Abstract
The ‘microgenderome’ provides a paradigm shift that highlights the role of sex differences in the host-microbiota interaction relevant for autoimmune and neuro-immune conditions. Analysis of cross-sectional self-report and faecal microbial data from 274 patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) suggests that commensal gut microorganisms may play both protective and deleterious roles in symptom expression. Results revealed significant sex-specific interactions between Firmicutes (Clostridium, Streptococcus, Lactobacillus and Enterococcus) and ME/CFS symptoms (including neurological, immune and mood symptoms), regardless of compositional similarity in microbial levels across the sexes. Extending animal studies, we provide support for the microgenderome in a human clinical population. Applied and mechanistic research needs to consider sex-interactions when examining the composition and function of human microbiota.
Our growing knowledge of the host-microbiota interaction is rapidly informing translational research and therapeutic approaches to an array of chronic health conditions. Flagged as ‘the microgenderome’, gender differences and the critical role of sex hormones has been emphasized within the brain-gut-enteric-microbial axis1. Using an animal model, Markle et al. confirmed the bidirectional relationship between commensal gut microbiota, sex hormones and the immune system and provided an explanation of sexual dimorphism in Type 1 diabetes2. Their results revealed evidence of sex-specific microbial communities, sex-specific responses to the same microbial communities, the role of sexual maturation impacting changes to microbial communities, and evidence that microbial communities can play a protective and therapeutic role by influencing hormonal, metabolic and immune pathways. Highlighting the need to examine sex-specificity in microbial composition and function, these findings and similar3,4 suggest that intestinal dysbiosis (marked alterations in gut microbiota5,6) may play causative and consequential roles in autoimmune diseases and other health conditions2.
Intestinal dysbiosis and increased intestinal permeability (aberrations in the mucosal lining and musculature of the gastrointestinal tract) have been observed in the neuro-immune condition, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS7,8,9). The core feature of post-exertional fatigue and multi-systemic symptomatology reflect dysfunction of the central nervous system (CNS), immune systems and inflammatory pathways10,11. Overlapping symptom presentation and the 2:1 female-dominant incidence rates are comparative to those found in autoimmune diseases12. Researchers have tended to shy away from investigating this vulnerable population since the xenotropic murine leukaemia virus-related virus (XMRV) controversies13. However, future research is required to clarify aetiology for this complex and debilitating condition10. Applying the microgenderome lens to ME/CFS may provide future opportunities to elucidate unconfirmed pathophysiology and differentiate treatment pathways for this heterogeneous clinical population.
Using a cross-sectional design with a retrospective clinical data sample (N = 274, 68.6% female, aged 6–81 years), we were able to provide sex comparisons for a) symptom presentation; b) microbial composition and; c) interactions between microbial communities and ME/CFS symptoms (see Method for detailed explanation).
Results
Sex Differences in Symptom Presentation
To assess sex differences in symptom presentation, self-reported symptoms were categorised into thirteen factors; with twelve factors categorized according to the International Consensus Criteria (ICC10), plus a mood symptoms factor (Table S1). Patients rated symptom severity (past 7 days) and frequency (past 12 months) using a 5-point Likert scale (0–4). Impact scores (frequency × severity of symptoms) were calculated as a measure of each factor with higher scores reflecting greater impairment. Mann-Whitney tests showed sex differences for nine of the thirteen factors with measures of central tendency indicating that females were more likely to report greater impairment (Table 1). Notwithstanding possible gender differences in self-reporting or coping styles, the upregulated serotonergic response observed in female patients with CFS14 and evidence in parallel clinical populations, e.g., pain (osteoarthritic15, migraine16, and deep tissue17, irritable bowel syndrome (IBS18); and depression19 indicate that an interaction between sex steroids, neuroendocrine and immune systems is a plausible explanation for increased symptom severity and associated functional impairment in women. These results prompted investigation of pathophysiological differences.
Table 1. Sex differences in self-reported ME/CFS symptoms.
