Abstract
The majority of vaccine candidates in clinical development are highly purified proteins and peptides relying on adjuvants to enhance and/or direct immune responses. Despite the acknowledged need for novel adjuvants, there are still very few adjuvants in licensed human vaccines. A vast number of adjuvants have been tested pre-clinically using different experimental conditions, rendering it impossible to directly compare their activity. We performed a head-to-head comparison of five different adjuvants Alum, MF59®, GLA-SE, IC31® and CAF01 in mice and combined these with antigens from M. tuberculosis, influenza, and chlamydia to test immune-profiles and efficacy in infection models using standardized protocols. Regardless of antigen, each adjuvant had a unique immunological signature suggesting that the adjuvants have potential for different disease targets. Alum increased antibody titers; MF59® induced strong antibody and IL-5 responses; GLA-SE induced antibodies and Th1; CAF01 showed a mixed Th1/Th17 profile and IC31® induced strong Th1 responses. MF59® and GLA-SE were strong inducers of influenza HI titers while CAF01, GLA-SE and IC31® enhanced protection to TB and chlamydia. Importantly, this is the first extensive attempt to categorize clinical-grade adjuvants based on their immune profiles and protective efficacy to inform a rational development of next generation vaccines for human use.
Beyond doubt, the advent of sequencing, rendering microbial genomes readily accessible, has been of utmost importance for vaccinology and has allowed for highly rational identification of vaccine antigens. Many novel vaccines currently in development are thus based on proteins or peptides predicted by computer databases or by screening antigen libraries1,2. Although these advances in the post-genomic era have enabled the design of highly pure, safe and simple vaccines, other challenges have emerged in parallel, including the inherent lack of immunostimulatory properties of proteins and peptides. Vaccine adjuvants are therefore considered key components in modern vaccinology since they provide the necessary help of enhancing the immune responses. In addition, adjuvants have many other favorable features including the ability to overcome immune senescence in elderly3, prolonging memory of the vaccines4, broadening the antibody repertoire5 and providing means for dose-sparing6. Since the adjuvants currently licensed for human-use almost exclusively induce antibody responses, there is a need for continued adjuvant research and development with the aim of expanding the repertoire of human adjuvants with the ability to induce cell-mediated immunity (CMI).
Early work in adjuvant discovery has been highly empirical and little in depth mechanistic insights is available for the adjuvants currently used in human vaccines. The scarce mechanistic insight is illustrated by the fact that although aluminum salt (commonly referred to as Alum) adjuvants have been used in a range of different vaccines in humans since the 1920s, their mechanism of action remains incompletely understood7. The growing insights into innate immunology and the development of a range of novel technical tools have provided a unique opportunity to take adjuvant research and development to a new level, avoiding previous empirical trial-and-error approaches. The recent clinical testing of several novel exploratory adjuvants with the ability to generate CMI responses8,9 clearly indicates that it is plausible to expand the range of adjuvants available for human use.
In addition to improving the vaccine technology, there is also an urgent need for a more rational preclinical development of adjuvants and better tools for predicting the protective potential of an adjuvant for a given antigen and/or disease target. However, many studies aimed at investigating the potential of novel adjuvants, are carried out with a range of different protocols, different antigens and in many cases with a lack of appropriate comparators e.g. Alum. Studies are also often carried out with non-clinically relevant model antigens, e.g. ovalbumin (OVA), or without animal challenge experiments, which could otherwise provide information on the efficacy. Furthermore, the problems with gaining access to adjuvants currently in clinical development make it difficult to perform comparisons with adjuvants for which we already know the immunological profile in humans. All of these parameters render it impossible to obtain an overview of the potency of different adjuvants and appreciate whether it is worthwhile to pursue further clinical development of a novel adjuvant, hence leaving the adjuvant field fragmented and dispersed10. This could potentially undermine valuable information about candidate adjuvants that could otherwise lead to identification of novel viable adjuvants for clinical development. Furthermore, this may lead to overlooking adjuvants with very distinct immunological profiles, which are markedly different from those already filling the clinical pipeline.
Herein, we performed a detailed comparison of five different clinical adjuvants in three different disease models (tuberculosis (TB), chlamydia, and influenza) using a standardized protocol that allowed comparison of results across different antigens and adjuvants. The main objectives of this study were to dissect and compare the immunological profile of these adjuvants and to test the efficacy in very different disease models with different requirements for protection; TB, which primarily requires a Th1 profile11, chlamydia for which an antibody response with a mixed Th1/Th17 profile is critical12, and influenza where measurement of antibody responses are accepted as a correlate of protection13. The proprietary adjuvants included in this study were obtained from partners involved in the EU-funded large collaborative project Advanced Immunization Technologies (ADITEC), and have the common denominator of already being advanced to clinical development (IC31®, CAF01, GLA-SE) or included in registered human vaccines (MF59® and Alum). This provided the possibility of comparing the mouse data reported herein to human data obtained from the clinical studies. The five adjuvants gave rise to very distinct immunological signatures irrespective of the target antigen tested, suggesting that the adjuvants when used together with antigens have potential in induction of protection against highly different diseases. These results on categorization of different clinical-grade adjuvants with proven excellent safety based on their immune profiles and their ability to mount protective immunity, lays the foundation for development of next-generation vaccines for human use.
Materials and Methods
Animals
Female CB6F1 (TB and influenza) and B6C3F1 (chlamydia), 6–8 weeks old, were ordered from Harlan Laboratories (The Netherlands, TB and chlamydia) or Charles River (Italy, influenza). TB- and chlamydia-infected mice were housed in animal facilities at Statens Serum Institut, Denmark, while influenza experiments were performed at Novartis Vaccines and Diagnostics s.r.l., Italy. Mice were provided standard food and water ad libitum. TB-infected animals were housed in a biosafety level 3 facility in cages contained within laminar flow safety enclosures (Scantainer, Scanbur).
Ethics Statement
The use of mice was conducted in accordance with the regulations set forward by the respective national animal protection committees and in accordance with European Community Directive 86/609 and the U.S. Association for Laboratory Animal Care recommendations for the care and use of laboratory animals. All the techniques/procedures have been refined to provide for maximum comfort/minimal stress to the animals. Experiments performed at Statens Serum Institut have been approved by the governmental Animal Experiments Inspectorate under licenses 2014-15-2934-01065 (TB) and 2013-15-2934-00978 (chlamydia), while experiments performed at Novartis Vaccines and Diagnostics s.r.l. have been approved by the Ministry of Health under license AEC201111.
Antigens
H56
H56 (Ag85B-ESAT6-Rv2660c) was recombinantly produced in E. coli K12 containing the H56 expression vector under IPTG induction, after which the recombinant H56 was harvested as inclusion bodies from disrupted host cells by centrifugation. The inclusion bodies were dissolved in 8 M Urea, 10 mM cysteine, pH 6 and filtered through 0.22 μm filters. The recombinant protein was purified using a two-stage ion exchange scheme (Sartobind® Q SingleSep and S SingleSep), at pH 4.9 and 6.0 respectively) after which the buffer was switched to 20 mM glycine, pH 8.8 using a tangential flow filtration system (Merck Millipore). Protein purity was assessed by SDS-PAGE followed by coomassie staining and western blot with anti-H56 (Polyclonal, rabbit serum) and anti-E. coli antibodies (DAKO) to detect contaminants. Furthermore, the protein was validated for residual DNA, endotoxins and bioburden in accordance with internal GMP standards.
CT681
A synthetic DNA construct containing ompA (CT681 – AA23-AA349, leaving out the signal peptide) was codon-optimized for expression in E. coli followed by insertion into the pJexpress 411 vector (DNA2.0), including a N-terminal histidine tag. Following protein expression, the protein was initially purified by metal chelate affinity chromatography as described in Follmann et al. 200814 and subsequently purified by IEC using HiTrap Q Sepharose HP (GE Healthcare). Purified rMOMP was refolded by a stepwise removal of buffer containing urea ending up in 20 mM Tris, 150 mM NaCl, 10% glycerol pH 8 which yielded soluble protein. Protein purity was assessed by SDS-PAGE followed by coomassie staining and western blot with anti-penta-His (Qiagen) and anti-E. coli antibodies to detect contaminants (DAKO). The Limulus Amebocyte Lysate test was used to determine the amount of endotoxin in the rMOMP preparation and was below 10 EU/mg.
