Abstract
Metal hypersensitivity in patients with a total knee arthroplasty (TKA) is a controversial topic. The diagnosis is difficult, given the lack of robust clinical validation of the utility of cutaneous and in vitro testing. Metal hypersensitivity after TKA is quite rare and should be considered after eliminating other causes of pain and swelling, such as low-grade infection, instability, component loosening or malrotation, referred pain, and chronic regional pain syndrome. Anecdotal observations suggest that two clinical presentations of metal hypersensitivity may occur after TKA: dermatitis or a persistent painful synovitis of the knee. Patients may or may not have a history of intolerance to metal jewelry. Laboratory studies, including erythrocyte sedimentation rate, C-reactive protein level, and knee joint aspiration, are usually negative. Cutaneous and in vitro testing have been reported to be positive, but the sensitivity and specificity of such testing has not been defined. Anecdotal reports suggest that, if metal hypersensitivity is suspected and nonsurgical measures have failed, then revision to components fabricated of titanium alloy or zirconium coating can be successful in relieving symptoms. Revision should be considered as a last resort, however, and patients should be informed that no evidence-based medicine is available to guide the management of these conditions, particularly for decisions regarding revision. Given the limitations of current testing methods, the widespread screening of patients for metal allergies before TKA is not warranted.
Keywords: total knee arthroplasty, metal hypersensitivity, metal allergy, persistent synovitis, failed knee arthroplasty
Orthopaedic surgeons have been implanting metallic hip and knee arthroplasty components for >40 years. Total knee arthroplasty (TKA) components—usually a femoral component of cobalt-chrome alloy that articulates with an ultra-high–molecular-weight polyethylene tibial component, with or without metal backing and a polyethylene patella component—have generally provided notable pain relief and improvement in function for a variety of patients with arthritic conditions. The complications of infection, instability, malalignment, and loosening cause recurrent pain, swelling, and disability after TKA and require revision.1 Almost 20% of patients with a well-fixed, properly aligned knee implant are “dissatisfied” with the procedure for a variety of reasons.2 In addition, some patients have pain and stiffness after TKA for which no readily available explanation exists.3 Although the idea of metal hypersensitivity as a cause of a painful knee implant was first introduced almost 18 years ago, it has been considered an insignificant cause of the failure of modern TKA.4
Basic Science and Metal Sensitivity
All metals that come into contact with biologic systems undergo some degree of corrosion, and metal ions released from TKA components intra-articularly may form complexes with native proteins. These metal-protein complexes may act as antigens or allergens and cause an immunologic response in the body or synovial joint. The most common metal sensitizer in humans is nickel, followed by cobalt and chromium.4,5 Polyethylene and polymethyl methacrylate particles are relatively large and do not elicit the same response as metal ions.4,5
The prevalence of metal sensitivity in the general population is approximately 10% to 15%. Nickel sensitivity has the highest prevalence, approximately 14%, and cross-reactivity between nickel and cobalt is most common.5 However, the prevalence of metal sensitivity in patients with well-functioning implants, mostly of the hip, is approximately 25%.5 In a review of studies of patients with a failed, loose, or poorly functioning implant, the average prevalence of metal sensitivity was 60% (range, 13% to 71%).5 It is not known whether this phenomenon is a cause or an effect.
The pathophysiology of metal hypersensitivity to orthopaedic implants has been described previously in great detail.5 This implant-related hypersensitivity is generally a type IV allergic reaction, a delayed cell-mediated response, with activation of specific T lymphocytes. These and other lymphocyte populations release a variety of cytokines that perpetuate the inflammatory response and trigger the participation of activated macrophages.5 This response can produce substantial tissue inflammation and eventual periprosthetic tissue damage. Although it is known that Langerhans cells in the dermis are associated with skin hypersensitivity reactions, the particular cells in the periprosthetic knee joint responsible for the presentation of the metal-protein antigen are not known but could be endothelial cells, macrophages, or other synovial tissue cells.5
No generally accepted and reliable test is available for the clinical diagnosis of metal hypersensitivity to the components used in total hip arthroplasty or TKA.5 Dermatologists routinely have used a panel of cutaneous patch testing to different metal-salt complexes to determine hypersensitivity to a particular metal (Figure 1). An erythematous reaction to the allergen can be rated only qualitatively. However, controversy exists over the validity of patch testing to determine deep-tissue or joint hypersensitivity to metals.5 In a retrospective, case-controlled study of the sensitivity to metals in TKA components, Granchi et al6 reported on 94 patients who underwent dermal (back) patch testing to 11 metals and haptens for bone cements. In 20 patients who had no knee implant but who were candidates for TKA, 15% had positive patch testing to at least one metal hapten. Positive patch testing was significantly greater in a group of 27 patients with a stable knee arthroplasty (44%; P = 0.05) and in a group of 47 patients with a loose knee arthroplasty (57%; P = 0.001). No predictive value of the patch testing was seen in determining the fixation status of the TKA.6 The medical history for metal allergy identified by previous skin testing or a questionnaire was found to be a risk factor for the loosening of a TKA because failure was four times more likely in patients with prior symptoms of metal hypersensitivity.6
Figure 1.
