Abstract
Children with autism spectrum disorders (ASD) imitate less than typically developing (TD) children; however, the specific features and causes of this deficit are still unclear. The current study investigates the role of joint engagement, specifically children's visual attention to demonstrations, in an object-directed imitation task. This sample was recruited from an early ASD screening study, which allows for an examination of these behaviors prior to formal diagnosis and ASD-specific intervention. Children with ASD imitated less than TD children; children with other developmental delays showed no significant difference from the two other screen-positive groups. Additionally, only the ASD group showed decreased visual attention, suggesting that early visual attention plays a role in the social learning of children with ASD.
Keywords: autism spectrum disorders, imitation, visual attention, social learning, developmental disabilities, cognitive development
Children with autism spectrum disorders (ASD) are less likely to imitate a variety of acts compared to typically-developing (TD) children, and in some studies, compared to children with other developmental disabilities (DD; Charman, Swettenham, Baron-Cohen, Cox, Baird, & Drew, 1997; DeMyer et al., 1972; Jones & Prior, 1985; Rogers, Hepburn, Stackhouse, & Wehner, 2003; Rogers & Pennington, 1991; Rogers, Young, Cook, Giolzetti, & Ozonoff, 2008; Young, Rogers, Hutman, Rozga, Sigman, & Ozonoff, 2011). Researchers are still struggling to characterize the imitation deficit observed in ASD and its causes (for review, see Sevlever & Gillis, 2010; Vivanti & Hamilton, 2014). Given that imitation provides a foundation for a range of social, cognitive, and communicative skills (Ingersoll & Schreibman, 2006), identifying the scope and nature of this difference is important for understanding ASD and for informing targeted interventions.
Imitation batteries for children with ASD often measure simple responses, such as reproducing body movements (e.g., hand clapping) or one-step acts (e.g., banging a spoon; e.g., Ingersoll, 2008; McDuffie, Turner, Stone, Yoder, Wolery, & Ulman, 2007; Rogers et al., 2003; Stone, Ousley, & Littleford, 1997; Toth, Munson, Meltzoff, & Dawson, 2006). Practically, these tasks are accessible to a wide age range. Very simple acts may also emphasize social interaction between the partners (Uzgiris, 1981), and such interactions may be particularly difficult for children with ASD. However, imitation is also important for learning about the physical environment, including how to use objects and tools. Past studies that have compared the imitation of physical (versus social) goals in ASD and TD groups show mixed results, with some reporting impairments in ASD and others finding no differences (for reviews, see Edwards, 2014; Sevlever & Gillis, 2010; Williams, Whiten, & Singh, 2004). One current goal is to test children's imitation of multi-step acts that lead to outcomes on objects.
The second goal is to investigate what defining features of ASD may underlie differences in imitation. A leading candidate is problems in attending to and processing social information – including dyadic interaction, social looking patterns, and joint attention (Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Sigman, Dijamco, Gratier, & Rozga, 2004; Vivanti & Hamilton, 2014). To date, eye-tracking studies with children (ages 4-15) have found no ASD-specific differences in total time spent looking at demonstrations in imitation tasks (Hobson & Hobson, 2007; Vivanti, Nadig, Ozonoff & Rogers, 2008; Vivanti, Trembath, & Dissanayake, 2014), though children with ASD have a tendency to focus on objects, rather than a demonstrator's face during such tasks (Vivanti & Hamilton, 2014). These findings extend research showing decreased orienting to social stimuli in 3- and 4-year-olds with ASD (Dawson, et al., 2004) and abnormal looking patterns in high-risk infants (Zwaigenbaum, Bryson, Rogers, Roberts, Brian, & Szatmari, 2005). Limited social attention could lead to fewer opportunities for social learning, which may be especially critical during the foundational social interactions of early childhood.
One limit of past imitation research with clinical populations (including ASD) is that it has been conducted with fairly old children. Most studies have tested children above the age of 4 years, and often children as old as age 12 (for review, see Table 1 of Williams et al., 2004; though, see also Rowberry, et al., 2015). Depending on the age of diagnosis and other external factors, children receive varying exposure to targeted interventions (e.g., the Early Start Denver Model), which may provide formal training in imitation and in how to direct attention during social interactions. In the current study, we ruled out effects of ASD-specific intervention by recruiting at-risk children before their families received diagnoses. This recruitment method also provided a stringent comparison for the ASD group, because we tested children who screened positive for ASD (and thus may exhibit similar atypical behavioral patterns) but who ultimately did not receive a diagnosis or were diagnosed with another developmental delay.
