Abstract
Cyanobacteria are photosynthetic prokaryotes that make major contributions to the production of the oxygen in the Earth atmosphere. The photosynthetic machinery in cyanobacterial cells is housed in flattened membrane structures called thylakoids. The structural organization of cyanobacterial cells and the arrangement of the thylakoid membranes in response to environmental conditions have been widely investigated. However, there is limited knowledge about the internal dynamics of these membranes in terms of their flexibility and motion during the photosynthetic process. We present a direct observation of thylakoid membrane undulatory motion in vivo and show a connection between membrane mobility and photosynthetic activity. High-resolution inelastic neutron scattering experiments on the cyanobacterium Synechocystis sp. PCC 6803 assessed the flexibility of cyanobacterial thylakoid membrane sheets and the dependence of the membranes on illumination conditions. We observed softer thylakoid membranes in the dark that have three-to four fold excess mobility compared to membranes under high light conditions. Our analysis indicates that electron transfer between photosynthetic reaction centers and the associated electrochemical proton gradient across the thylakoid membrane result in a significant driving force for excess membrane dynamics. These observations provide a deeper understanding of the relationship between photosynthesis and cellular architecture.
Cyanobacteria dominated Earth’s biosphere for billions of years and are the reason for the oxygen atmosphere on our planet1. Thylakoid membranes in cyanobacteria are not found in chloroplasts as in plants and eukaryotic algae2, but typically occupy the peripheral region of the cell interior3. In Synechocystis sp. PCC 6803, the thylakoid membranes form concentric layers that tend to follow the shape of the cell envelope. Electron tomography4 and other high-resolution structural studies like super-resolution microscopy with fluorescent tagging5, fluorescence recovery after photobleaching6 and NMR spectroscopy7 have given reliable information about thylakoid membrane structure and architecture within cells.
Among non-invasive techniques, Small Angle Neutron Scattering (SANS) in combination with other methods8,9,10,11,12 is able to provide spatially averaged structural information about membranes systems under different experimental conditions, in algal cells8,9,10,11 and in isolated thylakoid membrane sistems11,12. The SANS data in Liberton et al. (2013) showed that within wild type cyanobacterial cells, the thylakoid membranes are arranged in various architectures, with center-to-center distances between 630 Å and 110 Å. This complex spacing corresponds to membrane pairs of different organizations: from pairs with sufficient space to accommodate light-harvesting complexes (phycobilisomes) within the interthylakoidal space, up to very closely appressed thylakoid membrane pairs that cannot accommodate light-harvesting antennas. These findings were acknowledged by11 in their review article, and Nagy et al. (2011) observed similar architecture in eukaryotic algae and plant thylakoids9. Because SANS is a sensitive technique capable of detecting repeat distances in large populations of cells, even membrane architectures that are not readily seen in small samples of cells examined by TEM are revealed by SANS.
However, to date little information exists about the internal dynamics of thylakoids in terms of flexibility and mobility of the membranes in their native environment, since biophysical studies are typically carried out under defined conditions in dilute aqueous environments that do not reflect the complex crowded environment of a living cell. In this work, we observe the mobility of thylakoid membranes in live cyanobacterial cells by neutron spectroscopy. Neutron Spin-Echo13(NSE) is a high-resolution spectroscopic technique that has already proved to be a successful method of studying the structure and dynamics of model bilayer lipid membranes14,15,16,17 and proteins in solution18 but has thus far not been used to study the internal dynamics of intact living cell components. This technique allows simultaneous observation of correlation times and length scales and thereby can directly deduce the geometry and modes of motion observed, such as membrane undulation or diffusion, which are not possible using purely energy-resolved spectroscopic techniques.