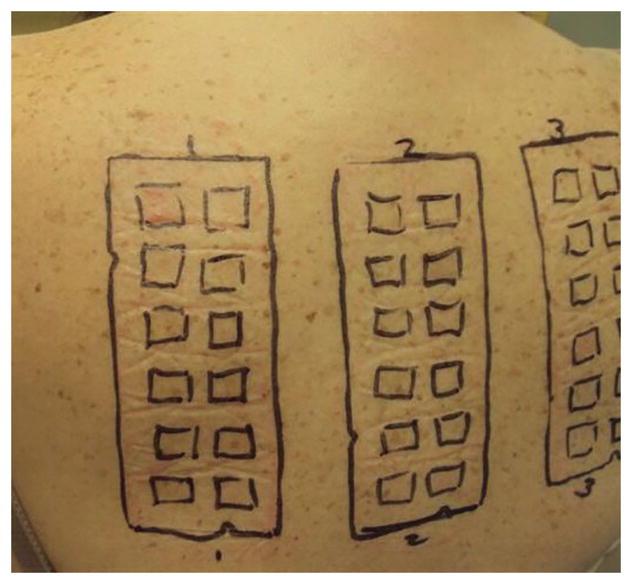
Clinical photograph demonstrating cutaneous patch testing on the upper back of a female patient who has persistent pain, synovitis, and stiffness after a total knee arthroplasty with an implant of a cobalt-chromium alloy. A mild allergic reaction to nickel, consisting of erythema and edema, is shown in the upper left box. (Courtesy of Audrey Echt, MD, Raleigh, NC.)
Another test for metal hypersensitivity is the in vitro lymphocyte transformation test, in which peripheral blood lymphocytes from the patient are challenged with a variety of metal salts and the uptake of a radioactive nucleotide is quantified after 6 days.5 The final test is the in vitro leukocyte migration inhibition test, which quantifies the migration of cells in the presence of a sensitizing metal antigen by one of four methods.4,5 Few data show the utility of these in vitro tests for TKA patients, however. In a prospective study of 92 patients undergoing TKA in Japan, a modified lymphocyte stimulation test (mLST) was performed preoperatively, and 24 patients (26%) had a positive mLST response to at least one tested metal.7 The most frequent sensitizer was nickel, followed by chromium, cobalt, and iron.7 The clinical significance of these findings is unknown. At the present time, no evidence supports the routine or widespread preoperative patch or in vitro lymphocyte testing of patients for metal hypersensitivity before primary TKA.
Clinical Syndromes Purportedly Related to Metal Hypersensitivity After Total Knee Arthroplasty
The association of metal orthopaedic implants with an eczematous dermatitis has been known for almost 40 years, with numerous case reports of reactions to stainless steel screws and cobalt-chromium alloy implants.4,5 Several reports of persistent localized or systemic skin reactions after TKA have appeared in the dermatology literature.8–10 In the largest series, Verma et al8 observed 30 patients over 3 years who had an erythematous, papular, scaly, and sometimes exudative eczema around the knee area after TKA. Of these patients, 13 women and 2 men were available for complete evaluation and patch testing. They had a mean age of 65 years, with dermatitis commencing 1 to 3 months after TKA. All implants were fabricated of a cobalt-chromium alloy femoral component and a titanium alloy tibial component. In all cases, the dermatitis was localized to the outer aspect of the knee lateral to the anterior midline incision.8 Patch testing showed that only seven patients had a 1+ or 2+ reaction to a metal: four to nickel, two to chromium, and one to cobalt. Both men had negative patch tests to all tested substances. All patients were treated with topical steroids, and the eruption cleared within 2 weeks.8
A prospective study of 92 patients who underwent TKA included 5 patients with eczema. Of the five patients, three had a localized eczema that developed in the skin over the knee, and two had a form that began in the skin over the knee and then extended over the entire body.7 Using a mLST, a significant association was seen between chromium sensitivity and the development of eczema. One patient had spontaneous resolution of the eczema, and the other four had the condition for >1 year postoperatively. Two of these patients underwent revision, one with cobalt-chrome beaded, noncemented components to ceramic revision knee components, and the other with a cobalt-chrome cemented unicondylar knee to titanium knee components. Both patients had resolution of the dermatitis within 2 months after revision.7 No good explanation was offered for the high prevalence of dermatitis after primary TKA in this series.
