Abstract
The liver is a unique lymphoid organ whose microenvironment is biased towards tolerance induction. We previously found that a proportion of CD4+ autoreactive recent thymic emigrants (RTEs) retained in the liver after thymic egress and acquired IL-10 producing capability. To investigate the tolerance of these liver persisting CD4+ RTEs in more detail and to study the liver stromal cell types that facilitate the tolerogenic changes in young T cells, the phenotype and function of liver RTEs were further characterized and the impact of liver sinusoidal endothelial cells (LSECs) and Kupffer cells on RTEs were examined using an in vitro co-culture system. More than 70% of CD4+ CD44hi RTEs in the liver acquired Foxp3-LAG3+ CD49b− regulatory phenotype and function. But higher ratio of apoptosis with enhanced FasL and Bim expression was also found in these CD4+ liver RTEs when compared to those in the lymph nodes and spleen. LSECs played an important role in RTEs’ acquisition of tolerogenic and regulatory phenotype. These results indicate an important role of liver microenvironment in enforcing peripheral tolerance to CD4+ thymic emigrants against self- and gut-derived antigens.
The liver is a unique lymphoid organ whose microenvironment is biased towards tolerance induction. Such tolerance occurs when mature T cells encounter self or foreign antigens in the liver1, including administration of foreign antigens via the portal vein or mouth2,3, allogeneic liver transplantation4, and hepatotropic virus infection5,6. Even in the absence of antigen, the liver is known to selectively sequester and delete CD8+ T cells activated at sites distant from the liver7,8,9,10. The microenvironment in the liver induces T cell apoptosis and the acquisition of anergic phenotype in CD8+ T cells and regulatory functions in CD4+ T cells11,12,13,14,15.
The liver contains many distinct cell subsets that manifest antigen presenting cell (APC) activity with regulatory or tolerogenic features. These include dendritic cells (DCs), Kupffer cells (KCs), sinusoidal endothelial cells (LSECs), hepatic stellate cells, and even hepatocytes. The unique architecture of the hepatic sinusoids allows circulating T cells to make direct contact with these APCs and recognize self-, neo-, and gut-derived antigens presented by them16,17. The tolerogenic APCs in the liver express inhibitory or immunoregulatory molecules including prostaglandin E2 (PGE2), PD-L1, Fas ligand (FasL), LSECtin, and IL-10 which down-regulate the numbers and effector functions of antigen-specific T cells17,18,19. In addition, LSECs constitutively express adhesion molecules such as integrin ligands, intercellular adhesion molecules 1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1), facilitating the sequestration of activated T cells, in particular, CD8+ T cells8.
We previously identified a unique population of CD4+ recent thymic emigrants (RTEs) in the liver20. When adoptively transferred into lymphopenic RAG1−/− mice, liver-derived RTEs induce more severe inflammatory cell infiltration in the lung, liver, and intestine than lymph node (LN) RTEs, suggesting that liver may retain a proportion of autoreactive CD4+ RTEs that just escape from negative selection in the thymus. The adoptive transfer of thymic RTE precursors (mature Qa2+ CD4+ single positive (SP) thymocytes) into MHC II−/−, Ltbr−/−, or wild type recipients, or direct analysis of GFP+ CD4+ RTEs from RAG2p-GFP transgenic mice reveal that these liver persisting autoreactive RTEs acquire activated phenotype (CD44hiCD62LloPD-1loFoxp3−) in a MHC II-dependent and secondary lymphoid organ-independent manner. When compared to RTEs in the lymph nodes, RTEs in the liver produce higher level of IL-10 upon activation and respond poorly to IL-7-induced survival, indicating that these RTEs acquire tolerogenic properties in the liver microenvironment. To investigate the mechanisms of hepatic tolerogenicity to autoreactive young T cells that just emigrate from the thymus, the current study characterized the phenotype and function of CD4+ liver RTEs in more details and then studied the impact of liver KCs and LSECs on RTEs’ acquisition of tolerogenic phenotype.
Methods
Mice
C57BL/6 congenic mice (CD45.1+ and CD45.2+) were purchased from Peking University Health Science Center (Beijing, China). FVB-Tg (Rag2-EGFP) 1Mnz/J mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were backcrossed 10 generations onto the C57BL/6 background (termed as RAG2p-GFP in this paper). Mice were used at 5-11 weeks of age unless stated otherwise. The animals were kept in a specific pathogen-free facility at Peking University Health Science Center (Beijing, China). The experimental procedures on use and care of animals had been approved by the ethics committee of Peking University Health Science Center. This study was carried out in accordance with these approved guidelines.
Reagents
Anti-Fas (15A7), anti-FasL (MFL3), anti-LAG3 (eBioC9B7W), anti-TIGIT (GIGD7) and anti-FoxP3 (FJK-16s) were purchased from eBioscience (San Diego, CA, USA). Anti-Qa-2 (695H1-9-9) and anti-F4/80 (BM8) were purchased from BioLegend (San Diego, CA, USA). Anti-C3 (RmC11H9) was purchased from Cedarlane (Canada). Annexin V and PI were purchased from Biosea (Beijing, China). All other antibodies used in the study were purchased from BD Biosciences (San Diego, CA, USA). Percoll and Ficoll were purchased from Solarbio (Beijing, China) and Huajing Biotechnology (Shanghai, China), respectively. Recombinant mouse IL-7, Flt3 ligand and recombinant human TGF-β1, IL-2 were purchased from R&D Systems (Minneapolis, MN, USA). Collagenase IV was purchased from Invitrogen (Grand Island, NY, USA). DNase was purchased from Roche (Basel, Switzerland). γ-secretase inhibitor (N-[N-(3.5-difluorophenacetyl)-L-alanyl]-S-phenyl glycinet-butyl ester, DAPT) was purchased from Calbiochem (San Diego, CA, USA). Mouse TGF-β Elisa Kit was purchased from eBioscience (San Diego, CA, USA). Transwell membrane (0.4 μm) was purchased from Corning (NY, USA).