| ME/CFS Symptom Factors (Possible range) | Females |
Males |
Sex Comparison |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mdn(Range) | M(SD) | n | Mdn(Range) | M(SD) | U | p | r | ||
| F1. | Exertion and Fatigue (0–48) | 169 | 31(0–48) | 30.01 (15.70) | 74 | 31.5 (0–48) | 27.77 (16.19) | 6806.0 | 0.269 | 0.07 |
| F2. | Neurocognitive Symptoms (0–144) | 161 | 47 (0–120) | 50.07 (33.52) | 72 | 43.5 (0–120) | 44.85 (30.13) | 6241.5 | 0.349 | 0.06 |
| F3. | Pain Symptoms (0–208) | 156 | 45.5 (0–179) | 54.02 (43.70) | 70 | 21 (0–160) | 31.74 (32.29) | 7219.0 | 0.000*** | 0.26 |
| F4. | Sleep Symptoms (0–64) | 167 | 29 (0–64) | 30.89 (18.66) | 74 | 24 (0–64) | 25.51 (18.41) | 7244.5 | 0.033* | 0.14 |
| F5. | Neurosensory Symptoms (0–112) | 167 | 24 (0–103) | 28.31 (21.98) | 74 | 17 (0–82) | 21.34 (18.88) | 7391.5 | 0.015* | 0.16 |
| F6. | Immunity Impairment (0–112) | 165 | 8 (0–72) | 13.5 (15.58) | 74 | 4 (0–70) | 9.76 (14.15) | 7002.0 | 0.068 | 0.12 |
| F7. | Gastrointestinal (GI) Symptoms (0–128) | 163 | 24 (0–113) | 27.93 (22.86) | 73 | 11 (0–112) | 19.71 (21.85) | 7344.0 | 0.004** | 0.19 |
| F8. | Genitourinary (GU) Symptoms (0–48) | 170 | 2 (0–44) | 6.54 (9.71) | 77 | 4 (0–48) | 8.00 (10.91) | 5959.5 | 0.249 | -0.07 |
| F9. | Sensitivities (0–32) | 168 | 12 (0–32) | 12.94 (9.71) | 72 | 4.5 (0–32) | 7.58 (8.24) | 8098.5 | 0.000*** | 0.27 |
| F10. | Energy Production/Transportation Impairments (0–112) | 167 | 22 (0–128) | 30.93 (28.67) | 72 | 12 (0–86) | 17.78 (19.12) | 7628.5 | 0.001*** | 0.21 |
| F11. | Mood (0–128) | 159 | 19 (0–113) | 27.16 (26.44) | 69 | 12 (0–116) | 20.25c (22.93) | 6424.5 | 0.040* | 0.14 |
| F12 | ICC Symptom Score [F1-F10] (0–1008) | 126 | 245.5 (2–826) | 268.37 (172.91) | 58 | 185.5 (11–607) | 207.66 (147.87) | 4480.0 | 0.014* | 0.18 |
| F13 | Total Symptom Score [F1-F11] (0–1136) | 120 | 291.5 (2–908) | 264.81 (193.41) | 57 | 196 (11–664) | 223.72 (161.07) | 4301.0 | 0.006** | 0.21 |
Descriptive statistics, Mann-Whitney U test statistics and effect sizes (r) comparing symptom scores across the sexes. *P < 0.05, **P < 0.01, ***P < 0.001.
Sex Similarity in Microbial Composition
Comparison between sexes for each genus relied on culture-based methods of assessing faecal microbial content. Metagenomic advances provide superior detection of microbial diversity, however, culture-based methods continue to have utility to examine viable count within clinical and applied research settings6. Genera were quantified by viable count (frequency as per cfu/g exponent) and relative abundance (RA; ratio of genera count divided by total detectable bacteria count expressed as a percentage). Anaerobic (Bacteroides, Bifidobacterium, Clostridium, Eubacterium, and Lactobacillus) and aerobic (Escherichia, Streptococcus, Enterococcus) genera were investigated.
Mann-Whitney tests revealed no significant sex differences in the frequency (count) or proportion (RA) of each genus (Table S2). Additionally, sex comparisons of the total detectable bacteria count (Total Bacteria: Mdnmales = 1010 cfu/g, Mdnfemales = 1010 cfu/g, U = 7097.5, P = 0.093, r = −0.10) and the ratio between all detectable aerobic and anaerobic bacteria (Aerobic:Anaerobic Ratio: Mdnmales = 1.21, Mdnfemales = 1.10, U = 6844.5, P = 0.088, r = 0.10) did not differ significantly between the sexes. These results suggest sex-consistency in microbial composition within this clinical sample.
Interactions between Microbial Community and Symptom Expression
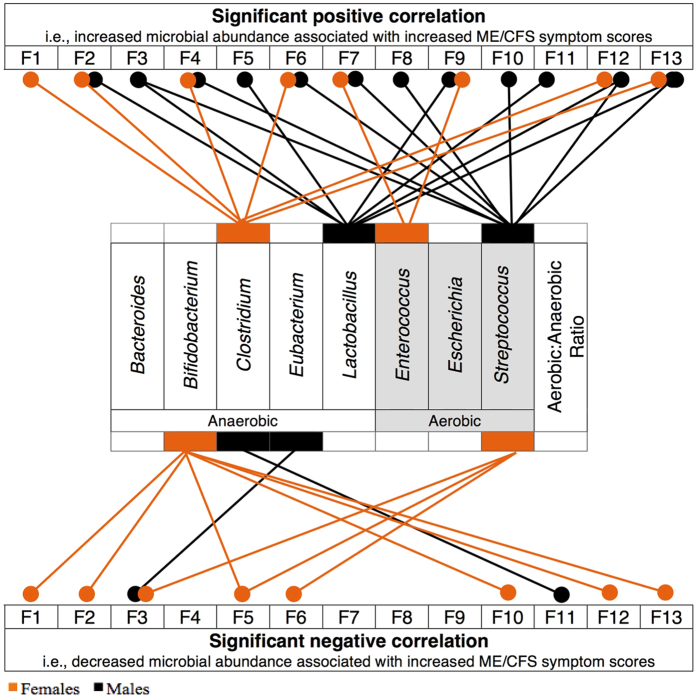
Spearman’s rank order correlations (rs) were used to investigate sex-interactions between microbial RA and ME/CFS symptom factors (Table S3). Multiple significant associations between genera and ME/CFS symptoms indicated a pattern of results diverging between the sexes (Fig. 1). The sex-specific interactions observed for Clostridium, Lactobacillus and Streptococcus are discussed.
