Abstract
The longstanding focus in chronic kidney disease (CKD) research has been on the glomerulus, which is sensible because this is where glomerular filtration occurs, and a large proportion of progressive CKD is associated with significant glomerular pathology. However, it has been known for decades that tubular atrophy is also a hallmark of CKD, and is superior to glomerular pathology as a predictor of GFR decline in CKD. Nevertheless there are vastly fewer studies that investigate the causes of tubular atrophy, and fewer still that identify potential therapeutic targets. The purpose of this review is to discuss plausible mechanisms of tubular atrophy, including tubular epithelial cell apoptosis, cell senescence, peritubular capillary rarefaction and downstream tubule ischemia, oxidative stress, atubular glomeruli, epithelial-to-mesenchymal transition, interstitial inflammation, lipotoxicity and Na+/H+ exchanger-1 (NHE1) inactivation. Upon obtaining a better understanding of tubular atrophy (and interstitial fibrosis) pathophysiology, it might then be possible to consider tandem glomerular and tubular therapeutic strategies, in a manner similar to cancer chemotherapy regimens, which employ multiple drugs to simultaneously target different mechanistic pathways.
Keywords: apoptosis, CKD, interstitial fibrosis, lipotoxicity, NHE1
Introduction
Over 26 million people in the US are afflicted with chronic kidney disease (CKD), as defined by persistent albuminuria and/or decline in glomerular filtration rate (GFR) [1]. These CKD criteria reflect that glomerular pathology is central to the pathogenesis for most types of CKD in adults and in a significant proportion of children, and that heavy albuminuria and decreased GFR are risks for mortality [2, 3]. In adults, in whom diabetes and hypertension are common, the majority of CKD patients in the US do not undergo a diagnostic renal biopsy and thus, the specific glomerular pathology is often unknown. As a result, some glomerular diseases, such as hypertensive nephrosclerosis, which should be diagnosed according to well-established pathologic criteria [4], are overrepresented in ESRD registries as clinical diagnoses of exclusion [5]. In contrast, in the pediatric population, biopsies are more routinely performed for the suspicion of glomerular disease, and the specific etiology is therefore more likely to be known in children. While the distribution of CKD diagnoses differs between children and adults, with a predominance of structural/inherited/tubular disorders, there is still a significant representation of glomerular etiologies in children (Table 1).
Table 1.
Incidence of ESRD by major causes in pediatric (ages 0-19) and adult (ages 20 and older) populations over the period 2008-2012. Data are derived from 2014 USRDS database and expressed as percentages.
| Pediatric (N=6,204) |
Adult (N=554,689) | |
|---|---|---|
| Diabetes | 1.0 | 45.0 |
| Glomerulonephritis | 34.3 | 8.4 |
| Interstitial nephritis/pyelonephritis | 5.0 | N/A |
| Hypertensive/large vessel disease | 4.5 | 28.7 |
| Hereditary/congenital/cystic diseases | 38.3 | 2.3 |
N/A; not available.
Regardless of the primary glomerular disease etiology, tubular atrophy, which is ultimately defined as the disappearance of either individual tubular epithelial cells or entire tubules, often in conjunction with interstitial fibrosis, is an important hallmark of CKD because tubular atrophy has repeatedly been shown to be superior to glomerular pathology as a predictor of CKD progression [6-11]. Prior to the actual disappearance of cells, the initial morphologic features include loss of brush border and apical mitochondria, followed by epithelial simplification to a more cuboidal appearance, which is associated with diminished transport functions [12]. The final degeneration phase is characterized by wrinkling of the tubular basement membrane, influx of inflammatory cells and macrophages, tubular epithelial cell apoptosis and ultimately replacement of the entire area with scar/fibrous matrix proteins [12].
