Abstract
Background
Recurrent atrial fibrillation (AF) occurs in up to 50 % of patients within 1 year after catheter ablation, and a clinical risk score to predict recurrence remains a critical unmet need. The aim of this study was to (1) develop a simple score for the prediction of rhythm outcome following catheter ablation; (2) compare it with the CHADS2 and CHA2DS2-VASc scores, and (3) validate it in an external cohort.
Methods
Rhythm outcome between 3 and 12 months after AF catheter ablation were documented. The APPLE score [one point for age>65 years, persistent AF, impaired eGFR (<60 ml/min/1.73 m2), LA diameter ≥43 mm, EF < 50 %] was associated with AF recurrence and was validated in an external cohort in 261 patients with comparable ablation and follow-up.
Results
In 1145 patients (60 ± 10 years, 65 % male, 62 % paroxysmal AF) the APPLE score showed better prediction of AF recurrences (AUC 0.634, 95 % CI 0.600–0.668, p < 0.001) than CHADS2 (AUC 0.538) and CHA2DS2-VASc (AUC 0.542). Compared to patients with an APPLE score of 0, the odds ratio for AF recurrences was 1.73, 2.79 and 4.70 for APPLE scores 1, 2, or ≥3, respectively (all p < 0.05). In the external validation cohort, the APPLE score showed similar results (AUC 0.624, 95 % CI 0.562–0.687, p < 0.001).
Conclusions
The novel APPLE score is superior to the CHADS2 and CHA2DS2-VASc scores for prediction of rhythm outcome after catheter ablation. It holds promise as a useful tool to identify patients with low, intermediate, and high risk for AF recurrence.
Keywords: Atrial fibrillation, Catheter ablation, AF recurrences, Risk scores
Introduction
Recurrence of atrial fibrillation (AF) occurs in up to 50 % of patients within 1 year after catheter ablation, and a clinical risk score to predict recurrence remains a critical unmet need. Several observational studies have investigated predictors of AF recurrence. Persistent AF and enlarged left atrial (LA) diameter are generally recognized as important baseline predictors for rhythm outcome [1]; however, other studies have generated considerable disagreement regarding which additional variables to include in AF recurrence models [2, 3]. Among several significant limitations of previously published studies are the inclusion of post-ablation variables (i.e., early recurrences within first weeks) [2], and the inclusion of populations who have undergone primarily cryoablation [2, 3], which may not be generalizable to the majority of procedures which are performed using radiofrequency ablation techniques. Finally, none of those scores have been validated in an external population.
Based on the results of a previous study [4], we developed a new scoring system for AF recurrences, i.e., APPLE score [one point for age>65 years, persistent AF, impaired eGFR (<60 ml/min/1.73 m2), left atrial diameter ≥43 mm, left ventricular ejection fraction<50 %, range from 0 to 5]. Here, we sought to (1) assess this score for the prediction of rhythm outcome following AF catheter ablation; (2) compare it with the CHADS2 and CHA2DS2-VASc scores, and (3) validate it in an external AF ablation cohort.
Methods
Study population
The study population consisted of two cohorts: a discovery set from Heart Center Leipzig (HCL), Germany, and a validation set from Vanderbilt University (VU), US. 1145 patients from The Heart Center Leipzig AF Ablation Registry and 261 from The Vanderbilt AF Ablation Registry were included in this study (Table 1). Patients underwent AF catheter ablation according to current guidelines at HCL between January 2007 and December 2011, and at VU between March 2004 and December 2011.
Table 1.