Cell isolation
CD4+ RTEs and CD4+ thymic RTE precursors were isolated as previously described20. Briefly, CD4+ CD8− CD25−NK1.1−GFP+ cells (RTEs) from the liver, lymph nodes or spleen were sorted using FACS Aria II (BD Biosciences). For the purification of CD4+ thymic RTE precursors, thymocytes were treated with anti-CD8 mAb and complement to remove CD8+ cells. The thymocytes were then stained with a series of antibodies and the cells with the following phenotype CD4+ CD8− Qa2+ CD69− CD25− CD44loNK1.1− were sorted. The purity of these T cell populations was >97% when analyzed by flow cytometry.
For isolation of non-parenchymal cells (NPCs) in the liver, C57BL/6 mice (CD45.2+) were sacrificed and the livers were perfused with 10-20 ml of 0.5 mg/ml collagenase IV in PBS. Livers were mechanically disrupted and incubated with 0.5 mg/ml collagenase IV and 0.25 mg/ml DNase in 20 ml PBS for 30-40 minutes at 37 °C with constant rotation (200 rpm). The resulting cell suspension was then passed through a 150 μm sterile stainless steel meshes. After centrifugation at 500 g for 5 min at 4 °C, the pellet was suspended in an isotonic 35% Percoll solution, and centrifuged at 1000 g for 10 min at room temperature. The resulting pellet was resuspended in ammonium chloride to remove red blood cells and NPCs in the liver were obtained. NPCs were further stained with CD146 and F4/80 to isolate sinusoidal endothelial cells (CD146+) and kupffer cells (F4/80+).
For isolation of stromal cells in the spleen, spleens from C57BL/6 mice (CD45.2+) were mechanically disrupted and incubated with 2 mg/ml collagenase IV and 0.5 mg/ml DNase in 8 ml PBS for 30 minutes at 37 °C with constant rotation (200 rpm). The resulting cell suspension was then passed through a sterile stainless steel meshes and centrifuged at 1500 rpm for 5 min at 4 °C. The cell pellet was resuspended in 4 ml of Ficoll and layered over 4 ml of Ficoll, with 2 ml of PBS further layered on top. After centrifugation at 1700 g for 10 min at 4 °C, stromal cells in the spleen at the interface between PBS and Ficoll were harvested. Flt3 ligand-induced DC (FLDC) was obtained as previously described21.
Low-dose TCR stimulation and TGF-β ELISA
CD4+ RTEs were cultured with plate-bound anti-CD3 (0.5 μg/ml) and soluble anti-CD28 (0.25 μg/ml). The supernatants were collected at 72 h of culture and the concentration of TGF-β was measured by Mouse ELISA Kit (eBioscience).
In vitro suppression assay
For iTreg induction, CD4+ CD8− CD25− CD62L+ CD44lo naive T cells were isolated from C57BL/6 mice (CD45.2+) and plated at 2 × 105 cells per well of a 96-well flat-bottom plate with plate-bound anti-CD3 (2 μg/ml), soluble anti-CD28 (1 μg/ml), rhIL-2 (2 ng/ml), rhTGF-β1 (1 ng/ml), anti-IFN-γ (5 μg/ml) and anti-IL-4 (5 μg/ml). The cells were harvested 3 days later and were used in the suppression assay. For the in vitro suppression assay, CFSE labeled CD4+ CD8− CD25− CD62L+ CD44lo naive T cells from CD45.1+ C57BL/6 mice were either cultured alone or co-cultured with iTregs, or with CD4+ RTEs from the liver or mesenteric lymph nodes of CD45.2+ congenic mice at the ratio of 1:1. Anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) were added in the co-culture system. The proliferation of T cells was determined by CFSE dilution of CD45.1+ T cells under various culture conditions 2 days later or BrdU incorporation and staining 3 days later.
T-APC co-culture
CD45.1+ CD4+ RTE precursors (5 × 105 per well) were stimulated with anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) in the presence of 1 × 105 CD45.2+ NPCs or splenic stromal cells, or 7 × 104 LSECs, or KCs, or FLDCs. On the third day, 2 ng/ml of rhIL-2 was added. After 5 days of co-culture, T cells were collected for further analysis of IL-7 responsiveness, FasL and LAG3 expressions by flow cytometry, and IL-10 production by flow cytometry after restimulation with plate-bound anti-CD3 (2 μg/ml) for one additional day. CD45.1+ T cells were gated in these analysis. The transwell membrane (0.4 μm) was used to separate LSEC from CD4+ RTE precursors when needed. γ-secretase inhibitor (5 μM) was used to block the Notch signaling.
IL-7 responsiveness
T cells were purified and cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT) with or without 1 ng/ml IL-7. Twenty-four hours later, the cells were collected and stained with anti-CD4, Annexin V, and PI.
Quantitative RT-PCR
RNA was extracted from the CD4+ RTEs from the lymph nodes and liver using TRIZOL (Invitrogen, Grand Island, NY, USA), and cDNA was obtained using the FastQuant RT Kit (TIANGEN, Beijing, China). Quantitative Real-Time PCR was performed using SYBR Green Supermix (Bio-Rad Hemel Hempstead, England, UK) on an iCycler real-time PCR system (Bio-Rad Ltd, Hemel Hempstead, England, UK), with each sample in triplicate. Primers were as follows: Bcl2 forward, 5′-CCATGTGGCTATGCGG-3′; Bcl2 reverse, 5′-ATCAGCCACGCCTAAA-3′; Bcl-xl forward, 5′-GGACCGCGTATCAGAG-3′; Bcl-xl reverse, 5′-GCATTGTTCCCGTAGAG-3′; Bim forward, 5′-CGACAGTCTCAGGAGGAACC-3′; Bim reverse, 5′-CCTTCTCCATACCAGACGGA-3′; Bax forward, 5′-GTGGTTGCCCTCTTCTACTTTG-3′; Bax reverse, 5′-CACAAAGATGGTCACTGTCTGC-3′; Bak forward, 5′-CGAGATGGACAACTTGCCCCTGG-3′; Bak reverse, 5′-CAGCTGATGCCACTCTTAAATAGGCT-3′. The quantifications were based on ΔΔCT calculations and were normalized to GAPDH as loading controls.