Pathology of tubular atrophy
The first widely recognized report to describe the relationship between tubular atrophy and GFR was a case series from the UK that included 50 patients with biopsy-proven primary glomerulonephritis (GN) [8]. Most patients had the nephrotic syndrome, including 12 with minimal change disease, and 20 with other causes. Fourteen subjects had proliferative GN, including two with fibrocellular crescents. Subjects with associated systemic diseases were excluded. Glomerular pathology was scored for presence of sclerosis on a 0 to 5 scale. Tubular atrophy was defined by the presence of tubular epithelial thinning, pyknotic nuclei or tubular dilation, with or without protein casts. Ten random fields per specimen were observed and scored as the percentage with tubular atrophy features. Tubular atrophy significantly correlated with serum creatinine, creatinine clearance, as well as ability to concentrate and acidify urine [8]. There was also a correlation between glomerular pathology and serum creatinine, creatinine clearance and urine concentration, though the P values were less significant compared to P values associated with tubular atrophy.
This landmark study was followed two years later by a widely cited case series from the US that contained 70 biopsies from patients ranging in age from 8 to 75 years [9]. In contrast to the UK report, this series included patients with underlying systemic diseases, such as diabetes and multiple myeloma. Detailed semi-quantitative histomorphometric analyses were conducted on glomerular and tubulointerstitial compartments [13], which were correlated with inulin and creatinine clearances (as indices of GFR), para-aminohippurate (PAH) clearance (as an index of renal plasma flow), and maximum osmolality and ammonium secretion, as indices of urinary concentration and acidification capacities, respectively. There was a tight correlation between tubular atrophy or interstitial fibrosis with inulin or creatinine clearances, and every patient with an inulin clearance below 60 ml/min/1.73 m2 showed evidence of some tubular atrophy on biopsy. In contrast, there was a weak relationship between glomerular histology and estimated GFR. Urinary concentration and acidification capacity also correlated with tubular atrophy, and to a lesser extent with glomerular pathology. PAH clearance correlated with tubular atrophy, particularly if accompanied by vascular disease.
More than 25 years elapsed before a larger, more definitive study was published by Bohle et al [14], which examined the relationship between GFR and quantitative histomorphometric analyses of glomerular and tubulointerstitial pathology. In this series, which contained over 3,000 biopsies with predominately chronic GN, but also included diabetic nephropathy, amyloidosis and primary tubulointerstitial diseases, there was a significant correlation between cortical interstitial volume and serum creatinine. Moreover, glomerular pathology was not associated with elevated creatinine, unless it was accompanied by tubular atrophy and interstitial fibrosis.
Tubular atrophy has also been associated with renal dysfunction in transplanted kidneys. In a study of 146 transplant biopsies performed for clinical indications, with multiple associated diagnoses, Bunnag et al examined a panel of 12 histologic parameters and identified tubular atrophy and interstitial fibrosis as the most predictive markers of eGFR decline [11]. Tubular atrophy has been associated with multiple transplant-specific processes, such as acute cellular rejection, anti-donor antibody-mediated injury, and chronic rejection due to non-immune mechanisms [15]. The prevalence of interstitial fibrosis and tubular trophy is so common in late allograft failure, that the abbreviation IF/TA has now replaced the term chronic allograft nephropathy. However, IF/TA is also an early predictor of renal allograft function. In a protocol biopsy study performed three months post-transplant in 280 patients, 10-year graft survival was 95 % in patients with normal histology, 82 % with IF/TA without transplant vasculopathy, and 41 % with IF/TA plus transplant vasculopathy [16].
One countervailing view is that the importance of tubular atrophy may be exaggerated in the evaluation of CKD pathology and pathophysiology because the tubulointerstitium comprises 90 % of the biopsy area, and features in this compartment are therefore easily observed and measured [17]. In addition, the capacity to examine renal tubular epithelial cells in an in vitro environment has been feasible for decades, which has permitted generation of large amounts of data to support roles for tubule cells in kidney disease pathogenesis. In contrast, the glomerulus encompasses only 5-10 % of biopsy area, which creates a greater probability of sampling error that could be amplified if diseased glomeruli are disproportionately replaced by scar matrix, and remnants of the previously connected nephron remain [12]. Furthermore, many specialized features of glomerular endothelial cells and podocytes still cannot be accurately modeled in cell culture systems, which may preclude generation of reliable in vitro data. These problems have been somewhat, but not wholly circumvented by strategies that employ glomerular-specific conditional knockout mice [18, 19]. Finally, the contribution of medullary tubulointerstitial pathology is largely unknown, since diagnostic biopsies are intended to sample only cortical tissue.