Results and Discussion
Assessment of the internal dynamics of thylakoids
We used the NSE spectrometer at the Spallation Neutron Source19 to study wild type cyanobacterial cells in a heavy water (deuterium oxide) solution. The high content of deuterium (D) in the heavy-water-filled spaces of the cyanobacterium provides excellent neutron contrast for the hydrogen-rich (H) lipid membranes and, therefore, the correlations between neighboring membranes dominate the coherent scattering signal in our probed window of observation, as shown previously8,9,10,11,12. Neutron data were collected at 20 °C during 12-hour light and dark alternating cycles to mimic the circadian clock. NSE measures the so-called intermediate scattering function which represents pair-correlation distances observed in reciprocal space (as in diffraction) and the time-dependent loss or relaxation of such correlations due to the mobility of the correlated spatial features. The reciprocal space coordinate or scattering vector magnitude, q, is inversely proportional to distances, d, in real space as d = 2π/q, and the corresponding time information is expressed as either relaxation times or inversely as relaxation rates Γ.
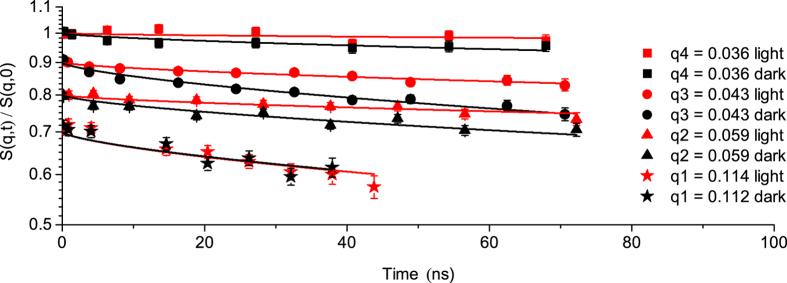
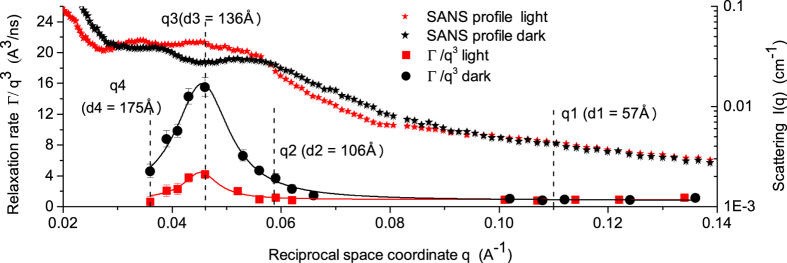
The normalized intermediate scattering functions of cyanobacteria (Fig. 1) show identical relaxation modes for both light and dark states at q values higher than 0.07 Å−1 (d ≤ 90 Å). Conversely, at q values lower than 0.07 Å−1 (d ≥ 90 Å) considerable difference exists between the two states, with slower decay in measurements collected in light compared to measurements collected in dark. NSE data were fitted by a stretched exponential function with a stretching exponent of 2/3, as predicted by Zilman & Granek20 for bilayer membranes (Table 1 and Methods). The spatial or q dependence of the relaxation rate Γ displayed in Fig. 2 is the major result of this study since its variations describe the dynamics of our system. Starting from the right side of Fig. 2 (high q values), a q3 dependence of the relaxation rate Γ is observed, which is a typical signature of the undulation motions in lipid bilayer membranes21 at length scales much smaller than the inter-membrane distance (the interthylakoidal space). In general, the tendency of decreasing magnitude of Γ with decreasing q is a common observation, as relaxations related to larger distances tend to take longer times. The absolute value of the relaxation rate at these high q values (short correlation distances) is similar for both light and dark measurements. More interestingly, we also observe a strong deviation from the Γ(q3) dependence when increasing the observed correlation distances to q values below 0.07 Å−1 (d ≥ 90 Å), peaks region in Fig. 2. This strong deviation from q3 dependence represents significant excess mobility that exists in addition to the underlying q3 dependent undulation dynamics. Between 0.07 Å−1 and 0.03 Å−1, Γ increases up to a maximum value at around 0.046 Å−1 and then decreases again slowly. This corresponds to a speed-up of the internal motion up to a very fast relaxation (peak in Γ relaxation rate represents a minimum of the relaxation time) followed by a slow-down process. This effect is more pronounced during the dark cycle compared to the light cycle.