Patients in whom a persistent dermatitis develops, without concurrent knee synovitis or component loosening, should be referred to a dermatologist for treatment with topical or systemic steroids, although little evidence-based medicine supports this recommendation. Revision of the components for severe dermatitis has been reported infrequently.7,11 The senior author (P.F.L.) has seen four TKA patients with a mild erythematous pruritic rash in the lateral parapatellar region and recommended topical steroids.
Severe Persistent Synovitis After Cobalt-chromium Total Knee Arthroplasty
Persistent painful synovitis and effusion after TKA typically is related to chronic infection, instability, loosening, polyethylene wear, or recurrent hemarthrosis. Crystalline arthropathy also can cause painful synovitis and effusions after TKA. Over the past several years, multiple reports have appeared of severe painful persistent synovitis in patients with cobalt-chromium TKA components12–16(Tables 1 and 2). These patients are more likely to be women than men, with the onset of symptoms extending from 2 months to 2 years after primary TKA. The presenting symptoms are persistent pain, swelling, and, usually, stiffness. On physical examination, these patients may or may not have lateral knee dermatitis, but all have an effusion, synovitis, and some limitation of range of motion. Radiography is typically unremarkable. Obviously, this clinical picture is indistinguishable from that of chronic or indolent infection of the TKA.12
Table 1.
Demographics and Workup of Reported Metal Hypersensitivity Cases After Total Knee Arthroplasty
| Study | Cases (Knees ) | Gende r | Age (yr) | Index TKA: Alloy (Manufacturer) | Time in Situ (mo) | Infection Workup | Cutaneous Findings | Metal Hypersensitivity Test, Outcome (Element) |
|---|---|---|---|---|---|---|---|---|
| Anand et al12 | 1 (1)a | M | 70 | Co-Cr (Zimmer) | 20 | Normal ESR, CRP level, IL-1, negative culture and PCR | None | Patch test, positive (Ni, Co) |
| Thomsen et al16 | 1 (1) | F | 60 | Co-Cr (Aesculap) | Approxi mately 48 | Normal CRP level, negative culture | Eczematous reaction | LTT, negative |
| Bergschmidt et al13 | 1 (1) | F | 58 | Co-Cr, Ti (Lima) | 3 | Normal CRP level, negative culture | None | Patch test, positive (Ni, Pd) |
| McMaster and Patel14 | 1 (1)b | M | 65 | Co-Cr (DePuy) | 20 | Elevated ESR, CRP level, serum Co, negative aspiration and culture | Rash | LTT, negative |
| Thakur et al15 | 5 (6) | F | 78 | Co-Cr (Stryker) | 3 | Normal ESR, elevated CRP level, negative culture | None | Patch test, positive (Ni) |
| M | 64 | Co-Cr (Stryker) | 24 | Normal ESR and CRP level, negative aspirate and culture | None | Patch test, positive (Co) Known Ni allergy | ||
| F | 56 | Co-Cr (Stryker) | 24/28 | Normal ESR and CRP level | None | Patch test, positive (Co, Mn) | ||
| F | 56 | Co-Cr (Stryker) | 5 | Normal ESR and CRP level, negative aspirate | None | None | ||
| F | 64 | Co-Cr (Stryker) | 24 | Normal ESR and CRP level, negative aspirate and culture | None | Patch test, negative |
Patient initially presented 2 months postoperatively with positive ESR, positive CRP level, and aspirate of 56,000 nucleated cells and underwent single-stage débridement with antibiotic treatment without resolution of symptoms.
Case report describing adverse metal reaction related to taper corrosion in revision rotating hinge prosthesis.
