Abstract
Background
The possibility of incorporating generics into combination antiretroviral therapy and breaking apart once-daily single-tablet regimens (STRs), may result in less efficacious medications and/or more complex regimens with the expectation of marked monetary savings. A modeling approach that assesses the merits of such policies in terms of lifelong costs and health outcomes using adherence and effectiveness data from real-world U.S. settings.
Methods
A comprehensive computer-based microsimulation model was developed to assess the lifetime health (life expectancy and quality adjusted life-years—QALYs) and economic outcomes in HIV-1 infected patients initiating STRs compared with multiple-table regimens including generic medications where possible (gMTRs). The STRs considered included tenofovir disoproxil fumarate/emtricitabine and efavirenz or rilpivirine or elvitegravir/cobicistat. gMTRs substitutions included each counterpart to STRs, including generic lamivudine for emtricitabine and generic versus branded efavirenz.
Results
Life expectancy is estimated to be 1.301 years higher (discounted 0.619 QALY gain) in HIV-1 patients initiating a single-tablet regimen in comparison to a generic-based multiple-table regimen. STRs were associated with an average increment of $26,547.43 per patient in medication and $1,824.09 in other medical costs due to longer survival which were partially offset by higher inpatients costs ($12,035.61) with gMTRs treatment. Overall, STRs presented incremental lifetime costs of $16,335.91 compared with gMTRs, resulting in an incremental cost-effectiveness ratio of $26,383.82 per QALY gained.
Conclusions
STRs continue to represent good value for money under contemporary cost-effectiveness thresholds despite substantial price reductions of generic medications in the U. S.
Introduction
Innovations in antiretroviral therapy (ART) have dramatically altered the natural history of HIV infection, transforming it into a manageable chronic disease [1]. ART has markedly increased survival in people living with HIV (PLH) and extended life expectancy such that there are little differences between those with and without HIV [2]. Nonetheless, PLH still have a slightly shorter lifespan relative to the general population with important challenges remaining [3]. Delayed access to treatment and suboptimal ART adherence crucially influence poor outcomes, including the increased risk of hospitalization and death [4].
Increasing life expectancy contribute to increased total lifetime costs of HIV care, posing challenges for funders despite annual costs of treating someone with HIV having remained fairly constant over the years [5]. Many different strategies have been proposed to reduce the economic burden of HIV [6]. One strategy is the incorporation of generic medications into ART regimens as soon as they become available. That has led to suggestions of potentially including less efficacious drugs as well as more complex regimens under the expectation of monetary savings [7].
At first glance, these strategies seem appealing because they appear to present significant economic savings. Caution is warranted in interpreting these results, however, because some of these economic analyses are focused mainly on ART costs, ignoring other important costs such as inpatient and outpatient costs, or even wider economic societal implications, that may account for up to 45% of the total lifetime costs of HIV care [8–10]. Moreover, there are compelling data from a meta-analysis that suggest that treating with recommended treatments, rather than alternative recommendations is associated with markedly improved outcomes [11].
There is evidence that antiretroviral pill burden and treatment adherence are key determinants of the risk of hospitalization and inpatients costs [4, 12]. Patients on a once-daily single-tablet regimen (STR) have been shown to be significantly more likely to be highly adherent (≥95%) to therapy than patients who received a multiple-tablet regimen (MTR), and that improved adherence among patients treated with STR is associated with a lower risk of hospitalization and reduce healthcare costs, including significantly lower inpatient costs [12]. These important factors are crucial for cost-effectiveness studies and should be incorporated into models to better inform efficient resource allocation.
Also of concern is the justification of monetary savings being considered at the expense of potential health losses without adequately addressing the fact that societal value for a health loss is regarded to be substantially higher than the societal value for a health gain, i.e. monetary saving required to move from optimal to sub-optimal therapy are higher than the maximum willingness to pay for a health gain obtained by moving from lower to a higher health status [13].
The present study includes a computer-based microsimulation model of HIV disease progression based on adherence and effectiveness data from real-world settings in the US to comprehensively assess the long-term health and economic outcomes of HIV infected patients initiating single-tablet regimens (STRs) compared with multiple-tablet regimens including generic medications when possible (gMTRs).
Materials and Methods
Overview
In a simulation study two treatment strategies were considered for HIV-1 infected adult patients initiating first-line ART (Table 1): a branded 1-pill strategy (STR), and a generic-based 3-pill strategy (gMTR). Evaluated STRs were based on a backbone of tenofovir and emtricitabine (TDF/FTC), whereas gMTRs were based on a backbone of tenofovir and generic lamivudine (TDF+g3TC). Third agents and the percentage of patients initiating them did not depend on the treatment strategy chosen, and consisted of efavirenz (branded for STR: EFV; generic for MTR: gEFV), rilpivirine (RPV) and cobicistat-boosted elvitegravir (EVG/COBI).
Table 1. First-line antiretroviral therapy strategies, regimen components and market shares.
| STR Strategy | gMTR Strategy | % Patients Initiating |
|---|---|---|
| EFV/TDF/FTC | gEFV+TDF+g3TC | 30.4%a [14] |
| RPV/TDF/FTC | RPV+TDF+g3TC | 26.1%a [14] |
| EVG/COBI/TDF/FTC | EVG/COBI+TDF+g3TC | 43.5%a [14] |
COBI, cobicistat; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; g3TC, generic lamivudine; gEFV, generic efavirenz; gMTR, generic multiple-tablet regimen; RPV, rilpivirine; STR, single-tablet regimen; TDF, tenofovir.
aPercentage of patients initiating corresponding ART regimen.
The expected long-term, comparative economic and clinical impact of the proposed treatment strategies were analyzed using a microsimulation cost-effectiveness model for HIV treatment. Given the chronic and progressive nature of the disease, the model considered a lifetime time horizon. The economic impact was measured in terms of the expected total per-person cost to the U.S. health system, whereas the clinical impact or effectiveness was measured in terms of corresponding expected quality-adjusted life-years (QALY). Following U.S. recommendations, an annual 3% discount rate was applied to both costs and effectiveness. Deterministic sensitivity analysis was used to assess parameter uncertainty, where possible.
Microsimulation model
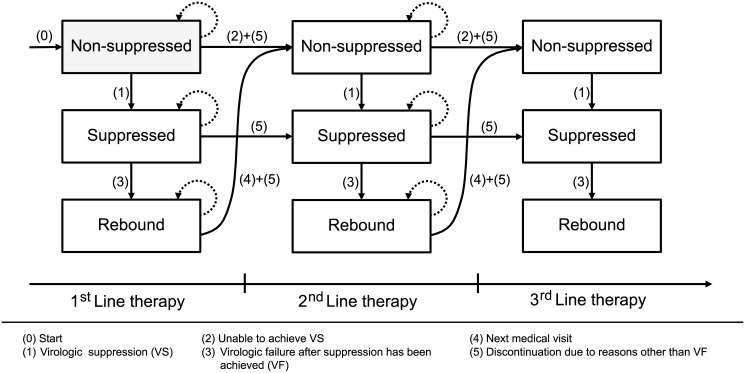
At the core of the microsimulation model lies a state-transition model (Fig 1), in which patients’ virologic response to ART is simulated, in monthly updates, to belong to one of 3 mutually exclusive health states: “non-suppressed”, “suppressed” and “rebound”.