Table 1.
Group Characteristics (MSEL domain scores reported as age equivalencies)
| ASD n = 15 | DD n = 10 | TD-SP n = 6 | TD n = 8 | F | |
|---|---|---|---|---|---|
| Chronological Age | 29.07 (6.24) | 29.55 (7.16) | 25.78 (3.70) | 28.56 (7.35) | 0.48 |
| Fine Motor Ability | 20.80 (5.36) | 22.20 (5.09) | 25.00 (1.55) | -- | 1.64 |
| Expressive Language | 15.73 (8.62) | 18.30 (8.03) | 23.50 (4.04) | -- | 2.13 |
| Receptive Language | 14.33 (10.46)a | 18.70 (7.07)a | 27.33 (3.72)b | -- | 4.96* |
| Visual Reception | 17.80 (7.49)a | 21.40 (5.06)a | 29.17 (7.22)b | -- | 6.08* |
| ELC† | 63.13 (18.67)a | 66.10 (12.76)a | 100.67 (10.80)b | -- | 13.03* |
| CARS2 Total Score | 32.90 (4.46)a | 21.75 (3.35)b | 17.50 (1.17)b | -- | 47.36* |
| ADOS(2) Severity Score | 6.60 (1.76)a | 1.50 (0.97)b | 1.17 (0.41)b | -- | 56.01* |
Note: MSEL = Mullen Scales of Early Learning; ASD = autism spectrum disorders; DD = developmental disabilities; TD-SP = typically-developing screen-positive; TD = typically-developing; ELC = Early Learning Composite; CARS2 = Childhood Autism Rating Scale, Second Edition; ADOS(2) = Autism Diagnostic Observation Schedule (Second Edition). Clinical measures not available for the TD group because they did not receive evaluations.
Means with different letters are significantly different at p < .05 level using Bonferroni-corrected pairwise comparison
Means with different letters are significantly different at p < .05 level using Bonferroni-corrected pairwise comparison
MSEL Early Learning Composite (M = 100, SD = 15)
p < .05
In the current study, we measure young children's performance in an object-directed task. In order to parallel the complexity and variety of behavior sequences that children observe in everyday life, each trial includes several different causally opaque acts leading to an outcome. These relatively long act sequences may also make group differences in social attention more pronounced. This design extends work with older children with ASD (Marsh, Pearson, Ropar, & Hamilton; 2013; Nielsen & Hudry, 2010; Nielsen, Slaughter, & Dissanayake, 2013; Vivanti, et al., 2014). We hypothesize that young children with ASD, who have not yet received a diagnosis nor any formal imitation training, will show less reproduction of both the outcome and the preceding steps relative to TD children. An additional measure of looking behavior will help clarify the role of visual attention in early imitation skills.
Method
Participants
Toddlers (N = 39; M = 28.58 months, SD = 6.31; range = 20.80 to 46.30; 28 males) were recruited from a larger study using the Modified Checklist for Autism in Toddlers – Revised, with Follow-Up (M-CHAT-R/F; Robins, Fein, & Barton, 2009; for information on the larger study see Robins, Casagrande, Barton, Chen, Dumont-Mathieu, & Fein, 2014). Parents completed the M-CHAT-R during regularly-scheduled pediatrician visits. Children who were considered at risk for ASD (based on M-CHAT-R/F scores, pediatrician concern, or a secondary autism screening) were invited to 1-2 sessions with a licensed psychologist and a doctoral student clinician that included research and clinical measures. Participants were recruited consecutively from these scheduled evaluations. Clinicians incorporated results from the Autism Diagnostic Observation Schedule (Second Edition; ADOS/ADOS2), Childhood Autism Rating Scale, Second Edition (CARS2), Vineland Adaptive Behavior Scales – II (VABS-II), and Mullen Scales of Early Learning (MSEL) with a parent interview of ASD symptoms to arrive at a clinical best estimate of diagnosis. All clinicians administering the ADOS(2) were research reliable. Parents provided informed consent, and the institutional review board provided oversight of the project.
