Abstract
Background
Prescription opioid misuse is a major public health concern in the US. Few resources exist to support community pharmacists engaging patients who misuse or are at risk for misuse.
Objectives
This report describes the results of the execution of the ADAPT-ITT model (a model for modifying evidence-based behavioral interventions to new populations and service settings) to guide the development of a behavioral health framework for opioid medication misuse in the community pharmacy setting.
Methods
Pharmacy, addiction, intervention, and treatment experts were convened to attend a one-day meeting to review the empirical knowledgebase and discuss adapting the screening, brief intervention, and referral to treatment (SBIRT) protocol for addressing opioid medication misuse in community pharmacy. Qualitative data gathered from the meeting were analyzed by 2 independent coders in a 2-cycle process using objective coding schemes. Percentage of agreement and Cohen’s Kappa were calculated to assess coder agreement.
Results
First-cycle coding identified 4 distinct themes, with coder percentage of agreement ranging from 93.5–99.6% and with Kappa values between 0.81–0.93. Second-cycle coding identified 10 sub-themes, with coder percentage of agreement ranging from 83–99.8% and with Kappa values between 0.58–0.93. Identified themes and sub-themes encompassed patient identification, intervention, prevention, and referral to treatment.
Conclusions
Focus of screening efforts in the emerging model should capitalize on pharmacists’ knowledge of medication management. Screening likewise should be multidimensional in order to facilitate patient-centered interventions that activate additional disciplines able to interface with patients at risk or involved in medication misuse.
Keywords: Opioid misuse, adherence, medication management, qualitative research
Introduction
The misuse of prescription opioids has reached epidemic proportions in the US and is a major concern for public health.1, 2 Opioid medication misuse involves diverse behaviors, including taking more medication than prescribed, doctor shopping, early refills, use for psychoactive effects, and/or use to relieve distress besides pain.3 These behaviors have been documented in clinical settings3 and health insurance claims.4 Regular opioid medication consumers who have mental, behavioral, and pain conditions have a heighted-risk for engaging in opioid medication misuse behaviors.4
The community pharmacy, a primary location for distribution of opioid medications,5, 6 is one potentially effective location to address misuse. The feasibility of this resource is supported by their ubiquitous presence throughout communities, and pharmacists are one of the most prevalent advanced-degreed health professionals in the nation.7 Notably, pharmacists are consistently ranked among the most trusted professionals.8 Furthermore, patients are receptive to receiving behavioral health information from pharmacists,9 who in turn have positive attitudes and motivation to deliver care to those who misuse opioid medications.10
The busy community pharmacy workflow may be especially adaptable to addressing opioid medication misuse by employing the well-established Screening, Brief Intervention, and Referral to Treatment (SBIRT) protocol.11–17 SBIRT integrates screening patients for substance use, with 1 or 2 30 -minute sessions to explore the patient’s motivation for change followed, if necessary, by referral to more intensive care. Studies in medical settings have shown that brief interventions can reduce prescription medication misuse, including opioid medication misuse.18,19 Considering that screening and brief counseling about medications are routine activities within community pharmacy, the SBIRT protocol could be a valuable yet untapped possibility for addressing misuse of opioid medications.20
This potential opportunity is, however, beset by specific challenges, including identification/operationalization of opioid misuse behaviors,21,22 co-occurring serious health risks such as overdose risk,23, 24 physical dependence,25 and legitimate pain management needs.4, 26–29 Thus, SBIRT models developed for other substances, such as alcohol and tobacco, cannot be simply applied in the community pharmacy setting for addressing opioid medication misuse. Skepticism is reinforced by emerging literature showing that brief motivational interventions for drug use in primary care settings has inconsistent impact on outcomes.17, 30–32 Accordingly, it is timely to modify SBIRT so as to be congruent with the spectrum and severity of problems associated with opioid medication misuse and its management in the community pharmacy setting.