Figure 1. Associations between microbiota relative abundance and ME/CFS symptoms (F1–F13) for females (nrange = 120–170, orange), and males (nrange = 57–77, black).
Only significant results from Spearman’s rank correlations are presented (P < 0.05). Anaerobic (white) and aerobic (grey) bacteria genera are distinguished. The Aerobic:Anaerobic Ratio: total detectable aerobic bacteria divided by total detectable anaerobic bacteria multiplied by 1000 (including but not limited to the selected genera presented above).
Clostridium
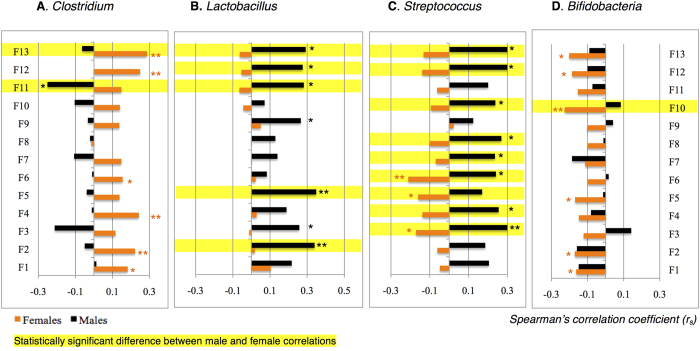
In females, the Clostridium genus was positively associated with eight of the thirteen ME/CFS symptoms. Significant small-medium positive correlations were shown for fatigue (F1: rs = 0.18, n = 166, p = 0.019), neurocognitive symptoms (F2: rs = 0.22, n = 158, p = 0.005), sleep (F4: rs = 0.24, n = 164, p = 0.002), immunity impairments (F6: rs = 0.16, n = 162, p = 0.049), total ICC symptoms (F12: rs = 0.25, n = 123, p = 0.006), and total symptoms score (F13: rs = 0.29, n = 117, p = 0.002). For males, an opposite association was found, with a significant negative correlation between Clostridium RA and mood symptoms (F11: rs = −0.25, n = 68, p = 0.039). Whilst not reaching significance, a similar pattern of results was observed for pain, gastro-intestinal, and energy production/transportation impairment factors for males (Fig. 2A and Table S3).
Figure 2. Microbial-dependent sex differences in ME/CFS symptoms (F1–F13) for females (nrange = 120–170, orange), and males (nrange = 57–77, black).
Spearman’s correlation coefficient are presented showing the size of the relationship between symptom factors and the relative abundance (RA) of A. Clostridium, B. Lactobacillus, C. Streptococcus, and D. Bifidobacterium. Positive correlations indicate that an increase in microbial relative abundance was monotonically associated with an increase in symptom scores. The direction of a positive association could also be explained in reverse. Negative correlations indicate an inverse monotonic relationship between the two variables. Correlations were classified as small (0.01), moderate (0.03) and large (0.05) effect sizes51. *P < 0.05, **P < 0.01. zobs values were calculated52 to examine whether there was a significant difference between male and female correlation coefficients. Statistically significant differences are highlighted when zobs < −1.96 or zobs > 1.96.
Lactobacillus
Figure 2B highlights the positive associations between the distribution of Lactobacillus and total ME/CFS symptom factors for males (F12: rs = 0.28, n = 58, p = 0.036; F13: rs = 0.29, n = 57, p = 0.028) in this sample (Table S3). However for females, no significant relationships were revealed between these variables. Notably, in males only, analyses recorded moderate effect sizes for neurocognitive (F2: rs = 0.34, n = 72, p = 0.003) and neurosensory factors (F5: rs = 0.35, n = 74, p = 0.002). Other symptoms associated with neurological impairment, including pain (F3: rs = 0.26, n = 70, p = 0.031) and mood factors (F11: rs = 0.28, n = 69, p = 0.019) also showed consistently significant associations and similar effect sizes for males. When considering the compositional similarity in the frequency and distribution of Lactobacillus across the sexes in this sample, the symptom expression differences in males may be best explained by a sex-specific response to the same microbial community.
Streptococcus
The sex-divergent pattern of associations between Streptococcus levels and ME/CFS symptoms was consistent across twelve of the thirteen symptom factors (Fig. 2C and Table S3). Correlations for Streptococcus RA suggested opposing protective or pathogenic qualities between the sexes. For males, analyses revealed small to moderate significant positive associations between Streptococcus RA and pain (F3: rs = 0.39, n = 70, p = 0.001), sleep (F4: rs = 0.26, n = 74, p = 0.028), immunity (F6: rs = 0.24, n = 74, p = 0.038), gastrointestinal (F7: rs = 0.24, n = 73, p = 0.44, genitourinary (F8: rs = 0.27, n = 77, p = 0.018), energy production/transportation impairments (F10: rs = 0.24, n = 72, p = 0.045), ICC symptom (F12: rs = 0.33, n = 58, p = 0.013), and Total symptom (F13: rs = 0.31, n = 57, p = 0.017) factors. Conversely for females, there were significant negative correlations between Streptococcus RA and pain (F3: rs = −0.17, n = 154, p = 0.034), neurosensory (F5: rs = −0.16, n = 165, p = 0.040), and immunity impairments (F6: rs = −0.21, n = 163, p = 0.007).