Mechanisms of tubular atrophy
Tubular epithelial cell apoptosis
Over the past 20 years it has repeatedly been shown, in both human biopsies and animal models of progressive kidney diseases, that renal tubular epithelial cells undergo apoptosis [20-29]. Multiple apoptosis pathways have been implicated in tubular atrophy, including recent reports linking proximal tubule endoplasmic reticulum (ER) stress in diabetic nephropathy [29, 30]. Until recently the most compelling argument that apoptosis is the cause of tubular atrophy had been kinetic experiments demonstrating that proximal tubule apoptosis precedes tubular atrophy in mouse models of focal and segmental glomerulosclerosis (FSGS) [22]. A more definitive link between apoptosis and tubular atrophy is provided in a study by Grgic et al, in which mice were genetically engineered to express the simian diphtheria toxin receptor exclusively in kidney epithelial cells, thereby allowing isolated effects of tubule cell apoptosis to be examined following systemic, sublethal diphtheria toxin administration [31]. A single dose resulted in significant proximal tubule apoptosis, most prominently in the S1 and S2 segments, as well as interstitial inflammation with macrophage, T cell and neutrophil infiltrates. These findings were followed by robust tubular epithelial proliferation and recovery, simulating the effects of a single episode of ischemic acute kidney injury (AKI). However, when the diphtheria toxin was given on three occasions over a two week period, which models persistent apoptotic insults from CKD, there was a significant, sustained increase in serum creatinine, and the resulting pathology included interstitial capillary rarefaction, tubular atrophy (characterized by simplified, flattened epithelia, and thick, wrinkled tubular basement membranes) and the appearance of inflammatory infiltrates, fibroblasts and fibrosis within the interstitium [31]. Although the mechanism for this more severe phenotype was not extensively investigated, the authors speculated that the atrophic and regenerating tubules secrete inflammatory and pro-fibrotic cytokines [32, 33], which may perpetuate progressive renal dysfunction (see following discussion). Intriguingly, the multiple dose diphtheria toxin regimen also caused glomerulosclerosis, and there was a statistical correlation between atrophic tubules and scarred glomeruli, elevated creatinine and albuminuria.
Cell senescence
A recent theory for the pathogenesis of a variety of diseases is acceleration of normal aging processes, such as oxidative stress. Senescent cells are defined by acquired absence of the ability to divide, which is associated with telomere shortening and initiation of a DNA damage response. Although not a diagnostic criterion, a lower threshold for apoptosis is generally recognized as a feature of senescent cells. Because multiple stimuli, such as reactive oxygen species (ROS) have subsequently been discovered to cause stress-induced cell senescence, which is characterized by p16Ink4a or p53-induced, p21-dependent cell cycle arrest, these pathways have increasingly been implicated in disease pathogenesis, including CKD [34]. The best example in the kidney literature may be the subset of AKI that proceeds to CKD [35]. Theoretically, in AKI that results in renal function recovery, injured tubular epithelial cells undergo cell death, and are replaced by regenerating cells. On the other hand, tubular epithelial cells that are injured, but survive may adopt a maladaptive, senescent phenotype, which includes secretion of inflammatory cytokines, such as TGF-β, that then perpetuate progression to CKD. Experimental evidence to support cell senescence as a mechanism of tubular atrophy is scant, but Braun et al showed that tubular atrophy was reduced following ischemic injury in mice with the p16Ink4a gene deleted (INK4a−/−) compared to wild-type mice, and mice transplanted with kidneys from INK4a−/− animals developed decreased tubular atrophy and interstitial fibrosis [36].
Autophagy is a cellular pathway that prevents cell senescence. In response to multiple cell stressors cytosolic proteins, lipids and carbohydrates, as well as damaged organelles are sequestered in double membrane organelles, called autophagosomes, which fuse with lysosomes, and the contents are degraded. In most instances the degradation products are recycled, which promotes cell survival. In rats that underwent unilateral nephrectomy plus induction of diabetes with streptozotocin, proximal tubule cells were noted to contain decreased autophagosomes after three days [37], suggesting that this hypertophic phase, which heralds tubular atrophy, is fueled by decreased autophagy. In several more recent reports, conditional deletion of a key autophagy gene (Atg5) has yielded data that suggest a role for autophagy in preventing tubular atrophy. Proximal tubule Atg5-null mice developed a modest phenotype at nine months, but significant renal dysfunction with tubular atrophy and renal fibrosis at 24 months [38]. In a separate study, combined Atg5 deletion from proximal and distal tubules led to a 50 % increase in serum creatinine at one and five months, with cellular evidence of ER and oxidative stress [39]. Finally, Atg5−/− mice subjected to intraperitoneal injection of fatty acid-loaded albumin to induce lipotoxicity (see discussion below) developed increased proximal tubule apoptosis and severe histopathology compared to identically treated wild-type mice [40].