Hemagglutinin (HA)
Influenza A/California/2009 (H1N1) subunit vaccine was produced by Novartis Vaccines. The vaccine was derived from embryonated eggs applying production procedures followed for clinical grade influenza vaccines and its major constituent is hemagglutinin (HA) from the indicated influenza strain.
Adjuvants
CAF01 from Statens Serum Institut (Copenhagen, Denmark), IC31® from Valneva Austria GmbH (previously Intercell AG, Vienna, Austria), GLA-SE from the Infectious Diseases Research Institute (Seattle, WA, United States), MF59® from Novartis Vaccines (Siena, Italy), Aluminium hydroxide (Al(OH)3) (2% Alhydrogel), from Brenntag Biosector (Frederikssund, Denmark) was used in the TB and Chlamydia experiment, while the Aluminium hydroxide suspension used in the Influenza experiment was produced at Novartis Vaccines and Diagnostics (Marburg, Germany).
Immunization
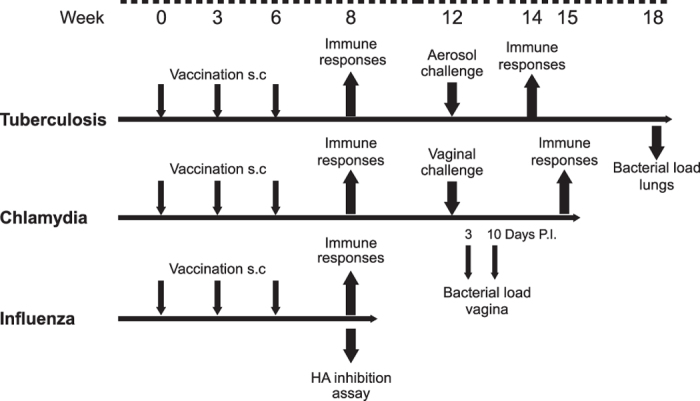
Mice were immunized subcutaneously (s.c.) at the base of the tail three times at three-week intervals. Adjuvants were mixed with 5 μg recombinant antigen or 1 μg of HA by vortexing to a final volume of 200 μl for each injection. CAF01 (dose 250 μg/50 μg (DDA/TDB)/200 μl)15, IC31® (dose 100 nmol/4 nmol (KLK/ODN1a)/200 μl)16, GLA-SE (dose 5 μg GLA and 2% v/v squalene in 200 μl)17, Alhydrogel 2.0% (dose 500 μg aluminum content /200 μl)18, MF59® (dose of 100 μl 4.3% w/v squalene, 0.5% w/v Tween 80, 0.5% w/v Span 85 mixed 1:1 with PBS)19. Control mice received three injections of 5 μg recombinant antigen or 1 μg HA in 200 μL PBS, respectively, while negative controls received three injections of sterile PBS (pH 7.4). The study designs for the three disease models are shown in Fig. 1.
Figure 1. Study design for the animal experiments.
ELISA for antigen-specific serum antibodies
Maxisorb Plates (Nunc) were coated with 0.05 μg/well H56 or MOMP in PBS overnight at 4 °C. Individual mouse sera from at least four mice per group were analyzed in duplicate, ten- (IgG1) or fivefold (IgG2a) dilution series, at least 8 times in PBS with 2% BSA, starting with a 20-fold dilution. HRP-conjugated secondary antibodies (rabbit anti-mouse IgG1 and IgG2a; Zymed) was diluted 1:2000 in PBS with 1% BSA. After 1 h of incubation, antigen-specific antibodies were detected using TMB substrate as described by the manufacturer (Kem-En-Tec Diagnostics). The absorbance values were plotted as a function of the reciprocal dilution of serum samples. Reciprocal plasma dilutions corresponding to a cut-off of 0.2 OD450 were computed using Excel (v.15.0.4693.1000). HA specific serum antibodies were determined by a two-step fully automated rapid ELISA (Hamilton Starlet System, Switzerland) with individual sera to titrate total HA specific IgG, IgG1 and IgG2 as described previously20.
Lymphocyte cultures, IL-17 ELISA and Flow cytometry for MOMP and H56
Peripheral blood mononuclear cells (PMBC) and splenocytes were isolated as described previously21,22. Cultures were adjusted to 2 × 105 cells/well (MSD/ELISA) or 1–2 × 106 cells/well in a total volume of 200 μl/well (IC-FACS) and stimulated with antigens at a final concentration of 2 μg/ml, whereas Con A was used at a concentration of 1 μg/ml as a positive control for cell viability. Culture supernatants were harvested after 72 h of incubation for the investigation of Multiplex cytokine assay and IL-17 ELISA (as previously described23). Splenocytes were stimulated at 37 °C in the presence of recombinant antigen (2 μg/ml) for 1 hour, and subsequently incubated for 5 hours after adding 10 μg/ml BFA (Sigma-Aldrich) and stained as previously described22. Responses were analyzed using a FACSCanto flow cytometer (BD Biosciences) and FlowJo v.10.0.7 (Tree Star Inc.).
Intra-cellular flow cytometry staining for splenocytes stimulated in vitro with HA antigen
Spleens were harvested and filtered through a 70 μm nylon mesh (BD Biosciences) and stimulated with 1 μg/ml HA O/N and subsequently incubated for 4 hours with BFA (2.5 μg/ml) as described previously24. Briefly, cells were stained with combinations of the following antibodies: CD4-V500, CD44-V450, CD3-PerCP-Cy5.5, CD8-Texas Red®, IL-4 and IL-13 Alexa Fluor® 488, IL-2 APC, TNF-Alexa Fluor® 700, IFN-γ PE, IL-17 PE Cy7 and LIVE/DEAD® Fixable Aqua. Flow cytometry was performed on FACS LSRII instruments using DIVA software (BD Biosciences) and data were then analyzed using Flowjo software (Tree Star Inc.).
Multiplex cytokine assay
The proinflammatory panel 1 (Mouse) 7-plex cytokine assay (IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-10, TNF-α) or the Th1/Th2 panel (mouse) 9-plex cytokine assay (IFN-γ, IL-1b, IL-2, IL-4, IL-5, IL-10, TNF-α, IL-12) or the mouse IL-17 assay (all from Meso Scale Discovery) were performed according to the manufacturer’s instructions. The plates were read on the Sector Imager 2400 system or 6000 system (Meso Scale Discovery) and calculation of cytokine concentrations in unknown samples was determined by 4-parameter logistic non-linear regression analysis of the standard curve.
Mycobacterial challenge
Six weeks after the third immunization animals were infected with Mycobacterium tuberculosis Erdman (ATCC) by the aerosol route as described previously15. Six weeks after infection, mice were sacrificed and organs homogenized in PBS for bacterial enumeration as described previously in Ref. 15.
Chlamydia challenge
Six weeks after the final vaccination the mice were challenged intra-vaginally with 4 × 105 inclusion forming units (IFU) Chlamydia trachomatis Serovar D (UW-3/Cx, ATCC VR-885) purchased from the American Type Culture Collection (ATCC) essentially as described previously15. The mice oestrus cycles were synchronized ten and 3 days before challenge by subcutaneous injection of 2.5 mg Medroxyprogesteronacetat (Depro-Provera; Pfizer). Vaginal swabs were obtained at day 3 and 10 after infection and analyzed as previously described18. Inclusions were enumerated by visual inspection using a fluorescence microscopy.