Fifteen children received a clinical diagnosis on the autism spectrum, based on DSM-IV-TR diagnostic criteria (American Psychiatric Association, 2000; Autistic Disorder, n = 12; PDD-NOS, n = 3). The DD group (n = 10) included children who received a diagnosis not on the autism spectrum (e.g., Global Developmental Delay, Developmental Language Disorder). Those children who received no diagnosis were placed in the typically-developing screen-positive group (TD-SP; n = 6). A random sample of children whose M-CHAT-R/F scores suggested no autism risk were invited to participate in a research session only. This typically developing group (TD; n = 8) did not receive a clinical evaluation, thus MSEL data and other clinical measures were not available. Seven additional children were excluded from the analyses because they a) did not complete evaluation (n = 2), b) refused to engage in the imitation task (n = 3), or c) exhibited sub-clinical autism-like deficits that precluded clear diagnostic group categorization (n = 2).
The four groups were matched for chronological age (see Table 1). The ASD, DD, and TD-SP groups did not differ in fine motor ability or expressive language, but there were significant differences in receptive language and visual reception, as measured by the MSEL (see Table 1). Early Learning Composite (ELC) scores from the MSEL indicated the ASD and DD groups were matched on overall cognition. The ASD group had significantly higher CARS2 total scores and ADOS(2) severity scores than the other two groups. These differences are characteristic of children with ASD or DD, and so are not corrected for in subsequent analyses (Dennis, Francis, Cirino, Schachar, Barnes, & Fletcher, 2009; Miller & Chapman, 2001). This analysis plan is consistent with past studies of imitation in ASD (e.g., Nielsen, et al., 2013; Vivanti, et al., 2014).
Materials
Six novel stimuli objects were used; each had four associated target acts (see Table 2).
Table 2.
Detailed Stimuli Descriptions Including Photos and Target Acts
| Stimuli | Photo | Target acts |
|---|---|---|
| Lift-top box (20 × 17 × 6 cm) |

|
1. flip switch 2. rotate box to align handle with tool 3. apply tool around handle 4. open box (with or without tool) to reveal toy |
| Music Machine (11 × 8 × 13 cm) |

|
1. press silver button at base 2. turn first round switch to the right and back 3. touch pick to strings 4. strum strings (with pick or hand) to produce sound |
| Snap Container (16 × 16 × 5 cm) |

|
1. lift handle 2. unsnap side latch 3. apply tool to lid 4. lift lid (with or without tool or unsnapping) to reveal toy |
| Mounted Light (14 cm tall, 13 cm diameter) |

|
1. remove band 2. press button 3. touch light with back of hand 4. press light to illuminate |
| Pop-up Canister (16 × 11 × 16 cm) |

|
1. twist latch 2. pull latch away from canister 3. press button to make it lift 4. open canister to reveal toy |
| Scarf Bottle (27 cm tall, 7 cm diameter) |

|
1. pull down blue tab 2. apply thumb to yellow button on front of bottle 3. press yellow button on front of bottle 4. open top to reveal scarf |
Note: The mean time it took to demonstrate each act sequence ranged from 9-12 s.
Procedure
Each child was tested and videotaped individually in a lab room. The child and experimenter sat across from each other at a small table. Each session included six imitation trials, one with each object, which were presented in two (counterbalanced) orders. For each trial, the experimenter purposefully performed the target acts on the object. Each act could be completed independently (e.g., the snap container could be opened without unsnapping the side or using the tool). After each demonstration, the experimenter removed the object from view, reset it, said, “Now it's your turn”, and gave the object to the child for 30 s.
Dependent Measures and Scoring
Our primary dependent measure, the imitation score, was scored from video. Children received zero (0) points for unattempted target acts, half (.5) of a point for unsuccessful attempts, and one (1) point for successful imitation of a target act. Sample unsuccessful attempts include touching a button without depressing it, or pulling on a band without removing it. There were four acts per object yielding a total imitation score that ranged from 0-24. Each score was converted into a percentage of acts imitated. Two children did not interact with all six objects and their scores were based on their object set size (four or five objects). All raters were blind to the diagnostic groups and research hypotheses. A randomly-chosen 28.21% of participants were rescored by secondary raters. Agreement between raters was high; ICC (1,11) = .99.
To test for differences in children's attention to the adult's demonstrations, a frame-by-frame analysis of the video was conducted. For each frame, children were scored as looking at or away from the demonstration (including the experimenter and object). The attention score was the percent of total demonstration time a child spent looking at the demonstration. Scoring reliability was assessed by re-coding a randomly-chosen 28.21% of participants; ICC (1,6) = .99.