Co = cobalt, Cr = chromium, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, IL-1 = interleukin 1, LTT = lymphocyte transformation test, Mn = manganese, Ni = nickel, PCR = polymerase chain reaction, Pd = palladium, Ti = titanium, TKA = total knee arthroplasty,
Table 2.
Surgical Findings and Outcomes of Revision Surgery for Reported Metal Hypersensitivity Cases After Total Knee Arthroplasty
| Study | Index TKA: Alloy (Manufacturer) | Time in Situ (mo) | Histopathology Findings | Revision TKA: Alloy (Manufacturer) | Follow-up (mo) | Clinical Results |
|---|---|---|---|---|---|---|
| Anand et al12 | Co-Cr (Zimmer) | 20 | Lymphoplasmacellular fibrinous tissue, consistent with a type IV allergic reaction | Ti (Biomet) | 14 | Improved motion, resolved pain |
| Thomsen et al16 | Co-Cr (Aesculap) | Approximately 48 | Not reported | Zr-and N-coated Co-Cr (Aesculap) | 18 | Improved motion, resolved pain, resolution of eczema |
| Bergschmidt et al13 | Co-Cr, Ti (Lima) | 3 | Lymphoplasmacellular fibrinous tissue, consistent with type IV allergic reaction | Ceramic femur, Ti tibia (CeramTec, Lima) | 12 | Improved motion, no pain at rest |
| McMaster and Patel14 | Co-Cr (DePuy) | 20 | Eosinophilic fibrinous tissue devoid of cells and corrosive metallic product consistent with ALTRa | Co-Cr (DePuy) | 8 | Resolved pain, wound healing with coverage |
| Thakur et al15 | Co-Cr (Stryker) | 3 | Synovial hypertrophy and hyperplasia with perivascular lymphoplasmacytic aggregates and myxomatous degeneration | Oxinium femur, Ti tibia (Smith & Nephew) | 12 | Improved motion, resolved pain |
| Co-Cr (Stryker) | 24 | Chronic histiocytic reaction to birefringent small-particular material | Oxinium femur, Ti tibia (Smith & Nephew) | 24 | Improved pain | |
| Co-Cr (Stryker) | 24/28 | Synovial hypertrophy and hyperplasia associated with diffuse mononuclear and giant cell reaction, perivascular chronic inflammation | Oxinium femur (Smith & Nephew) | 12 | Preserved motion, resolved pain | |
| Co-Cr (Stryker) | 5 | Synovial hyperplasia with chronic histiocytic response | Oxinium femur, Ti tibia (Smith & Nephew) | 12 | Preserved motion, resolved pain | |
| Co-Cr (Stryker) | 24 | Foreign body reaction to colorless refractile material with synovial cell hyperplasia and foci of myxoid degeneration | Oxinium femur (Smith & Nephew) | 4 | Improved motion, resolved pain |
Case report describing adverse metal reaction related to taper corrosion in revision rotating hinge prosthesis.
ALTR = adverse local tissue reaction, Co = cobalt, Cr = chromium, N = nitrogen, Ti = titanium, TKA = total knee arthroplasty, Zr = zirconium
The minimum workup should include laboratory studies, including C-reactive protein level and erythrocyte sedimentation rate, as well as a knee joint aspiration for white blood cell count, differential, and aerobic/anaerobic culture. These tests are usually negative for infection. The senior authors (P.F.L., J.J.J.) recommend a second knee aspiration for culture before excluding infection. Bacterial cultures should be kept for approximately 3 weeks to identify potentially slow-growing organisms. The authors do not routinely submit the knee fluid for acid-fast bacilli and fungal cultures unless a high clinical suspicion exists for these microorganisms. Serum metal levels of cobalt, chromium, and titanium typically are elevated in patients with well-functioning unilateral or bilateral TKA;17 therefore, the authors do not recommend obtaining serum metal levels if metal hypersensitivity to the knee arthroplasty is suspected. Patch testing and in vitro lymphocyte testing have been performed in these patients, but as stated, no standard of care exists for the diagnosis of deep-tissue metal hypersensitivity associated with the presence of metallic orthopaedic implants. No proven nonsurgical treatment is available for metal hypersensitivity suspected to be the cause of painful, persistent synovitis and effusion of the TKA. Treatment of symptoms with NSAIDs and physical therapy modalities may be attempted, but to our knowledge, no report exists of the use of a systemic or intra-articular steroid for this condition.