Toward this goal, this report describes the results of a meeting of an interdisciplinary panel of experts employing ADAPT-ITT (Assessment, Decision, Administration, Production, Topical Experts, Integration, Training, and Testing33) to guide modifications of SBIRT for opioid medication misuse in the community pharmacy setting. ADAPT-ITT was designed to serve as a framework for adapting evidence-based HIV interventions. Similar to other initiatives used to modify brief intervention models,34–37 ADAPT-ITT is a framework for modifying evidence-based behavioral interventions to new populations or service delivery settings.33 We describe herein results of the utilization of the “ADAPT” portion of the model to modify SBIRT for the community pharmacy setting to address opioid medication misuse. In addition, the results are synthesized into a conceptual framework (Figure 1) that is applicable for integrating patient identification, intervention, prevention, and referral to care for patients in the community pharmacy setting who are at risk for opioid medication misuse or who are already engaging in this hazardous behavior.
Figure 1.
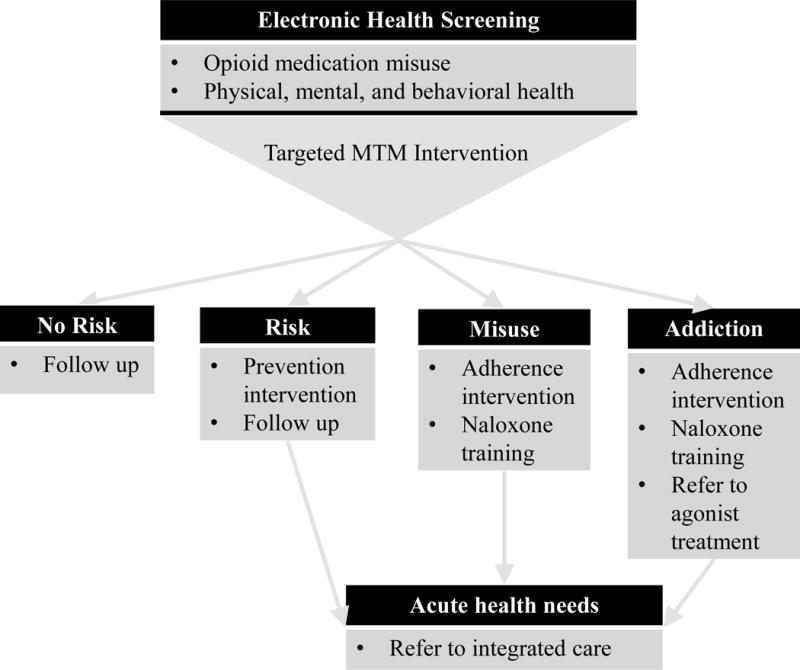
Targeted intervention framework within community pharmacy workflow
Material and Methods
The Consolidated Criteria for Reporting Qualitative Research (COREQ)38 was followed to ensure quality and transparency of the methods and results described in this report. COREQ is a checklist consisting of 32 items organized into 3 domains: 1) research team and reflexivity, 2) study design, and 3) analysis and findings. With the exception of items not applicable to our project (interview guides, repeated interviews, and data saturation), the following methods, results, and discussion sections satisfy the COREQ requirements.
Attendees
Eleven experts and 3 practicing pharmacists from the US and UK were invited to participate in a one-day intensive video-recorded meeting held at the University of Pittsburgh to discuss modification of SBIRT for use in community pharmacy to address opioid medication misuse. Attendees were purposively selected and invited based on several criteria, they were: 1) known to the lead author (GC) through collaborative academic/research associations (n=10), 2) identified through a search of the published literature (n=1), and 3) referred from other experts in the field (n=2). Attendants represented expertise in pharmacy, brief intervention, opioid addiction, behavioral interventions, and substance abuse treatment (Table 1). GC was also a participant in the meeting having expertise in brief intervention and addiction. The diverse professional and research backgrounds of the panel members thus enabled obtaining diverse perceptions that could be integrated into a SBIRT model appropriate to the community pharmacy setting.
Table 1.