Figure 1. S(q, t)/S(q, 0) of cyanobacteria at 20 °C for light and dark states.
All scattering functions start at unity and have been shifted for better visualization. Solid lines represent the stretched exponential function fitting with stretching exponent of 2/320; 0.035 Å−1, 0.05 Å−1 and 0.095 Å−1 minimum value of momentum transfer were measured according to the correlation peaks observed in the SANS experiment8. Five different groupings of the time channels were applied, leading to 15 q values. Only four q values for each state are shown here in relation to Figs 2 and 3.
Table 1. Characteristics of membrane relaxation and bending fluctuations.
| Sample | q (Å−1) | Γ/q3 (Å3/ns) | Γ (1/ns) | τ (ns) |
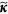 (kBT) ** (kBT) **
|
|---|---|---|---|---|---|
| PCC6803 light | 0.114 | 0.92 | 1.37E-3 | 729.9 | 7655.45 |
| PCC6803 dark | 0.112 | 0.94 | 1.33E-3 | 751.9 | 7304.45 |
| PCC6803 light | 0.059 | 1.19 | 2.44E-4 | 4098.3 | – |
| PCC6803 dark | 0.059 | 3.69 | 7.59E-4 | 1317.5 | – |
| PCC6803 light | 0.043 | 3.75 | 2.98E-4 | 3355.7 | – |
| PCC6803 dark | 0.043 | 14.34 | 1.14E-3 | 877.2 | – |
| PCC6803 light | 0.036 | 0.61 | 3.35E-5 | 29850.7 | – |
| PCC6803 dark | 0.036 | 4.61 | 2.29E-4 | 4366.8 | – |
A value of 0.00125Kg·m·s−1 was use as D2O viscosity at 20 °C for calculation of bending modulus. **Effective bending coefficient according to Zilman & Granek20 are valid when calculated only in the high q regime where 1/q « interthylakoidal distance (the linear dependence around q1 position in Fig. 2).
Figure 2. q3 dependence of the decay rate Γ for cyanobacteria during light and dark states.
The vertical dashed lines show the position of the corresponding observed distances within membrane pairs, with q3 at the peak of Γ function. With increasing distance observed (from q1 to q2), the influence of the neighboring membrane becomes apparent in the increase of the relaxation rate up to a maximum where the membranes repel each other. This leads to a decrease of the relaxation rate to the state where the influence of the neighboring membrane is no longer visible (q4). Small Angle Neutron Scattering profiles8 are super-imposed to observe the correlation of dynamics features with the structure.
The q3 dependent bending undulations describe correlations within a single membrane and are controlled by the elastic modulus with a higher bending coefficient or membrane stiffness corresponding to faster relaxation rates. From the q3 dependence of the relaxation rate Γ at high q values effective bending coefficients of 7655kBT for membrane structure in light and 7304kBT for membrane structure in dark were calculated20 (Table 1). The calculated bending coefficients show that during light measurement the membrane is stiffer compared to the dark state in which the bending coefficient shows a more flexible system. This is in agreement with a previous study on chloroplast remodeling within living cells of Chlamydomonas reinhardtii, which reports more stacked thylakoids with well-defined periodicity during the light harvesting state in comparison with a dark state in which the thylakoids are partially unstacked and undulated due to physiological responses of thylakoids to anaerobiosis22.