Baseline characteristics of the study populations with and without recurrences
| n (%) | AF recurrences Heart Center Leipzig (n = 1145)
|
p value | AF recurrences Vanderbilt University (n = 261)
|
p value | ||||
|---|---|---|---|---|---|---|---|---|
| Total population | No (n = 768) | Yes (n = 377) | Total population | No (n = 100) | Yes (n = 161) | |||
| Age, years | 60 ± 10 | 60 ± 10 | 61 ± 10 | 0.038 | 61 ± 10 | 58 ± 10 | 60 ± 10 | 0.031 |
| Females | 402 (35) | 261 (34) | 141 (37) | 0.255 | 76 (29) | 27 (27) | 49 (30) | 0.553 |
| Persistent AF | 433 (38) | 240 (31) | 193 (51) | < 0.001 | 136 (52) | 43 (43) | 93 (58) | 0.020 |
| BMI, kg/m2 | 29 ± 5.0 | 29 ± 5 | 29 ± 5 | 0.147 | 31 ± 6 | 32 ± 7 | 31 ± 6 | 0.223 |
| eGFR (ml/min/1.73 m2)a | 78 ± 17 | 79 ± 17 | 75 ± 17 | 0.005 | 58 ± 6 | 59 ± 4 | 58 ± 7 | 0.181 |
| eGFR < 60 ml/min/1.73 m2 | 225 (19) | 128 (17) | 97 (26) | < 0.001 | 43 (17) | 13 (13) | 30 (19) | 0.233 |
| Hypertension | 834 (73) | 557 (73) | 277 (74) | 0.734 | 152 (58) | 56 (56) | 96 (60) | 0.553 |
| Diabetes mellitus | 192 (17) | 121 (16) | 71 (19) | 0.190 | 47 (18) | 17 (17) | 30 (19) | 0.712 |
| Coronary artery disease | 168 (15) | 113 (15) | 55 (15) | 0.955 | 48 (18) | 18 (18) | 30 (19) | 0.909 |
| Chronic heart failure | 76 (7) | 40 (5) | 36 (10) | 0.006 | 25 (10) | 7 (7) | 18 (11) | 0.252 |
| Peripheral artery disease | 94 (8) | 54 (7) | 40 (11) | 0.038 | n/a | n/a | n/a | n/a |
| Previous stroke | 87 (8) | 53 (7) | 34 (9) | 0.204 | 15 (6) | 4 (4) | 11 (7) | 0.318 |
| CHADS2, median (IQR) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 0.013 | 1 (0–1) | 1 (0–1) | 1 (0–2) | 0.258 |
| CHA2DS2-VASc, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.006 | n/a | n/a | n/a | n/a |
| APPLE score, median (IQR) | 1 (1–2) | 1 (0–2) | 1 (1–2) | < 0.001 | 1 (0–2) | 1 (0–2) | 2 (1–2) | < 0.001 |
| EF, % | 59 ± 10 | 59 ± 10 | 58 ± 11 | 0.017 | 60 ± 12 | 62 ± 11 | 59 ± 12 | 0.021 |
| LVEDd, mm | 49 ± 7 | 49 ± 7 | 50 ± 7 | 0.361 | 47 ± 7 | 47 ± 7 | 47 ± 7 | 0.957 |
| LA diameter, mm | 43 ± 6 | 42 ± 6 | 44 ± 6 | < 0.001 | 40 ± 8 | 39 ± 8 | 41 ± 8 | 0.064 |
GFR estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (HCL) and modification of diet in renal disease formula (VU)
Paroxysmal and persistent AF was defined according to current guidelines [5]. Paroxysmal AF was defined as self-terminating within 7 days after onset documented by previous routine electrocardiograms (ECG) or Holter ECG. Persistent AF was defined as any AF episode either lasting longer than 7 days or requiring drug or direct current cardioversion for termination.
In all patients, transthoracic and transesophageal echocardiography was performed prior to ablation. At HCL, all class I or III antiarrhythmic medications with the exception of amiodarone were discontinued at least 5 half-lives before the procedure. At VU, antiarrhythmic medications were continued peri-procedurally at the discretion of the individual operator and discontinued 3 months after the procedure.
eGFR was estimated at HCL and VU according to the standard formulas used clinically at each institution. At HCL, the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation was used: eGFR = 141 × min(Scr/κ, 1)α × max(Scr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159[if Black], where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 [6]. At VU, eGFR was estimated using the MDRD (modification of diet in renal disease) formula: eGFR = 186 × Scr−1.154 × Age−0.203 × [1.210 if Black] ×[0.742 if female]. As standard clinical practice at VU, an eGFR cutoff of 60 ml/min/1.73 m2 is used where estimates greater than 60 ml/min/1.73 m2 were recorded as 60 ml/min/1.73 m2.
The study was performed according to the Declaration of Helsinki and Institutional Guidelines. Patients provided written informed consent.