Expertise of attendees (n)
| Opioid overdose prevention and harm reduction expert (1) |
| Health services pharmacy expert (1) |
| Pharmacologic opioid treatment expert (1) |
| Practicing addiction pharmacist (1) |
| Psychosocial addiction treatment expert (1) |
| Practicing community pharmacists (2) |
| Behavioral intervention experts (3) |
| Brief intervention and addiction experts (4) |
Procedures employed in the ADAPT Framework
Prior to convening the experts and again at its beginning, meeting goals were articulated to promote focused discussion and ensure acquisition of accurate comprehensive data. Table 2 lists the 8 ADAPT-ITT components, their procedures, and when each was implemented (or will be implemented in the case of 6, 7, 8, i.e., the “ITT” portion). The first component, assessment, consisted of presentations by a subset of attendees focusing on their areas of expertise as it pertains to the goal of the project. Presentation content included brief intervention for drug use in primary care, brief intervention for alcohol use in the community pharmacy setting, brief intervention for medication adherence in the community pharmacy setting, brief intervention for opioid medication misuse in the community pharmacy setting, agonist medication treatment, and naloxone-based opioid overdose prevention. The goal of the presentations was to align the attendees with respect to the empirical knowledgebase relating to prevention, intervention, and treatment of opioid medication misuse in the context of limitations/strengths of modifying the SBIRT model.
Table 2.
ADAPT-ITT framework for modifying SBIRT in the community pharmacy setting for opioid medication misuse
| Model Component | Objective | Executed |
|---|---|---|
| 1. Assessment | Presentations by attendees | Scientific working session |
| 2. Decision | Discussant summarization and roundtable discussion of needed intervention components | Scientific working session |
| 3 Administration | Topic specific discussion on needed changes to screening, intervention, prevention, and referral to treatment | Scientific working session |
| 4. Production | Gather written notes Plan review of video recordings Plan writing up meeting proceedings and adapted SBIRT model |
Scientific working session/post scientific working session |
| Analyzing and writing up meeting results | Post scientific working session | |
| 5. Topic Experts | Sending results to meeting attendees for review Revising manuscript based on feedback |
Post scientific working session |
| 6. Integration | Incorporate SBIRT model into research protocol | Post scientific working session |
| 7. Training | Training approach/methods in research protocol | Post scientific working session |
| 8. Testing | Pilot study methodology in research protocol | Post scientific working session |
The decision component, coordinated through a moderator/discussant, involved synthesizing the information contained in the presentations followed by a free-flowing roundtable discussion focusing on identifying novel intervention components as candidates for inclusion in the adapted SBIRT model. The administration component, conducted by the presenters, and guided by GC in the question/answer component of the presentations, examined the modifications proposed to adapt SBIRT to the community pharmacy setting. The production component, occurring toward the end of and after the meeting, involved GC collecting handwritten notes taken by the attendees, gathering input from other attendees who did not take notes but sent comments via email, and establishing a timeline and agenda to review the video transcript and notes. Next, in the topic experts component of the model, drafts of the results and summary were sent to the attendees for clarification, revision, and comment. The last 3 components; integration, training, and testing; are currently in preparation for a pilot study of the adapted SBIRT model for the community pharmacy setting.
Data Collection and Analysis
The data consisted of a video recording of the meeting (6 hours), notes from the meeting attendees, and additional notes sent by email. Meeting attendees offered a number of suggestions and comments that were related to opioid misuse, addiction, and pharmacy. The video transcript and meeting notes were analyzed in 2 cycles using a coding process led by GC in which specific content was identified and tallied.39 This coding and tallying process was facilitated by creating objective coding schemes40, 41 reflecting the thematic discussion (Table 3). The first cycle coding scheme was developed by GC based on his meeting participation and review of the data. This scheme encompassed 4 themes: patient identification, intervention, prevention, and referral to care. The second cycle coding scheme was developed by GC and a doctoral student research assistant (TY) after completing first cycle coding through reviewing and discussing themes and patterns that emerged from the first cycle. First cycle coding was carried out by GC and TY. Second cycle coding was carried out by TY and a masters-level student research assistant (JR). TY and JR were trained on coding schemes by GC in one-on-one meetings before and during the analysis process in which concepts were reviewed and discussed. Analysis of agreement between coders was conducted using percentage of agreement and Cohen’s Kappa (K). K agreement levels of 0.0–0.2 were considered slight, whereas those between 0.21–0.40, 0.41–0.60, 0.61–0.80, >0.80 were respectively considered fair, moderate, substantial, and near/perfect.42 In addition to these statistics, selected statements from within the dataset are presented to illustrate the various ideas and themes that emerged. Data were managed, coded, and analyzed using Nvivo 10.43
Table 3.