Correlation of dynamics with membrane flexibility
For model lipid bilayers, dynamic modes in addition to undulation have been interpreted as thickness fluctuations14,15,16,17. We propose a different explanation for our observations in cyanobacteria. The additional dynamics appear in the range of average length between 100 Å–180 Å that is rich with scattering intensity arising from correlations between very closely appressed thylakoid membrane pairs that cannot accommodate light harvesting antennas, as shown by8,11 and observed also by10 for other algal cells. The dynamics at this length scale therefore should contain strong contributions from the relative motion of neighboring membranes across the interthylakoid space (Fig. 3). In contrast to the q3 dependent bending undulations of individual membranes, motions of membrane pairs relative to each other in the direction across the interthylakoid space (large correlation distances) may be thought of as the local observation of a diffusive motion of large membrane areas. This diffusive component of the membranes motion is only observable through the time-dependent decay of correlations between membranes, and would not appear in the coherent signal of isolated single membranes. Importantly, although a stiffer membrane, submitted to a random undulation, relaxes faster to its flat state, this membrane is less efficient in exploring the available volume, in our case the interthylakoid space. The same space is sampled faster by a flexible membrane structure that is very efficient in exploring the available volume20 (Table 1). The membrane exhibits a speed-up/slow-down of undulation motion with different amplitude when two adjacent membranes feel each other’s influence in light or dark. Softer thylakoid membranes in the dark display three-to four-fold excess dynamics compared to membranes under light conditions. The more rigid membrane in light takes a longer time to relax which is the time necessary for the membrane structure to move through the available space. This represents the relaxation times we observe by NSE of about 2000 ns–3000 ns in light and only 600 ns–800 ns in the dark, for the peak position in Fig. 2.
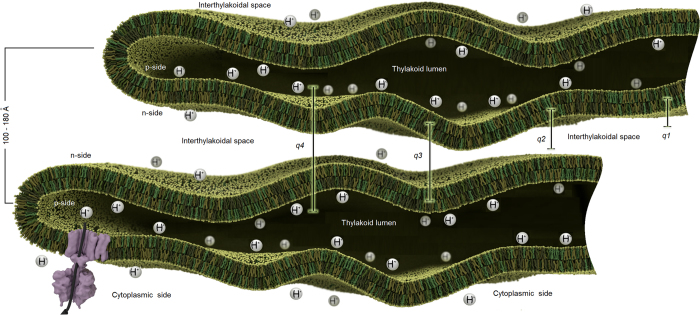
Figure 3. Thylakoid membrane sheet-like structure dynamics.
A small region of two adjacent closely appressed membranes that cannot accommodate phycobilisomes is presented undulating freely in the cytoplasm, and the corresponding interthylakoidal distances. From q1 to q4 NSE samples a larger distance (57 Å–175 Å) within the interthylakoidal space and different relaxation behavior is observed. The excess of protons (H + ) in the thylakoid lumen results in restricted membrane dynamics observed during light. At dark, H + pressure is alleviated by chemiosmosis and the membranes undulate freely resulting in higher relaxation rates and excess dynamics. Purple feature is the ATP-Synthase responsible for moving down the electrochemical gradient. The center-to-center distance of a membrane pair corresponds to SANS correlation peaks for closely appressed membranes in Fig. 2 (100 Å–180 Å).
The relation between excess dynamics and physiological processes within thylakoids
There could be several possible explanations for the difference in membrane mobility observed between light and dark. One reason could lie in the function of the photosynthetic apparatus in the thylakoid membrane. Absorption of light by photosystem II initiates water oxidation that results in transfer of photo-generated reducing equivalents to photosystem I. This results in an excess of protons in the lumenal space of the thylakoid membrane (Fig. 3) that is alleviated by moving an electrochemical gradient (H+) through the membrane by chemiosmosis23. In light, photosynthesis maintains the presence of a proton concentration gradient. The increased density of protons within the lumenal space could result in increased pressure upon membranes. Such pressurized membranes will appear stiffer when observed during light and display restricted motility. The protons move across the thylakoid membrane by the function of ATP-Synthase. They are the motive force to generate ATP. In the dark cycle when light harvesting and photosynthesis stop, the difference in the proton density between the lumenal and the interthylakoidal space is finally, equilibrated over time. Pressure is released, and the membrane becomes more relaxed and flexible and can move easily within the available space.
Furthermore, refinements to the chemiosmotic hypothesis have proposed that localized domains of protons are confined to the region near the thylakoid membrane, rather than being delocalized within the lumenal space24. Such organization suggests that in addition to the increased pressure by the presence of protons, the inner leaflet of the thylakoid membrane bilayer (the p-side, Fig. 3) has a higher positive charge than the outer leaflet (the n-side). The negative outer leaflets would therefore repel each other when the undulating motion brought two neighboring membranes in close proximity. This repelling will constrict the excess undulation motion during light. The electrochemical gradient is dissipated by chemiosmosis. The repelling will stop and the membranes recover their higher flexibility during dark when the proton density equilibrates.