Sirtuins are a highly conserved family of NAD+-dependent deacetylases, which defend against apoptosis, oxidative stress and inflammation by promoting autophagy [41]. Sirtuin-1 (Sirt1) has been extensively studied in the kidney, and is most highly expressed in the inner medulla [42], perhaps as a teliologic counterweight to the hyperosmolar and hypoxic environment. SIRT1+/− mice developed substantial apoptosis and interstitial fibrosis following ureteral obstruction, compared to wild-type mice, and treatment with the Sirt1 activator SRT1720 ameliorated the tubulointerstitial phenotype [42]. Another Sirt1 activator, resveratrol, has also been shown to limit interstitial fibrosis from ureteral obstruction, by deacetylation of Smad3, a downstream activator of TGF-β [43]. In mice that underwent chronic dietary calorie restriction, kidney Sirt1 levels were increased, and this was associated with enhanced proximal tubule autophagy and resistance to apoptosis and oxidative stress [44]. Proximal tubule Sirt1 expression was decreased in streptozotocin-induced type 1 diabetes or the db/db model of type 2 diabetes [45]. Importantly, Sirt1 expression changes preceded the advent of albuminuria, and proximal tubule-specific Sirt1 deletion or overexpression correlated with glomerular histology and albuminuria, implying that proximal tubule Sirt1 regulates glomerular function in diabetic nephropathy. The purported mechanism of crosstalk is through diffusion of nicotinamide mononucleotide (NMN, a NAD precursor) from proximal tubule to glomerulus, which normally preserves podocyte function by suppressing claudin-1. However, an early manifestation of diabetic nephropathy is decreased proximal tubule NMN synthesis, resulting in both impaired NMN secretion to the glomerulus, as well as decreased NAD+-dependent Sirt1 deacetylation [45].
Peritubular capillary rarefaction and altered blood flow
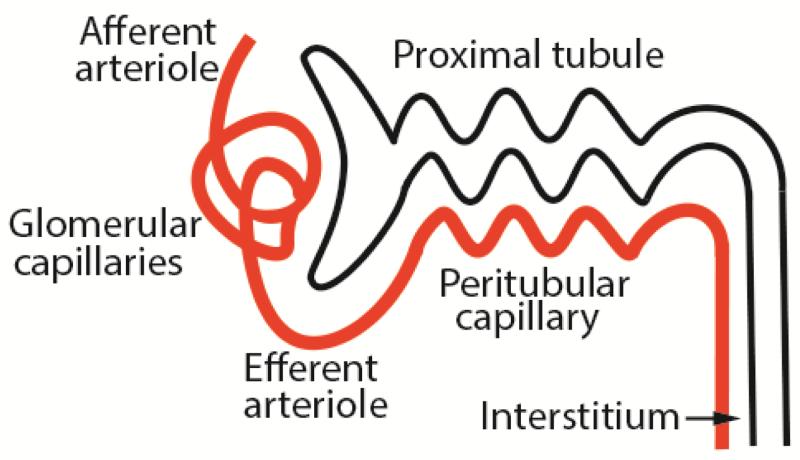
The vascular microanatomy for each nephron is essentially two sets of capillaries – glomerular and post-glomerular, which are separated by the efferent arteriole (Figure 1). Since the peritubular capillaries represent the sole blood supply to the tubules, glomerular scarring that damages the upstream capillary bed could have downstream consequences. In the previously cited studies by Bohle et al [14] an important additional finding was a significant inverse relationship between post-glomerular capillary area and serum creatinine, implying that obliteration of the peritubular capillary network led to tubular atrophy. These data have subsequently been corroborated in multiple rodent models of CKD [46-48]. Mechanisms of peritubular capillary regression in the context of chronic glomerular injury include reduction of angiogenic and survival factors (vascular endothelial growth factors (VEGFs), fibroblast growth factors (FGFs), angiopoietins, nitric oxide) and/or their receptors [49], secretion of anti-angiogenic factors (endostatin, thrombospondin-1), malfunction of endothelial cells and their progenitors, and impaired endothelial cell-pericyte interactions, leading to detachment of both cell types from basement membrane [50, 51]. Many of these mechanisms result in endothelial apoptosis, which can lead to thrombosis and inflammation.