Coding schemes and inter-rater agreement
| First cycle codes | Second cycle codes | % Agreement | Kappa | Total n of codes |
|---|---|---|---|---|
| Patient identification | 97.3 | 0.88 | 206 | |
| Operationalize target behavior | 93.7 | 0.63 | 66 | |
| Policing role | 99.8 | 0.84 | 12 | |
| Use EHR or electronic screen | 99.5 | 0.78 | 9 | |
| Comprehensive assessment | 96.9 | 0.63 | 40 | |
|
| ||||
| Intervention | 93.5 | 0.81 | 296 | |
| Building on pharmacy strengths | 87.6 | 0.60 | 67 | |
| Patient-centered intervention | 83.0 | 0.58 | 52 | |
|
| ||||
| Prevention | 99.6 | 0.92 | 19 | |
| Prevention of SUD | 99.7 | 0.91 | 23 | |
| Overdose prevention | 97.3 | 0.84 | 20 | |
|
| ||||
| Referral to care | 99.5 | 0.93 | 67 | |
| Refer to professionals to assist in follow through | 99.1 | 0.63 | 21 | |
| Refer back to prescribers for additional care | 99.8 | 0.86 | 14 | |
Results
First Cycle Codes
Table 3 displays inter-rater agreement from the first cycle and second cycle coding. As can be seen, patient identification, intervention, prevention, and referral to care, had a high level of agreement ranging from 93.5–99.6% with K values ranging from 0.81–0.93. The most frequent topic identified in the first cycle of coding was intervention (n=296), followed by patient identification (n=206), referral to care (n=67), and prevention (n=19). Results of the first cycle coding were thus deemed informative to be utilized for development of the second cycle coding scheme.
Second Cycle Codes
Patient identification
Second cycle coding identified 4 themes that were encompassed in the patient identification code (see Table 3). The first theme focused on defining and operationalizing the target behavior(s) for screening. Although the panelists recognized the importance of screening for a variety of medications and use patterns, the consensus recommendation was to focus screening within the pharmacist area of expertise, namely prescribed opioids with particular emphasis for risk of adverse drug events. With respect to opioid medications, the most important adverse events are misuse, addiction, and overdose.
Inquiring about consumption behavior and adherence to the prescribing regimen can, however, be challenging in the pharmacy setting. In particular, the attendees noted that community pharmacists have a dual role of screening/monitoring and “policing” aberrant behavior—that is to say—tracking and reporting illegal or suspicious behavior. This dual responsibility is stressful and formally extends the boundaries of professional practice. One participant commented: “I don’t think the pharmacist… should be in a legal role. Or, they can’t be in both [a helping and a legal role]. It’s going to be hard to be in both, manage both.”
The discussion within the meeting that could resolve this tension was the central point within the third identified theme. Specifically, electronic surveys in kiosks and/or health record screening to meet busy community pharmacy workflow demands were discussed as tools to lessen the “policing” role of the community pharmacist. Rather than directly interview the patient about opioid use patterns in context of adherence to the prescription regimen, the pharmacist would instead discuss with the patient the results of a health screening. As one participant noted: “…the pharmacist basically gets data from a person at a kiosk, sits down, and begins with the statement that, ‘this is what you are telling me about yourself. Where do we begin?’ The consultative role of the pharmacist is with the data.”
In the context of problem identification, the fourth theme thus addressed the need for comprehensive screening. The rationale for a broader health perspective rather than circumscribed focus on drug use behaviors was based on understanding that patients at risk or misusing opioid medications commonly evince a spectrum of problems. Comprehensive screening is therefore a requisite for pharmacists to document the factors predisposing to risk for or sustaining hazardous use of opioid medications.