Another explanation of the observed differences in membrane excess dynamics between light and dark could be the dynamic control of protein diffusion within the thylakoid lumen25. In higher plant thylakoids, the lumenal space undergoes a significant expansion in light. In a study involving electron microscopy, light scattering and theoretical modeling of the lumenal space in Arabidopsis, it was shown that reversible changes in the thylakoid lumen induced by alteration of the light environment would modulate the diffusion of proteins within the restricted space of the lumenal compartment. This is done in order to facilitate PC-mediated electron transport and to repair photo-damaged photosystem II complexes25. If similar effects are present in cyanobacterial thylakoids, then the expansion of the lumen restricts the undulation motions of the membranes, i.e. membranes observed during light will appear again stiffer.
Is not certain whether the observed changes may constitute a mechanism by which cyanobacteria regulate photosynthetic activity in response to light intensity, or may be a byproduct of photosynthetic activity, e.g., through charge gradients across lipid membranes, or through an unknown, more indirect chain of events. However, this study is the first direct measurement of the undulation dynamics of photosynthetic membranes in living prokaryotic cells and adds to the open question of whether other components of the photosynthetic machinery exhibit internal dynamics and how these dynamics might affect the yield of photosynthesis. The question of how the plasticity and dynamics of thylakoid membranes can be regulated, and related to the functions of sub-cellular architectures, is now an open field in biological research. This holds promise for important discoveries and we are giving a first demonstration of the possibility to observe directly such complex dynamic relations.
Methods
Sample preparation
Synechocystis sp. PCC 6803 was grown in BG11 medium at 20 °C and 30 μmole photons·m-2·s−1 light intensity. The cells were exchanged into a D2O based BG11 medium by three sequential centrifugation and resuspension steps8. For the final resuspension step the cells were suspended in 4 ml of D2O-based BG11 media (OD750nm = 41.6, 1 cm pathlength). A control sample from the same culture was maintained under conditions identical to the NSE sample (OD750nm = 36.5, 1 cm pathlength). There was no significant change in the OD750nm of the sample over the time course of the experiment.
Neutron Spin Echo experiment
NSE experiments were performed using the NSE spectrometer at the Spallation Neutron Source19, Oak Ridge National Laboratory. Measurements were carried out in 4 mm-path quartz cells. A closed sample environment was used to allow dark adaptation of the samples. The sample holder was equipped with a lighting apparatus that illuminated the samples with cool white LED lights set at 100 μmole photons·m−2·s−1. The NSE experiment was performed at incident wavelengths of 9 Å and 11 Å accessing a dynamical range of 0.1 ≤ τmax ≤ 130 ns at different momentum transfers for each sample according to correlation peaks observed in the SANS experiment8. Solid angles of 0.035 Å−1, 0.05 Å−1 and 0.095 Å−1 were measured (minimum value of momentum transfer). NSE spectra were taken at 20 °C under 12 h light and dark alternating cycles to mimic the circadian clock. One hour of equilibration was allowed between each cycle so that cells may adapt to the light or dark condition before data collection. Graphite foil was used as a standard elastic reference and solvent BG11 measurements were also necessary for proper data reduction. The entire experiment lasted 14 days: 7 days for cyanobacterial sample and 7 days for reference and background sample.