Fig 1. State-transition model at the core of the microsimulation model, consisting of 3 main health-states (‘non-suppressed’, ‘suppressed’ and ‘rebound’) within each subsequent therapy line.
Arrows indicate the possible transitions between states and therapy lines.
Patients enter the model in the “non-suppressed” state (transition 0), where they initiate first-line therapy. Within this state, a monthly probability of virologic suppression (VS), applied during the first 6 months of therapy, determines if and when a patient moves to the “suppressed” state, and can be considered a responder to therapy (transition 1). Patients in the “non-suppressed” state that do not achieve VS within the first 6 months of therapy move to the “non-suppressed” state of the subsequent therapy line at the next scheduled medical visit (transition 2).
Once transitioned to the “suppressed” state, from the 7th month of therapy onwards, patients are subject to a monthly probability of virologic failure after suppression has been achieved (VF). Upon VF, patients move to the “rebound” state (transition 3), where they stay until the next scheduled medical visit, upon which they move to the “non-suppressed” state of the subsequent therapy line (transition 4).
Irrespective of their state of virologic suppression, patients are continuously subject to a monthly probability of discontinuation due to reasons other than VF (transitions 5). Upon discontinuation while in the “suppressed” state, patients immediately transition to the “suppressed” state of the subsequent therapy line. For the remaining non-virologically suppressed states, patients transition to the “non-suppressed” state of the subsequent therapy line. Finally, patients are also continuously subject to a monthly probability of death (not shown in Fig 1).
The same rules as above, conditional on the health states patients are in and the time they have spent in them, are applied to each subsequent therapy line. Six active therapy lines are modelled. Upon failing or discontinuing sixth-line therapy, patients are assumed to move to a non-suppressed state, where they remain until death. Throughout the simulation, medical visits are scheduled every 3 months.
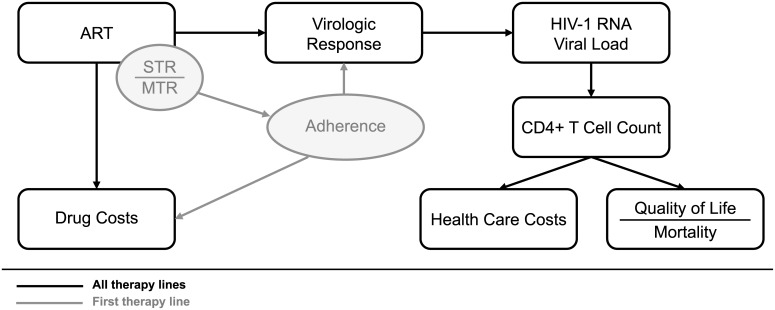
In each model run, a cohort of patients is simulated with key baseline characteristics (i.e. age, gender, HIV-1 RNA viral load and CD4+ T cell count) and first-line treatment strategy (STR vs. gMTR) dependent adherence levels. Every patient is simulated under both an STR and a gMTR first-line strategy (with equivalent third agent). Differential adherence between STRs and gMTRs is assumed to influence virologic response for first-line therapy only. Subsequent therapy lines are the same for all patients, irrespective of first-line STR or gMTR strategy. At each monthly cycle, the characteristics associated with each patient, including age, HIV-1 RNA viral load, CD4+ T cell count, quality of life (QoL), and health care and drug costs, are tracked and updated. An influence diagram relating the different model components can be found in Fig 2. Details are given below.
Fig 2. Model influence diagram.
Within each therapy line, the choice of ART regimen determines virologic response and drug costs. Virologic response, in turn, influences the evolution of HIV-1 RNA viral load and CD4+ T cell counts over time. Health care costs and QALY, through QoL and mortality, are dependent on CD4+ T cell counts. For first-line therapy, differential adherence between STR and MTR further influences virologic response and drug costs.
Model Parameterization
Baseline characteristics
With the aim of modeling a general ART-naïve HIV infected population, correlated baseline HIV-1 RNA viral load and CD4+ T cell count distributions were calibrated with data from Rodriguez et al. (2006) [15], presenting a mean viral load of 4.01 log10 HIV-1 RNA cps/mL and a mean CD4+ T cell count of 525 cells/mm3 (Table 2). Mean age at ART initiation was assumed to be 43 years, with 84% of the population being male (Table 2) [7].
Table 2. Baseline model characteristics.
| Variable | Value | Reference |
|---|---|---|
| Age—Mean (SD), years | 43 (12) | [7] |
| Gender—Male, % | 84 | [7] |
| Viral load × CD4 count—Mean (correlation matrix), log10 RNA cps/mL × cells/mm3 | [15] |
SD, standard deviation.
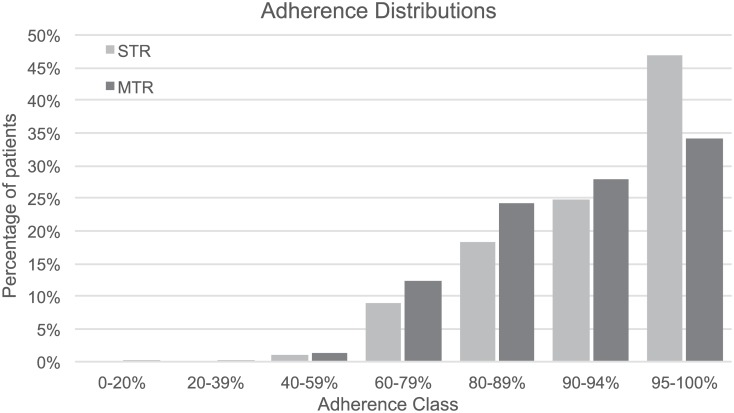
The proportions of patients receiving either of the three regimens considered in each of the treatment strategies (Table 1) were based on US market shares [14]. For patients receiving RPV-based ART, baseline viral load was simulated below 5 log10 RNA cps/mL, to match the corresponding label [16]. Real-world adherence levels to first-line therapy were based on Sweet et al. (2014) [17] and Sax et al. (2012) [4] and are presented in Fig 3.
Fig 3. Distribution of real-world average adherence levels, stratified by first-line treatment strategy.
MTR, multiple-tablet regimen; STR, single-tablet regimen.
State-transition probabilities
The monthly probabilities of virologic suppression and virologic failure for all first and subsequent therapy lines considered (Table 3), were calibrated on the basis of an external cohort state-transition model (corresponding to a single therapy line from Fig 1) as for the time-dependent percentage of patients in the “suppressed” state to approximate the time-dependent percentage of patients with HIV-1 RNA viral load below 50 cps/mL observed in the selected clinical trials. The treatment sequence from 2nd to 6th therapy line was based on expert opinion and efficacy derived from the corresponding trials.
