Abstract
The American Heart Association defined 7 ideal cardiovascular health (CVH) metrics and the benefits of them in reducing the incidence of stroke are well established, but it is unclear whether changes in them alter stroke risk. We calculated the changes of 7 ideal CVH metrics from 2006 to 2008 among 64,373 participants in the Kailuan study. We tested whether changes in the numbers and total scores for the CVH metrics were associated with the incidence of stroke in the 4.89 person-years follow-up. Cox regression modeling was used to estimate the risk of stroke. By year 2008, CVH metrics number of 32.54% participants improved (change ≥+1); 31.90% deteriorated (≤−1); 35.56% stayed the same; In the follow-up,we identified1,182 incident stroke events. Each increase in CVH metrics and every 1-point increase in total CVH score from 2006 to 2008 were associated with reduced odds of total stroke (hazard ratio = 0.87; 95% confidence interval; 0.83–0.92 and 0.89[0.86–0.92] respectively), after adjusting for age, gender, educational level, income and scores for the metrics of ideal CVH at baseline. Positive changes in ideal CVH metrics reduce the incidence of stroke. Our results support the concept that achieving ideal CVH helps to prevent stroke.
In 2010, the American Heart Association (AHA) released its 2020 Impact Goals for cardiovascular health (CVH) promotion and disease reduction1. The concept of ideal CVH is defined as the simultaneous presence of 4 ideal health behaviors (not smoking, having a normal body mass index [BMI], being physically active, and eating a healthy diet) and 3 ideal health factors (normal total cholesterol levels, blood pressure [BP], and fasting glucose levels). The presence of more ideal CVH metrics predicts a lower risk of cardiovascular disease (CVD), a lower risk of incident cancer and lower all-cause mortality2,3,4,5,6. Moreover, several studies have demonstrated the potentially combined protective impact of ideal CVH metrics on the incidence of stroke7,8,9,10. It is worthwhile to explore whether changes in health behaviors and factors affect the incidence of CVD and stroke.
Recent studies have demonstrated that changes in ideal CVH status are associated with subclinical atherosclerosis and arterial stiffness, which are potential predictors of CVD and stroke11,12. Currently, little is known about how well changes in ideal CVH metrics in the follow-up predict the incidence of stroke. Therefore, the present study aim to explore the relationships among changes in ideal CVH metrics and the risk of stroke.
Results
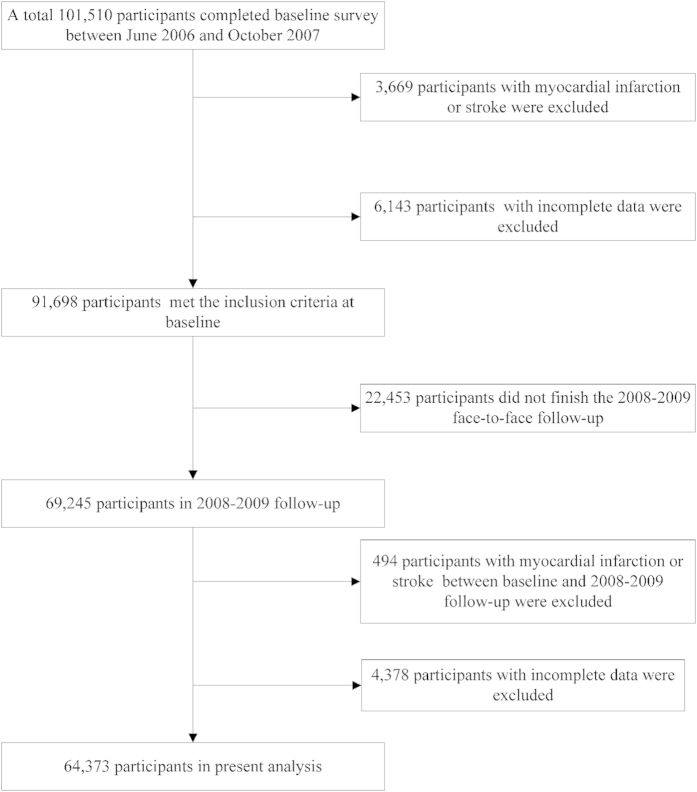
From June 2006 to October 2007, a total of 101,510 participants (81,110 men and 20,400 women, 18–98 years of age) were recruited to participate in the Kailuan study. At baseline, of the 101,510 participants, we excluded 3,669 with a history of myocardial infarction (MI) or stroke, and 6,143 with incomplete data regarding health factors or health behaviors. As 22,453 participants did not finish the 2008–2009 face-to-face follow-up,the remaining 69,245 participants were examined in 2008–2009. We also excluded 494 participants who had stroke or MI between the baseline and 2008–2009 follow-up and 4,378 with incomplete information. The analyses performed in this study were thus confined to the remaining 64,373 participants (Fig. 1).