Influenza HA inhibition assay
Serum samples were pre-treated with neuraminidase (Biogenetics) overnight at 37 °C, and HI was tested on individual sera. Briefly, 25 μL of two-fold serially diluted samples were incubated with 25 μL of strain-specific influenza antigen (whole virus inactivated, containing four hemagglutinating units) for 60 min at room temperature. A 0.5% (v/v) suspension of adult turkey red blood cells was added and incubated for 60 min. Outcomes were determined by visual inspection: a red dot indicated a positive reaction (inhibition), and a diffuse patch indicated a negative reaction (hemagglutination). All sera were tested in duplicates. The titer was defined as the reciprocal of the serum dilution at which the last complete agglutination inhibition occurred.
Statistical analysis
Prism 6 software (GraphPad v6.05) was used for all statistical analyses. TB CFU, Chlamydia IFU, IgG and HA inhibition titers were log-transformed before analysis. TB CFU were analyzed using one way ANOVA with Dunnett’s multiple comparisons test. Chlamydia IFU, IgG and HA inhibition titers were analyzed using Kruskal-Wallis test followed by Dunn’s post-test. Statistically significant differences are marked by asterisks in figures and explained in the figure legends.
Results
A panel of diverse adjuvants for comparative testing
As part of the EU-funded large collaborative project Advanced Immunization Technologies (ADITEC), several partners contributed with their proprietary adjuvants to create a panel of highly diverse adjuvants already in clinic (Table 1). The panel included squalene-based oil-in-water emulsions (MF59® and GLA-SE – the latter combined with a synthetic TLR4 agonist), cationic peptide KLK combined with the TLR9-stimulatory oligodeoxynucleotide ODN1a (IC31®), cationic liposomes formed of dimethyldioctadecylammonium (DDA) stabilized with synthetic mycobacterial cord factor (trehalosedibehenate) signaling through the C-type lectin receptor Mincle (CAF01), and conventional Alum delivered in the form of a 500 μg aluminum-content dose of diluted 2.0% Alhydrogel. The adjuvants were combined with antigens from three pathogens; H56 is an engineered fusion of three Mycobacterium tuberculosis (M.tb.) antigens, Ag85B, ESAT-6 and Rv2660c, MOMP is the recombinantly produced major outer membrane protein of Chlamydia trachomatis (C.t.), and HA is the purified egg-derived hemagglutinin (HA) from A/California/7/2009 (H1N1) influenza strain.
Table 1. .
| Adjuvant | Category | Immune profile* | Stage of development | References |
|---|---|---|---|---|
| Alum | Insoluble aluminum salts | TH2, antibody | In several licensed products | 62,63 |
| MF59® | Squalene oil-in-water emulsion | (TH1), TH2, antibody | Licensed for use in influenza vaccine in EU | 64,65,66 |
| CAF01 | Cationic liposomes formulated with a Mincle agonist | TH1, TH17 | Phase 1 | 45,67 |
| GLA-SE | Squalene emulsion combined with a TLR4 agonist | TH1, antibody | Phase 2 | 9,68 |
| IC31® | Cationic antimicrobial polypeptides combined with a TLR9 agonist | TH1 | Phase 2 | 8,69 |
*Immune profile reported in the literature, see references.
Comparative evaluation of the humoral immune response induced by the different adjuvants
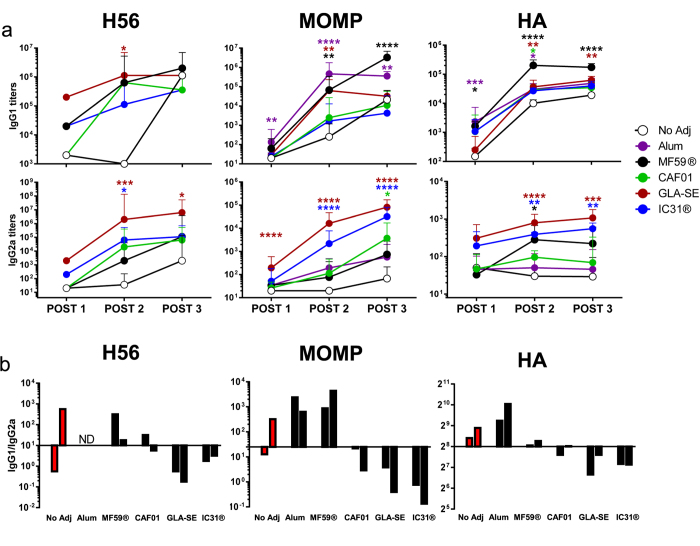
We first compared the ability of the five different adjuvants to induce humoral immune responses using the three model antigens. Groups of mice were vaccinated three times with H56, MOMP, or HA in combination with either CAF01, IC31®, GLA-SE, MF59® or Alum (H56 with Alum was not tested in this assay). Three vaccinations were chosen as previous optimization studies have shown this immunization regimen to induce stronger immune responses compared to two vaccinations using CAF01 (unpublished data) and GLA-SE25. Adding a fourth dose did not improve responses (unpublished data). Two weeks after each vaccination, the mice were partially bled and the vaccine-specific IgG1 and IgG2a antibody responses were measured in the serum using ELISA (Fig. 2). The kinetics of specific antibody induction after one, two or three vaccinations showed that GLA-SE in general induced the highest and earliest IgG2a antibody response, with a clear and detectable response after only a single vaccination (Fig. 2a). After the second vaccination, MF59® induced the highest IgG1 antibody response and was clearly superior to the other adjuvants in terms of generating HA-specific IgG1 titers in mice. Compared to vaccination with antigen H56 and MOMP alone (No Adj), there was a clear effect of adding adjuvants on the induction of IgG2a titers in particular after the second and third vaccination. In contrast, the benefit of adding adjuvants on the IgG1 titers was less evident and three vaccinations with recombinant H56 or MOMP alone showed comparable antibody titers as the adjuvanted groups, with the exception of MOMP combined with MF59® or Alum (P > 0.0001 and P > 0.01 compared to No Adj). HA titers followed a slightly different pattern with a more clear benefit of adding adjuvants on IgG1 titers, while the second and third vaccinations, even with adjuvants, had very limited boosting effect on the IgG2a antibody responses after the initial priming. This is in line with previously published data where adjuvanted influenza vaccines are prone towards IgG1 induction while inducing little IgG2a26. Common for all three antigens, after the second vaccination and particularly the third vaccination, an IgG1 bias was observed with the Th2-biased adjuvants Alum and MF59® (Fig. 2b), while the Th1-biased adjuvants GLA-SE and IC31® skewed the balance towards IgG2a. CAF01 had a slight bias towards IgG2a induction but with an overall more balanced IgG1/IgG2a ratio. When administered unadjuvanted, all three antigens induced IgG1 biased responses, which is in agreement with previously published data27,28,29.
Figure 2. Vaccine-specific humoral immune-responses after vaccination.
Groups of mice were immunized 3 times s.c. with H56, MOMP or HA formulated in Alum, MF59® CAF01, GLA-SE or IC31® with 3 week intervals (H56 with Alum was not tested in this assay). Two weeks after each vaccination serum samples from individual mice (n = 4 CB6F1 in TB, n = 12 B6C3F1 in chlamydia, n = 8 CB6F1 in influenza) were analyzed for antigen-specific IgG1 and IgG2a antibodies. Sera from vaccinated animals were analyzed in serial dilutions. H56 and MOMP IgG1/2a titers were determined at a cutoff of 0.2 OD450, while HA-specific IgG1/2a titers were determined from an internal standard. (a) IgG1 and IgG2a titers two weeks after 1st, 2nd and 3rd vaccination (Post 1, 2 and 3). Each point represents the geometric mean +95% CI of 4, 12 and 8 mice, for H56, MOMP and HA, respectively. Adjuvanted groups were compared to the no adjuvant group at each time-point using the Kruskal-Wallis test followed by Dunn’s post-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. (b) The ratio of IgG1/IgG2a titers after the second and third vaccination are shown. Bars represent the geometric means of 4, 12 and 8 mice, for H56, MOMP and HA, respectively. The bars are plotted from the median ratios within each antigen group (H56 10, MOMP 25, HA 256). Post 1 ratios have been omitted due to low titer responses resulting in large variations in ratios. The H56 and MOMP results are representatives of two independent experiments with similar results.