Intervention
Given the complexity of patient behaviors, meeting attendees discussed at length the variety of behaviors that might be targeted for intervention. Although clear consensus was not reached, second cycle coding of the intervention portion of the discussion identified two themes, which from the pharmacist perspective are essential to intervention. First, interventions must capitalize on the pharmacist’s strengths, such as medication review. This task builds on the core competencies of the pharmacists’ specific knowledge about medications and their interactions. Moreover, medication review aligns with community pharmacy workflow. One participant asserted: “…pharmacists [could]… support patient safety with respect to prescription opiate use based upon dose/type/other medications being used/disease status. This could be built from a Comprehensive Medication Review.”
The second theme explored the concept of patient-centered interventions. This discussion examined the importance of asking patients their preferences regarding the focus of any proposed intervention. One participant urged: “The sessions must be negotiated from a menu of alternatives, which are taken directly from the patient’s perception of the problem, or if possible, reframed by the interventionist in a way that is agreeable to patients.”
Prevention
The third theme in second cycle coding, prevention, identified 2 themes: first, preventing patients from proceeding to addiction, and second, averting overdose. Prevention of overdose was discussed primarily in relation to training pharmacists in naloxone rescue. One participant commented: “…we talk about prevention of overdose, of saying hey, if you are going to have [opioid] medications available to you; they’re going to be in your home; we need to get some ancillary type of medications in your home to reverse an overdose.”
Referral to treatment
The referral to treatment discussion also focused on 2 themes. The first was reconnecting patients to prescribers for higher levels of care (e.g., agonist treatment). The second was connecting patients with physical, behavioral, and/or mental health conditions to health professionals. One participant remarked: “If it’s going to get complicated really fast, you’re going to want to do something there [that] connects the person to a [health care] team that includes…the pharmacist, the primary care clinician, the nurse in the primary care clinician’s office, the social worker or behavioral specialist….”
Discussion
Themes coded in the first and second cycle coding process possessed high levels of inter-rater agreement and K values—thus demonstrating consistency and salience of the topics pertinent to pharmacist-based intervention for opioid medication misuse. Considered in aggregate, the results have several implications for a community pharmacy-based model for opioid medication misuse. Specifically, the components of an integrative framework, shown in Figure 1, underscore the importance of joining drug-specific and medical/psychological screening spanning 4 main levels of severity, ranging from no risk to addiction—thus setting the stage for a practical patient-centered work plan for addressing opioid medication misuse in the community pharmacy setting.
One of the most frequently discussed themes, patient identification, emphasized the importance of capitalizing on the pharmacist’s expertise, especially promoting adherence to the prescription regimen and preventing adverse drug events. Consuming more medication than prescribed, detecting early refills, and inappropriate use of medications are within the scope of pharmacists’ training and expertise. Opioid medication misuse often co-occurs with multiple health problems, which may lie outside pharmacists’ competencies. Accordingly, identification of high-risk or medication abusing patients must be comprehensive. Multidimensional information ideally would be captured using electronic methods such as kiosks and/or health record review. Electronic patient identification methods also have the benefit of removing the policing burden form the pharmacist, particularly considering that pharmacists report they believe patients would respond to electronic screening more favorably than face-to-face methods.10 Although not a strong theme of this project, attendees did note that comprehensive screening should employ measures that take into account burden of time on both the patient and the pharmacy.
Discussion also explored the role of the pharmacist on intervention. Medication management emphasizing adherence to the prescription regimen and safety are core professional activities that could readily be accommodated into intervention practice.20 Results from clinical trials demonstrate that pharmacists providing medication management can significantly improve the health behaviors of pateints.44, 45 A recent systematic meta-analysis located 44 medication therapy management (MTM) studies that suggested consistent improvement in behaviors such as medication adherence while lowering health care costs.44 MTM, consensually accepted in the pharmacy field as helpful to patients,45 has been codified into standardized guiding principles46, 47 and is supported by both some commercial insurance products and Medicare.48 Moreover, substance abuse screening and intervention can be easily incorporated in interventions that pharmacists provide within MTM.45 Brief “targeted” MTM interventions are also becoming more common and could likewise be implemented to address opioid medication misuse.49 Altogether, addressing opioid medication misuse by adapting the SBIRT model in the pharmacy setting entails conceptualizing the task as screening, intervening, prevention, and referring (if necessary) to manage medications so as to improve adherence and safety.