Statistics
The statistical analysis of the results was performed using GraphPad Prism 5 software (San Diego, CA, USA). Unpaired or two-tail paired Student t-test was used to evaluate the significance of the differences between two groups. The following terminology is used to denote the statistical significance: *p < 0.05, **p < 0.01, ***p < 0.005.
Results
Liver persisting CD4+ RTEs have regulatory functions
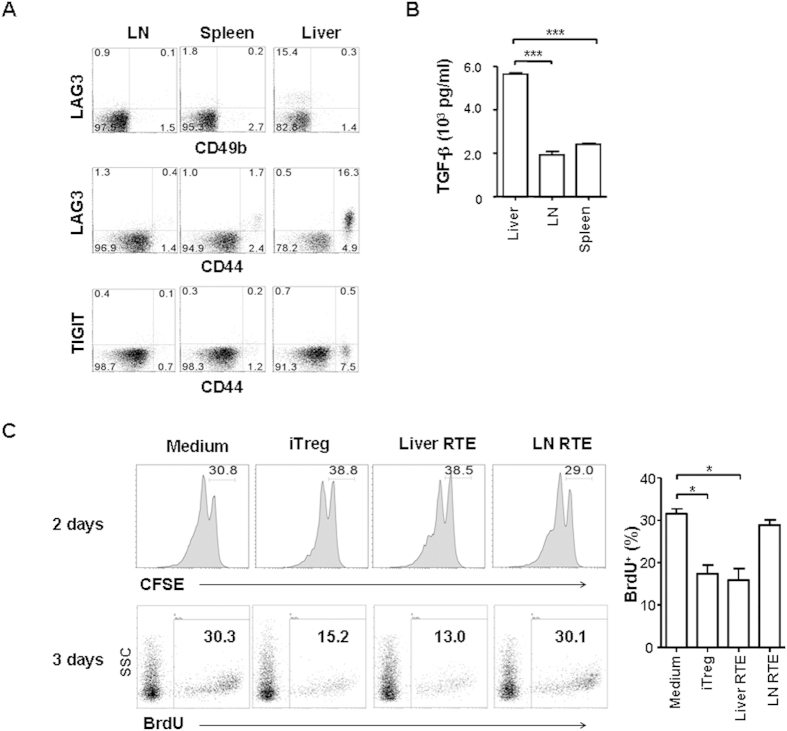
We previously found that liver persisting CD4+ RTEs with CD25− Foxp3− phenotype produced large amounts of IL-1020, reminiscent of peripherally derived CD4+ Foxp3- type 1 regulatory T cells (Tr1)22,23,24. We thus examined whether liver RTEs express important Tr1 surface markers. About 76.9% GFP+ CD4+ CD8− CD44hi RTEs in the liver of RAG2p-GFP transgenic mice expressed high level of lymphocyte activation gene (LAG)3 whereas 22.2% and 41.5% CD4+ CD44hi RTEs in the LN and spleen, respectively, expressed low level of LAG3 (Fig. 1A). No significant expression of CD49b was found in RTEs (Fig. 1A). The expression of inhibitory receptor TIGIT (T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain) was also measured and was found negative in RTEs (Fig. 1A). In addition to higher IL-10 production, CD4+ RTEs in the liver also secreted more TGF-β than RTEs in the LN and spleen (Fig. 1B).
Figure 1. The regulatory functions of liver persisting CD4+ RTEs.
(A) Higher expression of LAG3 by liver CD4+ RTEs. GFP+ CD4+ CD8− CD25-NK1.1- RTEs from mesenteric LN, spleen, and liver were examined for LAG3, CD49b, CD44, and TIGIT expression. Three independent experiments were performed and similar results were obtained. (B) The production of TGF-β by activated RTEs. GFP+ CD4+ CD8− CD25-NK1.1- RTEs from LN, spleen, and liver were stimulated with plate-bound anti-CD3 (0.5 μg/ml) and soluble anti-CD28 (0.25 μg/ml) for 3 days and the concentration of TGF-β in the supernatant was examined by ELISA. The statistical significance between any two tissues was calculated by Student t-test. (C) Liver CD4+ RTEs suppress the proliferation of naive T cells in vitro. CFSE labeled CD4+ CD8− CD25− CD62L+ CD44lo naive T cells purified from LN of CD45.1+ C57BL/6 mice were either cultured alone, or co-cultured with iTregs or CD4+ RTEs sorted from the liver or LN of CD45.2+ congenic mice at the ratio of 1:1. Anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) were added in the co-culture system. iTregs prepared in vitro was used as a positive control. The proliferation of CD45.1+ T cells was measured by CFSE dilution 2 days later or BrdU incorporation and staining 3 days later. The numbers in the plots with CFSE labeling indicated the cell ratio that did not enter cell cycle whereas the numbers in the plots with BrdU staining indicated the cell percentages that undergo proliferation. The average percentages of BrdU positive cells (with standard deviation) from three independent experiments were shown on the right and the statistical significance between any two groups was calculated by Student t-test.
To assess whether the expression of LAG3, IL-10, and TGF-β in CD4+ liver RTEs is associated with suppressor activity indicative of regulatory T cells, CD4+ RTEs purified from the liver and LN were co-incubated with anti-CD3- and anti-CD28-activated CD4+ CD25− naive T cells from LN. Induced regulatory T cells (Foxp3+ iTregs) prepared in vitro was used as a positive control. As shown in Fig. 1C, comparable suppression of naïve T cell proliferation, as measured by CFSE on day 2 or BrdU on day 3, was found in the wells co-cultured with liver RTEs and iTregs. LN RTEs had no effect on inhibiting T cell proliferation. These results indicate that some CD4+ RTEs acquire regulatory phenotype and function in the liver.