Figure 1. Flowchart of the study.
Characteristics at baseline
Characteristics were calculated for the 8 CVH groups (CVH score 0–7) in 2006. Participants with a greater number of ideal CVH metrics were more likely to be women and young, to have attained a higher educational level and to have a higher income (Table 1). The CVH metric scores were inversely related to total cholesterol levels, BP, BMI and fasting glucose levels.
Table 1. Baseline characteristics of participants by number of CVH metrics in 2006.
| Variable | Number of Ideal CVH Metrics in 2006 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | P Value | |
| No. of participants | 939 | 5795 | 14882 | 20700 | 15259 | 5859 | 860 | 79 | <0.001 |
| Age, year, mean (SD) | 50.88 ± 8.83 | 50.66 ± 10.27 | 51.38 ± 10.93 | 50.87 ± 11.72 | 49.77 ± 12.77 | 46.43 ± 13.64 | 47.5 ± 14.48 | 51 ± 12.23 | <0.001 |
| Women, n (%) | 11 (1.2) | 460 (7.9) | 1979 (13.3) | 3927 (19.0) | 4345 (28.5) | 2776 (47.4) | 439 (51.0) | 41 (51.9) | <0.001 |
| High school or above, n (%) | 173 (18.4) | 1134 (19.6) | 2770 (18.6) | 3955 (19.1) | 3557 (23.3) | 2167 (37.0) | 410 (47.7) | 37 (46.8) | <0.001 |
| Income > 800, RMB/month, n (%) | 153 (16.3) | 975 (16.8) | 2152 (14.5) | 2773 (13.4) | 2182 (14.3) | 1177 (20.1) | 219 (25.5) | 16 (20.3) | <0.001 |
| BMI, kg/m2, mean (SD) | 28.22 ± 2.54 | 27.6 ± 2.83 | 26.63 ± 3.17 | 25.24 ± 3.36 | 23.37 ± 2.86 | 22.33 ± 2.39 | 22.04 ± ± 2.11 | 21.79 ± 2.08 | <0.001 |
| TC,mmol/L, mean (SD) | 235.86 ± 37.56 | 224.77 ± 42.05 | 206.52 ± 45.94 | 187.76 ± 43.36 | 176.53 ± 37.29 | 170.26 ± 30.87 | 167.12 ± 26.95 | 169.81 ± 20.17 | <0.001 |
| SBP, mmHg, mean (SD) | 140.94 ± 20.75 | 138.31 ± 18.81 | 135.52 ± 18.93 | 131.67 ± 19.24 | 124.28 ± 19.27 | 112.53 ± 16.74 | 111.77 ± 16.26 | 105.99 ± 8.79 | <0.001 |
| DSP, mmHg, mean (SD) | 90.24 ± 11.87 | 88.76 ± 10.88 | 86.81 ± 10.75 | 84.34 ± 10.76 | 79.86 ± 10.69 | 72.80 ± 9.36 | 72.48 ± 8.70 | 69.93 ± 6.15 | <0.001 |
| FBG, mmol/L, mean (SD) | 143.45 ± 45.78 | 119.57 ± 41.56 | 104.87 ± 34.41 | 95.33 ± 23.89 | 90.89 ± 16.58 | 88.8 ± 12.98 | 87.89 ± 10.05 | 86.55 ± 9.97 | <0.001 |
Data are presented as n, n (%) or mean ± SD. ANOVAs and χ2 tests were performed.
CVH indicates cardiovascular health; TC, total cholesterol; SBP, systolic blood pressure; DSP, diastolic blood pressure; FBG, fasting blood glucose; BMI, body mass index.