Bifidobacterium: Possible sex consistency
Although only reaching significance in the female subgroup, analyses of Bifidobacterium RA provided an example of sex consistency in this sample (Fig. 2D and Table S3) and provided support for possible protective properties of these species. Significant, small negative correlations were shown between Bifidobacterium RA fatigue (F1: rs = −0.16, n = 166, p = 0.036), neurocognitive (F2: rs = −0.17, n = 158, p = 0.032), neurosensory (F5: rs = −0.17, n = 164, p = 0.030), energy/production and transportation impairments (F10: rs = −0.23, n = 164, p = 0.003), ICC symptoms (F12: rs = −0.19, n = 123, p = 0.044), and Total symptoms (F13: rs = −0.20, n = 117, p = 0.029) factors.
Discussion
Observations in this ME/CFS sample showed a) sex differences in symptom presentation; b) sex consistency in microbial communities and; c) sex-specific interactions with gut microbiota and symptom expression. Associations between symptom level and bacterial level, in the context of sex consistency in microbial communities, imply sex-specific interactions with gut microbiota. Precise mechanisms of sex interactions can only be hypothesized because the hormonal status of patients was not available for this sample. It has been suggested that changes in microbial composition and the associated imbalance in production of estrogen receptor agonists/antagonists may contribute to immune disturbances and other symptoms observed in ME/CFS20. Specific bacterial taxa (Firmicutes, Actinobacteria and Proteobacteria) metabolise and consequently modulate homeostasis of sex steroid hormones through genes that encode hydroxysteroid dehydrogenase (HSD) enzymes21. Particular species within the genera Clostridium, Bacteroides and Eubacterium are known to produce the enzymes 7α– and 7β–hydroxysteroid dehydrogenase22,23, deconjugating primary bile acids enabling humans and animals to absorb cholesterol, the precursor of steroid hormones. The results from this study however question our current understanding of these processes and suggest the need to examine the host relationship with intestinal organisms at the species level of each of the three genera.
The relationship between microbiota and hormones appears bidirectional. In populations with intestinal dysbiosis, the consequence of changes to hormonal metabolism and dysregulation may help explain symptom expression and variability. In reverse, hormonal imbalances may also perpetuate intestinal dysbiosis. The Firmicutes phylum of bacteria include Clostridium, Lactobacillus, and Streptococcus species, all of which showed interesting sex-interactions in our sample. Prospective studies should consider obtaining hormonal status and biomarkers to examine possible interactions with microbial composition in an attempt to delineate the physiology underlying these sex-differences.
Perhaps the associations between Clostridium composition and some ME/CFS symptoms in females may reflect the influence of diet and variation at the species level. An increase in Firmicutes has also been associated with a more typically ‘Western diet’ with opportunistic species Clostridium difficile and Clostridium perfringens flourishing with increased refined sugar intake24. The sex-specific associations in the current sample raise further questions about intestinal dysbiosis in ME/CFS, particularly investigating whether higher levels of Clostridium species exacerbate neurological symptoms in females and the potential benefits of targeting treatment to restore intestinal balance.
Observations across Lactobacillus and Streptococcus genera suggest support for D-lactate as a contributing factor to symptom expression, particularly in males. This hypothesis explains the neurological symptoms of ME/CFS as a consequence of neurotoxic effects of bacterial metabolites (i.e., D-lactic acid produced by most species of Lactobacillus and Streptococcus) on the brain and nervous system25. Increased D-lactic acid levels have been found in the serum of CFS patients with intestinal bacterial overgrowth7, associated with cognitive and neurological impairments26, and reduced in response to treatment in a sample of CFS patients27. The mechanisms of a sex-specific response to D-lactic acid have not been considered.
Potential sex differences in symptom expression as a consequential or contributing factor in microbial composition have clinical and research implications. Treatment aimed at restoring intestinal homeostasis, including faecal transplants, antibiotic and probiotic therapy require consideration of individual variation and potential sex difference affecting treatment responsiveness. Clinical trials need to be designed with appropriately sized samples to enable sex comparisons. Compositional similarity within a clinical population may be falsely interpreted without considering sex interactions. Notably, the findings for Lactobacillus spp. in males caution against premature probiotic supplementation with D-lactate producing bacteria. However, results support the health-promoting effects of Bifidobacteria as observed across diverse disease states including IBS28,29, cancer30, anxiety and depression31,32.
In combination, our results suggest support for the microgenderome in a human clinical population. The sex-interactions observed using genera analyses do not provide specificity and prompt the need for further examination at the species level. These results call for mechanistic research to examine the role of the sex steroid interaction with microbiota in the modulation of fatigue, pain, neural and immune responses seen within ME/CFS. This is a clinically complex but potentially advantageous research population with overlapping symptomatology relevant for diverse clinical groups. Research efforts that generate phenotypes and mechanistic understanding of the human microbiome require examination of potential sex and functional differences within compositionally similar communities.
Methods
Setting and Participants
The methods for this study were conducted in accordance with the approved guidelines for human experimental research. Ethics approval was obtained from Victoria University Human Research Ethics Committee in May 2013 (HRE13-109). As a retrospective sample, there was no direct contact with participants. Patients obtaining faecal assessment through Bioscreen (Aust.) signed informed consent to allow their microbial results and accompanying self-reported symptoms to be used for research purposes.