Comparative evaluation of the cellular immune responses induced by the different adjuvants
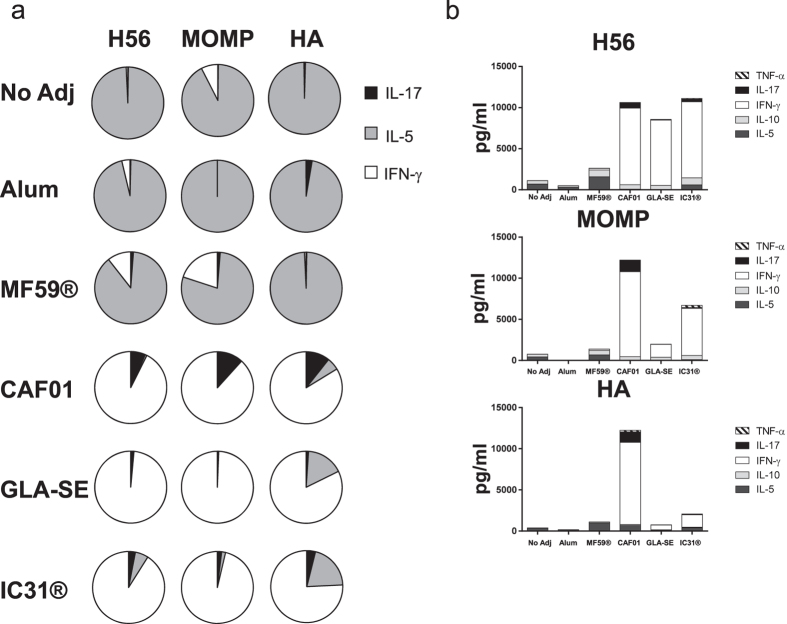
To evaluate CMI responses, splenocytes were isolated two weeks after the third vaccination and re-stimulated with the homologous recombinant protein. The levels of secreted cytokines released to the supernatant after 72 hours of in vitro antigen stimulation were measured using MSD technology. Depicting the overall profiles as the relative cytokine contribution demonstrated a breakdown into different classes of adjuvants (Fig. 3a). Thus, Alum and MF59® exhibited a Th2 bias whereas GLA-SE and IC31® induced a clear Th1-biased response with a strong IFN-γ component and negligible levels of IL-5. CAF01 induced a mixed Th1/Th17 phenotype and was the only adjuvant with a clear IL-17 response for all three antigens. Considering the absolute amount of cytokine secretion showed a more diverse pattern and magnitude depending on the type of antigen used (Fig. 3b). Thus, CAF01 was the only adjuvant consistently giving rise to high levels of cytokine secretion, primarily IFN-γ, with all three antigens whereas IC31® gave highest response in combination with H56, intermediate levels with MOMP and low amounts in combination with HA. GLA-SE had a prominent adjuvant effect for H56 whereas it showed a modest effect in adjuvanting HA and MOMP.
Figure 3. H56, MOMP and HA specific cellular immune response induced by vaccination with different adjuvants.
Groups of mice (n = 4 CB6F1 in TB and influenza, B6C3F1 in chlamydia) were vaccinated three times with H56, MOMP or HA formulated in Alum, MF59® CAF01, GLA-SE or IC31®. Two weeks after the third vaccination splenocytes from individual mice were isolated and stimulated with H56, MOMP or HA for 72 hours. The level of cytokines released to the culture supernatants were measured. (a) Pie-charts showing the relative contribution of antigen-specific IL-17, IL-5 and IFN-γ. Means of four mice, measured in triplicates and the background from samples stimulated with medium without antigen have been subtracted. (b) The levels of IFN-γ, TNF-α, IL-10, IL-5 and IL-17 released to the supernatant are shown as stacked bars, representing the means of four individual mice measured in triplicates and background from samples stimulated with medium without antigen have been subtracted. The H56 and MOMP results are representatives of two independent experiments.
The phenotype and cytokine subsets were further assessed by flow cytometry using the frequency of IFN-γ, IL-17 and IL-4/IL-13 for HA (Fig. S1) and the IFN-γ, IL-2, TNF-α distribution for H56 and MOMP (Fig. S2). Common for all adjuvants was the induction of CD4 T cell responses whereas no CD8 T cell responses were observed (results not shown). All groups receiving HA showed a measurable IL-4/IL-13 population, suggesting a Th2-bias response to this antigen in CB6F1 mice. This Th2 response was increased in combination with all adjuvants, albeit groups receiving HA in combination with the Th1 adjuvants, GLA-SE, IC31®, and CAF01 also had a population of IFN-γ producing CD4 T cells. CAF01 induced the overall highest recall response upon restimulation with HA and an additional IL-17 producing component. Overall, the phenotypes determined by flow cytometry were coherent with those determined by MSD. In the H56/MOMP analysis, IC31® induced the most vigorous response in terms of frequency of cytokine-producing CD4 T cells in particular when combined with H56. The response was dominated by triple-positive IFN-γ/IL-2/TNF-α T cells constituting ~50% of the cells, but also a considerable population of IL-2/TNF-α double producing cells usually considered to be the key trait of central memory T cells22. GLA-SE and CAF01 induced comparable T cell profiles with triple-positive, TNF-α/IL-2 double positive and TNF-α single producers. Furthermore, IFN-γ/TNF-α double producers were observed with CAF01, GLA-SE and IC31®, while IC31® was the only adjuvant that induced a small but noticeable population of IFN-γ single producers. IFN-γ/TNF-α and IFN-γ single producers are generally considered as more differentiated/effector subpopulations30. MF59® induced a strong Th2 bias response, based on the IL-5 secretion (see Fig. 3a,b), as well as distinct populations of TNF-α single producing CD4 T cells and IL-2/TNF-α co-producers.
In vitro evaluation of protective efficacy against influenza
In order to assess the adjuvants ability to induce protective immunity against influenza, the hemagglutination inhibition (HAI) titers were measured two weeks after each vaccination (Table 2). The HAI titer is a surrogate marker of influenza-specific neutralizing antibodies widely used for in vitro correlate of protection13. MF59® adjuvanted HA induced the highest levels of HAI titers, significantly higher than that of HA alone after the second vaccination (p < 0.0001, Kruskal-Wallis followed by Dunn’s post-test compared to no adjuvant). GLA-SE also elicited a significantly elevated HAI titer after the second vaccination (p < 0.05) while IC31®, CAF01 and Alum did not induce significantly higher HAI titers compared to that of antigen alone.
Table 2. .