Results
Preliminary analyses showed no significant effects of object presentation order, trial number, or gender on the scores; we collapsed across these factors for subsequent analyses. Across groups, imitation scores met assumptions of normality (Shapiro-Wilk p's > .05), but violated Mauchly's test of sphericity; χ2(5) = 18.99, p = .002. Greenhouse-Geisser adjusted degrees of freedom are reported.1
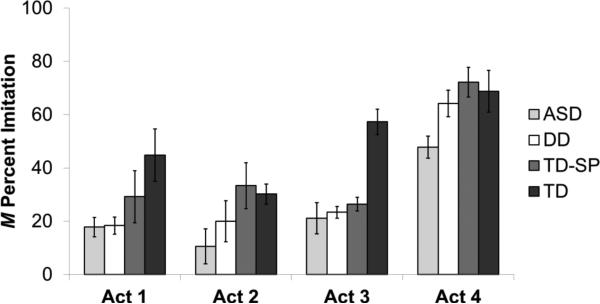
A repeated measures ANOVA, with act number (1-4) as a within subjects factor and diagnosis group as a between subjects factor, revealed two main effects (see Figure 1). First, there was a significant difference among groups in total imitation scores; F(3, 35) = 8.71, p < .001, ηp2 = .43. Specifically, the TD group (M = 50.26%, SD = 10.88), imitated significantly more target acts overall than both the ASD group (M = 24.31%, SD = 14.89; Tukey's HSD p < .001) and the DD group (M = 31.46%, SD = 9.38; p = .012). In addition, the TD-SP group (M = 40.28%, SD = 8.90) imitated more acts overall than the ASD group, p = .046. Unsuccessful attempts accounted for equal proportions of total imitation scores across the four groups, F(3, 38) = 1.54, p = .220, ηp2 = .12. Total imitation scores correlated with CARS2 total scores (Pearson r = -.47, p = .008) and MSEL ELC scores (Pearson r = .51, p = .003).
Figure 1.
Mean percentage of target acts imitated (+/− SE) as a function of demonstrated act number and diagnostic group. ASD = autism spectrum disorders; DD = developmental disabilities; TD-SP = typically-developing screen-positive; TD = typically-developing.
The ANOVA also showed a main effect of children's imitation of the different acts within each demonstration, F(2.25, 78.80) = 35.76, p < .001, ηp2 = .51. Children were significantly more likely to imitate the final act in the sequence (which produced the physical outcome) than they were to imitate any of the first three acts (the steps leading to the outcome); Bonferroni-corrected p values < .001. There were no significant differences in imitation of the first three acts, and no significant interaction between the act number and group, F(6.75, 78.80) = 1.38, p = .227, ηp2 = .11.
Attention scores were not normally distributed across groups (Shapiro-Wilk p's ≤ .002); nonparametric measures are reported. A Kruskal-Wallis test revealed significant differences between groups’ attention scores, χ2(3) = 15.01, p = .002. Subsequent Mann-Whitney U pairwise comparisons revealed that the ASD group (M = 80.13%, SD = 14.40) looked a significantly lower percentage of the time than the DD (M = 97.10%, SD = 3.98; U = 27.50), TD-SP (M = 98.83%, SD = 2.86; U = 11.50), and TD groups (M = 97.63%, SD = 5.21; U = 17.50), p's ≤ .008. There was also a significant correlation between total imitation scores and attention scores (Pearson r = .69, p < .001).
Discussion
The first aim of this study was to investigate the imitation of object-directed acts in young children with ASD who had not been previously diagnosed. Across all types of target acts, the ASD group imitated fewer acts than both of the TD groups, but did not differentiate from a matched DD group. This difference was observed for both acts producing physical outcomes and acts leading to those ends. These results support proposals that young children with ASD show a general impairment in imitating acts on objects relative to TD children (e.g., Rogers et al., 2003).
Regardless of diagnostic group, children were more likely to reproduce the final act, relative to the first three acts. This may reflect a recency effect; children may better remember the last act of a sequence. Alternately, children may have imitated the final act because it directly led to the physical outcome. This would suggest that on this task, all children prioritize the reproduction of outcomes relative to acts leading to outcomes (emulate), which is consistent with past reports from TD children (Bekkering, Wohlschlager, & Gattis, 2000; Elsner, 2007) as well some studies with older children with ASD (e.g. Marsh et al., 2013; Vivanti et al., 2014). Future research in which the order of the acts varies would help distinguish these alternatives. Future investigations could also examine imitation of different types of acts leading to outcomes (e.g., using a tool versus not), which was not systematically varied in the current study.