Changes in CVH metrics and stroke
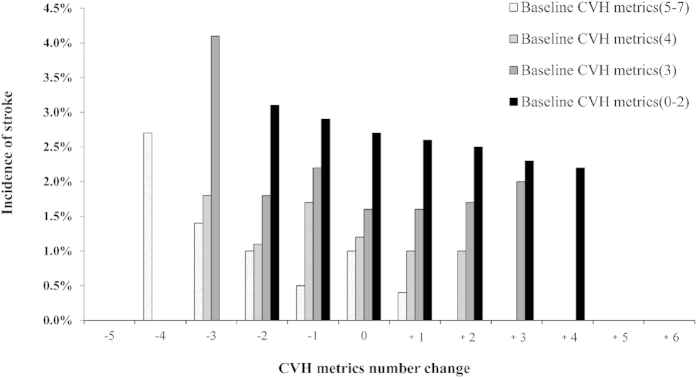
64373 participants were enrolled by year 2008, CVH metrics number of 32.54% participants improved (ideal CVH metrics number change ≥+1); 31.90% deteriorated (change ≤−1); 35.56% stayed the same. The relationship between the CVH metrics number change and the odds of having a stroke was graded; those with a greater increase in the CVH metrics number had a proportionally lower incidence of stroke (Fig. 2).
Figure 2. Relationship observed between change of ideal cardiovascular health (CVH) metrics number (from year 2006 to 2008) and the incidence of stroke according to different levels of CVH metrics number at baseline.
The horizontal axis indicates the change of CVH metrics number between year 2006 and 2008.
After adjusting for age, women, education, income, CVH metrics score at baseline, each increase in CVH metrics from 2006 to 2008 was associated with significantly reduced odds of experiencing a stroke (hazard ratios [HR] = 0.87; 95% confidence interval [CI], 0.83–0.92) (Table 2); i.e., such a positive change was associated with a 13% reduction in the odds of experiencing a stroke. Moreover, the interaction between gender and the change in CVH metrics number between 2006 and 2008 was not significant after adjusting for age, educational level, income, and CVH score at baseline (p = 0.727).
Table 2. Change in CVH metrics number from 2006 to 2008 predicting stroke in the follow-up.
| Variable | Stroke (n = 1182) |
Ischemic (n = 978) |
Intracerebral hemorrhagic (n = 196) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI)a | P Value | HR (95% CI)b | P Value | HR (95% CI)c | P Value | |
| Model 1 | 0.89 (0.85–0.94) | <0.001 | 0.87 (0.82–0.92) | <0.001 | 0.99 (0.87–1.13) | 0.87 |
| Model 2 | 0.87 (0.83–0.92) | <0.001 | 0.85 (0.80–0.90) | <0.001 | 0.97 (0.85–1.11) | 0.67 |
| Model 3 | 0.87 (0.83–0.92) | <0.001 | 0.85 (0.80–0.90) | <0.001 | 0.97 (0.85–1.11) | 0.67 |
CVH indicates cardiovascular health; HR, hazard ratios; CI, confidence interval; Cox proportional hazards regression was performed.
aModel 1: Adjusted for CVH metrics score at baseline.
bModel 2: Adjusted for age, women, CVH metrics score at baseline.
cModel 3: Adjusted for age, women, education, income, CVH metrics score at baseline.
Table 3 demonstrates that every 1-point increase in the total score from 2006 to 2008 resulted in a reduction in the odds of having a stroke after adjusting for age, women, education, income, CVH metrics score at baseline (HR = 0.89; 95% CI, 0.86–0.92).
Table 3. Change in CVH metrics score from 2006 to 2008 predicting stroke in the follow-up.
| Variable | Stroke (n = 1182) |
Ischemic (n = 978) |
Intracerebral hemorrhagic (n = 196) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI)a | P Value | HR (95% CI)b | P Value | HR (95% CI)c | P Value | |
| Model 1 | 0.91 (0.88–0.94) | <0.001 | 0.90 (0.87–0.94) | <0.001 | 0.93 (0.85–1.00) | 0.06 |
| Model 2 | 0.89 (0.86–0.92) | <0.001 | 0.88 (0.85–0.92) | <0.001 | 0.91 (0.84–0.99) | 0.03 |
| Model 3 | 0.89 (0.86–0.92) | <0.001 | 0.88 (0.85–0.92) | <0.001 | 0.91 (0.84–0.99) | 0.03 |
CVH indicates cardiovascular health; HR, hazard ratios; CI, confidence interval; Cox proportional hazards regression was performed.
aModel 1: Adjusted for CVH metrics score at baseline.
bModel 2: Adjusted for age, women, CVH metrics score at baseline.
cModel 3: Adjusted for age, women, education, income, CVH metrics score at baseline.