A patient-centered approach to intervention emphasizes the unique circumstances of each patient. Whereas the community pharmacy may be a readily accessible starting point for intervention, many factors beyond pharmacists’ scope of practice spanning addiction, medical problems, social adjustment, and family problems may also be undergirding hazardous use of opioid medications. Consequently, the pharmacist needs to be included within a team-based model of patient care that includes physicians, nurses, physical/occupational therapists, and social workers/behavioral health providers. A team-based approach to health care is an emerging model that is currently at varying levels of adoption within the larger health care environment. The new idea suggested in this project is that the community pharmacy should be included as an entry point of patient engagement.
Limitations
Several limitations of this study should be noted. The attendees represented a variety of training and practice backgrounds that, although producing rich discussion, may have under-emphasized the importance of the pharmacist’s perspective. These rich discussions therefore did not allow for the group to come to a clear consensus regarding details of a pharmacy-based intervention for opioid misuse. However, a clear framework arose as a guide to future delineation of a specific intervention protocol. Furthermore, the attendees in the Topical Experts portion of the ADAPT-ITT process were not required to review the entirety of the data in their assessment of these results. Rather, the attendees were asked to review only the results presented herein. All files were made available to attendees for their review if they wished to examine raw data. However, given the high level of agreement between the coders, the schemes and the data are considered to be accurate representations of the discussion.
Conclusion
A one-day intensive meeting was convened to review the empirical knowledgebase and discuss adapting the SBIRT protocol for opioid medication misuse in the community pharmacy setting. Discussions explored patient identification, including comprehensive assessment using electronic methods to fit within community pharmacy workflow and to avoid involving the pharmacist in a policing role. Adaptation of SBIRT for community pharmacy concentrated on capitalizing on the pharmacist’s knowledge of medication management, particularly related to adverse events and medication adherence. However, patients who misuse opioid medication often have problems that exceed the core competencies of the pharmacist. Interventions for acute needs should be team-based and encompass the range of disciplines that interface with medication misuse. Furthermore, a patient-centered intervention model is recommended whereby the factors that uniquely contribute to individual onset and maintenance of opioid misuse are taken into account.
Synopsis.
Prescription opioid misuse is a major public health concern, yet few models exist to support engaging community pharmacy patients who misuse opioids. This report describes qualitative results from a meeting of experts aimed at developing a behavioral health framework for opioid medication misuse in the community pharmacy setting. Themes included screening, intervention, prevention, and referral to treatment that capitalize on pharmacists’ knowledge of medication management. Interventions should be patient-centered, multidimensional, and multidisciplinary in order to interface with patients at risk or involved in medication misuse.
Acknowledgments
Role of Funding Source: This project was supported by a grant from the Staunton Farm Foundation, which had no involvement in the conceptualization, execution, or submission of this work.