The diagnosis of metal hypersensitivity as a cause of painful persistent synovitis and effusion of a cobalt-chromium TKA is one of exclusion. A causal association between these symptoms and metal allergy still remains unproven. No evidence has shown that patients with a known metal allergy have a higher rate of failure or revision of primary TKA than do those without such a history. Thus, the decision to recommend and proceed with revision of a TKA for severe dermatitis or painful persistent synovitis should be performed with great caution and appropriately guarded patient counseling.
Periprosthetic membrane and synovial tissue surrounding cobalt-chromium implants at the time of revision for painful persistent synovitis will most often show chronic inflammation, with lymphocyte and plasma cell predominance (Figure 2). Most case reports or case series describe revision of these well-fixed cobalt-chromium alloy components to a femoral component fabricated of zirconium or titanium alloy with a nitride coating. If a cobalt-chromium alloy tibial component is present, it likely also should be revised to one fabricated of titanium alloy. After revision with these components, patients in these reported cases generally have had resolution of the systemic or localized eczema and the painful persistent synovitis. However, reported follow-up times after these revisions are quite short.
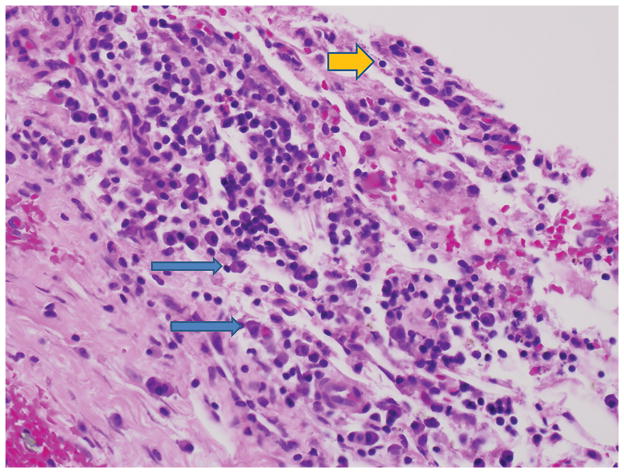
Figure 2.
High-power photomicrograph (hematoxylin-eosin, magnification ×125) demonstrating a membrane adjacent to a femoral component of a cobalt-chromium alloy in a patient with painful, persistent synovitis after total knee arthroplasty. Chronic inflammation is present, and the predominant cells are lymphocytes (yellow arrow) and plasma cells (blue arrows). No multinuclear giant cells and no polyethylene fragments are evident. The synovial biopsies showed the same pattern. (Courtesy of Maureen Bauer, MD, Durham, NC.)
Metal debris generated from stainless steel surgical instruments, particularly saw blades, may be an important source of bioavailable nickel. For example, in grade 316L stainless steel, the nickel content ranges from 10% to 14%. The presence of stainless steel debris in the periprosthetic tissues of patients with implants that are not fabricated from stainless steel has been documented.18 This finding confounds the management of patients who provide a history of nickel allergy or who have undergone cutaneous or in vitro testing that shows hypersensitivity to nickel or chromium.
Summary
Currently, insufficient evidence exists to recommend routine or widespread cutaneous or in vitro hypersensitivity testing before routine primary TKA. In addition, no evidence-based medicine is available to recommend any type of screening questionnaire related to metal hypersensitivity. Only anecdotal information exists to recommend a femoral component of zirconium or titanium alloy if a patient volunteers a medical history of severe dermatitis after contact with metal jewelry. This issue should be discussed preoperatively with the patient. Localized dermatitis of lateral knee skin after TKA typically is treated successfully with topical steroids. After exclusion of all other causes of failure, patients with severe systemic dermatitis or painful persistent synovitis may be offered revision to zirconium or titanium alloy components when nonsurgical methods have failed to improve symptoms. However, the evidence base to support revision surgery in this setting is limited to anecdotal reports or uncontrolled small case series. Such patients should be informed that the outcome of revision surgery for presumed metal allergy is unpredictable and should be considered only as a last resort.
Acknowledgments
The authors thank Audrey Echt, MD for the photograph, Maureen Bauer, MD, for the photomicrographs and assistance with their interpretation, and Stephen Perlman, MLS, for assistance with the literature search.
Dr. Jacobs acknowledges support by the National Institutes of Health.