We ran separate models predicting stroke in the follow-up for every 1-point increase of each CVH metric score (Table 4). Every 1-point increase in the total score of physical activity/ total cholesterol/blood pressure/fasting blood glucose from 2006 to 2008 resulted in a reduction in the odds of having a stroke ([HR = 0.91; 95% CI, 0.83–0.99], [HR = 0.84; 95% CI, 0.77–0.92], [HR = 0.59; 95% CI, 0.53–0.66], [HR = 0.81; 95% CI, 0.73–0.89], respectively) after adjusting for age, women, education, income, CVH metrics score at baseline. However, changes in total score of smoke, salt intake and BMI were not significantly associated with the occurrence of stroke in the follow-up.
Table 4. Change in each CVH metric score from 2006 to 2008 predicting stroke in the follow-up.
| CVH metric | Stroke (n = 1182) |
Ischemic (n = 978) |
Intracerebral hemorrhagic (n = 196) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI)a | P Value | HR (95% CI)a | P Value | HR (95% CI)a | P Value | |
| Smoke | 1.00 (0.92–1.07) | 0.89 | 0.97 (0.89–1.05) | 0.45 | 1.17 (0.97–1.41) | 0.11 |
| Salt intake | 1.06 (0.94–1.19) | 0.33 | 1.03 (0.91–1.17) | 0.60 | 1.14 (0.86–1.50) | 0.38 |
| Physical activity | 0.91 (0.83–0.99) | 0.045 | 0.93 (0.84–1.03) | 0.15 | 0.84 (0.67–1.05) | 0.12 |
| TC | 0.84 (0.77–0.92) | <0.001 | 0.82 (0.75–0.91) | <0.001 | 0.92 (0.73–1.15) | 0.45 |
| BP | 0.59 (0.53–0.66) | <0.001 | 0.61 (0.54–0.69) | <0.001 | 0.52 (0.39–0.69) | <0.001 |
| FBG | 0.81 (0.73–0.89) | <0.001 | 0.79 (0.71–0.88) | <0.001 | 0.83 (0.65–1.06) | 0.14 |
| BMI | 0.89 (0.79–1.01) | 0.07 | 0.90 (0.79–1.03) | 0.14 | 0.86 (0.64–1.16) | 0.31 |
CVH indicates cardiovascular health; HR, hazard ratios; CI, confidence interval; TC, total cholesterol; BP, blood pressure; FBG, fasting blood glucose ; BMI, body mass index; Cox proportional hazards regression was performed.
aAdjusted for age, gender, education, income, CVH score at baseline.
To determine whether any single CVH metric accounted for the association between the combined CVH score and stroke, we removed each CVH metric one at a time and then reexamined the association (Table 5). The residual composite CVH metric remained significantly associated with stroke.
Table 5. Hazard ratios (P Value) for CVH metrics score change when 1 CVH metric is omitted.
| Omitted CVH metric | Stroke (n = 1182) |
Ischemic (n = 978) |
Intracerebral hemorrhagic (n = 196) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI)a | P Value | HR (95% CI)a | P Value | HR (95% CI)a | P Value | |
| Smoke | 0.87 (0.84–0.90) | <0.001 | 0.87 (0.83–0.91) | <0.001 | 0.87 (0.79–0.95) | 0.002 |
| Salt intake | 0.87 (0.84–0.90) | <0.001 | 0.87 (0.83–0.90) | <0.001 | 0.89 (0.82–0.91) | 0.01 |
| Physical activity | 0.89 (0.86–0.92) | <0.001 | 0.88 (0.85–0.92) | <0.001 | 0.92 (0.85–1.02) | 0.11 |
| TC | 0.89 (0.85–0.92) | <0.001 | 0.88 (0.85–0.92) | <0.001 | 0.90 (0.83–0.99) | 0.03 |
| BP | 0.91 (0.88–0.95) | <0.001 | 0.90 (0.87–0.94) | <0.001 | 0.95 (0.87–1.05) | 0.32 |
| FBG | 0.90 (0.86–0.93) | <0.001 | 0.89 (0.86–0.93) | <0.001 | 0.92 (0.84–1.01) | 0.07 |
| BMI | 0.88 (0.85–0.91) | <0.001 | 0.87 (0.84–0.91) | <0.001 | 0.91 (0.83–0.99) | 0.03 |
CVH indicates cardiovascular health; HR, hazard ratios; CI, confidence interval; TC, total cholesterol; BP, blood pressure; FBG, fasting blood glucose; BMI, body mass index; Cox proportional hazards regression was performed.