Table 3. Calibrated monthly virologic suppression and virologic failure probabilities.
| Therapy Line | ART Regimen | Monthly Probability of Virologic | Source | |
|---|---|---|---|---|
| Suppression | Failure | |||
| 1st Line | EFV/TDF/FTC | 42.38% | 0.03% | GS-236-102 [18–20] |
| STR | RPV/TDF/FTC | 56.92% | 0.34% | STAR Study [21, 22] |
| EVG/COBI/TDF/FTC | 44.63% | 0.03% | GS-236-102 [18–20] | |
| 1st Line | gEFV+TDF+g3TC | 33.27% | 0.16% | Study 903 [23, 24] |
| gMTR | RPV+TDF+g3TC | 47.25% | 1.69% | Assumptiona |
| EVG+COBI+TDF+g3TC | 35.33% | 0.16% | Assumptiona | |
| 2nd Line | ATV/r + TDF/FTC | 9.12% | 0.82% | BMS Study 045 [25, 26] |
| 3rd Line | DRV/r + TDF/FTC | 12.46% | 0.33% | POWER 1–2 [27–31] |
| 4th Line | DTG + TDF/FTC | 28.70% | 2.09% | SAILING [32, 33] |
| 5th Line | DRV/r + DTG + TDF/FTC | 21.14% | 1.65% | VIKING [34] |
| 6th Line | ENF + OBRb | 43.69% | 1.13% | TORO 1–2 [35, 36] |
/r, ritonavir boosted; ATV, atazanavir; COBI, cobicistat; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ENF, enfuvirtide; EVG, elvitegravir; FTC, emtricitabine; g3TC, generic lamivudine; gEFV, generic efavirenz; OBR, optimized background regimen; RPV, rilpivirine; TDF, tenofovir.
aDetermined by applying the odds ratio between the corresponding probabilities of the gEFV+TDF+g3TC and EFV/TDF/FTC regimens to the probabilities of the RPV/TDF/FTC and EVG/COBI/TDF/FTC regimens.
bCalibrated on the percentage of patients remaining on treatment, assuming that for a multi-drug resistant end-of-line population, reaching a viral load below 50 RNA cps/mL is not the criteria on which viral failure is determined [35].
Simplified selection criteria adopted for the incorporated clinical trials were: 1) phase II or III randomized clinical trials, with preference to phase III studies; 2) patient characteristics in terms of the number of previous therapy lines that best fitted the therapy line in question; 3) follow-up data in at least two points in time, with preference for the most recent studies with more available time-points and longer follow-up (e.g.: efficacy and safety results at 48, 96 and 144 weeks).
First-line gMTR, EFV+TDF+3TC has been studied in clinical trial setting in Study 903 [23, 24] and results from this trial, as in other publish cost-effectiveness analysis [7], were assumed to reflect those of gEFV+TDF+g3TC. RPV+TDF+g3TC and EVG/COBI+TDF+g3TC, have not been studied in a clinical trial setting. To overcome this lack of information, viral suppression and failure probabilities for these regimens were determined by applying the odds ratio between the corresponding probabilities of the gEFV+TDF+g3TC and EFV/TDF/FTC regimens to the probabilities of the RPV/TDF/FTC and EVG/COBI/TDF/FTC regimens.
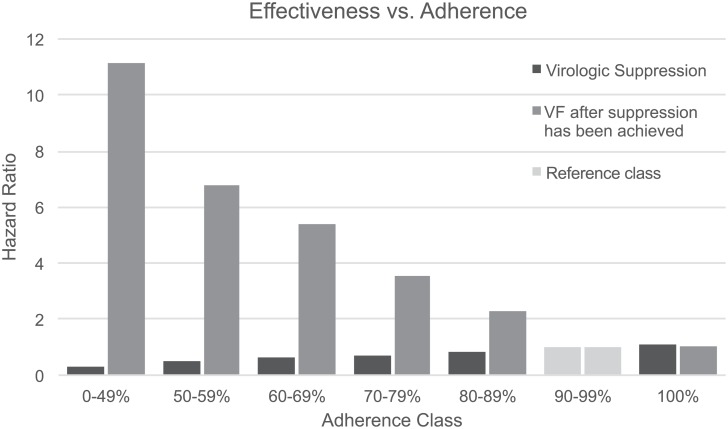
The first-line therapy virologic suppression and failure probabilities presented in Table 3 were considered for high levels of average adherence usually observed in clinical trial settings. To accommodate for declining levels of virologic response typically associated with real-life lower adherence levels (Fig 3), monthly suppression and failure probabilities were adjusted using hazard ratios adapted from Nachega et al. (2007) [37] (Fig 4), using the 90–99% adherence class as a reference class for the base-case values in Table 3.
Fig 4. Hazard ratios of virologic suppression and virologic failure for different adherence classes.
VF, virological failure.
Monthly probabilities for discontinuation due to other reasons were determined from the clinical trials used for the calibration of virologic response probabilities, considering discontinuations for all reasons (including adverse events) except virologic failure (lack of efficacy) and death (Table 4).
Table 4. Calibrated monthly virologic suppression and virologic failure probabilities.
| Therapy Line | ART Regimen | Monthly Probability of Discontinuation | Source | |
|---|---|---|---|---|
| First year | Subsequent Years | |||
| 1st Line | EFV/TDF/FTC | 1.09% | 0.50% | GS-236-102 [18–20] |
| STR | RPV/TDF/FTC | 0.91% | 0.61% | STAR Study [21, 22] |
| EVG/COBI/TDF/FTC | 0.84% | 0.56% | GS-236-102 [18–20] | |
| 1st Line | gEFV+TDF+g3TC | 1.36% | 0.46% | Study 903 [23, 24] |
| gMTR | RPV+TDF+g3TC | 1.14% | 0.56% | Assumptiona |
| EVG+COBI+TDF+g3TC | 1.05% | 0.52% | Assumptiona | |
| 2nd Line | ATV/r + TDF/FTC | 0.71% | 0.79% | BMS Study 045 [25, 26] |
| 3rd Line | DRV/r + TDF/FTC | 1.04% | 1.28% | POWER 1–2 [27–31] |
| 4th Line | DTG + TDF/FTC | 0.94% | 0.10% | SAILING [32, 33] |
| 5th Line | DRV/r + DTG + TDF/FTC | 1.15% | 1.22% | VIKING [34] |
| 6th Line | ENF + OBR | 1.83% | 2.13% | TORO 1–2 [35, 36] |
/r, ritonavir boosted; ATV, atazanavir; COBI, cobicistat; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ENF, enfuvirtide; EVG, elvitegravir; FTC, emtricitabine; g3TC, generic lamivudine; gEFV, generic efavirenz; OBR, optimized background regimen; RPV, rilpivirine; TDF, tenofovir.
aSimilar procedure as for the assumptions in Table 3.
Monthly probabilities of death were determined by multiplying CD4+ T cell count dependent standardized mortality ratios (SMR) to gender and age specific base mortality rates reported by the Center for Disease Control and Prevention (CDC) national vital statistics (Table 5) [38].
Table 5. Standardized mortality ratios as a function of CD4+ T cell count.
| CD4+ T cell count (cells/mm3) | Standardized mortality ratio |
|---|---|
| ≥500 | 2.5 |
| 350–499 | 3.5 |
| 200–349 | 5.6 |
| ≤199 | 30.3 |
Evolution of HIV-1 RNA viral load and CD4+ T cell count
While in the “suppressed” state (Fig 1), patients have their viral load set, and maintained, at a constant low level (below 50 cps/mL). Meanwhile, CD4+ T cell counts increase over time in a logarithmic fashion. The monthly logarithmic rate of increase for the different therapy lines and regimens considered were determined on the reported 48 week mean change from baseline in CD4+ T cell count observed in the clinical trials used for virologic response calibration (Table 6).