Catheter ablation
LA catheter ablation was performed using a well-documented approach [7]. At HCL, patients presenting with AF at the beginning of the procedure were electrically cardioverted and ablation was performed during sinus rhythm (SR) (i.e., AF termination with ablation was not attempted). At VU, patients presenting in AF underwent ablation during AF and were electrically cardioverted to SR if they remained in AF following completion of circumferential pulmonary vein isolation, linear ablation, and ablation of complex fractionated atrial electrograms (CFAE). In all patients, circumferential LA ablation lines were placed around the antrum of the ipsilateral pulmonary veins (irrigated tip catheter, pre-selected tip temperature of 48 °C, and maximum power of 30–50 W). In patients with persistent AF, additional linear lesions were added at the LA roof, the basal posterior wall and the LA (mitral) isthmus. At the end of procedure, linear block was confirmed across the roof and the mitral isthmus. Ablation of CFAEs was not performed at HCL, and was performed in patients with persistent AF according to operator discretion at VU.
After circumferential line placement, voltage and pace mapping along the ablation lines were used to identify and close gaps. The isolation of all pulmonary veins with bidirectional block was verified with a multipolar circular mapping catheter and was defined as the procedural endpoint.
After ablation, class I and III antiarrhythmic drugs were not reinitiated at HCL, but continued until 3 months following ablation at VU. Proton pump inhibitors were added for 4 weeks. According to the current guidelines [5], oral anticoagulation was prescribed for 3–6 months after catheter ablation and depending on risk stratification of stroke using the CHADS2 or CHA2DS2-VASc score thereafter [8].
Follow-up
All patients were followed in the outpatient clinic for at least 12 months after catheter ablation. During this follow-up period, at HCL 7-day Holter ECG recordings were performed (immediately, 3, 6 and 12 months after the ablation). At VU, a 48-h Holter ECG was obtained at 3 months, and a 14–30 days event recorder was obtained at 6 and 12 months. Additional ECGs and Holter ECG recordings were obtained when patients’ symptoms were suggestive of AF. AF recurrences were defined as any atrial arrhythmia lasting >30 s between 3 and 12 months after ablation. If electrical or pharmacological cardioversion and/or repeat procedure were needed after 3 months blanking period, this was also considered as an AF recurrence, i.e., study endpoint.
Statistical analysis
Data are presented as the mean and standard deviation for normally distributed continuous variables and as proportions for categorical variables. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. The differences between continuous values were assessed using an unpaired two-tailed t test for normally distributed continuous variables, a Mann–Whitney test for skewed variables, and a Chi-square test for nominal variables.
ROC (receiver operating characteristic) curves were generated for graphical illustration of CHADS2, CHA2-DS2-VASc and APPLE scores’ performance in predicting rhythm outcome, with the area under the curve (AUC) being equivalent to the c index for determining the predictive value for a score. The c indices (i.e., areas under the ROC curves) for the 3 scores were compared by using DeLong’s method [9].
A p value <0.05 was considered as statistically significant. Statistical analyses were performed with SPSS statistical software version 17 and with R statistics [10].
Results
APPLE score as predictor for AF recurrences in the discovery set
At HCL, 379 (33 %) patients experienced AF recurrences between 3 and 12 months after catheter ablation. At 3, 6 and 12 months follow-up, 100 patients (8.8 %), 109 (9.5 %) and 79 (6.8 %) were on antiarrhythmic drugs, respectively. Patients with recurring AF are compared to patients without recurring AF in Table 1. Patients with AF recurrences were older, more likely to have persistent AF, impaired renal function, had larger LA diameter and lower EF (all p < 0.005).