| HA inhibition titers | ||||||
|---|---|---|---|---|---|---|
| Adjuvant | POST 1 |
POST 2 |
POST 3 |
|||
| GMT | 95% CI | GMT | 95% CI | GMT | 95% CI | |
| PBS | <10 | — | <10 | — | <10 | — |
| No Adj | 62 | [19–203] | 1050 | [434–2543] | 1076 | [548–2113] |
| Alum | 538* | [227–1274] | 1810 | [1327–2468] | 2915 | [1636–5198] |
| MF59® | 320 | [133–768] | 19612**** | [10087–38177] | 8611**** | [5890–12582] |
| CAF01 | 336 | [117–800] | 2690 | [2143–3964] | 2436 | [2213–2716] |
| GLA-SE | 269 | [179–405] | 5346* | [3242–8822] | 5263* | [2078–13333] |
| IC31® | 381 | [145–1001] | 2792 | [1765–4416] | 2560 | [1878–3491] |
Protective efficacy in an aerosol TB challenge model
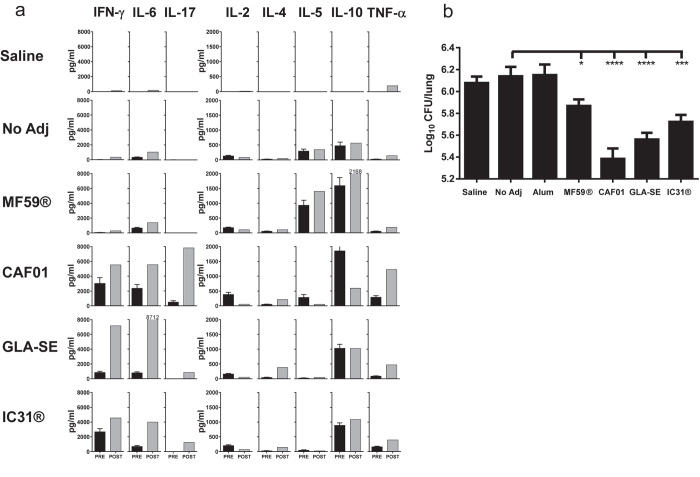
The potential of different adjuvants in induction of protective immunity to M.tb. infection was then studied. Six weeks after the third vaccination, H56 vaccinated mice were challenged with M.tb. Erdman through the aerosol route. Two weeks after challenge, PBMCs were isolated and stimulated with H56 for 72 hours and analyzed for vaccine-specific secretion of IFN-γ, IL-10, IL-2, IL-4, IL-5, IL-6, TNF-α and IL-17 (Fig. 4a). The post-infection H56 responses measured in the PBMC’s (post) were compared to the pre-infection responses in the splenocytes (pre) with the differences in target organ kept in mind. While infection alone induces limited responses at this early time point after challenge, all groups previously primed with an adjuvanted vaccine were able to mount significant responses. H56 in combination with CAF01, IC31® and GLA-SE all primed IFN-γ, IL-6 and TNF-α responses prior to infection and all of these cytokine responses remained two weeks after challenge. The strongest amplification of IFN-γ and IL-6 responses was observed in the GLA-SE vaccinated group. MF59® maintained the same profile with no IFN-γ secretion after infection. CAF01 was the only group where an IL-17 response was observed after immunization and this response was strongly elevated after infection. In contrast, the IL-17 response was induced only after infection in the IC31® and GLA-SE groups. H56-specific IL-10 was seen in all groups independent of their Th-profile and was not augmented by the infection.
Figure 4. H56 specific immune responses and protective efficacy against challenge with Mycobacterium tuberculosis.
Groups of mice (CB6F1) were immunized three times s.c. with 5 μg of H56 formulated in Alum, MF59® CAF01, GLA-SE or IC31® with 3 week intervals. Six weeks after the third vaccination, mice received an aerosol challenge with M.tb. Erdman (~50 CFU/mouse). Two weeks after challenge, PBMC’s were isolated from a pool of 12 individual mice and stimulated with recombinant H56 (2 μg/ml) for 72 hours. Culture supernatants were analyzed for released cytokines (TNF-α, IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-10, IL-17). (a) Grey bars indicate the levels of cytokines released from PBMCs after infection (Post) and the black bars represent the levels of cytokines measured in splenocytes prior to infection (Pre). Black bars represent mean + SEM of four mice measured in triplicates (Pre), while grey bars are means of triplicates measured of PBMC’s pooled from 12 mice within groups (Post). (b) After six weeks of infection, mice were euthanized and the bacterial loads in the lungs (CFUs) of individual mice was assessed. Each bar represents the mean + SEM Log10 CFU levels and represents 14–16 mice (Alum n = 8). The results are pooled from two experiments with same overall results. Adjuvanted groups were compared to the “no adjuvant” group using one way ANOVA with Dunnett’s multiple comparisons test. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Six weeks after infection, the mice were sacrificed and the numbers of viable colony-forming units (CFU) in the lungs were enumerated in order to assess protective efficacies. (Fig. 4b). The highest levels of protection were seen upon vaccination with H56 in CAF01/GLA-SE (p < 0.0001) and IC31® (p < 0.001), whereas MF59® gave rise to a relatively modest, but significant, reduction of 0.27 log10 CFU (p < 0.05). As with the related fusion protein H1 (Ag85B-ESAT-6), H56 combined with Alum did not mount protective immunity to TB challenge with comparable levels of bacterial numbers as the H56 alone group15. There was no significant difference between the PBS control group and H56 antigen alone (No adj).
Protective efficacy in a murine chlamydia challenge model
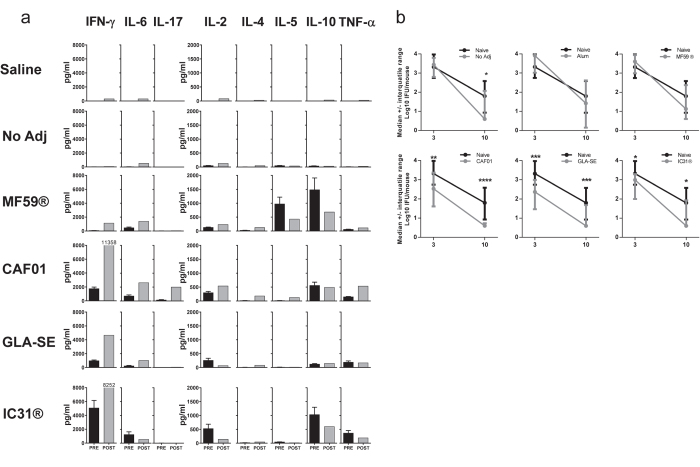
Next, we sought to evaluate the efficacy of different vaccine formulations in induction of protective immunity to a vaginal C.t. challenge. Groups of mice, vaccinated with MOMP singly or in combination with different adjuvants, were challenged vaginally with C.t. six weeks after the last vaccination. Three weeks after challenge, PBMCs were stimulated with recombinant MOMP for 72 hours for analysis of IFN-γ, IL-10, IL-2, IL-4, IL-5, IL-6, TNF-α and IL-17 in the supernatant. The post-infection PBMC responses (post) as well as the pre-infection splenocyte responses were examined (pre) (Fig. 5a). Like for H56, the cytokines IFN-γ, IL-6 and TNF-α were among the dominating cytokines in the CAF01, GLA-SE, and IC31® groups but only IFN-γ seemed to be selectively increased upon chlamydia infection. The CAF01-induced IL-17 response was shown to be clearly boosted by the infection, although not to the same extent as that observed with the TB infection and further, no IL-17 responses are seen in any other groups.
Figure 5. MOMP specific immune responses and protective efficacy against challenge with Clamydia trachomatis.
Groups of mice (B6C3F1) were immunized three times s.c. with 5 μg of MOMP formulated in Alum, MF59® CAF01, GLA-SE or IC31® with 3 week intervals. Six weeks after the third vaccination, mice received a vaginal challenge with C. trachomatis. Three weeks after challenge, PBMC’s were isolated from individual mice (n = 12), pooled within groups and stimulated with recombinant MOMP for 72 hours. Culture supernatants were analyzed for released cytokines (TNF-α, IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-10, IL-17). (a) The levels of cytokines released from PBMCs after infection (post) were compared to the levels of cytokines prior to infection, measured in splenocytes (pre). Black bars represent mean + SEM of four mice measured in triplicates (Pre), while grey bars are means of triplicates measured of PBMC’s pooled from 12 mice within groups (Post). (b) On day 3 and 10 after infection, mice were vaginally swabbed and the vaginal bacterial load (IFUs) of 28 individual mice (Naïve n = 56) was assessed. Each point represents the median Log10 ± interquatile range. Adjuvanted groups were compared to the naïve controls at each time-point using the Kruskal-Wallis test followed by Dunn’s post-test. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Vaginal swabs were collected at day 3 and 10 post challenge and protection was assessed by isolating C.t. from vaginal swabs and comparing the number of IFUs recovered at the indicated time points (Fig. 5b). CAF01, IC31® and GLA-SE were able to significantly reduce the bacterial shedding at day 3 and 10 post challenge, whereas MOMP adjuvanted with MF59® or Alum was not able to induce significant protection at any time-point. The un-adjuvanted MOMP vaccinated group did not show any significant reduction in their bacterial loads on day 3 after challenge, albeit a significant reduction was seen at day 10 post challenge.