Table 6. 48 Week CD4+ T cell count increase.
| Therapy Line | ART Regimen | CD4+ T cell count increase at 48 weeks | Source |
|---|---|---|---|
| 1st Line | EFV/TDF/FTC | 206 | GS-236-102 [18–20] |
| STR | RPV/TDF/FTC | 200 | STAR Study [21, 22] |
| EVG/COBI/TDF/FTC | 239 | GS-236-102 [18–20] | |
| 1st Line | gEFV+TDF+g3TC | 205 | Study 903 [23, 24] |
| gMTR | RPV+TDF+g3TC | 199 | Assumptiona |
| EVG+COBI+TDF+g3TC | 230 | Assumptiona | |
| 2nd Line | ATV/r + TDF/FTC | 110 | BMS Study 045 [25, 26] |
| 3rd Line | DRV/r + TDF/FTC | 102 | POWER 1–2 [27–31] |
| 4th Line | DTG + TDF/FTC | 162 | SAILING [32, 33] |
| 5th Line | DRV/r + DTG + TDF/FTC | 110 | VIKING [34] |
| 6th Line | ENF + OBR | 119 | TORO 1–2 [35, 36] |
/r, ritonavir boosted; ATV, atazanavir; COBI, cobicistat; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ENF, enfuvirtide; EVG, elvitegravir; FTC, emtricitabine; g3TC, generic lamivudine; gEFV, generic efavirenz; OBR, optimized background regimen; RPV, rilpivirine; TDF, tenofovir.
aSimilar procedure as for the assumptions in Table 3.
For patients in the “rebound” state, viral load is reset to the highest level ever observed since treatment initiation (≥ 50 RNA cps/mL), after which it keeps increasing on a log10 scale, by an annual rate of 0.11 while CD4+ T cell counts are greater than or equal to 200 cells/mm3 and by an annual rate of 0.17 otherwise [39]. CD4+ T cell counts are similarly reset to the lowest level ever observed, afterwards further decreasing as a function of viral load, determined on the basis of data from the Multicenter AIDS Cohort Study [40].
For patients in the “non-suppressed” state that will never suppress, viral load and CD4+ T cell count evolution is equivalent to that of patients in the “rebound” state. For the remaining patients, viral load and CD4+ T cell count are equivalent to the evolution discussed for patients in the “suppressed” state.
Costs and Quality of Life
Costs accounted for in the model include ART costs, hospitalizations (inpatient) costs, and other medical expenses (cost of prophylaxis therapy for opportunistic infections, outpatient treatment, emergency department visits, non-HIV medication and laboratory testing [8]). All costs were inflated, where necessary, to 2014 USD using publically available health-care consumer price indices [41].
Unit ART costs were based on data from First Databank, (April 1st, 2015; Table 7). The cost of generic EFV was assumed to be 25% of its branded equivalent. The cost for the optimized background regimen (OBR) of sixth-line therapy was based on the OBR composition presented in Hill et al. (2011) [42].
Table 7. Annual ART costs (First Databank, April 1st, 2015).
| Therapy Line | ART Regimen | AnnualART Cost |
|---|---|---|
| 1st Line | EFV/TDF/FTC | $25,874.00 |
| STR | RPV/TDF/FTC | $24,975.86 |
| EVG/COBI/TDF/FTC | $29,896.54 | |
| 1st Line | gEFV+TDF+g3TC | $17,143.14 |
| gMTR | RPV+TDF+g3TC | $24,167.86 |
| EVG+COBI+TDF+g3TC | $29,088.55 | |
| 2nd Line | ATV/r + TDF/FTC | $34,159.88 |
| 3rd Line | DRV/r + TDF/FTC | $34,049.88 |
| 4th Line | DTG + TDF/FTC | $31,649.88 |
| 5th Line | DRV/r + DTG + TDF/FTC | $66,123.88 |
| 6th Line | ENF + OBR | $59,193.30 |
Inpatient costs and other medical costs were calculated based on the patient’s CD4+ cell count (Table 8). Real-world evidence suggests a 45% decrease in hospitalizations for patients on STR therapy as compared to those on gMTR therapy [43], as such, it was assumed that first-line STR therapy inpatient costs were 55% of the corresponding first-line gMTR therapy costs. This value was subject to sensitivity analysis to reflect other studies evidence [4, 12].
Table 8. Annual inpatient and other medical costs as a function of CD4+ T cell count.
| CD4+ T cell count (cells/mm3) | Inpatient Costs | Other Medical Costs |
|---|---|---|
| ≤ 50 | $32,018.95 | $8,529.76 |
| 51–200 | $12,463.18 | $5,919.56 |
| 201–350 | $5,660.61 | $4,433.19 |
| 351–500 | $3,198.01 | $4,224.74 |
| > 500 | $1,386.67 | $4,182.01 |
CD4+ T cell count dependent health-related QoL utility values for quality adjusted life year calculation were taken from Kauf et al. (2008) [44] and are presented in Table 9.
Table 9. Quality of life utility values as a function of CD4+ T cell count.
| CD4+ T cell count (cells/mm3) | QoL Utility |
|---|---|
| ≥ 500 | 0.946 |
| 350–499 | 0.933 |
| 200–349 | 0.931 |
| 100–199 | 0.853 |
| < 100 | 0.781 |
Results
The model simulated 200,000 hypothetical individuals initiating ART (100,000 for STRs and 100,000 for gMTRs) using real-world evidence. All results represent the averages of each group of hypothetical individuals (STR vs. gMTR) and are discounted at 3.0% per annum.
After incorporating real-world evidence, over the lifetime of a patient an additional discounted 0.619 QALYs (14.466 vs. 13.847) were estimated to be gained with STRs compared with gMTRs (Table 10). Single-tablet regimens were associated with an incremental $26,547.43 per patient in medication costs and $1,824.09 in other medical costs due to longer survival in comparison to gMTRs (Table 10). STRs, however, were estimated to reduce inpatient costs by $12,035.61 relative to gMTRs (Table 10). Overall, STRs were associated with incremental lifetime costs of $16,335.91 compared with gMTRs, resulting in an incremental cost-effectiveness ratio (ICER) of $26,383.82 per QALY gained (Table 10).
Table 10. Cost-effectiveness of STRs versus gMTRs for the treatment of HIV-1 infection in the United States.
| STR | gMTR | Δ STR-gMTR | |
|---|---|---|---|
| Total Costs | $547,540.20 | $531,204.29 | $16,335.91 |
| Medication Costs | $450,474.20 | $423,926.76 | $26,547.43 |
| Inpatient Costs | $31,258.18 | $43,293.79 | -$12,035.61 |
| Other Costs | $65,807.83 | $63,983.73 | $1,824.09 |
| Life Years | 15.400 | 14.785 | 0.614 |
| QALY | 14.466 | 13.847 | 0.619 |
| ICER ($/QALY) | $26,383.82 |
gMTR, generic multiple-tablet regimen; ICER, Incremental cost-effectiveness ratio; QALY, Quality adjusted life years; STR, Single-tablet regimen.