Figure 1.
Schematic diagram of the initial portion of the nephron. Note that the peritubular capillary emanates from the efferent arteriole, and provides blood supply to a parallel tubule.
The benefits of ACE inhibitors and ARBs for CKD therapies are predicated upon reduction of efferent arteriolar tone, resulting in commonly cited mechanisms of normalization of intraglomerular hypertension and amelioration of extracellular matrix deposition. However, it is not as widely recognized that angiotensin blockade increases peritubular capillary blood flow and interstitial pO2 [52], with a potential manifestation of preventing tubular atrophy.
Tubule ischemia
Peritubular capillary rarefaction logically leads to reduced blood flow, which results in tubule hypoxia and tubular epithelial cell death [22, 53-55]. The S3 segment of the proximal tubule is especially vulnerable, due to the fine balance between low O2 tension and high metabolic demands, even under ambient conditions. CKD patients are often anemic, which contributes to tissue hypoxia by further reducing the O2-carrying capacity (blood flow × Hgb concentration × O2 saturation). Interstitial fibrosis and tubular basement membrane thickening increase the distance for O2 diffusion, thereby amplifying the effects of diminished blood flow on tubule damage. Disproportionate interstitial fibrosis compared to glomerular pathology may provide an explanation for the previously discussed discordance between glomerular and tubular pathology as predictors of CKD progression.
One consequence of tissue hypoxia is the activation of hypoxia inducible factors (HIFs). The HIF-1 protein is composed of α and β subunits, and HIF-1α undergoes constitutive proteasomal degradation under normoxic conditions. In response to hypoxia, HIF-1αβ heterodimers evade degradation, and act as transcription factors, to stimulate expression of hypoxia-sensitive target genes, such as VEGF and erythropoietin. Multiple animal models of CKD have demonstrated HIF-1 upregulation [56, 57], but it remains unclear whether enhanced HIF-1 activity is salutary or deleterious [50, 58]. For example, kidney-specific deletion of HIF-1α abrogates tubulointerstitial disease from unilateral ureteral obstruction [57], whereas HIF-1α stimulation with cobalt chloride was recently shown to be beneficial in the rat model of streptozotocin-induced diabetic nephropathy [59]. Explanation for conflicting HIF-1 effects could be related to cell type, context (disease model, kinetics of HIF-1 regulation, timing of interventions), the vast number of HIF-1 target genes (>200), some of which have contradictory functions, as well as opposing effects of individual target molecules. For example, VEGF promotes angiogenesis, which may improve hypoxia, but also triggers inflammatory cascades [50].
Oxidative stress
A mechanism related to hypoxia (and inflammation), which has been implicated in tubular atrophy, is oxidative stress. The most important, and potentially toxic ROS are the superoxide anion (·O2−), the hydroxyl radical (·OH), and hydrogen peroxide (H2O2), which can then react with substrates to form other oxidants, such as peroxynitrite (ONOO−). Oxidative stress can also be accelerated by oxidized target molecules, most notably advanced glycation end products in diabetic nephropathy. During oxidative phosphorylation and electron transport in mitochondria, electrons from reduced forms of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) are transferred to O2, the vast majority of which is reduced to H2O. Under normal circumstances, ROS are generated when a very small percentage of electrons are leaked at electron transport chain complexes I and III, and transferred to O2 to create ·O2−. In pathologic states additional intracellular sources of ROS include (a) redox-activated flavin proteins, and leakage of electrons at the coenzyme Q-complex III interface [60]; (b) oxidases (also known as oxidoreductases), such as acyl CoA oxidase in peroxisomes, NADPH oxidases [61], which localize to phagosomes and plasma membrane compartments in resident cells of glomeruli, tubules and the interstitium [62], and xanthine oxidase; (c) uncoupled nitric oxide synthase, which leads to nitrosylation of intracellular proteins.