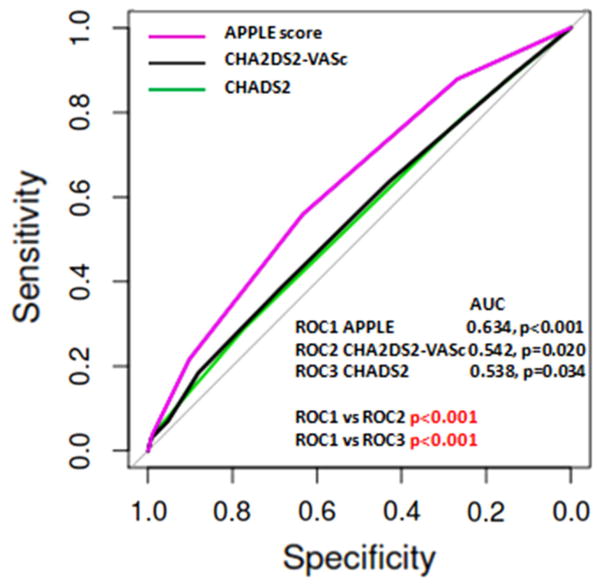
Using the logistic regression analyses all three scores—the CHADS2, CHA2DS2-VASc and APPLE—were significant predictors of AF recurrences between 3 and 12 months (OR 1.18, 95 % CI 1.04–1.35, p = 0.013, OR 1.12, 95 % CI 1.03–1.22, p = 0.007, and 1.64, 95 % CI 1.45–1.86, p < 0.001, respectively). Based on ROC curve analysis, the APPLE score had a better predictive value (c index 0.634) compared with CHADS2 and CHA2DS2-VASc (0.538 and 0.542, respectively) with highly significant differences among the scores (p < 0.001) (Fig. 1).
Fig. 1.
ROC curves for the CHADS2, CHA2DS2-VASc and APPLE scores in predicting AF recurrences
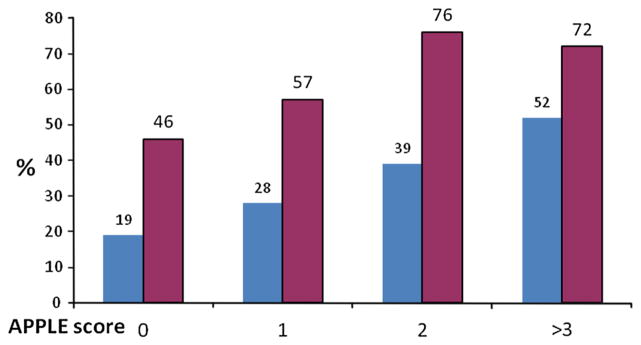
The proportion of patients with an APPLE score of 0, 1, 2 and ≥3 was 21, 34, 30 and 15 %, respectively. AF recurrence rates according to APPLE score was 19 % (APPLE score 0), 28 % (1), 39 % (2), and 52 % (≥3) (p < 0.001) (Table 2; Fig. 2). Compared to patients with an APPLE score of 0, the risk (OR) for AF recurrences was 1.73 (95 % CI 1.17–2.55, p = 0.006), 2.79 (95 % CI 1.90–4.12, p <0.001) and 4.70 (95 % CI 3.03–7.30, p <0.001) for APPLE scores 1, 2, or ≥3, respectively.
Table 2.
APPLE score distribution in the study populations
| n (%) | APPLE score Heart Center Leipzig | Heart Center Leipzig n = 1145
|
p value | APPLE score Vanderbilt | Vanderbilt University n = 261
|
p value | ||
|---|---|---|---|---|---|---|---|---|
| No (n = 768) | Yes (n = 377) | No (n = 100) | Yes (n = 161) | |||||
| 0 | 245 (21) | 199 (81) | 46 (19) | < 0.001 | 67 (26 %) | 36 (54 %) | 31 (46 %) | 0.003 |
| 1 | 392 (34) | 281 (72) | 111 (28) | 84 (32 %) | 36 (43 %) | 48 (57 %) | ||
| 2 | 340 (30) | 207 (61) | 133 (39) | 63 (24 %) | 15 (24 %) | 48 (76 %) | ||
| ≥3 | 168 (15) | 81 (48) | 87 (52) | 47 (18 %) | 13 (28 %) | 34 (72 %) | ||
Fig. 2.
Distribution of AF recurrences within study population according to the APPLE score
APPLE score as predictor for AF recurrences in an external validation set
Baseline characteristics of the population in an external validation set (n = 261, VU) are presented in Table 1. 185 (61.7 %) patients suffered AF recurrences between 3 and 12 months after catheter ablation. As in the discovery cohort, patients with AF recurrences were older, more likely to have persistent AF, had lower EF (all p < 0.05) and a trend towards larger LA diameter (p = 0.061), and consequently higher APPLE score (p < 0.001).