aAdjusted for age, gender, education, income, CVH score at baseline and 6 indicator variables representing the 7 possible baseline CVH metrics.
Discussion
Positive changes in ideal CVH metrics numbers and in overall CVH scores were significantly associated with a lower risk of stroke. These associations remained after adjusting for demographic variables and for baseline values for CVH metrics. To our knowledge, this is the first study to explore the relationship between changes in CVH metrics and the incidence of stroke. The Northern Manhattan Study (NOMAS)8 previously showed that an increase in the number of ideal health factors was associated with a reduced risk of stroke. Kulshreshtha9 et al. expanded on these findings by considering the full range of poor, intermediate, and ideal scores. These authors demonstrated that an improvement in one component of an ideal health metric by one level (e.g., from poor to intermediate or from intermediate to ideal) was associated with an 8% lower risk of stroke. Zhang10 et al. further demonstrated the potentially combined protective impact of ideal CVH metrics on ischemic and intracerebral hemorrhagic stroke in a Chinese population. Moreover, several studies7,13,14,15 have demonstrated a combined effect of several healthy lifestyle factors on stroke risk, which indicates that changes in lifestyle may reduce the incidence of stroke.
Previous studies have assessed this association at a single time point. Our findings demonstrate the benefits of increasing the number of ideal CVH indicators and of improving CVH score levels on the risk of stroke during the follow-up period. Furthermore, the effectiveness persisted when any 1 CVH metric was omitted, indicating the utility of the 7 CVH metrics as a whole. Because shifts in the distribution of risk factors within a population can have a dramatic impact on reducing the burden of disease, clinicians can help to reduce the risk of stroke in their patient populations by helping patients to maximize the number of ideal CVH metrics and by helping patients with poor health to transition to intermediate or ideal health.
Recent studies have suggested possible mechanisms responsible for reductions in the risk of stroke. Spring12 et al. observed that alterations in health behaviors are linked to alterations in the burden of subclinical atherosclerosis (coronary artery calcification and carotid intima-media thickness) after adjusting for demographic variables, medications, and baseline health and life factors. Because previous studies16,17,18 have demonstrated that carotid intima-media thickness is predictive of incident clinical stroke, it is reasonable to hypothesize that healthier behaviors, together with lower intima-media thickness, may contribute to a lower risk of stroke. In addition, Aatola11 et al. demonstrated that changes in ideal CVH status (both from childhood to adulthood and from young adulthood to middle age) were independent predictors of adult pulse-wave velocity (PWV), which is an index of arterial stiffness. A significant association between PWV and cerebral small vessel disease (SVD) has already been reported in several studies19,20; PWV exposes the small vessels in the brain to highly pulsatile pressure and flow, which may contribute to the pathogenesis of cerebral SVD. The consequences of SVD for the brain parenchyma are primarily lesions, such as lacunar infarcts, white matter lesions, large hemorrhages, and microbleeds, in subcortical structures21. In our study, we explored the relationship between changes in ideal CVH metrics and cerebrovascular disease, including ischemic and hemorrhagic stroke. It will be of great value to determine the association between changes in ideal CVH metrics and the incidence of cerebral SVD.
Consistent with previous studies9,15,22 that have demonstrated significant associations between ideal health behaviors or factors and the risk of total stroke, we also observed favorable effects of increased numbers of ideal CVH metrics on the incidence of total stroke. Although some studies7,10 have also observed a graded, inverse association between the number of healthy lifestyle indicators and the risk of ischemic and/or hemorrhagic stroke, the separate effects on ischemic or hemorrhagic stroke were not significant in our study. A possible reason for this difference may be that the relatively lower incidence of stroke that we observed precluded the association from reaching significance when stroke subtypes were considered individually.