The dataset included 274 patients who had signed consent to participate in research during faecal microbial assessment (FMA) through the NATA (National Association of Testing Authorities) accredited laboratory, Bioscreen. All patients received a diagnosis of CFS in accordance with the Canadian Definition Criteria33 or Fukuda criteria34 during treatment from one of the co-authors (DPL) between January 2011 and April 2013. Only the earliest available data were included when multiple FMA results were available for the same patient.
Sex representation within this study was equivalent to prevalence ratios amongst clinical ME/CFS populations10 with 86 male (31.4%) and 188 female (68.6%) participants. The age range of 6 to 81 years (M = 39.25, SD = 14.81) is consistently representative of the occurrence of ME/CFS across developmental stages10. Age was not provided for two participants. Additional demographic information or information about comorbid diagnoses were not available. Therefore, no additional exclusion criteria were applied.
Data sources/measurement
Faecal Microbial Analysis. Sample collection: Prior to faecal sample collection, patients were instructed to cease antibiotic and/or probiotic treatment for four and two weeks, respectively. Patients collected a sample of their first morning bowel motion in a faecal container (anaerobic pouch system) with a perforated lid to aid anaerobiasis (achieved by activating Anaero Gen Compact (Oxoid, Thermo Fisher Scientific, Australia)). Samples were immediately transported to the laboratory in cold conditions (<12 °C) for analysis within 48 hours after collection. Laboratory protocol rejects samples subjected to inaccurate collection, transportation, anaerobiasis or refrigeration procedures. Internal quality assurance investigations validated the anaerobic transport and culture methods (see35).
Microbial identification and quantification: After removal from the anaerobic pouch system, all faecal samples were processed within 10–15 minutes. Between 0.5–1.0 g was transferred to 10 mL of 1% glucose-saline buffer36. Dilution factor was determined by the difference in the weight of the glucose-saline buffer with and without the sample. One hundred and one thousand fold dilutions (beginning from 10−1 to 10−7) of homogenised faecal samples were prepared37. Dilutions (10 and/or 1 μL amounts) were transferred onto dried Columbia horse blood agar (Oxoid), chromogenic medium (Oxoid), colistin and nalidixic acid blood selective agar (Oxoid), and chloramphenicol-gentamicin selective Sabouraud agar for aerobic incubation. Anaerobic incubation (4 day duration) in anaerobic jars (Oxoid) utilised pre-reduced Columbia horse blood haemin agar and Raka Ray medium. Aerobic media were incubated at 35 °C for 48 hours. A stereomicroscope was used to examine both aerobic and anaerobic culture plates for a minimum of 20 min/plate before bacterial identification. Each colony from each medium was microscopically examined and the colony/viable count were quantified for each plate. To assess purity prior to identification, similar morphotypes were sub-cultured onto horse blood agar.
Identification using MALDI-TOF MS analysis: Following overnight purity checks, index bacterial colonies were transferred to a target polished steel plate (MSP 96, Bruker Daltonics Inc.) for drying under exhaust ventilation in a Class II Biohazard Hood (Gelman Sciences Australia) at room temperature. Air-dried samples were subjected to protein extraction with 1 μL 70% formic acid (Sigma). After repeat air-drying under exhaust ventilation, samples were overlaid with 1 μL of matrix solution (saturated solution of α-cyano-4-hydroxycinnamic acid (HCCA) in a mixture of 47.5% ultra-pure water, 2.5% trifluoroacetic acid, and 50% acetonitrile). Dried samples were analysed using Microflex MALDI-TOF mass spectrometer (Bruker Daltonik GmbH, Leipzig, Germany) equipped with a 60 Hz nitrogen laser. Spectra were recorded in the positive linear mode for the mass range of 2,000–20,000 Da at maximum laser frequency. The MALDI Biotyper 3.0 software package (default settings; Daltonik GmbH, Bremen, Germany) was used to automatically analyse and measure raw spectra without user intervention. This technology can detect approximately 5000 species. The most prevalent microorganisms are quantified (viable count detection limits include anaerobes >108 CFU/g, facultative anaerobes >105).
Data Used for Statistical Analysis. Genera investigated: Anaerobic (Bacteroides, Bifidobacterium, Clostridium, Eubacterium, and Lactobacillus) and aerobic genera (Escherichia, Streptococcus, Enterococcus) were investigated. Species identified during FMA were classified according to genera (data provided is the combined total of species identified within each genus). Species-level analyses were not included due to the heterogeneity of species identified during MALDI-TOF MS assessment and insufficient power to correlate less common species. Whilst genera-level investigations lack specificity, some evidence suggests similar metabolic and functional capacity within taxa and genera38.
Justifications for selected genera: A priori selection of genera was grounded in the literature. Some of the most abundant strains of enteric microbiota within healthy human samples fall within Bacteroides, Clostridium, Eubacterium, and Prevotella as the anaerobic genera and Escherichia and Streptococcus as the aerobic genera39. Within infants, some of the dominant enteric microbiota include Lactobacillus, Bifidobacterium and Streptococcus species40. Whilst the abundance of specific microbiota does not necessarily equate to their purpose, function or importance39, they provide an initial direction for examining specific genera.