Discussion
Herein, we report for the first time a comprehensive head-to-head comparison of immunogenicity and protective efficacy of five different clinically tested/practiced adjuvants; Alum, MF59®, CAF01, GLA-SE and IC31®. The adjuvant panel is highly diverse, but with the common denominator of having been tested in human subjects offering a unique opportunity to compare the mouse data from the current study with already available human data. Alum was the first adjuvant to be used in human vaccines and since the 1920s, billions of doses have been administered primarily with the purpose of adsorbing antigen and inducing antibodies. From numerous human trials, it is known that Alum is a poor inducer of cellular immune responses. In line with the human data and as reported previously in several animal studies, the single characteristic of Alum in our study was its ability to enhance antibody titers. MF59® has been used in licensed vaccines in Europe since 1997 and primarily induces antibodies and an increased CD4 T cell response with an unbiased Th-profile in humans31,32,33. Overall, this is in agreement with our findings in mice showing strong antibody responses but also induction of a CD4 response with a Th2 profile along with CD4 T cells co-producing TNF-α/IL-2. GLA-SE, CAF01, and IC31® are in the early stages of clinical development but it is clear from the emerging data that they are able to induce strong and long-lived CMI responses in humans and for CAF01 and IC31® phenotypic profiles with a remarkable resemblance between mouse and man.
MF59® was originally developed as a delivery system for immunomodulators but pre-clinical studies suggested that this emulsion had potential on its own with influenza antigens34. MF59® was the most effective adjuvant in an influenza vaccine assessed as increased IgG1 and protective HI titers and indeed numerous clinical trials in humans show the same overall profile and also a clear efficacy of MF59® in influenza vaccines35 (reviewed by Reed et al. 201336). However, in humans with no pre-existing immune response e.g. against H5N1, two vaccinations seem to be required in order to enhance IgG titers (Galli et al. 2009, Fig. 237). Since the discovery of the adjuvant activity of aluminum compounds, there have been several attempts to use different aluminum forms for augmenting the effect of influenza vaccines but with very limited success38 (reviewed by Tetsutani et al. 201239). In a clinical study directly comparing the effect of Alum and MF59®, there was an 8–9 fold increase in frequency of subjects reaching the predefined endpoint (HAI titers ≥ 40), when vaccinated with equivalent doses of subvirion influenza A/H5N1 in MF59® compared to Alum40. The superiority of MF59® over Alum in induction of influenza specific antibody responses was also observed in our study with a 2.5–10 fold increase in HAI for MF59® compared to Alum and in a recent study aimed at investigating the efficacy of emulsion adjuvants in influenza subunit vaccines24.
It is well-known that the antibodies raised by current inactivated influenza vaccines are mainly strain-specific antibodies directed against highly variable surface proteins resulting in protection against antigenically matching virus strains. With the threat of novel pandemic influenza variants and the continuous emergence of drifted strains, there has been considerable interest in the development of influenza vaccines with broader coverage. Several clinical trials have shown that MF59® can induce responses of increased breadth compared to un-adjuvanted influenza vaccines, which mainly relates to the antibody repertoire, whereas there is limited information on the ability of MF59® to induce CMI responses to the vaccine (reviewed in O´Hagan et al. 201141). Thus, it has been suggested that the potency of emulsion-based adjuvants could be improved by combining them with additional immunomodulators capable of inducing CD8 and/or CD4 T cell responses, with the ability to induce influenza cross-protection42,43. Accordingly, it has been shown in preclinical studies that GLA-SE containing the TLR4-ligand GLA, and not the stable emulsion (SE) alone, was capable of inducing protection against infection with a heterologous virus strain44. As in the studies herein, GLA-SE was found to enhance IFN-γ secretion and induce a shift from IgG1 to IgG2 production. In our study, GLA-SE also elicited significantly elevated HAI titers, which is in line with recent human data where GLA-SE induced HAI titers ≥1:40 in more than 66% of the subjects45. In the direct comparison of the HAI titers against the homologous virus strains, MF59® gave rise to stronger responses but it is plausible that changing the experimental set-up e.g. assessing survival upon challenge with a heterologous strain would offer advantages to GLA-SE.
The adjuvants IC31® and CAF01 were developed with the purpose of generating Th1 responses and both induce lower HAI titers in direct comparison to emulsion-based adjuvants. Paralleling the design of GLA-SE, it may be possible to benefit from their Th1-inducing ability e.g. by combining their individual immunomodulators into a stable emulsion formulation. Although the major purpose would be to augment the induction of neutralizing antibodies, it is important to maintain their ability to generate memory T cells that not only recognize conserved viral proteins, and thereby provide cross-protective responses, but also provide long-lived protection, which is of particular relevance for prophylactic vaccines. In this context, CAF01 in combination with trivalent inactivated vaccines (TIV) has previously been shown to promote significant T cell responses in the ferret model, which is considered to be the most accepted model of influenza disease, and importantly provided higher levels of protection compared to TIV alone and even TIV in a squalene emulsion46. A detailed characterization of the murine immune response afforded by a trivalent influenza vaccine administered in IC31® showed a very pronounced induction of IFN-γ that was maintained even 200 days after a single vaccination, at least in part, due to the formation of a vaccine depot at the injection site47,48.
CAF01-based TB vaccines were also capable of inducing long-term protective responses with a high frequency of vaccine-induced CD4 T-cells retrieved more than one year after vaccination in mice4. The T-cells had a strong proliferative potential presumably facilitated by the specific physicochemical properties of the delivery vehicle (DDA) that supports a slow-release of antigen from the site of vaccination49. The immunogenicity results from human trials have so far been comparable for IC31® and CAF01, both of which were capable of inducing very persisting T cell responses as late as 2½ (IC31®8) and 3 years (CAF019) after vaccination. Assessing the phenotype of the vaccine-induced CD4 T-cells at this late-stage post vaccination showed that more than 50% of the cells co-secreted TNF-α and IL-2; a phenotype which has been associated with central memory T cells (Tcm)22. In our studies, all three adjuvants with the ability to induce highly significant protection against TB, induced phenotypic profiles very similar to what was observed previously in humans with a substantial proportion of IL-2/TNF-α co-producers, most pronounced in the CAF01 group where app. 50% of the cells were of this phenotype. Indeed, of the relatively few Th1 cells induced by MF59®, the majority were IL-2/TNF-α co-producers, which were reported to represent the main population induced by MF59® adjuvanted influenza vaccine in children33, and this could explain the modest, albeit significant, protection induced by a MF59®-adjuvanted TB vaccine. There is an increasing amount of evidence that Tcm’s may play an important role in protection against TB. Most recently, the Kaufmann lab demonstrated that the protection afforded by a recombinant BCG primarily resided in the Tcm population50, but also in humans this phenotype was shown to be associated with responses in individuals capable of controlling infection51. Whether this profile directly mediates the superior TB disease control in humans, including vaccine-promoted protection, remains to be investigated.