Our study has several strengths, and it demonstrated, for the first time, the relationship between changes in CVH metrics and the incidence of stroke, with consecutive follow-up examinations, validated diagnostic methods and rigorous ascertainment of stroke outcomes. However, some limitations to our results should be considered. First, the Kailuan study was not a nationwide study, and a large proportion of the participants were manual workers. Thus, our findings may not be directly generalizable to other Chinese populations. Second, we used modified definitions of physical activity and diet to compute scores for the relevant CVH metrics. Salt intake is consistently associated with a risk of stroke23, and excessive intake of salted food is a serious problem in China. Moreover, the assessments of diet, smoking habits, and physical activity levels were all based on self-reports; thus, these assessments may not provide fully accurate information about exposure levels. Third, the mean follow-up period may not have been long enough to observe a sufficiently large number of stroke events to be able to classify these events into different subtypes for further discussion. Finally, we cannot completely exclude the effects of residual confounding factors that may be related to unmeasured lifestyle factors, such as socioeconomic or neuropsychological covariates (e.g., stress and depression), that may be associated with stroke risk.
In conclusion, our study demonstrates the favorable effect of positive changes in the number of ideal CVH metrics and in the levels of CVH scores on the risk of stroke in a Chinese population. Our findings provide evidence in support of the importance of promoting ideal health behaviors and factors for preventing stroke. Future prospective studies with larger sample sizes, longer follow-up periods and more detailed imaging are needed to determine whether our results hold in larger populations and over longer time periods and to explore the potential relationships between changes in CVH metrics and stroke subtypes.
Methods
Study Design and Population
The Kailuan study4 was a prospective cohort study conducted in the community of Kailuan in Tangshan, which is a large, modern city southeast of Beijing.
All participants underwent questionnaire assessments and clinical and laboratory examinations conducted in the 11 hospitals responsible for healthcare of this community. Measurements of parameters were made biennially, and we recorded adverse events annually. The study was performed in accordance with the guidelines of the Helsinki Declaration and was approved by the Ethics Committees of the Kailuan General Hospital, the Beijing Chaoyang Hospital, and the Beijing Tiantan Hospital. Every participant will sign a informed-consent before his/her participantion.
Ideal CVH metrics
Compared with the concept of ideal CVH in 20101, we used alternative measures for the dietary and physical activity metrics in this study. Ideal health behaviors and factors (no history of smoking, consumption of salt <6 g/d, moderate or vigorous physical activity for >80 min per week, BMI <25 kg/m2, untreated systolic BP <120 mmHg and diastolic BP <80 mmHg, untreated fasting blood glucose level <100 mg/dL, and untreated total cholesterol level <200 mg/dL) were defined and described in our previous study10.
In accordance with AHA definitions, scores for the 7 CVH metrics were classified as ideal, intermediate, or poor. We generated a total score that ranged from 0 to 14 by assigning a value of 0 for poor health, 1 for intermediate health, and 2 for ideal health for each metric. Demographic data (age, sex, household income and educational level) were collected using questionnaires.
Changes in number and total score of 7 ideal CVH metrics
Changes in the number of ideal CVH metrics (range −7 to +7) and in the total score (range −14 to +14) were calculated by subtracting the number or total score for the metrics obtained in 2006 from the number or total score obtained in 2008.
Follow-up and Stroke Assessment
The participants were followed-up in face-to-face interviews conducted at every 2-year routine medical examination until December 31, 2013 or to the event of interest, or death. The follow-ups were performed by trained physicians who were blinded to the baseline data that have been described in our previous study10. The primary outcome was the first occurrence of stroke, which was either the first nonfatal stroke or a stroke that resulted in death without a preceding nonfatal event. Stroke was diagnosed according to World Health Organization criteria24, and brain computed tomography (CT) or magnetic resonance (MR) scans were performed to confirm diagnoses. Strokes were classified into 3 main types: cerebral infarction, intracerebral hemorrhage, and subarachnoid hemorrhage. All stroke outcomes were validated by the Data Safety Monitoring Board and by the Arbitration Committee for Clinical Outcomes. Because of the small number of patients with subarachnoid hemorrhage (n = 33) and the differing etiologies of this type of stroke, we did not include subarachnoid hemorrhage in this study.
Statistical Analyses
Continuous variables were described as means ± SD and were compared using ANOVAs. Categorical variables were described as percentages and were compared using χ2 tests. For trends, we assigned a numeric value to the number of ideal CVH metrics for each subject and analyzed this value as a continuous variable.