Undiscounted life expectancy after ART initiation was estimated to be 22.4 years for STR and 21.1 years, with a benefit of 1.3 years in favor of the STR treatment. The undiscounted QALY gain for STR over gMTR was 1.3 QALY for a marginal lifetime cost of $41,188.85 resulting in an undiscounted ICER of $31,910.87 per QALY gained.
Sensitivity Analysis
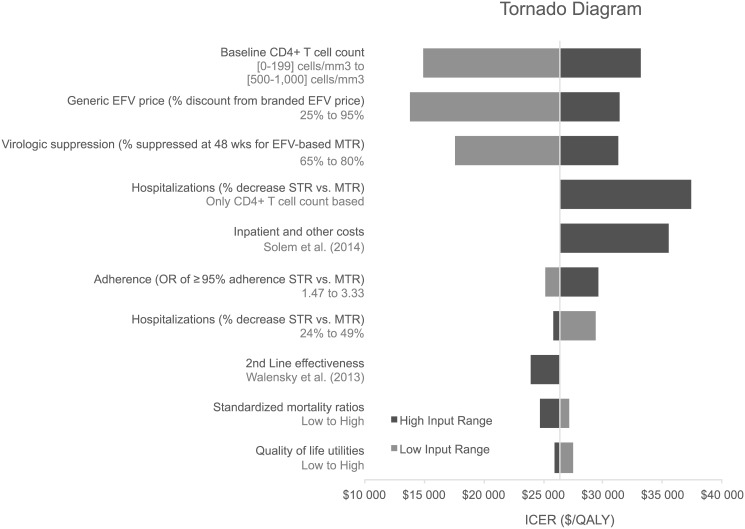
A deterministic sensitivity analysis was conducted to determine the factors that had the greatest influence on the ICER. The range of variation to each parameter is described in the S1 Table. The discounted lifetime ICER for HIV treatment initiation with STRs compared with gMTRs was $26,384 per QALY in the base case and ranged from $13,790 to $37,438 per QALY (Fig 5).
Fig 5. Tornado diagram of univariate analyses showing the degree to which uncertainty in individual variables affects ICER ($/QALY).
EFV, efavirenz; ICER, incremental cost-effectiveness ration; MTR, multiple-tablet regimen; QALY, quality adjusted life years; STR, single-tablet regimen.
As shown in the Tornado diagram, the ICER increased when baseline CD4 cell count, gEFV price discount, percentage of patients suppressed at 48 weeks for EFV-based gMTR and odds ratio of ≥95% adherence are assumed higher than in the base case. The opposite is true for a higher percentage decrease in hospitalizations on STR vs. MTR, higher standardized mortality ratios and higher quality of life utilities.
Considering second-line therapy effectiveness equal to that considered in Walensky et al. (2013) [7] decreased the ICER to $23,895 per QALY. Allowing hospitalization costs to be driven exclusively by CD4 counts for both STR and MTR results in an ICER of $37,438 per QALY. Considering a different source of inpatient and other costs [10] similarly increases the ICER to $35,521 per QALY. Finally, assuming, upon failing 6th-line therapy, a successive sequence of further therapy lines with efficacy, safety and cost parameterization equal to that of the 6th-line, results in a dominant scenario for STR as compared to gMTR: an increase of 0.49 discounted QALY, accompanied by $14,348 in discounted total cost savings.
The cost-effectiveness results were most sensitive to baseline CD4+ T cell count, generic EFV price (% discount from branded EFV price) and 48 weeks virologic suppression rates for EFV-based gMTR. Sensitivity analysis to the values of other variables like the odds ratio of ≥95% adherence between STR and MTR, hospitalizations rates, second line-treatment effectiveness, standardized mortality ratios, quality of life utilities and costs revealed minor uncertainty in the range of 2% to 12% relative to baseline ICER.
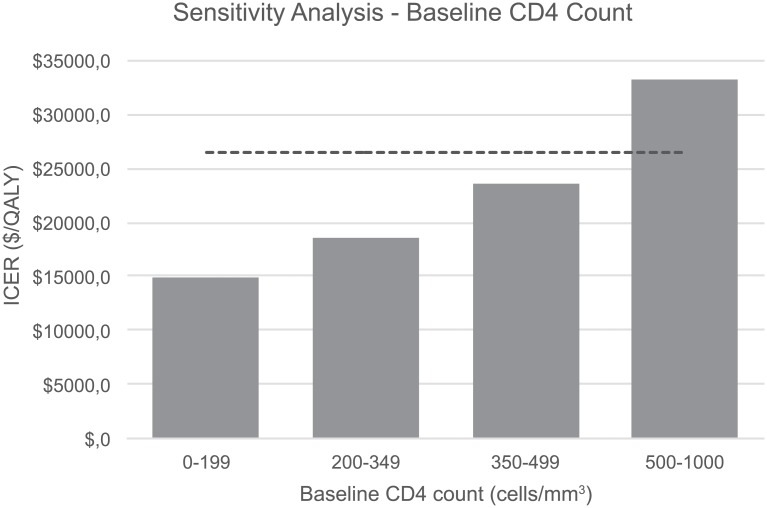
The assumption about the value of baseline CD4 count had the most impact in the variability of the ICER of STR over gMTR (Fig 6). It ranged between $14,911/QALY and $33,211/QALY in patients with baseline CD4 count of 0–199 cells/mm3 to 500–1000 cells/mm3, respectively.
Fig 6. One-way sensitivity analysis for baseline CD4 count by class.
ICER, incremental cost-effectiveness ration; QALY, quality adjusted life years.
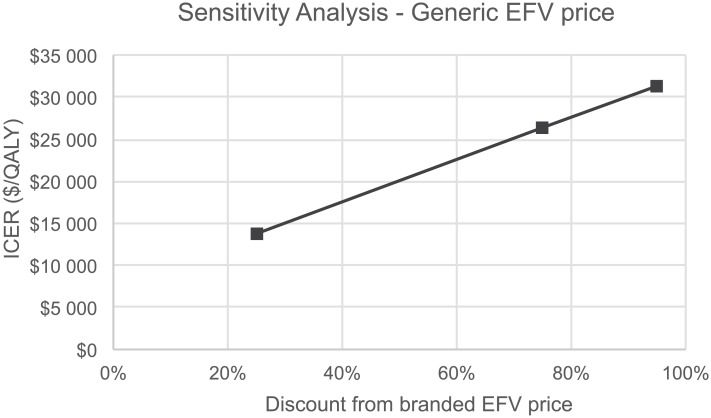
The base case analysis already incorporated a substantial discount of 75% on the price of generic relative to branded efavirenz. Varying the price generic efavirenz up to a 95% discount was associated with a maximum ICER of $31,421/QALY (Fig 7). This value is still well below commonly used cost-effectiveness thresholds in US [45].
Fig 7. One-way sensitivity analysis for generic efavirenz price reduction.
EFV, efavirenz; ICER, incremental cost-effectiveness ration; QALY, quality adjusted life years.
Discussion
In this study we introduce a comprehensive model to assess the lifetime health and economic outcomes of HIV infected patients initiating STRs compared with MTRs including generic medications where possible. STRs considered are those composed of TDF/FTC and efavirenz or rilpivirine or elvitegravir/cobicistat. MTRs considered included the counterparts to STRs substituting emtricitabine by generic lamivudine (g3TC) and branded efavirenz by the generic. The underlying motivation was to understand the value for money of branded STRs in comparison to MTRs composed of generic medications available in the U.S. market.