Liver persisting CD4+ RTEs undergo apoptosis
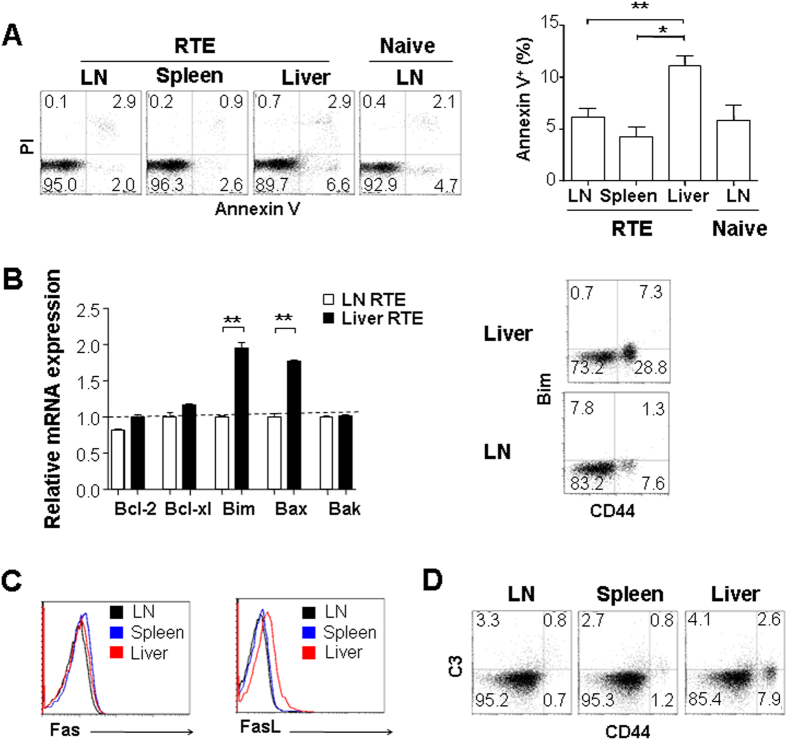
We previously found that the survival of liver CD4+ RTEs could not be improved by the addition of IL-720. To examine the homeostasis of these RTEs with regulatory phenotype, CD4+ RTEs in the liver, LN, and spleen of RAG2p-GFP transgenic mice were compared for their ex vivo apoptosis with Annexin V staining. As shown in Fig. 2A, the highest ratio of cell apoptosis was found in liver RTEs. Consistently, liver RTEs expressed about 2-fold higher levels of pro-apoptotic molecules Bim and Bax than LN RTEs (Fig. 2B). The expressions of anti-apoptotic molecules including Bcl-2 and Bcl-xL were similar in RTEs obtained from the liver, LN, and spleen.
Figure 2. The apoptosis of liver persisting CD4+ RTEs.
(A) RTEs in the liver undergo more apoptosis than those in other lymphoid tissues. GFP+ CD4+ CD8− CD25- RTEs from mesenteric lymph nodes (LN), spleen, and liver of RAG2p-GFP transgenic mice were stained with Annexin V and Propidium iodide (PI) and analyzed by flow cytometry. The average percentages of annexin V+ cells were plotted and the statistical significance between any two tissues was calculated by Student t-test. (B) CD4+ RTEs in the liver express higher level of pro-apoptotic molecules Bim and Bax when compared to RTEs in LN. Total RNA was extracted from CD4+ RTEs purified from LNs and liver and quantitative RT-PCR was performed to compare the transcription of Bcl-2, Bcl-xl, Bim, Bax, and Bak (left panel). The comparison of Bim protein level by flow cytometry was also performed in liver and LN RTEs (right panel). (C) Expression of FasL, but not Fas in CD4+ RTEs is higher in the liver than in LNs and spleen. (D) Similar levels of complement C3 deposition on the surface of CD4+ RTEs obtained from the liver, LN, and spleen. Two or three independent experiments were performed and similar results were obtained.
Although lowered IL-7 responsiveness in liver RTEs may partially explain the higher death rate of liver RTEs20, other death pathways were further examined. Fas/FasL regulates immune homeostasis and tolerance via inducing apoptosis of activated T cells25,26,27,28. Compared to CD4+ RTEs obtained from LN and spleen, cells from the liver expressed higher level of FasL but not Fas (Fig. 2C).
Hsu, et al. found that immature RTEs, particularly NKAP-deficient RTEs, could be eliminated by the classical complement activation pathway29. We thus examined whether more complement C3 was bound to liver RTEs. As shown in Fig. 2D, similar levels of C3 were found on the surface of RTEs obtained from the liver, LN, and spleen, suggesting that complement-mediated cell deletion may not be specifically involved in the regulation of RTEs in the liver. Together, these results suggest that Fas/FasL pathway and Bim-mediated mitochondria pathway may together lead to the increased apoptosis of autoreactive CD4+ RTEs in the liver.
LSECs induce the tolerogenic phenotype of CD4+ RTEs
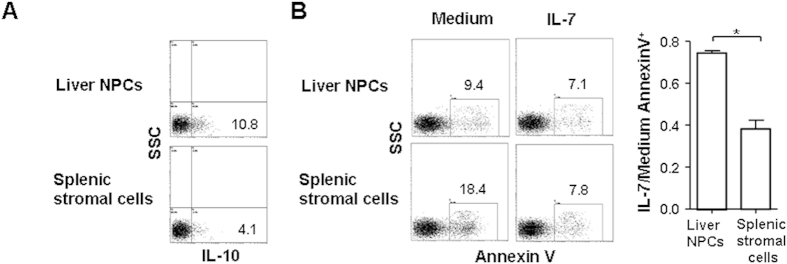
Using Ltbr−/− mice with defects in secondary lymphoid organ, we previously found that the retention and activation of a proportion of CD4+ RTEs mainly occurred in the liver, independent of the signals from LN and spleen. This suggests that CD4+ RTEs’ acquisition of tolerogenic phenotype depends on liver microenvironment20. To investigate the tolerogenic role of cellular components in the liver, an in vitro T-APC co-culture system was applied and the mixed population of liver stromal cells was first tested. Stromal cells purified from the spleen were used as controls. As RTEs from LN, spleen, and liver showed different properties that may be acquired during their presence in these organs, we purified thymic CD4+ RTE precursors (Qa2+ CD69− CD44lo CD25− CD4+ CD8− mature SP thymocytes) for the T-APC co-culture20. The IL-7 responsiveness, the expression of IL-10, FasL, and LAG3 in T cells 4 days after co-culture were examined. Undetectable level of FasL and LAG3 expression in CD4+ RTE precursors was found before the in vitro co-culture (data not shown). Compared to splenic stromal cells, T cells co-cultured with liver non-parenchymal cells (NPCs) showed nearly 2.5-fold more IL-10 production (Fig. 3A). The survival of T cells co-cultured with liver NPCs was better than that of T cells with splenic stromal cells (Fig. 3B). However, the addition of IL-7 reduced the apoptosis of T cells by half in the wells co-cultured with splenic stromal cells whereas it did not decrease the apoptosis of T cells co-cultured with liver NPCs (Fig. 3B). These results indicate the important roles of liver NPCs in inducing IL-10 expression and reducing IL-7 responsiveness in RTEs.