The accumulation of ROS results from an imbalance between production and consumption by antioxidant scavengers, such as glutathione, or detoxification by enzymes, such as superoxide dismutase, which reduces ·O2− to H2O2, and catalase, which metabolizes H2O2 to H2O + O2. Under normal conditions low ambient concentrations of ROS have intended, homeostatic functions by oxidizing cysteine residues on proteins, especially kinases and phosphatases, which can alter secondary structure, protein-protein interactions, and ultimately catalysis [63]. At higher intracellular concentrations ROS can modify accidental targets, such as DNA, membrane lipids (through lipid peroxidation) and structural proteins, resulting in apoptosis and necrosis [29, 64]. In some instances, such as ROS derived from NADPH oxidase-4 (NOX4), dual deleterious and beneficial properties have been described with implications for tubular atrophy and interstitial fibrosis [42, 65-67], and the divergent phenotypes may be related to the concentration of ROS generated.
Although a definitive role for oxidative stress in the pathogenesis of tubular atrophy has not been established, the best supportive evidence may be derived from catalase-overexpressing transgenic mice, which showed reduced proximal tubule apoptosis in models of diabetic nephropathy and angiotensin II-mediated hypertensive renal disease [27, 28, 68]. There is also some suggestion that oral antioxidant therapies may be beneficial for CKD progression in humans, though the number of trials is small [69]. On the other hand, a recent trial with the Nrf2 activator bardoxolone methyl, which has antioxidant and anti-inflammatory properties, demonstrated no effect on progression to ESRD and increased cardiovascular mortality in patients with diabetic CKD [70].
Atubular glomeruli
Another possible mechanism of tubular atrophy in the context of chronic glomerular diseases is related to the disconnection of proximal tubules from the associated glomeruli, which was first observed in ultrastructure experiments from microdissected nephrons [71]. This phenomenon, which is variably referred to as aglomerular tubules, or more commonly, atubular glomeruli, is most often observed with primary tubular disorders. The prototype may be hereditary cystinosis, which results in proximal tubule lysosome accumulation of cystine and apoptosis, followed by development of glomerulotubular stenosis (“swan neck deformity”), and ultimately severing of the glomerulotubular junction [72]. Atubular glomeruli are presumably associated with peritubular capillary disruption, although this has not been investigated in detail, perhaps due to the tedious methods that would be required for such experiments.
In a hypothesis formulated by Kriz to explain tubular atrophy resulting from primary glomerular diseases, “degenerative” diseases are caused by fibrocellular crescents that accumulate along the outer/basolateral surface of the proximal tubule, and can entrap extravasated ultrafiltrate [12]. “Inflammatory” glomerular diseases are characterized by encroachment of crescents upon the glomerulotubular junction. According to both models, atubular glomeruli develop as an intermediate step between the expanding crescent and complete loss of nephron function [12]. The guiding principle is that tubular atrophy directly results from a diseased, adjacent glomerulus, and interstitial inflammation and fibrosis are secondary, rather than causal processes.
Epithelial-to-mesenchymal transition (EMT)
EMT is a process whereby epithelial cells lose apical-basal polarity and cell-cell adhesion, and acquire migratory and invasive properties to become mesenchymal stem cells, which are multipotent stromal cells capable of differentiating into fibroblasts. The EMT concept was described many years ago, and has been unequivocally implicated in embryonic development programs and tumor metastasis [73]. More recently EMT has been hypothesized as a possible mechanism to explain tubular atrophy. The appeal of this hypothesis is that tubule epithelial cells are not actually deleted, but instead convert to a myofibroblast phenotype, and then contribute to scar matrix deposition [74, 75] – one process would therefore explain several aspects of CKD pathogenesis. Because EMT is associated with enhanced ATP generation under hypoxic conditions [76, 77], and pro-fibrotic cytokines generally activate transcription factors that are associated with protection against apoptosis, EMT might therefore convey short-term advantages to a cell submerged in an ischemic state, as described above. Fibrogenic cytokines, most notably TGFβ1, have been detected at increased concentrations in CKD tissue, and multiple studies have demonstrated that exposure of these cytokines to tubular epithelial cells maintained in cell culture results in acquisition of fibroblast features [78]. However, an EMT hypothesis for the pathophysiology of CKD has been difficult to prove in vivo, with most studies revealing only rare transitioning cells, as demonstrated by simultaneous expression of epithelial and mesenchymal markers [79, 80]. Furthermore, detection of such cells does not permit distinction between EMT versus mesenchymal-to-epithelial transition (MET). There are even fewer examples of cells with concomitant epithelial and mesenchymal features migrating across the tubular basement membrane to the interstitium, where myofibroblasts reside.