It is estimated that initial treatment for HIV infection with STRs would result in an enhanced life expectancy of 1.3 years (undiscounted) in comparison to use gMTRs. Lifetime health care costs are estimated to be higher with STR and partially offset by higher inpatients costs expected with gMTR treatment. We estimate a mean increment of $16,336 in lifetime health care costs with STR treatment over gMTR corresponding into an incremental cost of $26,384 per QALY. A sensitivity analysis to the key parameters of the model showed low level of uncertainty with ICER ranging from $13,790 to $33,211 per QALY.
Our estimates of discounted lifetime medical costs of $547,540 in HIV-infected individuals starting up-to-date STRs are comparable with other recently published model based estimates ($591,400) using a similar set of assumptions in patients initiating standard of care [46]. Also, mean life expectancy of 65.4 years and discounted quality adjusted life years of 14.5 QALY after ART initiation at the age of 43 years predicted by our model are within the range of previous research suggesting life expectancy values between 63 and 68.5 years and gains of 12.5 to 16.4 QALY [7, 46, 47]. Nonetheless, substantially lower discounted lifetime treatment costs ranging from $326,500 to $342.800 can be found in other studies, contrasting with our results [7, 47]. These differences may be explained by the non-inclusion of costs other than ART [7] or different assumptions about ART adherence or the risk of mortality modeling leading to a likely underestimation of lifetime medical costs [47].
Our study is subject to some limitations. The gMTR comparator in our study includes generic efavirenz and lamivudine in substitution of branded efavirenz and emtricitabine of EFV/TDF/FTC, RPV/TDF/FTC and EVG/COBI/TDF/FTC single-tablet regimens. In the literature, no direct evidence was found comparing these STRs and an MTR of RPV or EVG/COBI. In this case a simplifying assumption was made assuming the same relative measure of efficacy and safety between gEFV+TDF+g3TC and EFV/TDF/FTC (Study GS-236-102 and Study 903) [18–20, 23, 24], without performing any indirect comparison, nor controlling for any imbalances in study populations or trial protocols and definitions. This assumption was assessed in sensitive analysis by changing the virologic suppression rate for EFV-based MTR, which changes the odds ratios.
Although the gMTR comparators are not recommended options by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents [48], as the present analysis aimed at highlighting the losses associated with strategies driven by costs, this means that non-recommended strategies could be selected, as long as they lead to short-term cost-savings. In fact, some authors already reported the utilization of generics as a strategy to reduce costs with ART medication [6]. Other limitation that could be pointed is the non-inclusion of the new STR composed of dolutegravir, abacavir and lamivudine (DTG/ABC/3TC) as a first-line option. This is easily explained by the recent marketing of this STR which was not already in the market at the time of the model implementation. Nevertheless, besides DTG/ABC/3TC still having a low market share, if it was considered in the model it would only improve the results as it is a regimen with only one generic substitute, unlike EFV/TDF/FTC which has two.
Reported efficacy was assumed valid irrespective of the high levels of adherence observed and different measurement techniques used in studies GS-236-102 and 903. In first- and subsequent-line therapies no pooling of efficacy and safety data was performed if different studies were identified reporting on the same ART regimen. Virologic failure probabilities calibrated on our study are relatively low as compared to virologic failure probabilities of other first- and subsequent-line regimens. This can be expected to influence the average time spent in first-line therapy and in consequence, lifetime results.
Because little is known about the adherence-effectiveness relationship in integrase inhibitor (INI) based regimens for modeling purpose this relationship was based on results of populations mainly treated with EFV-based therapy (>60%) and variable NRTI-backbones [37, 49]. Differential adherence between STRs and gMTRs is assumed to influence virologic response for first-line therapy only. It has been suggested, however, that non-adherence to second-line regimens is associated with non-adherence to first-line regimens [50].
The STR associated benefit on adherence over MTR is based on Sweet et al. (2014) [17] and Sax et al. (2012) [4], who compare adherence of STR to adherence of any MTR, including not only generic based equivalent drugs of the STR, but also regimens with other drugs. As such, it was assumed that the observed STR benefit on adherence results from the STR formulation, and not, for instance, from a potentially better tolerability profile of the STR components as compared to the components of the regimens included in the MTR considered in Sweet et al. (2014) [17] and Sax et al. (2012) [4]. As more reliable adherence data become available, these could robustify the current analysis.
Patients discontinuing sixth-line therapy due to virologic failure or other reasons move to a seventh-line, where they are modelled as being in an ART-free non-suppressive state, with HIV-1 RNA viral load levels increasing from their highest value ever observed, and CD4+ T cell counts decreasing from their lowest value ever observed, until death. One might question the assumption used to model this seventh-line, in that it is not clear if patients failing multiple therapy-lines in reality move to an ART-free state. Furthermore, depending on the average time spent in previous therapy-lines and age reached when entering this seventh-line, its influence on overall results might be larger than intended. This was dealt with in a sensitivity analysis, assuming a successive sequence of further therapy lines with efficacy, safety and cost parameterization equal to that of the sixth-line therapy.
Due to the computational expensiveness of the model, traditional probabilistic sensitivity analysis through a large number of model runs at different random input values was deemed unfeasible. Solutions to this problem have been suggested in the literature, a common one of which is through regression meta-modelling [51]. The application of these types of methods was outside of the scope of the current project and will be dealt with in future research.
Consistent with other model estimates [52, 53] and real-world economic analyses [54] we also found that STR is a cost-effective therapeutic option in comparison to gMTR. Following Italian guidelines for the initial HIV treatment, Colombo et al. (2011) found that TDF/FTC/EFV one pill per day was the most cost-effective treatment strategy, compared with the other recommended therapeutic regimens, being also cost-effective (ICER €9,189/QALY) relative to other MTR with lower strength of evidence like TDF + 3TC + EFV [52]. A review conducted by the World Health Organization suggests for the clinical interchangeability of FTC and 3TC, but does not pronounce itself on their interchangeability when comparing single to multiple tablet regimens [55]. It further alerts for the fact that development of M184V/I mutations is associated to a greater extent with the use of a 3TC rather than FTC regimen, though the clinical implications of this difference are difficult to predict [55].
Most notably, real-word evidence suggests cost-savings in HIV-infected patients starting ART with STR in addition to health gains [12, 54, 56]. In a study assessing outcomes and costs of ART as a once-daily STR (n = 1,797) or two or more pills per day (MTR, n = 5,584) of Medicaid patients with an HIV diagnosis from 2005 to 2009 the authors report significantly lower total healthcare costs for STR patients (monthly costs of $2,959 vs $3,544 in MTR, p<0.001) due to lower pharmacy costs, fewer hospitalizations and lower hospital costs, explained by lower pill burden and enhanced adherence with STR [12]. An incremental cost-effectiveness analysis of efavirenz, tenofovir, and emtricitabine as a single-tablet regimen versus a multi-pill regimen, with reference to untreated HIV-infected patients, from the perspective of the Italian National Health Service, identified that a 24% price decrease would be needed for MTR to be comparable with STR in terms of the same ICER relative to no treatment [53].