References
- 1.CDC. Morbidity and Mortality Weekly Report. Vital Signs: Overdoses of Prescription Opioid Pain Relievers — United States, 1999–2008. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [PubMed] [Google Scholar]
- 2.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain physician. 2008;11(2 suppl):S63–88. [PubMed] [Google Scholar]
- 3.Knisely JS, Wunsch MJ, Cropsey KL, Campbell ED. Prescription Opioid Misuse Index: a brief questionnaire to assess misuse. J Subst Abuse Treat. 2008;35(4):380–386. doi: 10.1016/j.jsat.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inciardi JA, Surratt HL, Kurtz SP, Cicero TJ. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med. 2007;8(2):171–183. doi: 10.1111/j.1526-4637.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicero TJ, Kurtz SP, Surratt HL, et al. Multiple Determinants of Specific Modes of Prescription Opioid Diversion. J Drug Issues. 2011;41(2):283–304. doi: 10.1177/002204261104100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Bureau of Labor Statistics. Occupational Outlook Handbook. Washington, DC: U.S. Bureau of Labor Statistics; 2014. [Google Scholar]
- 8.Redman R. Pharmacists receive high marks on trust. Chain Drug Review. 2012;34(21):2. [Google Scholar]
- 9.Dhital R, Whittlesea CM, Norman IJ, Milligan P. Community pharmacy service users’ views and perceptions of alcohol screening and brief intervention. Drug Alcohol Rev. 2010;29(6):596–602. doi: 10.1111/j.1465-3362.2010.00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Cochran G, Field C, Lawson K, Erickson C. Pharmacists’ knowledge, attitudes and beliefs regarding screening and brief intervention for prescription opioid abuse: a survey of Utah and Texas pharmacists. J Pharm Health Serv Res. 2013;4(2):71–79. [Google Scholar]
- 11.Nilsen P, Baird J, Mello MJ, et al. A systematic review of emergency care brief alcohol interventions for injury patients. J Subst Abuse Treat. 2008;35(2):184–201. doi: 10.1016/j.jsat.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Havard A, Shakeshaft A, Sanson-Fisher R. Systematic review and meta-analyses of strategies targeting alcohol problems in emergency departments: interventions reduce alcohol-related injuries. Addiction. 2008;103(3):368–376. doi: 10.1111/j.1360-0443.2007.02072.x. discussion 377–368. [DOI] [PubMed] [Google Scholar]
- 13.Beich A, Thorsen T, Rollnick S. Screening in brief intervention trials targeting excessive drinkers in general practice: systematic review and meta-analysis. BMJ. 2003;327(7414):536–542. doi: 10.1136/bmj.327.7414.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins RJ, McAlaney J, McCambridge J. Change over time in alcohol consumption in control groups in brief intervention studies: systematic review and meta-regression study. Drug Alcohol Depend. 2009;100(1–2):107–114. doi: 10.1016/j.drugalcdep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Kaner EF, Dickinson HO, Beyer F, et al. The effectiveness of brief alcohol interventions in primary care settings: a systematic review. Drug Alcohol Rev. 2009;28(3):301–323. doi: 10.1111/j.1465-3362.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- 16.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: Comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99(1–3):280–295. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107(5):957–966. doi: 10.1111/j.1360-0443.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- 18.Zahradnik A, Otto C, Crackau B, et al. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104(1):109–117. doi: 10.1111/j.1360-0443.2008.02421.x. [DOI] [PubMed] [Google Scholar]
- 19.Heather N, Bowie A, Ashton H, et al. Randomised controlled trial of two brief interventions against long-term benzodiazepine use: outcome of intervention. Addict Res Theory. 2004;12(2):141–154. [Google Scholar]
- 20.Cipolle RJ, Strand L, Morley P. Pharmaceutical care practice: the patient centered approach to medication management. United States: The McGraw-Hill Companies, Inc; 2012. [Google Scholar]
- 21.Cochran G, Woo B, Lo-Ciganic W, Gordon AJ, Donohue J, Gellad W. Defining Non-Medical Use of Prescription Opioids within Health Care Claims: A Systematic Review. Subst Abus. doi: 10.1080/08897077.2014.993491. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154(11):2287–2296. doi: 10.1016/j.pain.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 24.Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796–801. doi: 10.1001/jamainternmed.2013.12711. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 26.Braker LS, Reese AE, Card RO, Van Howe RS. Screening for potential prescription opioid misuse in a michigan medicaid population. Fam Med. 2009;41(10):729–734. [PubMed] [Google Scholar]