Data analysis
The data analysis was performed with the standard reduction software package of the NSE-SNS instrument called ECHODET. Data were acquired with sufficient statistics so the entire wavelength spectra could be divided in 5 different solid angles for a better discretization of the q dependence. The intermediate scattering functions were fitted by a stretched exponential function with a stretching exponent of 2/3 predicted by Zilman & Granek20 for single membrane (Fig. 1):
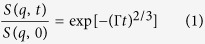 |
were Γ is the relaxation rate. Using this approach for fitting data one might argue that the translational diffusion of the entire cyanobacteria cell has also a contribution to the overall dynamics of the system. However, the cyanobacteria cell has a diameter of about 3.8 – 5.2 μm and the diffusion coefficient of such a large cell is outside the NSE window we can access for measurements. Therefore, translational diffusion was neglected from further analysis. To account for the bending motion of the membranes we applied the single membrane fluctuation model of Zilman & Granek20. This model, based on mode coupling theory, explains the dependence with q3 of the relaxation rate for systems where the hydrodynamic interactions dominate and the wavelengths are shorter than the characteristic correlation length, in our case 1/q « interthylakoidal distance21:
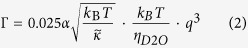 |
with 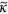 as the effective bending modulus; α is a scaling factor equal unity; kBT is the thermal energy; ηD2O is the viscosity of the solvent at T = 20 °C. Γ/q3 represents in fact the linear dependence observed at q values higher than 0.07 Å−1 (d ≤ 90 Å) in Fig. 2. The calculated values of bending coefficients are displayed in Table 1.
as the effective bending modulus; α is a scaling factor equal unity; kBT is the thermal energy; ηD2O is the viscosity of the solvent at T = 20 °C. Γ/q3 represents in fact the linear dependence observed at q values higher than 0.07 Å−1 (d ≤ 90 Å) in Fig. 2. The calculated values of bending coefficients are displayed in Table 1.
Additional Information
How to cite this article: Stingaciu, L.-R. et al. Revealing the Dynamics of Thylakoid Membranes in Living Cyanobacterial Cells. Sci. Rep. 6, 19627; doi: 10.1038/srep19627 (2016).
Acknowledgments
Neutron beam time for this research has been allocated by the Jülich Center for Neutron Science, JCNS1, Jülich Forschungszentrum GmbH, Germany, outstation to the Spallation Neutron Source (SNS), Oak Ridge, TN. The authors acknowledge Malcolm Cochran for the construction of the illumination setup and technical support during measurements, Dr. Piotr Zolnierczuk for providing automatic control of the illumination setup and software support during measurements, Rhonda Moody and Dr. Kevin Weiss from SNS biochemistry lab support, William B. O’Dell for assistance with Small-Angle Neutron Scattering (SANS) data, Dr. Jonathan Nickels, Prof. Dr. Dieter Richter and Dr. Michael Monkenbusch for discussion on data interpretation. The generation of neutrons for this research at ORNL’s Spallation Neutron Source and High Flux Isotope Reactor was sponsored by the Scientific User Facilities Division, Office of Basic Energy Sciences, Office of Biological and Environmental Research, U.S. Department of Energy. M.L., H.B.P., H.O.N. and V.S.U. were supported by the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science and Office of Basic Energy Sciences, under Award Number DE-SC 0001035.
Footnotes
Author Contributions L.-R.S. designed, performed, analyzed the NSE measurements and wrote the manuscript. M.L. and H.O.N. grew and prepared the cyanobacterial cells for the experiment. M.O., V.S.U. and H.B.P. supervised the project, initiated and directed this research. All authors discussed the results, reviewed and edited the manuscript.