Figure 3. Liver NPCs induce the tolerogenic phenotype in CD4+ RTEs.
CD4+ RTE precursors (CD4+ CD8− Qa2+ CD69− CD44lo thymocytes, 5 × 105 cells per well) were stimulated with anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) in the presence of 1 × 105 NPCs or 1 × 105 splenic stromal cells. On the third day, 2 ng/ml rhIL-2 was added. After 5 days of co-culture, the T cells were collected for further analysis. (A) Increased IL-10 production in T cells co-cultured with liver NPCs. T cells were re-stimulated with plate-bound anti-CD3 (2 μg/ml) for one day and IL-10 production was measured by flow cytometry. (B) Reduced IL-7 responsiveness in T cells co-cultured with liver NPCs. T cells were cultured in the presence or absence of 1 ng/ml IL-7 for one day and Annexin V staining was used to measure the apoptosis of T cells. The apoptosis of T cells cultured in the presence of IL-7 was calculated against that of cells cultured in medium and the mean ratios of three independent experiments were shown on the right.
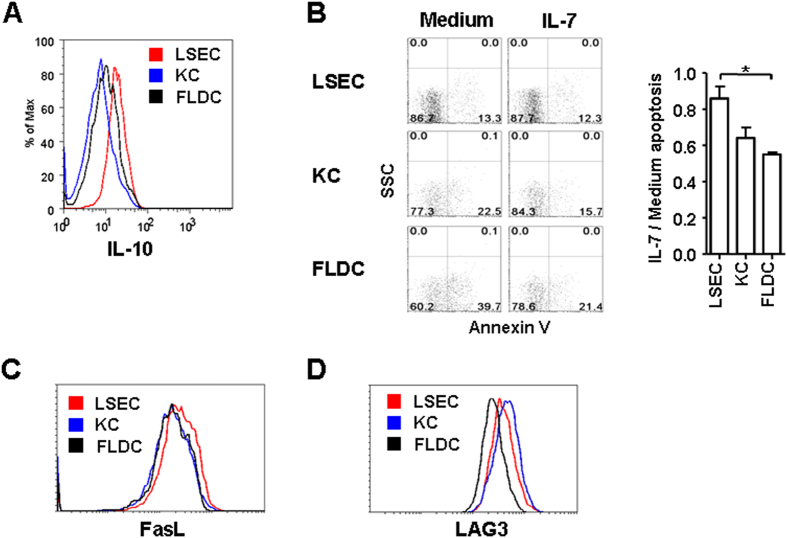
LSECs and KCs are two main stromal cell types involved in the liver tolerogenic regulation. To investigate their roles on RTE tolerance, thymic RTE precursors were co-cultured with purified liver LSECs and KCs. The co-culture with Flt3 ligand induced DCs (FLDCs) was used as a control. As shown in Fig. 4A, CD4+ RTE precursors co-cultured with LSECs revealed highest level of IL-10 production whereas those cultured with KCs produced lowest IL-10. In the absence of IL-7, LSECs showed highest capability in improving the survival of RTE precursors (Fig. 4B). In the presence of IL-7, however, the survival of T cells co-cultured with KCs and FLDCs was greatly improved whereas that of T cells with LSECs was not. After 4-day culture with LSECs, RTE precursors expressed high level of FasL (Fig. 4C). No significant FasL up-regulation was found in T cells co-cultured with KCs and FLDCs. Both KCs and LSECs induced the expression of suppressor marker LAG3 in RTE precursors whereas FLDCs did not (Fig. 4D). These results suggest that LSECs may be the main contributors in the acquisition of tolerogenic phenotype in autoreactive CD4+ RTEs and KCs may also participate in the induction of suppressor function.
Figure 4. LSECs contribute to the tolerogenic induction of CD4+ RTEs.
CD4+ RTE precursors (5 × 105 per well) were stimulated with anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) in the presence of 7 × 104 LSECs, KCs or FLDCs. On the third day, 2 ng/ml of rhIL-2 was added. After 5 days of co-culture, T cells were collected for further analysis. (A) Enhanced IL-10 production in T cells co-cultured with LSECs. T cells were restimulated with plate-bound anti-CD3 (2 μg/ml) for one day and IL-10 production by T cells was measured by flow cytometry. (B) Reduced IL-7 responsiveness in T cells co-cultured with LSECs. The apoptosis of T cells cultured in the presence of IL-7 was calculated against that of cells cultured in medium and the mean ratios of three independent experiments were shown on the right. (C) Increased expression of FasL in T cells co-cultured with LSECs. (D) Increased expression of LAG3 in T cells co-cultured with LSECs and KCs. Data are representative of two independent experiments.