Footnotes
Dr. Lachiewicz or an immediate family member has received royalties from Innomed; is a member of a speakers’ bureau or has made paid presentations on behalf of Mallinckrodt Pharmaceuticals and Pacira Pharmaceuticals; serves as a paid consultant to Gerson Lehrman Group, Guidepoint Global Advisors, and Pacira Pharmaceuticals; has received research or institutional support from Zimmer; and serves as a board member, owner, officer, or committee member of The Hip Society and the Orthopaedic Surgery and Trauma Society. Dr. Jacobs or an immediate family member has stock or stock options held in Implant Protection; has received research or institutional support from Medtronic Sofamor Danek, NuVasive, and Zimmer; and serves as a board member, owner, officer, or committee member of The Hip Society. Neither Dr. Watters nor any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article.
References
Evidence-based Medicine: Levels of evidence are described in the table of contents. In this article, reference 1 is a level II study. References 2–18 are level IV studies. References printed in bold type are those published within the past 5 years.
- 1.Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;446(446):45–50. doi: 10.1097/01.blo.0000214421.21712.62. [DOI] [PubMed] [Google Scholar]
- 2.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133–138. doi: 10.1016/j.arth.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 4.Merritt K, Rodrigo JJ. Immune response to synthetic materials: Sensitization of patients receiving orthopaedic implants. Clin Orthop Relat Res. 1996;326:71–79. [PubMed] [Google Scholar]
- 5.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83(3):428–436. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Granchi D, Cenni E, Tigani D, Trisolino G, Baldini N, Giunti A. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29(10):1494–1500. doi: 10.1016/j.biomaterials.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 7.Niki Y, Matsumoto H, Otani T, et al. Screening for symptomatic metal sensitivity: A prospective study of 92 patients undergoing total knee arthroplasty. Biomaterials. 2005;26(9):1019–1026. doi: 10.1016/j.biomaterials.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Verma SB, Mody B, Gawkrodger DJ. Dermatitis on the knee following knee replacement: A minority of cases show contact allergy to chromate, cobalt or nickel but a causal association is unproven. Contact Dermatitis. 2006;54(4):228–229. doi: 10.1111/j.0105-1873.2006.0775o.x. [DOI] [PubMed] [Google Scholar]
- 9.Handa S, Dogra S, Prasad R. Metal sensitivity in a patient with a total knee replacement. Contact Dermatitis. 2003;49(5):259–260. doi: 10.1111/j.0105-1873.2003.0225b.x. [DOI] [PubMed] [Google Scholar]
- 10.Beecker J, Gordon J, Pratt M. An interesting case of joint prosthesis allergy. Dermatitis. 2009;20(2):E4–E9. [PubMed] [Google Scholar]
- 11.Gao X, He RX, Yan SG, Wu LD. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011;26(4):665.e13–665.e16. doi: 10.1016/j.arth.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Anand A, McGlynn F, Jiranek W. Metal hypersensitivity: Can it mimic infection? J Arthroplasty. 2009;24(5):826.e25–826.e28. doi: 10.1016/j.arth.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Bergschmidt P, Bader R, Mittelmeier W. Metal hypersensitivity in total knee arthroplasty: Revision surgery using a ceramic femoral component. A case report Knee. 2012;19(2):144–147. doi: 10.1016/j.knee.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 14.McMaster WC, Patel J. Adverse local tissue response lesion of the knee associated with Morse taper corrosion. J Arthroplasty. 2013;28(2):375.e5–e8. doi: 10.1016/j.arth.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Thakur RR, Ast MP, McGraw M, Bostrom MP, Rodriguez JA, Parks ML. Severe persistent synovitis after cobalt-chromium total knee arthroplasty requiring revision. Orthopedics. 2013;36(4):e520–e524. doi: 10.3928/01477447-20130327-34. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen M, Rozak M, Thomas P. Pain in a chromium-allergic patient with total knee arthroplasty: Disappearance of symptoms after revision with a special surface-coated TKA. A case report. Acta Orthop. 2011;82(3):386–388. doi: 10.3109/17453674.2011.579521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luetzner J, Krummenauer F, Lengel AM, Ziegler J, Witzleb WC. Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res. 2007;461:136–142. doi: 10.1097/BLO.0b013e31806450ef. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JJ, Urban RM, Schajowicz F, Gavrilovic J, Galante JO. Particulate-associated endosteal osteolysis in titanium-base alloy cementless total hip replacement. In: StJohn KR, editor. Medical Implants: Mechanisms of formation and biological consequences ASTM STP 1144. Philadelphia, PA: American Society for Testing and Materials; 1992. pp. 52–60. [Google Scholar]