Although differences between the ASD and both TD groups persisted on these tasks, the DD group's scores were not significantly different from the ASD or TD-SP groups. A general limitation of this study is that the sample size is very small (and may be underpowered), although other imitation studies have tested groups of comparable sizes (e.g., Nielsen, et al., 2013). This may be particularly important in consideration of the DD group's imitation scores, which did not differ from the ASD group, despite a large effect (ηp2 = .43). Some prior studies of imitation of acts leading to physical outcomes have also struggled to elicit differences between children with ASD and children with mixed developmental disorders (Charman & Baron-Cohen, 1994; Wu, Chiang, & Hou, 2011); the lack of difference in this study may be exacerbated by the fact that all children in the DD group screened positive on an autism screening tool.
The second goal of this study was to examine whether differences in joint engagement, specifically looking behavior, might relate to imitation performance. The analysis of attention scores revealed that children with ASD looked at the demonstrations significantly less than the other three groups. This supports proposals that visual attention may play a role in imitation performance in children with ASD (Vivanti, et al., 2008). Fine motor ability (as measured by the MSEL) was also not significantly different among groups, which lends support to the idea that children with ASD fail on the front end of imitation (e.g., inattentive to demonstrations) rather than primarily in the stage of execution due to motor limitations (see Dziuk, et al., 2007; McDuffie, et al., 2007; Mostofsky, et al., 2006; Vanvuchelen, Roeyers, & De Weerdt, 2007).
Overall cognitive ability (measured by MSEL ELC scores) also correlated significantly with total imitation scores. Past studies have found associations between imitation and mental age, and particularly in the language domain (Poon, Watson, Baranek, & Poe, 2012; Roeyers, Van Oost, & Bothuyne, 1998; Sigman & Ungerer, 1984; Toth, et al., 2006; though see also, Stone, et al., 1997; Vanvuchelen, et al., 2007). This aligns with the results of the current study, however, it is unclear whether the cognitive deficits observed here explain decreased imitation, or are only co-occurring features of the ASD profile.
This study shows that, prior to diagnosis or intervention, young children with ASD show differences in imitation performance and visual attention in an object-directed imitation task. Deficits in imitation in ASD have been well-documented, and recent work exploring the relationship between imitation and visual attention finds that older children with ASD are less likely to look at the social elements of a demonstration (Hobson & Hobson, 2007; Vivanti, et al., 2008; Vivanti, et al., 2014). The current results show a decrease in total attention that is specific to toddlers with ASD. It is possible that young children with ASD fail to attend to social learning opportunities like DD and TD children do, and that these differences become more defined with age or as the result of intervention. This finding reaffirms the importance of engaging visual attention in targeted interventions for ASD, and especially when teaching imitation and other social skills.
However, the answer may not be so straightforward. The above-referenced work also shows that imitation deficits in ASD persist, even when differences in looking behavior are not present. It is possible that the early pedagogical experience accessed by visual attention to others is critical for later imitation development. Future research would help to elucidate the role of visual attention in early imitation, as well as the longitudinal influence of early looking patterns on social and communicative skills.
Acknowledgements
This research was supported by grant # HD039961 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, grant # 8368 from Autism Speaks, and the Georgia State University Second Century Initiative in Primate Social Cognition, Evolution, and Behavior (2CI-PSCEB).
We would like to thank the members of the early detection project team at Georgia State University, especially Karís Casagrande and Kiauhna Haynes for assistance with data management. We also thank the families and children who participated in our study sessions.
Footnotes
Ethical Standards
This study was approved by the Institutional Review Board of Georgia State University and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All parents and guardians provided informed consent prior to children's inclusion in the study.
Conflicts of Interest
Anna Gonsiorowski and Rebecca A. Williamson declare that they have no conflicts of interest. Diana L. Robins is a co-owner of M-CHAT LLC which receives royalties for commercial products that incorporate the M-CHAT-R/F.
Nonparametric analyses revealed the same findings. A Kruskal-Wallis test showed a significant difference among groups in total imitation scores, χ2(3) = 16.36, p = .001. Subsequent Mann-Whitney U pairwise comparisons revealed that the TD group imitated significantly more overall than the DD group (p = .002) and ASD group (p = .002), but not the TD-SP group (p = .135). The TD-SP group imitated significantly more than the ASD group (p = .022), but not the DD group (p = .082). There was no significant difference in overall imitation between the DD and ASD groups (p = .127).