Cellular interaction is required for and Notch signaling pathway is partially involved in the tolerance induction of CD4+ RTEs by LSECs
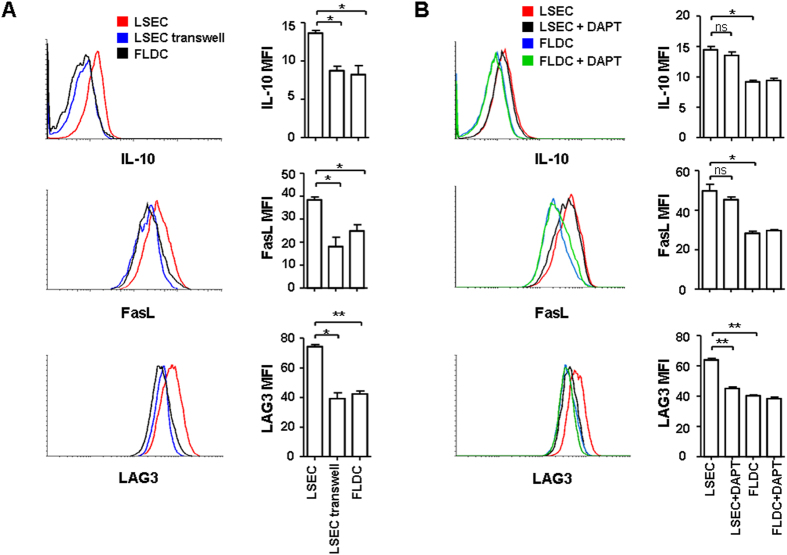
To investigate whether cellular interaction is required for the induction of CD4+ RTE tolerance by LSECs in the liver, we compared the phenotype of RTE precursors cultured with LSECs in the same well or separately in the transwell system. As shown in Fig. 5A, the T cells’ up-regulation of IL-10, FasL, and LAG3 was only found in the wells having CD4+ RTE precursors and LSECs cultured together. This suggests that direct cellular interaction with LSECs is required for the induction of tolerogenic phenotype of CD4+ RTEs.
Figure 5. Cellular interaction between LSECs and T cells and Notch signaling are important in the tolerance induction of CD4+ RTEs.
(A) Cellular interaction is required for the tolerance induction of CD4+ RTEs by LSECs. CD4+ RTE precursors were either cultured with LSECs in the same well, or in the upper well of the transwell system, with LSECs seeded on the bottom of the well. The T cell culture with FLDCs in the same well was used as a control. Anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) were added in the co-culture system. On the third day, 2 ng/ml of rhIL-2 was added. After 5 days of co-culture, T cells were collected and restimulated with plate-bound anti-CD3 (2 μg/ml) for one additional day and IL-10 expression in T cells was measured by flow cytometry. T cells were also examined for their expressions of FasL and LAG3. The mean fluorescence intensities (and standard deviation) of IL-10, FasL, and LAG3 in three experiments were shown on the right panel. (B) The Notch signaling pathway contributes to CD4+ RTEs’ tolerance induction by LSECs. CD4+ RTE precursors were cultured with LSECs or FLDCs in the same well in the presence of anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml). DAPT, a γ-secretase inhibitor (5 μM) was added in the co-culture system to block the Notch signaling. On the third day, 2 ng/ml of rhIL-2 was added. After 5 days of co-culture, CD4+ RTE precursors were collected and analyzed for the expressions of IL-10, FasL, and LAG3. The mean fluorescence intensities (and standard deviation) of IL-10, FasL, and LAG3 in three experiments were shown on the right panel.
Recently, the Notch signaling pathway activated by hepatocytes or LSECs has been reported to induce IL-10 production in Th1 cells30,31. We thus examined whether the phenotype of CD4+ RTE precursors acquired from T-LSEC co-culture depends on Notch. As shown in Fig. 5B, the addition of γ-secretase inhibitor (DAPT) which could block Notch activation prevented LAG3 upregulation in T cells. The induction of IL-10 and FasL expression, however, was not affected. These results suggest that the Notch pathway partially contributes to the liver tolerance of CD4+ RTEs.
Discussions
We have previously found a unique population of CD4+ RTEs in the liver that could induce severe inflammation and T cell infiltration in the lung and colon following transfer into RAG−/− recipients20. These autoreactive CD4+ liver RTEs have activated phenotype (CD44hiCD62Llo) and more than 40% of CD44hiCD4+ RTEs expressed CXCR3. The majority of these cells is Foxp3− and does not express inhibitory molecules PD-1, CD73, and FR4. The acquirement of this unique phenotype is independent of secondary lymphoid organs and relies on RTEs entering the liver. Upon low concentration of anti-CD3 and anti-CD28 stimulation, CD4+ RTEs in the liver proliferated faster and produced higher levels of IL-10 as well as IL-2, TNF-α, IFN-γ, and IL-4 than RTEs in the lymph nodes and spleen20. The current study further found high expression of LAG3, FasL, and TGF-β but not CD49b in CD4+ liver RTEs. Compared to iTregs, these liver RTEs showed similar level of inhibition of naïve T cell proliferation. Thus, the regulatory features of CD44hiFoxp3− CD4+ RTEs acquired in the liver under steady state condition are distinct from those of the conventional CD4+ Foxp3+ Treg cells, CD4+ Foxp3-LAG3+ CD49b+ Tr1 cells, and IL-10-producing T helper 1 (Th1) cells22,23,24,31,32,33,34.
The modulation of immune responses in the liver, in particular, the induction of tolerance is mediated by specialized liver-resident APCs, including LSECs, KCs, tolerogenic DCs, and hepatocytes35,36,37. Among them, LSECs have been shown to promote the anergy of CD8+ T cells, the differentiation of Foxp3+ CD4+ or Foxp3−CD4+ regulatory T cells, and the differentiation of IL-10+ IFN-γ+ Th1 cells 11,12,13,14,15,38. In our results, LSECs were more efficient than KCs in inducing IL-10 and FasL expressions and down-regulating IL-7 responsiveness in CD4+ RTEs. LSECs could also induce LAG3 expression in CD4+ RTEs, agreeing well with the findings that LSECs induce the differentiation of LAG3+ Foxp3− Tr1 cells14,39.