Patients with APPLE score of 0 (26 %), 1 (32 %), 2 (24 %), and ≥3 (28 %) had AF recurrence rates of 46, 57, 76, and 72 %, respectively (p = 0.003) (Table 2; Fig. 2). Compared to patients with an APPLE score of 0, the risk (OR) for AF recurrences was 1.5 (95 % CI 0.8–3.0, p = 0.185), 3.7 (95 % CI 1.8–7.9, p = 0.001) and 3.0 (95 % CI 1.4–6.8, p = 0.006) for APPLE scores 1, 2, or ≥3, respectively.
Discussion
Main findings
To the best of our knowledge, this is the first study demonstrating the predictive value of a new scoring system for the prediction of rhythm outcomes after AF catheter ablation in a large contemporary AF ablation cohort. The APPLE score, which is based on clinical variables, is a novel and simple tool with better predictive value compared to CHADS2 and CHA2DS2-VASc scores and was validated in an external validation set showing similar predictive ability.
APPLE score as predictor for AF recurrences
In a recent meta-analysis, D’Ascenzo et al. [1] demonstrated that persistent AF, LA diameter >50 mm and AF recurrence within the first month after procedure are the most powerful predictors of AF ablation failure. Several studies evaluated the predictive value of different scoring systems that were not specifically designed to predict rhythm outcomes after AF ablation (HATCH, CHADS2, CHA2DS2-VASc). While the HATCH score [11] revealed no value in prediction of AF recurrences after catheter ablation, it has been shown that CHADS2 and CHA2DS2-VASc scores are modest predictors for rhythm outcome after radiofrequency catheter ablation [4, 12, 13]. More recently, two other scores have been developed to predict rhythm outcomes after invasive AF treatment. First, the ALARMEc score (acronym for AF type, left atrium size, renal insufficiency, metabolic syndrome, cardiomyopathy) has been presented as a useful tool to predict AF recurrence after catheter ablation and shown to be superior to CHADS2 and CHA2DS2-VASc scores [3]. However, the results of this study are difficult to interpret as renal dysfunction was not defined in accordance with current NKF K/DOQI guidelines [14] and definitions of metabolic syndrome were not provided. Another score—BASE-AF2 (acronym for body mass index>28 kg/m2, atrial dilatation >40 mm, current smoking, early recurrence, AF duration >6 years, AF type)—was developed to predict rhythm outcomes after AF cryoablation and demonstrated very high predictive value with an AUC of 0.94 if the score was >3 points [2]. Similar to the ALARMEc study, BMI was not defined in accordance with current definitions [15, 16]. Furthermore, because AF often begins with asymptomatic episodes, the unclear cutoff of AF duration>6 years along with the influence of previous smoking complicate the interpretation and the implementation of this score in clinical practice. Finally, early recurrence is included as a variable, which obviously cannot be used for baseline prediction.
In contrast to other scores, the APPLE score included easily obtainable and clearly defined parameters. Based on results from the multivariable analysis of our previous study [4], we found that age, persistent AF, renal dysfunction, impaired EF and enlarged LA were significant, independent predictors of recurrence. Summarizing these findings, we developed the APPLE score. The APPLE score had a predictive value that was superior to both the CHADS2 and CHA2DS2-VASc scores (p < 0.001). In our study, the CHADS2 and CHA2DS2-VASc scores performed in accordance with previously published studies [3] (AUC 0.543 vs. AUC 0.550), highlighting the validity of our findings and the need for an improved rhythm outcomes prediction tool.
APPLE score in external validation cohort
Although recent rhythm outcomes prediction scores—ALARMEc and BASE-AF2—demonstrated better predictive value than the APPLE score (c indices 0.657 and 0.940 vs. 0.630) in their discovery datasets, the results of our study have been confirmed in an external validation set with similar predictive value (c index 0.624). Despite the differences in ablation protocols, peri-procedural management, and baseline patient characteristics that contributed to an overall difference in the rate of recurrence, the APPLE score remained a highly significant predictor for response to ablation. The simplicity of the APPLE score and inclusion of well-established parameters related to AF recurrence (e.g., AF type, LA size, renal dysfunction) make this score highly generalizable to a wide variety of populations undergoing catheter ablation for AF. The score-based peri- and/or post-procedural management could reduce the necessity for repeated procedures and/or select patients with high risk for more advanced ablation protocols (surgical or hybrid ablations). Prospective studies would be needed to confirm this hypothesis.