The D-lactate hypothesis and relationship between increased D-lactate levels and neurocognitive impairment26 further guided selection of genera investigated in this research. An association between D-lactic acidosis and an overgrowth of enteric lactic acid bacteria (including some species of Lactobacillus, Bifidobacterium, Enterococcus and Streptococcus) has been shown7. An Australian sample of patients with ME/CFS showed significantly higher levels of Enterococcus and Streptococcus genera viable count compared with healthy controls7. This study also showed variable levels of Escherichia coli amongst ME/CFS samples compared with controls, hence the Escherichia genus was also investigated.
A possible cause of D-lactic acidosis is from abnormal metabolism of carbohydrate by enteric microbiota41. Although not a primary byproduct, Eubacterium species can also produce lactic acid42. Evidence of dietary influences on microbial composition supported the rationale for including examination of Eubacterium (associated with dietary fibre and starch43); and Clostridium (associated with increased refined sugar inake26). Additionally, some strains of Clostridium have been associated with health44 and others with pathology45.
Some strains of Lactobacillus and Bifidobacteria are frequently associated with optimal health and used for probiotic supplementation28,29,30,31,32,46. Health-promoting functions of these microbiota contrast the D-lactic hypothesis and provided further justification for examining these genera.
The abundance of Prevotella as well as evidence of an association between colonic overgrowth and neurological symptoms47 suggests the need to further investigate this genus. Unfortunately, Prevotella species were excluded from the analysis due to variable microbial identification and quantification methods during the data collection period.
Selection of the eight genera was supported by post-hoc analyses of the current dataset showing that they accounted for large proportions of detectable microbiota in the majority of stool samples. To assess the level of representation of selected genera within this ME/CFS sample, the Total RA was calculated as the combined proportion of the eight genera investigated within the total detectable bacteria (i.e., including all genera and not specifically limited to those analyzed in this study). From the 270 samples that were assessed for both aerobic and anaerobic bacteria, the eight genera represented between 5–100% of detectable microbiota (M = 92.60%, SD = 16.80%). The most common Total RA score was 100% with 90% of the sample showing a Total RA of equal to or above 72%. Sex comparisons of the Total RA indicated similarity in representation of the eight genera investigated (Mdnmales = 99.67%, Mdnfemales = 99.77%, U = 8529.0, P = 0.263, r = 0.068).
Count: Microbial frequency of each genus was measured in colony-forming units per gram (CFU/g). Genera exponent values were used as a measure of each microbial count per patient.
Total Bacteria: Exponent values for the microbial frequency of all detectable bacteria as measured in CFU/g.
Aerobic:Anaerobic Ratio: Total detectable aerobic bacteria divided by total detectable anaerobic bacteria multiplied by 1000. This includes all genera and not specifically limited to those selected for data analysis.
Relative abundance (RA): Percentages were calculated by dividing the viable count of each genus by the total detectable bacteria count (methods akin to39,48). The expanded whole numbers for both counts were used in this calculation.
Total RA: The sum of RA percentages for the eight selected genera.
Patient Questionnaire: Concurrently to faecal sample collection, patients completed an 88-item Bioscreen Patient Questionnaire (BPQ). The BPQ is used for all referring patients regardless of clinical presentation. Items address diverse symptomatology similar to the Symptom Checklist-90-Revised49 and Beck Depression Inventory-II50. Patients rated symptom severity (past 7 days) and frequency (past 12 months) using a 5-point Likert scale (0–4). Frequency scores ranked from none at all (0) to extreme (4, severity) or constant (4, frequency). The BPQ showed high internal consistency within this ME/CFS population (Cronbach’s α = 0.974).
ME/CFS Symptom Factors: Seventy-six BPQ items were clinically classified into 13 factors reflecting ME/CFS symptoms in accordance with the ICC (F1-F1010) and mood symptoms (F11; Table S1). Seventeen items were omitted that were inconsistent with the ICC, could be misinterpreted as representative of two or more factors, or did not pertain to mood symptoms. Whilst psychological or mood symptoms are not specified under the ICC, high comorbidity with depression and anxiety symptoms in the ME/CFS population provided the rationale for further investigation of mood symptoms (predominantly depressive and anxiety symptoms). An impact score (severity × frequency) was calculated for each item (possible range 0–16) and relevant items were added to form corresponding factors. As measures of combined symptomatology, an ICC Symptoms Score (summation of F1-10) and Total Symptoms Score (summation of F1-F11) were calculated.
Bias: To reduce item selection bias, the factor classification was performed according to face validity as assessed by A.W., D.B. and M.B. and confirmed by consultation with clinician, D.P.L. No changes to the factor structure were made after commencing data analysis.
As a retrospective data sample, FMA results were initially performed for clinical purposes. Hence, no a priori hypotheses influenced data collection methods reducing the potential for investigator bias or falsification of data.
Statistical Methods. Descriptive statistics were performed for all ME/CFS symptom (Table 1 and Table S1) and microbial variables (Table S2) for the total sample, males and females. No outliers were found for microbial variables. The heterogeneity of symptom scores influenced the decision to include any clusters of outliers identified by SPSS on the ME/CFS Symptom Factors. Pairwise exclusion was used for missing data. All variables defied normality, therefore, nonparametric analyses were employed.