PD-1-PD-L1 interaction, LSECtin-CD44 interaction, membrane-bound TGF-β, and Notch pathway have been reported to contribute to LSEC-induced T cell tolerance11,13,40,41,42,43,44. As liver RTEs are PD-1lo IL-2+ IFN-γ+, PD-L1 and LSECtin may not play a major role in RTEs’ tolerance induction. It has been found that LSECs could express all four Notch ligands, Jagged1, Jagged2, Dll1, and Dll415,40. The interaction of Notch with its ligands induced IL-10 production in activated T cells or differentiated Th1 cells or PD-1 expression in activated T cells, resulting in the suppression of immune responses or maintenance of T cell exhaustion15,40,45,46. Interestingly, our results with γ-secretase inhibitor suggest for the first time that the activation of Notch pathway is involved in LSECs’ capability of LAG3 induction in CD4+ RTEs. Whether membrane-bound TGF-β is involved and coordinates with the Notch pathway to induce other tolerogenic phenotype in RTEs awaits further investigation. Together, the fenestrated LSECs that line the hepatic sinusoids may provide antigen-specific and Notch signals and likely other signals during transmigration of CD4+ RTEs across the endothelium, and induce the responding RTEs to become tolerogenic and express regulatory functions. KCs or even other liver APCs may be also involved in this tolerogenic regulation.
The regulatory functions of CD4+ liver RTEs to other T cells or even APCs are probably via IL-10 and/or FasL expression. IL-10 may activate STAT3 and its target gene expression in other T cells or even autoreactive CD4+ RTEs themselves and eventually limiting the activation and inflammatory cytokine production of these T cells47,48,49. FasL expression in CD4+ RTEs may facilitate the binding to its receptor Fas/CD95 on T cells and induce extrinsic pathway of cell apoptosis. The interaction of FasL and Fas leads to the recruitement of Fas-associated protein with death domain (FADD) and the activation of caspase 8. Activated caspase 8 can either directly cleave pro-caspase-3 and -7, or cleave the BH3-only protein Bid to activate Bax/Bak-mediated mitochondrial apoptotic pathway50. Whether high expression of IFN-γ by CD4+ liver RTEs plays an important regulatory role remains elusive. However, IFN-γ was reported to contribute to antigen-specific regulatory cell differentiation, development, or clonal deletion, thus maintaining immune tolerance 51,52.
Notably, the impact of the regulatory function of CD44hiCD4+ liver RTEs may be limited under steady state condition as these cells expressed high levels of FasL and Bim, responded poorly to IL-7-mediated cell survival, an indication of deletion of these CD4+ RTEs in the liver. Indeed, after being transferred into lymphoreplete mice via tail vein injection, the number of donor thymic CD4+ RTE precursors in the liver was decreased within 2-4 weeks20, suggesting that apoptotic cell death is the final fate of CD44hiCD4+ RTEs in the liver after a transient activation and tolerance induction. However, current results do not exclude the possibility that under special conditions these CD4+ CD44hi LAG3+ FasL+ IL-10+ IFN-γ+ RTEs in the liver may participate in the regulation of immune responses20,53.
Taken together, our data reveal that some newly generated CD4+ thymic emigrants acquire tolerogenic and suppressor phenotype in the liver and eventually undergo apoptosis. LSECs and the activation of Notch signaling pathway contribute to RTEs’ tolerance induction.
Additional Information
How to cite this article: Xu, X. et al. Liver sinusoidal endothelial cells induce tolerance of autoreactive CD4+ recent thymic emigrants. Sci. Rep. 6, 19861; doi: 10.1038/srep19861 (2016).
Acknowledgments
The authors wish to thank Prof. Li Tang (Beijing Institute of Radiation Medicine) for helpful discussion. This work was supported by grants from the National Natural Science Foundation of China (31270935 and 81471525), Beijing Natural Science Foundation (5152010, Q.G., 7132114, R.J.).
Footnotes
Author Contributions X.X., designed and performed the experiments, prepared figures; M.L., K.W., S.Z. and R.J. performed the experiments; J.H. and X.S. prepared the reagents and samples; Y.Z. helped with experimental design; H.W. helped with flow cytometry experiments and figure preparation; Z.J. analyzed the data; Q.G., designed the experiments and wrote the manuscript. All authors reviewed the manuscript.
References
- Benseler V. et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci USA 108, 16735–16740 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Liu Q., Grosfeld J. L. & Pescovitz M. D. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J Pediatr Surg 29, 1145–1148 (1994). [DOI] [PubMed] [Google Scholar]
- Cantor H. M. & Dumont A. E. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature 215, 744–745 (1967). [DOI] [PubMed] [Google Scholar]
- Calne R. Y. Immunosuppression in liver transplantation. N Engl J Med 331, 1154–1155 (1994). [DOI] [PubMed] [Google Scholar]
- Leroy V. et al. Phenotypic and functional characterization of intrahepatic T lymphocytes during chronic hepatitis C. Hepatology 38, 829–841 (2003). [DOI] [PubMed] [Google Scholar]
- Cooper S. et al. Analysis of a successful immune response against hepatitis C virus. Immunity 10, 439–449 (1999). [DOI] [PubMed] [Google Scholar]
- Kuniyasu Y., Marfani S. M., Inayat I. B., Sheikh S. Z. & Mehal W. Z. Kupffer cells required for high affinity peptide-induced deletion, not retention, of activated CD8+T cells by mouse liver. Hepatology 39, 1017–1027 (2004). [DOI] [PubMed] [Google Scholar]
- John B. & Crispe I. N. Passive and active mechanisms trap activated CD8+T cells in the liver. J Immunol 172, 5222–5229 (2004). [DOI] [PubMed] [Google Scholar]
- John B., Klein I. & Crispe I. N. Immune role of hepatic TLR-4 revealed by orthotopic mouse liver transplantation. Hepatology 45, 178–186 (2007). [DOI] [PubMed] [Google Scholar]
- John B. & Crispe I. N. TLR-4 regulates CD8+T cell trapping in the liver. J Immunol 175, 1643–1650 (2005). [DOI] [PubMed] [Google Scholar]
- Diehl L. et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+T cell tolerance. Hepatology 47, 296–305 (2008). [DOI] [PubMed] [Google Scholar]
- Limmer A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+T cells results in antigen-specific T-cell tolerance. Nat Med 6, 1348–1354 (2000). [DOI] [PubMed] [Google Scholar]