References
- Herrero A. & Flores E. (eds.) The Cyanobacteria: Molecular Biology, Genomics and Evolution. ISBN: 978-1-904455-15-8 (Caister Academic Press, 2008). [Google Scholar]
- Liberton M. & Pakrasi H. B. in The Cyanobacteria: Molecular Biology, Genomics, and Evolution (Herrero A. & Flores E. eds) 217–287 (Horizon Scientific Press, Norwich, United Kingdom, 2008). [Google Scholar]
- Mullineaux C. W. The thylakoid membranes of cyanobacteria: structure, dynamics and function. Aust. J. Plant Physiol. 26, 671–677 (1999). [Google Scholar]
- Liberton M., Austin J. R. 2nd, Berg R. H. & Pakrasi H. B. Unique thylakoid membrane architecture of a unicellular N2-fixing cyanobacterium revealed by electron tomography. Plant Physiol. 155, 1656–1666 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S., Gunzenhauser J. & Olivier N. A starter kit for point-localization super-resolution imaging. Curr. Opin. Chemical Biol. 15, 813–821 (2011). [DOI] [PubMed] [Google Scholar]
- Mullineaux C. W., Tobin M. J. & Jones G. R. Mobility of photosynthetic complexes in thylakoid membranes. Nature 390, 421–424 (1997). [Google Scholar]
- Tochio H. Watching protein structure at work in living cells using NMR spectroscopy. Curr. Opin. Chemical Biol. 16, 609–613 (2012). [DOI] [PubMed] [Google Scholar]
- Liberton M. et al. Organization and flexibility of cyanobacterial thylakoid membranes examined by neutron scattering. J. Biol. Chem. 288(5), 3632–3640 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G. et al. Reversible membrane reorganizations during photosynthesis in vivo: revealed by small-angle neutron scattering. Biochem. J. 436, 225–230 (2011). [DOI] [PubMed] [Google Scholar]
- Nagy G. et al. Modulation of the multilamellar membrane organization and of the chiral macrodomains in the diatom Phaeodactylum tricornutum revealed by small-angle neutron scattering and circular dichroism spectroscopy. Photosyn. Res. 111, 71–79 (2012). [DOI] [PubMed] [Google Scholar]
- Ünnep R., Nagy G., Markó M. & Garab G. Monitoring thylakoid ultrastructural changes in vivo using small-angle neutron scattering. Plant Physiology and Biochemistry 81, 197–207 (2014). [DOI] [PubMed] [Google Scholar]
- Nagy G. et al. Kinetics of structural reorganizations in multilamellar photosynthetic membranes monitored by small-angle neutron scattering. Eur. Phys. J. E Soft Matter 36(7), 69–80 (2013). [DOI] [PubMed] [Google Scholar]
- Mezei F. ed. Neutron Spin Echo. Lecture Notes in Physics. Berlin, Heidelberg, New York: Springer 128, 1980). [Google Scholar]
- Yi Z., Nagao M. & Bossev D. Bending elasticity of saturated and monosaturated phospholipid membranes studies by neutron spin echo technique. J. Phys. Condens. Matter 21, 155104 (2009). [DOI] [PubMed] [Google Scholar]
- Nagao M. Observation of local thickness fluctuations in surfactant membranes using neutron spin echo. Phys. Rev. E. 80, 031606 (2009). [DOI] [PubMed] [Google Scholar]
- Nagao M. Temperature and scattering contrast dependencies of thickness fluctuations in surfactant membranes. J. Chem. Phys. 135, 074704 (2011). [DOI] [PubMed] [Google Scholar]
- Woodka A. C. et al. Lipid Bilayers and Membrane Dynamics: Insight into Thickness Fluctuations. Phys. Rev. Lett. 109, 058102 (2012). [DOI] [PubMed] [Google Scholar]
- Biehl R. & Richter D. Slow internal protein dynamics in solution. J. Phys. Condens. Matter 26, 503103 (2014). [DOI] [PubMed] [Google Scholar]
- Ohl M. et al. The spin-echo spectrometer at the Spallation Neutron Source (SNS). Nuclear Instruments and Methods A 696, 85–99 (2012). [Google Scholar]
- Zilman A. G. & Granek R. Undulations and dynamic structure factor of membranes. Phys. Rev. Lett. 77, 4788–4791 (1996). [DOI] [PubMed] [Google Scholar]
- Ohta T. & Kawasaki K. Mode coupling theory of dynamic critical phenomena for classical liquids I. Progress of Theoretical Physics 55(5), 1384–1395 (1976). [Google Scholar]
- Nagy G. et al. Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by noninvasive techniques in vivo. PNAS 13(111), 5042–5047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191(4784), 144–148 (1961). [DOI] [PubMed] [Google Scholar]
- Dilley A. & Ewy R. G. Distinguishing between Luminal and Localized Proton Buffering Pools in Thylakoid Membranes. Plant Physiol. 122, 583–595 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H. et al. Dynamic control of protein diffusion within the granal thylakoid lumen. PNAS 108(50), 20248–20253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]