Examining Subgroups and Interactions. Sex comparisons on ME/CFS symptom factors: Descriptive statistics confirmed that each symptom factor (total, females and males separately) defied normality. A series of Mann-Whitney tests were used to compare the distribution of female and male symptom scores for each factor.
Sex comparison for microbial levels: Descriptive statistics confirmed that each microbial genus (count and RA) defied normality. A series of Mann-Whitney tests were used to compare the distribution of female and male microbial levels for count and percentage distribution independently. Effect sizes were calculated using equation:
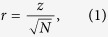 |
where N was the total sample used in the analysis. Effect sizes were classified as small (0.01), moderate (0.03) and large (0.05) according to Cohen’s (1988) guidelines51.
Associations between microbial level and ME/CFS symptoms: Spearman’s rank order correlations (rs) were used to investigate sex-interactions between microbial RA and ME/CFS symptom factors (Table S3). Missing data were excluded pairwise from the analyses. Correlations were deemed statistically significant at P < 0.05. Positive correlations indicated an increase in microbial relative abundance was monotonically associated with an increase in symptom scores. The direction of a positive association could also be explained in reverse. Negative correlations indicate an inverse monotonic relationship between the two variables. Correlations were classified as small (0.01), moderate (0.03) and large (0.05) effect sizes51.
Observed z scores were calculated using equation (2)52 to examine whether there was a statistically significant difference between the sexes for the strength of the correlation between symptom factor and microbial RA. Differences were deemed statistically significant when zobs < −1.96 or zobs > 1.9652.
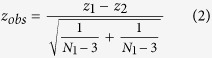 |
Design Limitations and Advantages
We caution against over-interpretation of these findings considering the limitations of cross-sectional, observational research design (unable to establish causation or consequence, difficulty excluding confounding variables53) and categorical analysis of genera rather than species. Other genera that were not selected during this investigation may also have relevance for ME/CFS symptomatology. Technological advancement enabling 16S amplicon sequencing of viable count will be able to identify and compare a broader range of genera and species. This will then allow comparison with other ME/CFS samples (e.g. 20) and the ability to examine the representative nature of these results whilst considering the impact of ethnic and geographic diversity on microbial composition. Applied human research has clinical relevance54 and can appropriately direct the pursuits in animal investigations where mechanistic studies are needed21. A symbiotic, interdisciplinary approach that integrates sex differences in clinical observational data and mechanistic data will inform therapeutic directions and treatment utility.
Additional Information
How to cite this article: Wallis, A. et al. Support for the Microgenderome: Associations in a Human Clinical Population. Sci. Rep. 6, 19171; doi: 10.1038/srep19171 (2016).
Supplementary Material
Acknowledgments
We thank S. Gauci and M. Scott for preliminary data entry and analysis.
Footnotes
Bioscreen (Aust.) Pty Ltd. and Victoria University provided post-graduate scholarship funding to A.W. without restriction on publication. D.B., M.B., H.B. and D.P.L. declare no competing financial interest.
Author Contributions A.W. wrote the manuscript; A.W., D.B. and M.B. conducted data analysis; H.B. and D.P.L. co-ordinated data collection; and all authors contributed to study design, data interpretation and manuscript editing.
References
- Flak M. B., Neves J. F. & Blumberg R. S.. Welcome to the microgenderome. Science 339, 1044–1045 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle J. G. M. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013). [DOI] [PubMed] [Google Scholar]
- Berer K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011). [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney R. D. et al. The microbiome: stress, health and disease. Mamm. Genome 25, 49–74 (2014). [DOI] [PubMed] [Google Scholar]
- Sekirov I. et al. Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 (2010). [DOI] [PubMed] [Google Scholar]
- Sheedy J. R. et al. Increased D-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 23, 621–628 (2009). [PubMed] [Google Scholar]
- Maes M. & Leunis J. C.. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuroend. Letters 29, 101–109 (2008). [PubMed] [Google Scholar]
- Maes M. et al. Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J. Affect. Disord. 136, 909–917 (2012). [DOI] [PubMed] [Google Scholar]
- Carruthers B. M. et al. Myalgic encephalomyelitis: International consensus criteria. J Intern Med 270 327–328 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. & Twisk F. N.. Chronic fatigue syndrome: Harvey and Wessely’s (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Medicine 8, 35–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre C. C.. Sex differences in autoimmune disease. Nature Immun. 2, 777–780 (2001). [DOI] [PubMed] [Google Scholar]
- Cohen J. & Enserink M.. Virology. False positive. Science 333, 1694–1701 (2011). [DOI] [PubMed] [Google Scholar]
- Weaver S. A. et al. Sex differences in plasma prolactin response to tryptophan in chronic fatigue syndrome patients with and without comorbid fibromyalgia. Journal of Women’s Health 19, 951–958 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka K. et al. Neural and psychosocial contributions to sex differences in knee osteoarthritic pain. Biol. Sex Diff. 3, 26–37 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. et al. Mechanisms of pain modulation by sex hormones in migraine. Headache: J. of Head & Face Pain 51, 905–922 (2011). [DOI] [PubMed] [Google Scholar]