In contrast to this evidence and to our results is the study by Walensky et al. (2013) estimating that the incremental cost-effectiveness for first-line branded STR in United States is over $100,000/QALY relative to gMTR. The authors suggest that starting or switching to generic based regimens would initially yield annual savings approaching $1 billion for programs that fund HIV treatment in the United States, at the expense of a slightly less effective generic alternative (1 QALY loss per patient). But because non-medication costs and the costs for other medications were not included, and adherence to ART was not explicitly modeled, we anticipate that these results may be subject to a high degree of uncertainty and potentially underestimate the total costs associated with gMTR. In fact, breaking down once-daily fixed-dose ART into their single components and switching emtricitabine by less efficacious lamivudine [57] may lead to lower adherence and virologic suppression [7]. Higher pill burden is associated with both significantly lower adherence rates and worse virological suppression [58]. Poor control of HIV [59], greater pill burden [12] and lower adherence [4] are all associated with higher risk of hospitalization and higher inpatient costs and other non-ART costs that may account up-until 45% of total lifetime costs of current HIV Care [10, 47].
Under these circumstances, until a once-a-day STR generic version is available it is likely that current gMTR possibilities may still be unattractive under contemporary cost-effectiveness thresholds ($100,000/QALY) [45], and may not provide sufficient economic savings to justify the permanent health losses, raising significant legal and ethical questions [60].
It should be noted that at the time of analysis in the U.S. no generic fixed dosed combinations (FDCs) were marketed yet. The current analysis is only applicable to scenarios where switching from an STR to generics means a therapy complication. In countries where generic FDCs are available, differences between generic and non-generic STRs will mainly be due to costs differences between both.
In conclusion, our modeled analysis demonstrates that STRs provided to HIV-1-infected treatment-naive individuals is cost-effective compared with gMTRs. We hope the findings from this study will provide further insight and help guide decisions.
Supporting Information
(DOCX)
Acknowledgments
The authors would like to thank the reviewers for their valuable comments, Analysis Group Inc. and Exigo Consultores for the economic modeling and Jorge Félix and Marta Afonso-Silva, from Exigo Consultores for the writing assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Gilead Sciences, Foster City, CA. DES, FLA, CJC and BV (prior to January 2015) are employees of independent academic institutions, research organizations or consulting firms and maintained independent scientific control over the study, including data analysis and interpretation of final results. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The funder provided support in the form of salaries (after January 2015) for CJC. Gilead contracted with Exigo Consultores for the development of the model. Exigo Consultores provided support in the form of salaries for BV, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4): 387–402. 10.1001/jama.2012.7961 [DOI] [PubMed] [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the Gap: Increases in Life Expectancy among Treated HIV-Positive Individuals in the United States and Canada. PLoS ONE. 2013;8(12): e81355 10.1371/journal.pone.0081355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2): 87–95. [DOI] [PubMed] [Google Scholar]
- 4.Sax PE, Meyers JL, Mugavero M, Davis KL. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7(2): e31591 10.1371/journal.pone.0031591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sloan CE, Champenois K, Choisy P, Losina E, Walensky RP, Schackman BR, et al. Newer drugs and earlier treatment: impact on lifetime cost of care for HIV-infected adults. AIDS. 2012;26(1): 45–56. 10.1097/QAD.0b013e32834dce6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazzard B, Moecklinghoff C, Hill A. New strategies for lowering the costs of antiretroviral treatment and care for people with HIV/AIDS in the United Kingdom. Clinicoecon Outcomes Res. 2012;4: 193–200. 10.2147/CEOR.S12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walensky RP, Sax PE, Nakamura YM, Weinstein MC, Pei PP, Freedberg KA, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med. 2013;158(2): 84–92. 10.7326/0003-4819-158-2-201301150-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebo KA, Fleishman JA, Conviser R, Hellinger J, Hellinger FJ, Josephs JS, et al. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24(17): 2705–2715. 10.1097/QAD.0b013e32833f3c14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44(11): 990–997. [DOI] [PubMed] [Google Scholar]
- 10.Solem CT, Snedecor SJ, Khachatryan A, Nedrow K, Tawadrous M, Chambers R, et al. Cost of treatment in a US commercially insured, HIV-1-infected population. PLoS One. 2014;9(5): e98152 10.1371/journal.pone.0098152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee FJ, Amin J, Carr A. Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks' follow-up. PLoS One. 2014;9(5): e97482 10.1371/journal.pone.0097482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIV. BMJ Open. 2013;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guria J, Leung J, Jones-Lee M, Loomes G. The Willingness to Accept Value of Statistical Life Relative to the Willingness to Pay Value: Evidence and Policy Implications. Environmental & Resource Economics. 2005;32: 113–127. [Google Scholar]
- 14.Gilead. Market share data, quarter 4. 2013.
- 15.Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296(12): 1498–1506. [DOI] [PubMed] [Google Scholar]
- 16.FDA. Emtricitabine/rilpivirine/tenofovir disoproxil fumarate full prescribing information. 2011.
- 17.Sweet D, Kim Y, Zhong Y, Zhuo D, Signorovitch J. Real-World Adherence among Patients Receiving Single versus Multiple Tablet Regimens for HIV-1 Infection, and Associations between Adherence and Viral Suppression: A Systematic Literature Review and Meta- Analysis. 20th International AIDS Conference Melbourne, Australia, 2014; 2014.
- 18.Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835): 2439–2448. 10.1016/S0140-6736(12)60917-9 [DOI] [PubMed] [Google Scholar]
- 19.Wohl DA, Cohen C, Gallant JE, Mills A, Sax PE, Dejesus E, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. Journal of acquired immune deficiency syndromes. 2014;65(3): e118–120. 10.1097/QAI.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 20.Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. Journal of acquired immune deficiency syndromes. 2013;63(1): 96–100. 10.1097/QAI.0b013e318289545c [DOI] [PubMed] [Google Scholar]
- 21.Cohen C, Wohl D, Arribas JR, Henry K, Van Lunzen J, Bloch M, et al. Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected adults. AIDS. 2014;28(7): 989–997. 10.1097/QAD.0000000000000169 [DOI] [PubMed] [Google Scholar]
- 22.Porter DP, Kulkarni R, Fralich T, Miller MD, White KL. Characterization of HIV-1 drug resistance development through week 48 in antiretroviral naive subjects on rilpivirine/emtricitabine/tenofovir DF or efavirenz/emtricitabine/tenofovir DF in the STaR study (GS-US-264-0110). Journal of acquired immune deficiency syndromes. 2014;65(3): 318–326. 10.1097/QAI.0000000000000017 [DOI] [PubMed] [Google Scholar]
- 23.Cassetti I, Madruga JV, Suleiman JM, Etzel A, Zhong L, Cheng AK, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV clinical trials. 2007;8(3): 164–172. [DOI] [PubMed] [Google Scholar]
- 24.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2): 191–201. [DOI] [PubMed] [Google Scholar]
- 25.Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19(2): 153–162. [DOI] [PubMed] [Google Scholar]
- 26.Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20(5): 711–718. [DOI] [PubMed] [Google Scholar]
- 27.Clotet B, Bellos N, Molina JM, Cooper D, Goffard JC, Lazzarin A, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369(9568): 1169–1178. [DOI] [PubMed] [Google Scholar]
- 28.Katlama C, Esposito R, Gatell JM, Goffard JC, Grinsztejn B, Pozniak A, et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS. 2007;21(4): 395–402. [DOI] [PubMed] [Google Scholar]
- 29.Haubrich R, Berger D, Chiliade P, Colson A, Conant M, Gallant J, et al. Week 24 efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients. AIDS. 2007;21(6): F11–18. [DOI] [PubMed] [Google Scholar]
- 30.Arasteh K, Yeni P, Pozniak A, Grinsztejn B, Jayaweera D, Roberts A, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antiviral therapy. 2009;14(6): 859–864. [DOI] [PubMed] [Google Scholar]
- 31.Janssen. POWER 1 and 2: 96-Week Pooled Analysis 2015 [19 March 2015]. Available: http://www.prezista.com/healthcare/claims/power-study.
