Abstract
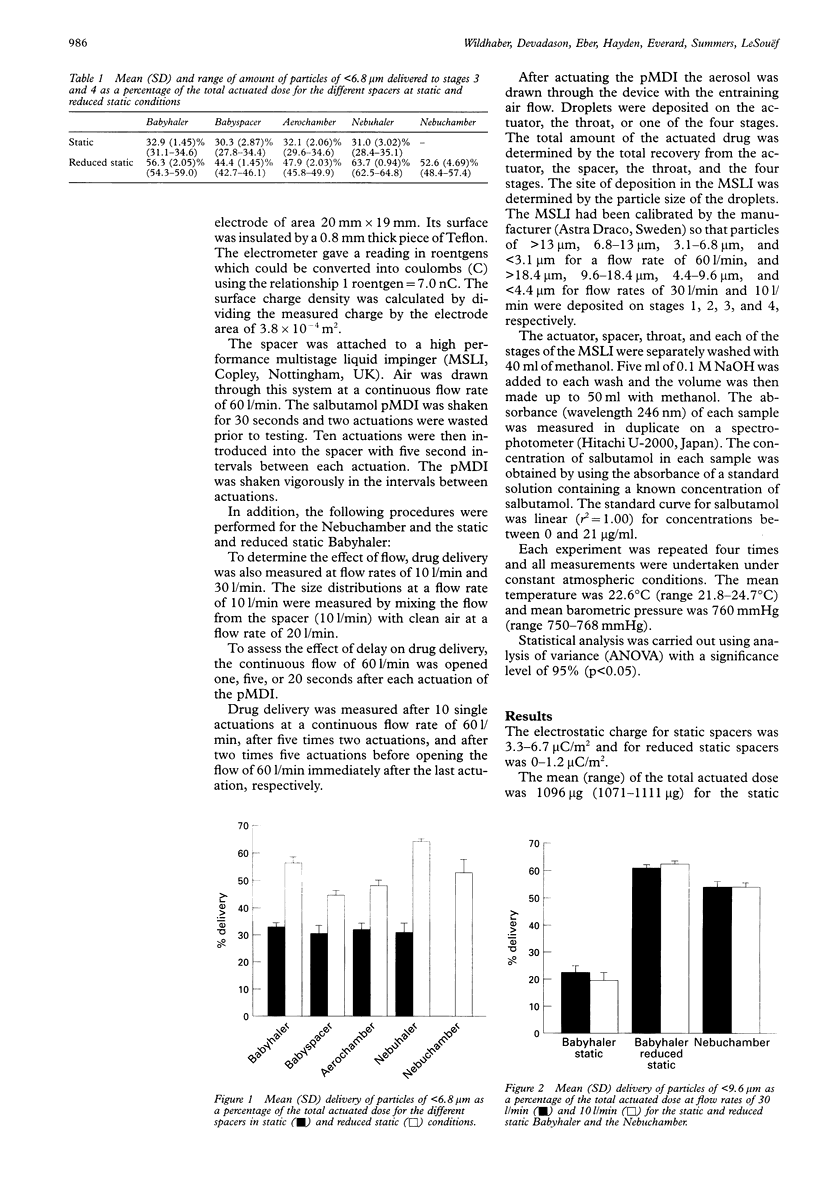
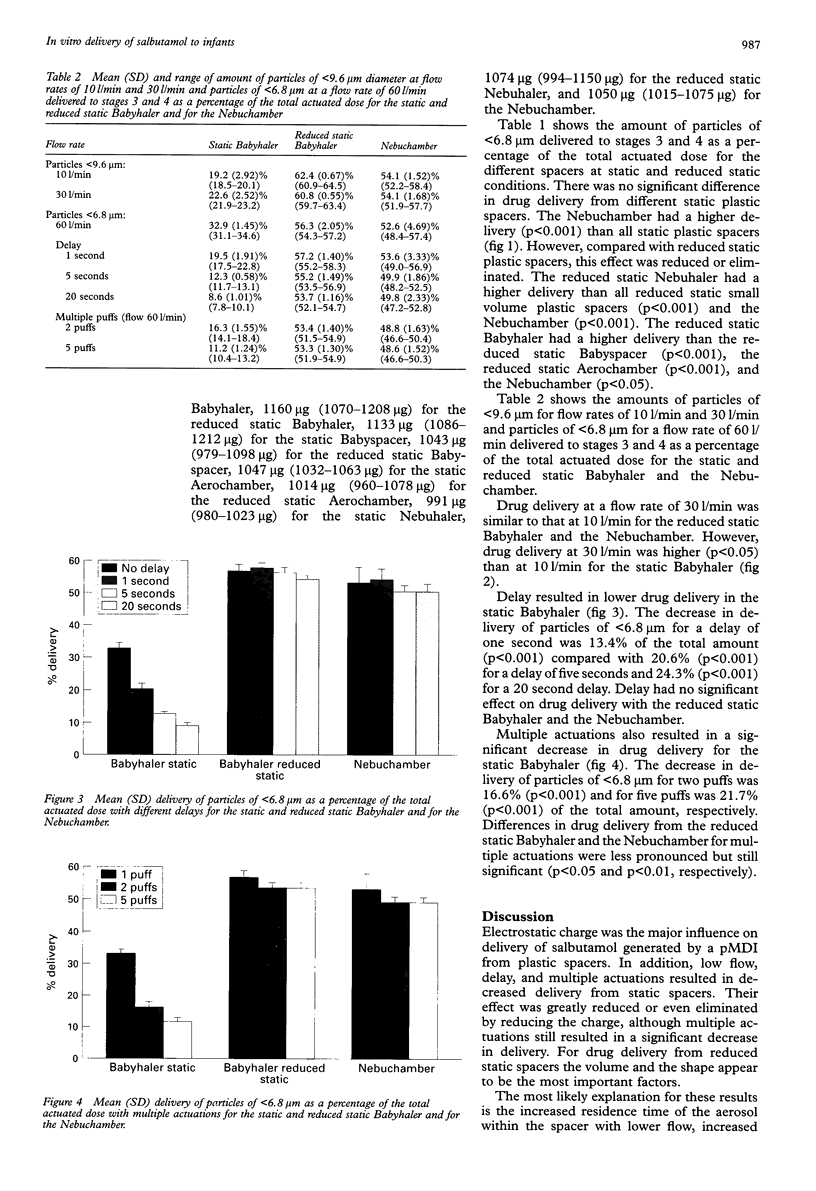
BACKGROUND: A study was undertaken to determine the influences of electrostatic charge, flow, delay, and multiple actuations on the in vitro delivery of salbutamol generated by a pressurised metered dose inhaler (pMDI) from small volume spacers used in infants. METHODS: Ten actuations from a salbutamol pMDI were drawn at different flow rates after either single or multiple actuations, with or without delay, through either static or reduced static spacers. An ionic detergent was used to reduce the charge of plastic spacers (Babyhaler, Babyspacer, Aerochamber, Nebuhaler). Electrostatic charge was measured using an electrometer. A multistage liquid impinger was used to determine the particle size distribution of the output of the pMDI through the spacers. RESULTS: Electrostatic charge on the surface of plastic spacers had the greatest influence on delivery, causing a decrease in drug delivery. Reducing charge by coating the surface with ionic detergent resulted in an increase of 46.5-71.1% (p < 0.001) in small (< 6.8 microns) particle delivery from small volume plastic spacers. Lower flow, delay, and multiple actuations resulted in decreased delivery from static spacers. Lower flow resulted in a decrease of 15% in small (< 9.6 microns) particle delivery. Delay and multiple actuations resulted in a decrease of 40.7% and 76.0%, respectively, in small (< 6.8 microns) particle delivery. The influences of lower flow, delay, and multiple actuations were greatly reduced or even eliminated by reducing charge. However, multiple actuations still resulted in a significant decreased delivery (p < 0.05). The reduced static Nebuhaler had a higher delivery than all small volume spacers. CONCLUSIONS: Electrostatic charge has a major influence on the delivery of salbutamol from small volume spacers. Using a metal spacer or ionic detergent coating of plastic spacers resulted in no or reduced charge and hence in improved delivery. Lower flow, delay, and multiple actuations played a major part only in static spacers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry P. W., O'Callaghan C. Multiple actuations of salbutamol MDI into a spacer device reduce the amount of drug recovered in the respirable range. Eur Respir J. 1994 Sep;7(9):1707–1709. doi: 10.1183/09031936.94.07091707. [DOI] [PubMed] [Google Scholar]