- Traub R. J. & Ji Y. P.. Sex differences and hormonal modulation of deep tissue pain. Frontiers in Neuroend. 34, 350–366 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulak A., Taché Y. & Larauche M.. Sex hormones in the modulation of irritable bowel syndrome. WJG 20, 2433–2448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth C. et al. Not all depression is created equal: sex interacts with disease to precipitate depression. Biol. Sex Diff. 4, 8–17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frémont M. et al. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe. 22, 50–56 (2013). [DOI] [PubMed] [Google Scholar]
- Markle J. G. & Fish E. N.. SeXX matters in immunity. Trends Immun. 35, 97–104 (2014). [DOI] [PubMed] [Google Scholar]
- Chikaia T., Nakaob H. & Uchida K.. Deconjugation of bile acids by human intestinal bacteria implanted in germ-free rats. Lipids 22, 669–671 (1987). [DOI] [PubMed] [Google Scholar]
- Lepercq P. et al. Isolates From Normal Human Intestinal Flora but Not Lactic Acid Bacteria Exhibit 7α- and 7β-hydroxysteroid Dehydrogenase Activities. Microb. Ecol. Health & Disease 16, 195–201 (2004). [Google Scholar]
- Brown K. et al. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 4, 1095–1119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L. The gut microbiome and the brain. J. Medicinal Food 17, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J., Oh M. S. & Carroll H. J.. D-lactic acidosis: a review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine. 77, 73–82 (1998). [DOI] [PubMed] [Google Scholar]
- Maes M., Mihaylova I. & Leunis J. C.. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut–intestinal permeability. J. Affect. Disord. 99, 237–240 (2007). [DOI] [PubMed] [Google Scholar]
- Ford A. C. et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am. J. Gastroent. 109, 1547–1561 (2014). [DOI] [PubMed] [Google Scholar]
- Mckernan D. et al. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroent. Motility 22, 1029–1035 (2010). [DOI] [PubMed] [Google Scholar]
- Reddy B. S. & Rivenson A.. Inhibitory effect of Bifidobacterium longum on colon, mammary, and liver carcinogenesis induced by 2-amino-3-methylimidazo[4,5-f]quinoline, a food mutagen. Cancer Research 53, 3914–3918 (1993). [PubMed] [Google Scholar]
- Desbonnet L. et al. Cognitive, Behavioral, and Systems Neuroscience: Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188 (2010). [DOI] [PubMed] [Google Scholar]
- Messaoudi M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutrition 105, 755–764 (2011). [DOI] [PubMed] [Google Scholar]
- Carruthers B. M. et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J. Chronic Fatigue Syndrome 11, 7–116 (2003). [Google Scholar]
- Fukuda K. et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann. Internal Med. 121, 953–959 (1994). [DOI] [PubMed] [Google Scholar]
- Coulson S. et al. Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacol. 21, 79–90 (2013). [DOI] [PubMed] [Google Scholar]
- Willis A.. Anaerobic Culture Methods in Aerobic Microbiology: A Practical Approach. (IRL Press, Oxford Univ. Press, Oxford, 2007). [Google Scholar]
- Thrupp L., Susceptability testing of antibiotics in liquid media in Antibiotics in Laboratory Medicine, Lorain V., (eds Williams & Wilkins) 73–113 (Baltimore, 1980). [Google Scholar]
- Hollister E. B., Gao C. & Versalovic J.. Basic concepts in the mammalian gut microbiome: Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 146, 1449–1458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal L. et al. The Prevalence of Species and Strains in the Human Microbiome: A Resource for Experimental Efforts. PLoS ONE. 9, 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M., Beuerlein M. & Danayan K.. An approach to the patient with a life-threatening acid-base disturbance: The acidemias. Uni. Toronto Med. J. 78, 122–128 (2001). [Google Scholar]
- Duncan S. H., Louis P. & Flint H. J.. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. App. & Environ. Microbiology. 70, 5810–5817 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albenberg L. G. & Wu G. D.. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 146, 1564–1572 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 331, 337–341 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatheway C. L.. Toxigenic clostridia. Clin. Microb. Rev. 3, 66–98 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested A. C., Logan A. C. & Selhub E. M.. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part II- contemporary contextual research. Gut Pathog. 5, 1–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan S. E. & Kirchgessner A.. Gut inflammation in chronic fatigue syndrome. Nutrition & Metabolism. 7, 79 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppé E. et al. Relative fecal abundance of extended-spectrum-β-lactamase-producing Escherichia coli strains and their occurrence in urinary tract infections in women. Antimicrobial Agents & Chemotherapy 57, 4512–4517 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L. R. Symptom Checklist-90-R: Administration, Scoring, and Procedures 14–15 (NCS Pearson, 1994). [Google Scholar]
- Beck A. T., Steer R. A. & Brown G. K. Manual for the Beck Depression Inventory-II 7–16 (Psychological Corporation, San Antonia, TX, 1996). [Google Scholar]
- Fritz C. O., Morris P. E. & Richler J. J.. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychology: General 141, 2–18 (2012). [DOI] [PubMed] [Google Scholar]
- Pallant. J. SPSS Survival Manual 132–134 (Allen & Unwin, 2005). [Google Scholar]
- Vandenbroucke J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Int. J. Surgery. 12, 1500–1524 (2014). [DOI] [PubMed] [Google Scholar]
- Kostic A. D., Howitt M. R. & Garrett W. S.. Exploring host-microbiota interactions in animal models and humans. Genes and Development 27, 701–718 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.