- 32.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893): 700–708. 10.1016/S0140-6736(13)61221-0 [DOI] [PubMed] [Google Scholar]
- 33.Pozniak A, Mingrone H, Shuldyakov A, Brites C, Federico Andrade-Villanueva J, Hagins D, et al. editors. Dolutegravir (DTG) Versus Raltegravir (RAL) in ART-Experienced, Integrase-Naive Subjects: 24-Week Interim Results From SAILING (ING111762) 20th Conference on Retroviruses and Opportunistic Infections; 2013; Atlanta, GA
- 34.Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3): 354–362. Epub 2014 Jan 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson M, Arasteh K, Clotet B, Cooper DA, Henry K, Katlama C, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. Journal of acquired immune deficiency syndromes. 2005;40(4): 404–412. [DOI] [PubMed] [Google Scholar]
- 36.Trottier B, Walmsley S, Reynes J, Piliero P, O'Hearn M, Nelson M, et al. Safety of enfuvirtide in combination with an optimized background of antiretrovirals in treatment-experienced HIV-1-infected adults over 48 weeks. Journal of acquired immune deficiency syndromes. 2005;40(4): 413–421. [DOI] [PubMed] [Google Scholar]
- 37.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8): 564–573. [DOI] [PubMed] [Google Scholar]
- 38.CDC. United States Life Tables, 2010. National Vital Statistics Reports. 2014;63(7). [PubMed] [Google Scholar]
- 39.Sabin CA, Devereux H, Phillips AN, Hill A, Janossy G, Lee CA, et al. Course of viral load throughout HIV-1 infection. Journal of acquired immune deficiency syndromes. 2000;23(2): 172–177. [DOI] [PubMed] [Google Scholar]
- 40.Cook J, Dasbach E, Coplan P, Markson L, Yin D, Meibohm A, et al. Modeling the long-term outcomes and costs of HIV antiretroviral therapy using HIV RNA levels: application to a clinical trial. AIDS research and human retroviruses. 1999;15(6): 499–508. [DOI] [PubMed] [Google Scholar]
- 41.Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers (CPI-U): U. S. city average, by expenditure category 2015 [cited 2015]. Available: http://www.bls.gov/news.release/cpi.t01.htm.
- 42.Hill AM, Cho M, Mrus JM. The costs of full suppression of plasma HIV RNA in highly antiretroviral-experienced patients. AIDS reviews. 2011;13(1): 41–48. [PubMed] [Google Scholar]
- 43.Rao G, Sutton S, Hardin J, Bennett C, editors. Impact of HAART Regimen on Adherence and Risk of Hospitalization in HIV/AIDS Patients Using VA Data ICAAC; 2013. September 10–13; Denver, CO. [Google Scholar]
- 44.Kauf TL, Roskell N, Shearer A, Gazzard B, Mauskopf J, Davis EA, et al. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2008;11(7): 1144–1153. [DOI] [PubMed] [Google Scholar]
- 45.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9): 796–797. 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 46.Sax PE, Sypek A, Berkowitz BK, Morris BL, Losina E, Paltiel AD, et al. HIV cure strategies: how good must they be to improve on current antiretroviral therapy? PLoS One. 2014;9(11): e113031 10.1371/journal.pone.0113031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schackman BR, Fleishman JA, Su AE, Berkowitz BK, Moore RD, Walensky RP, et al. The lifetime medical cost savings from preventing HIV in the United States. Med Care. 2015;53(4): 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DHHS. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2015.
- 49.Gardner EM, Hullsiek KH, Telzak EE, Sharma S, Peng G, Burman WJ, et al. Antiretroviral medication adherence and class- specific resistance in a large prospective clinical trial. AIDS. 2010;24(3): 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB. Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. The Journal of infectious diseases. 2013;207 Suppl 2: S49–56. 10.1093/infdis/jit107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Hagan A. Bayesian analysis of computer code outputs: A tutorial. Reliability Engineering & System Safety. 2006;91(10–11): 1290–1300. [Google Scholar]
- 52.Colombo GL, Colangeli V, Di Biagio A, Di Matteo S, Viscoli C, Viale P. Cost-effectiveness analysis of initial HIV treatment under Italian guidelines. Clinicoecon Outcomes Res. 2011;3: 197–205. 10.2147/CEOR.S24130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colombo GL, Di Matteo S, Maggiolo F. Antiretroviral therapy in HIV-infected patients: a proposal to assess the economic value of the single-tablet regimen. Clinicoecon Outcomes Res. 2013;5: 59–68. 10.2147/CEOR.S38977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maggiolo F, Colombo GL, Di Matteo S, Bruno GM, Astuti N, Di Filippo E, et al. Cost-effectiveness analysis of antiretroviral therapy in a cohort of HIV-infected patients starting first-line highly active antiretroviral therapy during 6 years of observation. Patient related outcome measures. 2015;6: 53–60. 10.2147/PROM.S63586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.WHO. Pharmacological equivalence and clinical interchangeability of lamivudine and emtricitabine: a review of current literature. 2012.
- 56.Colombo GL, Castagna A, Di Matteo S, Galli L, Bruno G, Poli A, et al. Cost analysis of initial highly active antiretroviral therapy regimens for managing human immunodeficiency virus-infected patients according to clinical practice in a hospital setting. Therapeutics and clinical risk management. 2014;10: 9–15. 10.2147/TCRM.S49428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rousseau FS, Wakeford C, Mommeja-Marin H, Sanne I, Moxham C, Harris J, et al. Prospective randomized trial of emtricitabine versus lamivudine short-term monotherapy in human immunodeficiency virus-infected patients. The Journal of infectious diseases. 2003;188(11): 1652–1658. [DOI] [PubMed] [Google Scholar]
- 58.Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(9): 1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnett PG, Chow A, Joyce VR, Bayoumi AM, Griffin SC, Nosyk B, et al. Determinants of the cost of health services used by veterans with HIV. Med Care. 2011;49(9): 848–856. 10.1097/MLR.0b013e31821b34c0 [DOI] [PubMed] [Google Scholar]
- 60.Ramiro MA, Llibre JM. Legal, ethical, and economic implications of breaking down once-daily fixed-dose antiretroviral combinations into their single components for cost reduction. Enfermedades infecciosas y microbiologia clinica. 2014;32(9): 598–602. 10.1016/j.eimc.2013.06.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.