Whereas there is concordance between murine and human data in regards to effector and memory T cell phenotypes, the specific induction of IL-17 producing CD4 T cells seen upon vaccination with the CAF01 adjuvant in this and several other studies is not directly mirrored in humans. In a recent phase I study, vaccination with the TB fusion-protein H1 in CAF01 was unable to induce high levels of IL-17 following two immunizations in healthy individuals9. In this study, we compared the post vaccination splenic responses to post infection PBMC’s, and although cytokine levels from these two different compartments are not directly comparable, some key differences between the adjuvants became evident from the relative comparison between groups. The vaccine-induced IL-17 primed by CAF01 vaccination was strongly boosted upon M.tb. infection (Fig. 4a). In groups vaccinated with GLA-SE or IC31®, no apparent IL-17 responses were detected after vaccination in the splenocytes, but upon M.tb. infection both exhibited measurable IL-17 responses in the PBMC’s. It is possible that CAF01-based vaccines are also able to induce IL-17 in humans but at a very low and un-detectable level and that the response only becomes apparent after infection. This boosting was not observed with MF59®, H56 alone, or in the saline control group. The immunomodulator in CAF01, TDB, was shown to signal through the C-type lectin receptor Mincle in mice, which in turn elicits a unique Th1/Th17-biased immune response through the Syk-Card9 signaling pathway52. Mincle is expressed on a variety of cell types e.g. neutrophils, dendritic cells, and macrophages and a human variant of the Mincle receptor has also been identified53. The recent study showing that TDB is capable of binding to human Mincle and activate APCs in vitro54 indicates that Mincle-activation may also be important for induction of immune response in humans. However, the use of more sensitive techniques and studying more immunologically competent compartments other than the PBMCs might be needed to detect Th17 responses in humans.
It is well-established that CMI responses, and in particular IFN-γ producing CD4 T-cells, play a major role in protection against chlamydia infection55,56. In agreement with this, vaccination with all three Th1 adjuvants (IC31®, GLA-SE, CAF01) reduced bacterial shedding in the genital tract at both day 3 and 10 (Fig. 5b). Testing different adjuvants, Dr. Robert Brunham’s lab demonstrated a superior effect of an adjuvant-formulation giving rise to a combined IFN-γ/IL-17 response57, however in our studies we did not see a superior effect of the IL-17 inducing CAF01 compared to the sole Th1 inducing adjuvant GLA-SE. The Th2 inducing adjuvant MF59® in combination with MOMP had no protective effect, which is in line with previous reports showing reduced migration to the genital mucosa of a chlamydia-specific Th2 clone compared to its Th1 counterpart58. It has been shown that antibodies play an important role for providing early clearance of infection using the same chlamydia model as in this study59 as well as against reinfection60. Somewhat surprising, we could observe that three vaccinations with un-adjuvanted MOMP induced an antibody response comparable to those of adjuvanted MOMP, which may explain the significant protection observed at day 10 in the un-adjuvanted MOMP group.
While it is generally accepted that a combination of humoral and cell mediated immunity is protective against chlamydia and that protection when using HI titers as influenza readout is mediated by a humoral response, the contribution of antibodies to protection against M.tb. is still debated. The comparison of the IgG1/2a titers (Fig. 2a) and TB bacterial burdens (Fig. 4b) indicates that protection against TB does not depend on antibody induction. While this fits with the general notion that a CMI Th1 response is crucial for TB protection, it does not elaborate on the possibility of a synergetic effect between cell mediated and humoral immunity. In particular, the induction of IgA titers by vaccination might be relevant as this isotype previously has been associated with protection against TB61. Currently, there are no vaccines building on the concept of inducing humoral immune responses in the clinical TB pipeline but this specific topic has generated interest from the funding bodies and it is possible that we will see new types of TB vaccines in the coming years.
It should be noted that the head-to-head evaluation of a wide range of different adjuvants for their ability to enhance immunogenicity of different antigens is a challenging task. In this regard, it is highly satisfying to observe that each individual adjuvant produced similar antigen-specific immunological profiles for three unique vaccine antigens. Thus, the distribution of T-cell phenotypes are the same whether using e.g. a TB antigen or the chlamydia MOMP protein, MF59® induces IL-5 independent of the antigen of choice, and GLA-SE generates the highest IgG2a titers with antigens produced in E. coli as well as with an antigen produced by embryonated eggs. Despite this remarkable consistency in adjuvant activity across different pathogen targets, there are, however, some key limitations in the current study that need to be taken into account; In order to perform this head-to-head comparison a standardized protocol was employed across the three models. This included unifying the number of vaccinations, amount of antigen, dose of adjuvant, timing between vaccinations, administration route and resting period before challenge and subsequent determination of protection. Nevertheless, all of these factors would presumably have a different optimum for different combinations of adjuvants and antigens, implying that some of these factors might not have been optimal for the tested adjuvants, hence preventing the adjuvants to exert their full adjuvanticity in the head-to-head comparison. However, due to the consistency in the immunological profiles of the different adjuvants across antigens, which are in line with the previously published human data, we are confident that these results could inform rational selection of suitable adjuvants to mount desired protective immune responses.
To our knowledge, this is the first report of a comprehensive non-clinical comparison of clinically tested/practiced adjuvants from different stakeholders, including academia and the private sector, and it is our hope that results obtained from this study will provide a platform for how adjuvants should be compared and thereby help accelerating adjuvant research and development.
Additional Information
How to cite this article: Knudsen, N. P. H. et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci. Rep. 6, 19570; doi: 10.1038/srep19570 (2016).
Supplementary Material
Acknowledgments
We appreciate the excellent technical assistance provided by Sharmila Subratheepam, Patricia Grenés, Linda Christensen, Merete Henriksen and the staff at the experimental animal facilities at Statens Serum Institut.This work was supported by the European Commission through contract FP7-HEALTH-2011.1.4-4-280873 (ADITEC).
Footnotes
PA is coinventor on a patent application covering the use of H56 and CAF01 in vaccines. All rights have been assigned to Statens Serum Institut, a Danish nonprofit governmental institute. NPK, RB, EA, PA, FF and AO are employees at Statens Serum Institut. RC and CF are employees at the Infectious Disease Research Institute. CB, AB, DC, UD, EDG and RR are employees of GSK vaccines. AM is an employee of Valneva Austria GmbH.
Author Contributions A.O., R.B., C.B., F.F., R.C., A.M., U.D., E.D.G., R.R., A.H., P.A., E.A. and N.P.K. contributed to the design of the experiments, N.P.K. performed and evaluated the tuberculosis experiments supervised by P.A. and E.A. R.B. performed parts of the experiments related to T.B. A.O. performed and evaluated the chlamydia associated experiments supervised by F.F. C.B., U.D., D.C. and A.B. performed and evaluated the influenza experiments supervised by E.D.G. and R.R. A.M., R.C., C.F., U.D. and Y.Z. contributed reagents/materials/analysis tools and expertise. N.P.K. and E.A. wrote the manuscript. A.H. contributed to the study design and writing the manuscript. F.F., A.O., E.D.G., U.D., C.B., C.F. and R.C. contributed to the manuscript. All authors reviewed the manuscript.