A Friedman test revealed significant differences in the frequency of completion of Acts 1-4, χ2(3) = 51.21, p < .001. Subsequent Wilcoxon-signed ranks tests revealed that, across groups, Act 4 was completed more frequently than the three other acts (p's < .001). Additionally, Act 2 was completed more frequently than Act 3 (p = .020)
AG participated in the conception and coordination of the study, analyzed and interpreted data, and helped draft the manuscript; RAW conceived the study and its design, participated in data analyses and interpretation, and helped draft the manuscript; DLR participated in conception and coordination of the study, acquired clinical data, and helped draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Anna Gonsiorowski, Department of Psychology, Georgia State University, Atlanta, GA P. O. Box 5010, Atlanta, GA 30302.
Rebecca A. Williamson, Department of Psychology, Georgia State University, Atlanta, GA P. O. Box 5010, Atlanta, GA 30302
Diana L. Robins, A.J. Drexel Autism Institute, Drexel University, Philadelphia, PA 3020 Market Street, Suite 560, Philadelphia, PA 19104
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text revision American Psychiatric Association; Arlington, VA: 2000. [Google Scholar]
- Bekkering H, Wohlschlager A, Gattis M. Imitation of gestures in children is goal-directed. The Quarterly Journal of Experimental Psychology. 2000;53(1):153–164. doi: 10.1080/713755872. [DOI] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S. Another look at imitation in autism. Development and Psychopathology. 1994;6(3):403–413. [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants with autism: An investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology. 1997;33(5):781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- DeMyer MK, Alpern GD, Barton S, DeMyer WE, Churchill DW, Hingtgen JN, Bryson CQ, Pontius W, Kimberlin C. Imitation in autistic, early schizophrenic, and non-psychotic subnormal children. Journal of Autism and Childhood Schizophrenia. 1972;2(3):264–287. doi: 10.1007/BF01537618. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Larson JCG, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Edwards LA. A meta-analysis of imitation abilities in individuals with autism spectrum disorders. Autism Research. 2014;7(3):363–380. doi: 10.1002/aur.1379. [DOI] [PubMed] [Google Scholar]
- Elsner B. Infants’ imitation of goal-directed actions: the role of movements and action effects. Acta Psychologica. 2007;124(1):44–59. doi: 10.1016/j.actpsy.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Hobson RP. Identification: The missing link between joint attention and imitation? Development and Psychopathology. 2007;19(2):411–431. doi: 10.1017/S0954579407070204. [DOI] [PubMed] [Google Scholar]
- Ingersoll B. The effect of context on imitation skills in children with autism. Research in Autism Spectrum Disorders. 2008;2(2):332–340. [Google Scholar]
- Ingersoll B, Schreibman L. Teaching reciprocal imitation skills to young children with autism using a naturalistic behavioral approach: Effects on language, pretend play, and joint attention. Journal of Autism and Developmental Disorders. 2006;36(4):487–505. doi: 10.1007/s10803-006-0089-y. [DOI] [PubMed] [Google Scholar]
- Jones V, Prior M. Motor imitation abilities and neurological signs in autistic children. Journal of Autism and Developmental Disorders. 1985;15(1):37–46. doi: 10.1007/BF01837897. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Marsh L, Pearson A, Ropar D, Hamilton A. Children with autism do not overimitate. Current Biology. 2013;23(7):R266–R268. doi: 10.1016/j.cub.2013.02.036. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Turner L, Stone W, Yoder P, Wolery M, Ulman T. Developmental correlates of different types of motor imitation in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(3):401–412. doi: 10.1007/s10803-006-0175-1. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society. 2006;12(3):314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Hudry K. Over-imitation in children with Autism and Down Syndrome. Australian Journal of Psychology. 2010;62(2):67–74. [Google Scholar]
- Nielsen M, Slaughter V, Dissanayake C. Object-directed imitation in children with high-functioning autism: Testing the social motivation hypothesis. Autism Research. 2013;6(1):23–32. doi: 10.1002/aur.1261. [DOI] [PubMed] [Google Scholar]