Cox proportional hazards regression was performed with changes in the number and total scores of CVH metrics as the covariate, to obtain the hazard ratios (HR) and two-sided 95% confidence intervals (CI) of each increase in CVH metrics and every 1-point increase in total CVH score for the primary outcome of the first occurrence of stroke. In the model, we adjusted for age, gender, educational level and the average monthly income of each family member.
Because 11 hospitals participated in the study, we used a Cox proportional hazards model with a sandwich covariance matrix as a random effect to account for the potentially confounding effect of multiple hospitals participating in the study. All statistical tests were 2-sided, and the significance level was set at 0.05. Statistical analyses were performed using SAS 9.3 (SAS Institute; Cary, NC).
Additional Information
How to cite this article: Yang, X. et al. Positive changes in ideal CVH metrics reduce the incidence of stroke. Sci. Rep. 6, 19673; doi: 10.1038/srep19673 (2016).
Acknowledgments
This study was funded by the Ministry of Science and Technology and the Ministry of Health of the People’s Republic of China (National 11th & 12th Five-year S & T Major Project grant numbers 2006BAI01A11, 2011BAI08B01, and 2011BAI08B02).
Footnotes
Author Contributions X.M.Y. and A.X.W. analyzed the data and wrote the manuscript. X.X.L., S.S.A. and S.H.C. performed research. Y.L.W., Y.J.W. and S.L.W. conceived, designed and supervised the study.
References
- Lloyd-Jones D. M. et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 121, 586–613 (2010). [DOI] [PubMed] [Google Scholar]
- Ford E. S., Greenlund K. J. & Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 125, 987–995 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen T. T. et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 125, 1971–1978 (2012). [DOI] [PubMed] [Google Scholar]
- Wu S. et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 5, 487–493 (2012). [DOI] [PubMed] [Google Scholar]
- Yang Q. et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 307, 1273–1283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen-Torvik L. J. et al. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 127, 1270–1275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Lifestyle factors on the risks of ischemic and hemorrhagic stroke. Arch Intern Med. 171, 1811–1818 (2011). [DOI] [PubMed] [Google Scholar]
- Dong C. et al. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 125, 2975–2984 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshreshtha A. et al. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 44, 1909–1914 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. et al. Ideal Cardiovascular Health Metrics and the Risks of Ischemic and Intracerebral Hemorrhagic Stroke. Stroke. 44, 2451–2456 (2013). [DOI] [PubMed] [Google Scholar]
- Aatola H. et al. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. J Am Heart Assoc. 3, e000532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B. et al. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 130, 10–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth T. et al. Healthy lifestyle and the risk of stroke in women. Arch Intern Med. 166, 1403–1409 (2006). [DOI] [PubMed] [Google Scholar]
- Chiuve S. E. et al. Primary prevention of stroke by healthy lifestyle. Circulation. 118, 947–954 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S. C., Akesson A. & Wolk A. Healthy diet and lifestyle and risk of stroke in a prospective cohort of women. Neurology. 83, 1699–1704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary D. H. et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 340, 14–22 (1999). [DOI] [PubMed] [Google Scholar]
- Chambless L. E. et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 151, 478–487 (2000). [DOI] [PubMed] [Google Scholar]
- Rosvall M., Janzon L., Berglund G., Engstrom G. & Hedblad B. Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis. 179, 325–331 (2005). [DOI] [PubMed] [Google Scholar]
- Poels M. M. et al. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke. 43, 2637–2642 (2012). [DOI] [PubMed] [Google Scholar]
- Rosano C. et al. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension. 61, 160–165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701 (2010). [DOI] [PubMed] [Google Scholar]
- Tikk K. et al. Primary preventive potential for stroke by avoidance of major lifestyle risk factors: the European Prospective Investigation into Cancer and Nutrition-Heidelberg cohort. Stroke. 45, 2041–2046 (2014). [DOI] [PubMed] [Google Scholar]
- Li X. Y., Cai X. L., Bian P. D. & Hu L. R. High salt intake and stroke: meta-analysis of the epidemiologic evidence. CNS Neurosci Ther. 18, 691–701 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 20, 1407–1431 (1989). [DOI] [PubMed] [Google Scholar]