008). [DOI] [PubMed] [Google Scholar]
- Fritsch B. et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66, 198–204 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya Vetencourt J. F. et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320, 385–388 (2008). [DOI] [PubMed] [Google Scholar]
- Liebetanz D., Nitsche M. A., Tergau F. & Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125, 2238–2247 (2002). [DOI] [PubMed] [Google Scholar]
- Sawtell N. B. et al. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron 38, 977–985 (2003). [DOI] [PubMed] [Google Scholar]
- Antal A., Kincses T. Z., Nitsche M. A., Bartfai O. & Paulus W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest. Ophthalmol. Vis. Sci. 45, 702–707 (2004). [DOI] [PubMed] [Google Scholar]
- Accornero N., Li Voti P., La Riccia M. & Gregori B. Visual evoked potentials modulation during direct current cortical polarization. Exp. Brain Res. 178, 261–266 (2007). [DOI] [PubMed] [Google Scholar]
- Strigaro G., Mayer I., Chen J. C., Cantello R. & Rothwell J. C. Transcranial Direct Current Stimulation Effects on Single and Paired Flash Visual Evoked Potentials. Clin. EEG Neurosci. 46, 208–213 (2015). [DOI] [PubMed] [Google Scholar]
- Antal A., Nitsche M. A. & Paulus W. External modulation of visual perception in humans. Neuroreport 12, 3553–3555 (2001). [DOI] [PubMed] [Google Scholar]
- Kraft A. et al. Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Exp. Brain Res. 207, 283–290 (2010). [DOI] [PubMed] [Google Scholar]
- Costa T. L. et al. Contrasting effects of transcranial direct current stimulation on central and peripheral visual fields. Exp. Brain Res. 233, 1391–1397 (2015). [DOI] [PubMed] [Google Scholar]
- Peters M. A., Thompson B., Merabet L. B., Wu A. D. & Shams L. Anodal tDCS to V1 blocks visual perceptual learning consolidation. Neuropsychologia 51, 1234–1239 (2013). [DOI] [PubMed] [Google Scholar]
- Krause B. & Cohen Kadosh R. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 8, 25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D. P. et al. Transcranial direct current stimulation enhances recovery of stereopsis in adults with amblyopia. Neurotherapeutics 10, 831–839 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]