- Carambia A. et al. TGF-beta-dependent induction of CD4+ CD25+ Foxp3+ Tregs by liver sinusoidal endothelial cells. J Hepatol 61, 594–599 (2014). [DOI] [PubMed] [Google Scholar]
- Kruse N. et al. Priming of CD4+T cells by liver sinusoidal endothelial cells induces CD25low forkhead box protein 3- regulatory T cells suppressing autoimmune hepatitis. Hepatology 50, 1904–1913 (2009). [DOI] [PubMed] [Google Scholar]
- Neumann K. et al. Liver sinusoidal endothelial cells induce immunosuppressive IL-10-producing Th1 cells via the Notch pathway. Eur J Immunol 45, 2008–2016 (2015). [DOI] [PubMed] [Google Scholar]
- Warren A. et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 44, 1182–1190 (2006). [DOI] [PubMed] [Google Scholar]
- Thomson A. W. & Knolle P. A. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 10, 753–766 (2010). [DOI] [PubMed] [Google Scholar]
- Crispe I. N. The liver as a lymphoid organ. Annu Rev Immunol 27, 147–163 (2009). [DOI] [PubMed] [Google Scholar]
- Kern M., Popov A., Kurts C., Schultze J. L. & Knolle P. A. Taking off the brakes: T cell immunity in the liver. Trends Immunol 31, 311–317 (2010). [DOI] [PubMed] [Google Scholar]
- Xu X. et al. Retention and tolerance of autoreactive CD4+ recent thymic emigrants in the liver. J Autoimmun 56, 87–97 (2015). [DOI] [PubMed] [Google Scholar]
- Dong J. et al. Homeostatic Properties and Phenotypic Maturation of Murine CD4+ Pre-Thymic Emigrants in the Thymus. PLoS One 8, e56378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Zhang R., Jin B. & Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol 12, 566–571 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura T. et al. CD4+ CD25−LAG3+regulatory T cells controlled by the transcription factor Egr-2. Proc Natl Acad Sci USA 106, 13974–13979 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N. et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 19, 739–746 (2013). [DOI] [PubMed] [Google Scholar]
- Hao Z. et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity 29, 615–627 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges P. B. et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26, 629–641 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk I. et al. Prevention of autoimmunity and control of recall response to exogenous antigen by Fas death receptor ligand expression on T cells. Immunity 29, 922–933 (2008). [DOI] [PubMed] [Google Scholar]
- Turbyville J. C. & Rao V. K. The autoimmune lymphoproliferative syndrome: A rare disorder providing clues about normal tolerance. Autoimmun Rev 9, 488–493 (2010). [DOI] [PubMed] [Google Scholar]
- Hsu F. C. et al. Immature recent thymic emigrants are eliminated by complement. J Immunol 193, 6005–6015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K. et al. Liver sinusoidal endothelial cells induce immunosuppressive IL-10-producing Th1 cells via the Notch pathway. Eur J Immunol 45, 2008–2016 (2015). [DOI] [PubMed] [Google Scholar]
- Burghardt S. et al. Hepatocytes contribute to immune regulation in the liver by activation of the Notch signaling pathway in T cells. J Immunol 191, 5574–5582 (2013). [DOI] [PubMed] [Google Scholar]
- Anderson C. F., Oukka M., Kuchroo V. J. & Sacks D. CD4+ CD25− Foxp3− Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med 204, 285–297 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D. et al. Conventional T-bet+ Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 204, 273–283 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Madan R., Karp C. L. & Braciale T. J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 15, 277–284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G. & Lohse A. W. Immune tolerance: what is unique about the liver. J Autoimmun 34, 1–6 (2010). [DOI] [PubMed] [Google Scholar]
- Crispe I. N. Liver antigen-presenting cells. J Hepatol 54, 357–365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne C. N. & Kubes P. Immune surveillance by the liver. Nat Immunol 14, 996–1006 (2013).24048121 [Google Scholar]
- Schurich A. et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol 184, 4107–4114 (2010). [DOI] [PubMed] [Google Scholar]
- Xu L., Yin W., Sun R., Wei H. & Tian Z. Liver type I regulatory T cells suppress germinal center formation in HBV-tolerant mice. Proc Natl Acad Sci USA 110, 16993–16998 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz S. et al. Notch regulates IL-10 production by T helper 1 cells. Proc Natl Acad Sci USA 105, 3497–3502 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology 137, 1498–1508 e1491-1495 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. et al. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity 20, 327–336 (2004). [DOI] [PubMed] [Google Scholar]
- Liu B. et al. Liver sinusoidal endothelial cell lectin inhibits CTL-dependent virus clearance in mouse models of viral hepatitis. J Immunol 190, 4185–4195 (2013). [DOI] [PubMed] [Google Scholar]
- Carambia A. et al. Inhibition of inflammatory CD4 T cell activity by murine liver sinusoidal endothelial cells. J Hepatol 58, 112–118 (2013). [DOI] [PubMed] [Google Scholar]
- Mathieu M., Cotta-Grand N., Daudelin J. F., Thebault P. & Labrecque N. Notch signaling regulates PD-1 expression during CD8+ T-cell activation. Immunol Cell Biol 91, 82–88 (2013). [DOI] [PubMed] [Google Scholar]
- Ostroukhova M. et al. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest 116, 996–1004 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt A., Biburger M., Papadopoulos T. & Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology 45, 475–485 (2007). [DOI] [PubMed] [Google Scholar]
- Wang H., Lafdil F., Kong X. & Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci 7, 536–550 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M. & Thornton A. M. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 259, 88–102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T., Strasser A. & Jost P. J. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ 19, 42–50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10, 595–602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. O. et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37, 511–523 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle P. A., Bottcher J. & Huang L. R. The role of hepatic immune regulation in systemic immunity to viral infection. Med Microbiol Immunol 204, 21–27 (2015). [DOI] [PubMed] [Google Scholar]