Limitations and strength of the study
This study is limited by its observational, retrospective design. Differences in the rate of persistent AF between the HCL and VU cohorts (more persistent AF at VU) existed and may, in-part, account for the difference in observed recurrence rates. Other inherent differences in ablation technique, patient selection, and peri-operative management likely existed between centers which are unavoidable in an observational setting, but approximate the real-world performance of AF ablation. We believe the differences observed between the discovery and validation cohorts demonstrate the generalizability of the APPLE score over a range of realistic clinical practice settings. As AF recurrence can be asymptomatic and under-detected, further studies with continuous rhythm monitoring during long-term follow-up are needed to confirm our findings. Nevertheless, this study is the largest to date that analyzes the predictive value of a novel and simple scoring system based on clinically relevant variables that are closely correlated with rhythm outcome. Furthermore, stratification of AF patients into different risk strata for post-interventional AF recurrences could be helpful for clinical decision as more aggressive ablation procedure and/or addition of antiarrhythmic drugs within blanking period could be an optimal choice in patients with higher APPLE score.
Future directions may evaluate the performance of the APPLE score in a prospective cohort and explore the combination of other variables into different risk scores.
Conclusions
The novel and simple APPLE score is superior to the CHADS2 and CHA2DS2-VASc scores for prediction of rhythm outcome after catheter ablation. It may provide a useful clinical tool to identify patients with low, intermediate or high risk of AF recurrence.
Acknowledgments
Dr. Kornej was supported by the German Cardiac Society St. Jude Medical Stipend. Dr. Husser was supported by the Volkswagen Foundation (#84 901). At Vanderbilt this work was supported by Grants from the American Heart Association to Dr. Shoemaker (11CRP7420009), Dr. Darbar (EIA 0940116N), and Grants from the NIH to Dr. Darbar (HL092217). This project was also supported by a Clinical and Translational Science Award (UL1TR000445) from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the NIH.
Footnotes
Conflict of interest There are no conflicts of interest.
References
- 1.D’Ascenzo F, Corleto A, Biondi-Zoccai G, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation? A meta-analysis. Int J Cardiol. 2013;167:1984–1989. doi: 10.1016/j.ijcard.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Canpolat U, Aytemir K, Yorgun H, et al. A proposal for a new scoring system in the prediction of catheter ablation outcomes: promising results from the Turkish Cryoablation Registry. Int J Cardiol. 2013;169(3):201–206. doi: 10.1016/j.ijcard.2013.08.097. [DOI] [PubMed] [Google Scholar]
- 3.Wojcik M, Berkowitsch A, Greiss H, et al. Repeated catheter ablation of atrial fibrillation: how to predict outcome? Circ J. 2013;77:2271–2279. doi: 10.1253/circj.cj-13-0308. [DOI] [PubMed] [Google Scholar]
- 4.Kornej J, Hindricks G, Kosiuk J, et al. Comparison of CHADS2, R2CHADS2 and CHA2DS2-VASc scores for prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014;7:281–287. doi: 10.1161/CIRCEP.113.001182. [DOI] [PubMed] [Google Scholar]
- 5.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornej J, Kosiuk J, Hindricks G, et al. Sex-related predictors for thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Clin Res Cardiol. 2015 doi: 10.1007/s00392-015-0823-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 9.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 10.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang RB, Dong JZ, Long DY, et al. Efficacy of catheter ablation of atrial fibrillation beyond HATCH score. Chin Med J (Engl) 2012;125:3425–3429. [PubMed] [Google Scholar]
- 12.Chao TF, Tsao HM, Lin YJ, et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012;5:514–520. doi: 10.1161/CIRCEP.111.968032. [DOI] [PubMed] [Google Scholar]
- 13.Letsas KP, Efremidis M, Giannopoulos G, et al. CHADS2 and CHA2DS2-VASc scores as predictors of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace. 2013 doi: 10.193/europace/eut210. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]