- 27.Novak SP, Herman-Stahl M, Flannery B, Zimmerman M. Physical pain, common psychiatric and substance use disorders, and the non-medical use of prescription analgesics in the United States. Drug Alcohol Depend. 2009;100(1–2):63–70. doi: 10.1016/j.drugalcdep.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amari E, Rehm J, Goldner E, Fischer B. Nonmedical prescription opioid use and mental health and pain comorbidities: a narrative review. Can J Psychiatry. 2011;56(8):495. doi: 10.1177/070674371105600808. [DOI] [PubMed] [Google Scholar]
- 29.Hudson TJ, Edlund MJ, Steffick DE, Tripathi SP, Sullivan MD. Epidemiology of regular prescribed opioid use: results from a national, population-based survey. J Pain Symptom Manage. 2008;36(3):280–288. doi: 10.1016/j.jpainsymman.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitz R, Palfai TP, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312(5):502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wingood GM, DiClemente RJ. The ADAPT-ITT model: a novel method of adapting evidence-based HIV Interventions. J Acquir Immune Defic Syndr. 2008;47 Suppl 1(Supplement 1):S40–46. doi: 10.1097/QAI.0b013e3181605df1. [DOI] [PubMed] [Google Scholar]
- 33.Pirotte MJ, Buckley BA, Lerhmann JF, Tanabe P. Development of a screening and brief intervention and referral for treatment for ED patients at risk for undiagnosed hypertension: a qualitative study. J Emerg Nurs. 2014;40(1):e1–9. doi: 10.1016/j.jen.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Klimas J, Cullen W, Field CA, Problem Alcohol/Drug Use Guideline Development G Problem alcohol use among problem drug users: development and content of clinical guidelines for general practice. Ir J Med Sci. 2014;183(1):89–101. doi: 10.1007/s11845-013-0982-2. [DOI] [PubMed] [Google Scholar]
- 35.Ornelas IJ, Allen C, Vaughan C, Williams EC, Negi N. Vida PURA: A Cultural Adaptation of Screening and Brief Intervention to Reduce Unhealthy Drinking among Latino Day Laborers. Subst Abus. 2014;0 doi: 10.1080/08897077.2014.955900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nygaard P, Aasland OG. Barriers to implementing screening and brief interventions in general practice: findings from a qualitative study in Norway. Alcohol Alcohol. 2011;46(1):52–60. doi: 10.1093/alcalc/agq073. [DOI] [PubMed] [Google Scholar]
- 37.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 38.Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. 3rd. Los Angeles: Sage; 2014. [Google Scholar]
- 39.Engel RJ, Schutt RK. The practice of research in social work. Los Angeles: SAGE; 2009. [Google Scholar]
- 40.Stake RE. The art of case study research. Thousand Oaks: Sage Publications; 1995. [Google Scholar]
- 41.Hallgren KA. Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutor Quant Methods Psychol. 2012;8(1):23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nvivo 10 [computer program] Burlington, MA: QSR International; 2012. [Google Scholar]
- 43.Viswanathan M, Kahwati LC, Golin CE, et al. Medication therapy management interventions in outpatient settings: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(1):76–87. doi: 10.1001/jamainternmed.2014.5841. [DOI] [PubMed] [Google Scholar]
- 44.Avalere Health LLC. Exploring Pharmacists’ Role in a Changing Healthcare Environment. Washington, DC: Avalere Health LLC; 2014. [Google Scholar]
- 45.American Pharmacists Association, National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model (version 2.0) J Am Pharm Assoc (2003) 2008;48(3):341–353. doi: 10.1331/JAPhA.2008.08514. [DOI] [PubMed] [Google Scholar]
- 46.Bluml BM. Definition of medication therapy management: development of professionwide consensus. J Am Pharm Assoc (2003) 2005;45(5):566–572. doi: 10.1331/1544345055001274. [DOI] [PubMed] [Google Scholar]
- 47.Perlroth D, Marrufo G, Montesinos A, et al. Medication Therapy Management in Chronically Ill Populations: Final Report. Burlingame, CA: Acumen, LLC; 2013. [Google Scholar]
- 48.Bacci JL, McGrath SH, Pringle JL, Maguire MA, McGivney MS. Implementation of targeted medication adherence interventions within a community chain pharmacy practice: The Pennsylvania Project. J Am Pharm Assoc (2003) 2014;54(6):584–593. doi: 10.1331/JAPhA.2014.14034. [DOI] [PubMed] [Google Scholar]