References
- Giefing C. et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 205, 117–131 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R., Pizza M., Del Giudice G. & De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci USA 111, 12288–12293 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington M. G. & Bowdish D. M. Immunosenescence and novel vaccination strategies for the elderly. Front Immunol. 4, 171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenstrom T. et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 182, 8047–8055 (2009). [DOI] [PubMed] [Google Scholar]
- Khurana S. et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2, 15ra15 (2010). [DOI] [PubMed] [Google Scholar]
- Dietrich J., Andreasen L. V., Andersen P. & Agger E. M. Inducing dose sparing with inactivated polio virus formulated in adjuvant CAF01. PLoS One. 9, e100879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., McKee A. S. & Munks M. W. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 9, 287–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dissel J. T. et al. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine. 28, 3571–3581 (2010). [DOI] [PubMed] [Google Scholar]
- van Dissel J. T. et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 32, 7098–7107 (2014). [DOI] [PubMed] [Google Scholar]
- Harandi A. M., Davies G. & Olesen O. F. Vaccine adjuvants: scientific challenges and strategic initiatives. Expert Rev Vaccines. 8, 293–298 (2009). [DOI] [PubMed] [Google Scholar]
- Redford P. S. et al. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 40, 2200–2210 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y. et al. Interleukin-22 promotes T helper 1 (Th1)/Th17 immunity in chlamydial lung infection. Mol Med. 20, 109–119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmit S. E., Petrie J. G., Cross R. T., Johnson E. & Monto A. S. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis. 204, 1879–1885 (2011). [DOI] [PubMed] [Google Scholar]
- Follmann F. et al. Antigenic profiling of a Chlamydia trachomatis gene-expression library. J Infect Dis. 197, 897–905 (2008). [DOI] [PubMed] [Google Scholar]
- Agger E. M. et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One. 3, e3116 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard C. et al. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One. 4, e5930 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windish H. P. et al. Protection of mice from Mycobacterium tuberculosis by ID87/GLA-SE, a novel tuberculosis subunit vaccine candidate. Vaccine. 29, 7842–7848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsen J. et al. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 1718, 22–31 (2005). [DOI] [PubMed] [Google Scholar]
- Calabro S. et al. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine. 31, 3363–3369 (2013). [DOI] [PubMed] [Google Scholar]
- Hekele A. et al. Rapidly produced SAM((R)) vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infect. 2, e52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T. et al. ESAT-6 (EsxA) and TB10.4 (EsxH) based vaccines for pre- and post-exposure tuberculosis vaccination. PLoS One. 8, e80579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenstrom T., Knudsen N. P., Agger E. M. & Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol. 190, 6311–6319 (2013). [DOI] [PubMed] [Google Scholar]
- Lindenstrom T. et al. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun. 80, 3533–3544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caproni E. et al. MF59 and Pam3CSK4 boost adaptive responses to influenza subunit vaccine through an IFN type I-independent mechanism of action. J Immunol. 188, 3088–3098 (2012). [DOI] [PubMed] [Google Scholar]
- Orr M. T. et al. Immune subdominant antigens as vaccine candidates against Mycobacterium tuberculosis. J Immunol. 193, 2911–2918 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraedts F. et al. Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs. Influenza Other Respir Viruses. 2, 41–51 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daifalla N. S., Bayih A. G. & Gedamu L. Immunogenicity of Leishmania donovani iron superoxide dismutase B1 and peroxidoxin 4 in BALB/c mice: the contribution of Toll-like receptor agonists as adjuvant. Exp Parasitol. 129, 292–298 (2011). [DOI] [PubMed] [Google Scholar]
- O’Meara C. P. et al. Immunization with a MOMP-based vaccine protects mice against a pulmonary Chlamydia challenge and identifies a disconnection between infection and pathology. PLoS One. 8, e61962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff P. H. et al. Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One. 8, e79194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Darrah P. A. & Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 8, 247–258 (2008). [DOI] [PubMed] [Google Scholar]
- Fabbiani M. et al. HIV-infected patients show impaired cellular immune response to influenza vaccination compared to healthy subjects. Vaccine. 31, 2914–2918 (2013). [DOI] [PubMed] [Google Scholar]
- Galli G. et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci USA. 106, 3877–3882 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedda L. et al. Dissecting the immune response to MF59-adjuvanted and nonadjuvanted seasonal influenza vaccines in children less than three years of age. Pediatr Infect Dis J. 34, 73–78 (2015). [DOI] [PubMed] [Google Scholar]
- Ott G., Barchfeld G. L. & Van Nest G. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine. 13, 1557–1562 (1995). [DOI] [PubMed] [Google Scholar]
- Vesikari T. et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 365, 1406–1416 (2011). [DOI] [PubMed] [Google Scholar]
- Reed S. G., Orr M. T. & Fox C. B. Key roles of adjuvants in modern vaccines. Nat Med. 19, 1597–1608 (2013). [DOI] [PubMed] [Google Scholar]
- Galli G. et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci USA. 106, 7962–7967 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport F. M., Hennessy A. V. & Askin F. B. Lack of adjuvant effect of AlPO4 on purified influenza virus hemagglutinins in man. J Immunol. 100, 1139–1140 (1968). [PubMed] [Google Scholar]
- Tetsutani K. & Ishii K. J. Adjuvants in influenza vaccines. Vaccine. 30, 7658–7661 (2012). [DOI] [PubMed] [Google Scholar]
- Bernstein D. I. et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis. 197, 667–675 (2008). [DOI] [PubMed] [Google Scholar]
- O’Hagan D. T., Rappuoli R., De Gregorio E., Tsai T. & Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 10, 447–462 (2011). [DOI] [PubMed] [Google Scholar]
- Sridhar S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 19, 1305–1312 (2013). [DOI] [PubMed] [Google Scholar]
- Wilkinson T. M. et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 18, 274–280 (2012). [DOI] [PubMed] [Google Scholar]
- Clegg C. H. et al. Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci USA. 109, 17585–17590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor J. J. et al. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE+GLA) adjuvant. Vaccine. 31, 5760–5765 (2013). [DOI] [PubMed] [Google Scholar]
- Martel C. J. et al. CAF01 potentiates immune responses and efficacy of an inactivated influenza vaccine in ferrets. PLoS One. 6, e22891 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl K., Riedl R., von Gabain A., Nagy E. & Lingnau K. The novel adjuvant IC31 strongly improves influenza vaccine-specific cellular and humoral immune responses in young adult and aged mice. Vaccine. 26, 3461–3468 (2008). [DOI] [PubMed] [Google Scholar]
- Schellack C. et al. IC31, a novel adjuvant signaling via TLR9, induces potent cellular and humoral immune responses. Vaccine. 24, 5461–5472 (2006). [DOI] [PubMed] [Google Scholar]
- Christensen D. et al. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J Control Release. 160, 468–476 (2012). [DOI] [PubMed] [Google Scholar]
- Vogelzang A. et al. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC::hly vaccine’s superior protection against tuberculosis. J Infect Dis. 210, 1928–1937 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C. L. et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 187, 2222–2232 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenen H. et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 184, 2756–2760 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flornes L. M. et al. Identification of lectin-like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics. 56, 506–517 (2004). [DOI] [PubMed] [Google Scholar]
- Ostrop J. et al. Contribution of MINCLE-SYK Signaling to Activation of Primary Human APCs by Mycobacterial Cord Factor and the Novel Adjuvant TDB. J Immunol. 195, 2417–2428 (2015). [DOI] [PubMed] [Google Scholar]
- Brunham R. C. et al. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J Infect Dis. 173, 950–956 (1996). [DOI] [PubMed] [Google Scholar]
- Igietseme J. U. et al. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 5, 317–324 (1993). [PubMed] [Google Scholar]
- Yu H. et al. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect Immun. 78, 2272–2282 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Rank R. G. & Kelly K. A. A Chlamydia trachomatis-specific Th2 clone does not provide protection against a genital infection and displays reduced trafficking to the infected genital mucosa. Infect Immun. 70, 5132–5139 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A. W., Follmann F., Erneholm K., Rosenkrands I. & Andersen P. Protection Against Chlamydia trachomatis Infection and Upper Genital Tract Pathological Changes by Vaccine-Promoted Neutralizing Antibodies Directed to the VD4 of the Major Outer Membrane Protein. J Infect Dis. 212, 978–989 (2015). [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Feilzer K. & Tumas D. B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 63, 4661–4668 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu S. et al. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol. 186, 3113–3119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N. & Aguilar J. C. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 82, 488–496 (2004). [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vaccine excipient and media summary in Epidemiology and prevention of vaccine-preventable diseases. 13th edn (eds Hamborsky J., Kroger A. & Wolfe S.) App. B-7 (Centers for Disease Control and Prevention, 2015). [Google Scholar]
- Podda A. & Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines. 2, 197–203 (2003). [DOI] [PubMed] [Google Scholar]
- El Sahly H. MF59™; as a vaccine adjuvant: a review of safety and immunogenicity. Expert Rev Vaccines. 9, 1135–1141 (2010). [DOI] [PubMed] [Google Scholar]
- O’Hagan D. T., Ott G. S., De Gregorio E. & Seubert A. The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine. 30, 4341–4348 (2012). [DOI] [PubMed] [Google Scholar]
- Coler R. N. et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One. 5, e13677 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werninghaus K. et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med. 206, 89–97 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agger E. M. et al. Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine. 24, 5452–5460 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.