- Barry P. W., O'Callaghan C. The effect of delay, multiple actuations and spacer static charge on the in vitro delivery of budesonide from the Nebuhaler. Br J Clin Pharmacol. 1995 Jul;40(1):76–78. doi: 10.1111/j.1365-2125.1995.tb04538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H. A metal aerosol holding chamber devised for young children with asthma. Eur Respir J. 1995 May;8(5):856–860. [PubMed] [Google Scholar]
- Bisgaard H., Anhøj J., Klug B., Berg E. A non-electrostatic spacer for aerosol delivery. Arch Dis Child. 1995 Sep;73(3):226–230. doi: 10.1136/adc.73.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard M. L., Clark A. R., Milner A. D. Drug delivery from holding chambers with attached facemask. Arch Dis Child. 1992 May;67(5):580–585. doi: 10.1136/adc.67.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. S., Eldridge M. A., Sackner M. A. Oropharyngeal deposition and delivery aspects of metered-dose inhaler aerosols. Am Rev Respir Dis. 1987 Jan;135(1):157–164. doi: 10.1164/arrd.1987.135.1.157. [DOI] [PubMed] [Google Scholar]
- Kraemer R., Birrer P., Modelska K., Aebischer C. C., Schöni M. H. A new baby-spacer device for aerosolized bronchodilator administration in infants with bronchopulmonary disease. Eur J Pediatr. 1992 Jan;151(1):57–60. doi: 10.1007/BF02073894. [DOI] [PubMed] [Google Scholar]
- Kraemer R., Frey U., Sommer C. W., Russi E. Short-term effect of albuterol, delivered via a new auxiliary device, in wheezy infants. Am Rev Respir Dis. 1991 Aug;144(2):347–351. doi: 10.1164/ajrccm/144.2.347. [DOI] [PubMed] [Google Scholar]
- König P. Spacer devices used with metered-dose inhalers. Breakthrough or gimmick? Chest. 1985 Aug;88(2):276–284. doi: 10.1378/chest.88.2.276. [DOI] [PubMed] [Google Scholar]
- Levison H., Reilly P. A., Worsley G. H. Spacing devices and metered-dose inhalers in childhood asthma. J Pediatr. 1985 Nov;107(5):662–668. doi: 10.1016/s0022-3476(85)80389-9. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C., Cant M., Robertson C. Delivery of beclomethasone dipropionate from a spacer device: what dose is available for inhalation? Thorax. 1994 Oct;49(10):961–964. doi: 10.1136/thx.49.10.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C., Lynch J., Cant M., Robertson C. Improvement in sodium cromoglycate delivery from a spacer device by use of an antistatic lining, immediate inhalation, and avoiding multiple actuations of drug. Thorax. 1993 Jun;48(6):603–606. doi: 10.1136/thx.48.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanon P. Inhalation anti-asthma therapy with spacers: technical aspects. Monaldi Arch Chest Dis. 1994 Jun;49(3):258–264. [PubMed] [Google Scholar]