- Poon KK, Watson LR, Baranek GT, Poe MD. To what extent do joint attention, imitation, and object play behaviors predict later communication and intellectual functioning in ASD? Journal of Autism and Developmental Disorders. 2012;42(6):1064–1074. doi: 10.1007/s10803-011-1349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Casagrande K, Barton ML, Chen C, Dumont-Mathieu T, Fein D. Validation of the Modified Checklist for Autism in Toddlers-Revised with Follow-Up (M-CHAT-R/F). Pediatrics. 2014;133(1):37–45. doi: 10.1542/peds.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton M. The Modified Checklist for Autism in Toddlers – Revised, with Follow-up (M-CHAT-R/F) 2009. Self-published. [DOI] [PMC free article] [PubMed]
- Roeyers H, van Oost P, Bothuyne S. Immediate imitation and joint attention in young children with autism. Development and Psychopathology. 1998;10(3):441–450. doi: 10.1017/s0954579498001680. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44(5):763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3(2):137–162. [Google Scholar]
- Rogers SJ, Young GS, Cook I, Giolzetti A, Ozonoff S. Deferred and immediate imitation in regressive and early onset autism. Journal of Child Psychology and Psychiatry. 2008;49(4):449–457. doi: 10.1111/j.1469-7610.2007.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowberry J, Macari S, Chen G, Campbell D, Leventhal JM, Weitzman C, Chawarska K. Screening for autism spectrum disorder in 12-month-old high-risk siblings by parental report. Journal of Autism and Developmental Disorders. 2015;45(1):221–229. doi: 10.1007/s10803-014-2211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevlever M, Gillis JM. An examination of the state of imitation research in children with autism: Issues of definition and methodology. Research in Developmental Disabilities. 2010;31(5):976–984. doi: 10.1016/j.ridd.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dijamco A, Gratier M, Rozga A. Early detection of core deficits in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(4):221–223. doi: 10.1002/mrdd.20046. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ungerer JA. Cognitive and language skills in autistic, mentally retarded, and normal children. Developmental Psychology. 1984;20(2):293–302. [Google Scholar]
- Stone WL, Ousley OY, Littleford CD. Motor imitation in young children with autism: What's the object? Journal of Abnormal Child Psychology. 1997;25(6):475–485. doi: 10.1023/a:1022685731726. [DOI] [PubMed] [Google Scholar]
- Toth K, Munson JN, Meltzoff A, Dawson G. Early predictors of communication development in young children with autism spectrum disorder: Joint attention, imitation, and toy play. Journal of Autism and Developmental Disorders. 2006;36(8):993–1005. doi: 10.1007/s10803-006-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgiris IC. Two functions of imitation during infancy. International Journal of Behavioral Development. 1981;4(1):1–12. [Google Scholar]
- Vanvuchelen M, Roeyers H, De Weerdt W. Nature of motor imitation problems in school-aged boys with autism. Autism. 2007;11(3):225–240. doi: 10.1177/1362361307076846. [DOI] [PubMed] [Google Scholar]
- Vivanti G, Hamilton A. Imitation in autism spectrum disorders. In: Volkmar FR, Paul R, Rogers SJ, Pelphrey KA, editors. Handbook of autism and pervasive developmental disorders. Vol. 1. John Wiley & Sons, Inc.; Hoboken, NJ: 2014. pp. 278–301. [Google Scholar]
- Vivanti G, Nadig A, Ozonoff S, Rogers SJ. What do children attend to during imitation tasks? Journal of Experimental Child Psychology. 2008;101(3):186–205. doi: 10.1016/j.jecp.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti G, Trembath D, Dissanayake C. Mechanisms of imitation impairment in autism spectrum disorder. Journal of Abnormal Child Psychology. 2014;42(8):1395–1405. doi: 10.1007/s10802-014-9874-9. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Singh T. A systematic review of action imitation in autism spectrum disorder. Journal of Autism and Developmental Disorders. 2004;34(3):285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Wu C, Chiang C, Hou Y. A two time point study of imitative abilities in children with autism spectrum disorders. Journal of Applied Research in Intellectual Disabilities. 2011;24(1):39–49. [Google Scholar]
- Young GS, Rogers SJ, Hutman T, Rozga A, Sigman M, Ozonoff S. Imitation from 12 to 24 months in autism and typical development: A longitudinal rasch analysis. Developmental Psychology. 2011;47(6):1565–1578. doi: 10.1037/a0025418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Journal of Developmental Neuroscience. 2005;23(2-3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]