The most definitive approach to address the role of EMT in tubular atrophy is with cell lineage tracing techniques, which allow the fate of a cell to be mapped from early development onward. If a heritable marker and expression system is carefully selected (in this case for tubular epithelial cell precursors), then once activated in vivo, the marker will be indefinitely detectable irrespective of subsequent pathologic perturbations. Using such an approach in a unilateral ureteral obstruction (UUO) model, Iwano et al demonstrated that approximately 30 % of injury-induced kidney myofibroblasts were derived from proximal tubule cells [75]. In stark contrast is the report by Humphreys et al, which detected zero proximal tubule-derived myofibroblasts following UUO [81]. This study instead identified the pericyte (when localized to peritubular capillaries) or resident fibroblast (when localized to the interstitium), rather than the tubular epithelial cell, as the source of scar-producing myofibroblasts. So why the discrepancy? The report by Humphreys et al offers some technical advantages that permit firmer conclusions to be drawn, such as (a) knock-in rather than transgenic overexpression of Cre recombinase, (b) expression of LacZ, as well as a fluorescently-labeled fate marker, which do not require immunohistochemical localization, and (c) detection of more faithful myofibroblast antigens (α-smooth muscle cell actin, FSP-1 and type I collagen [82] versus FSP-1 and HSP47 [75]). However, this topic remains controversial, and there are abundant data to support and refute EMT as a mechanism of tubular atrophy [83]. On balance, we conclude that EMT cannot be dismissed as a contributor to CKD pathogenesis, particularly for primary glomerular diseases, where it has not been tested as rigorously, but it is unlikely to represent a major mechanism.
Inflammation
Progressive glomerular diseases are generally not accompanied by large interstitial inflammatory infiltrates. Nevertheless, in FSGS interstitial inflammation more tightly correlates with CKD progression than glomerular pathology [84]. Furthermore, there are ample data to support a role for specific inflammatory cells, arising from a pool that includes both resident and recruited circulating inflammatory cells, in the pathogenesis of interstitial fibrosis [17, 85]. Studies implicating inflammatory cells, and associated cytokines and chemokines as direct causes of tubular atrophy are much less abundant. The most commonly invoked inflammatory cells in CKD tubulointerstitial pathophysiology are from the monocyte/macrophage lineage. The purpose of kidney macrophages may be for phagocytosis of damaged cells and matrix after injury, and although this macrophage subpopulation has not been well characterized, some common features include abundant lysosomes and immunomodulation by IL-10 [86, 87]. However, when the inflammation becomes unbridled, other populations of kidney macrophages may be injurious, either through secretion of inflammatory cytokines (e.g., IL-1β, IL-18, TNF-α and C-X-C chemokines), which can stimulate apoptosis and further inflammation [88-91], or an array of cytokines (e.g., TGF-β, PDGF, IGF-1), which directly stimulate myofibroblast differentiation. As a result it is difficult to determine solely from histologic studies whether inflammatory infiltrates are beneficial or detrimental. The role of specific cytokines and associated signaling pathways has been implicated from microarray studies in human biopsies with tubulointerstitial disease from diabetic nephropathy [92], and has been extensively reviewed recently [93]. There are some data indicating that systematic macrophage depletion is protective for tubular epithelial cells in models of ischemic AKI [94, 95], but similar data for CKD are lacking.
Lipotoxicity
The intracellular accumulation of excess non-esterified fatty acids (NEFA) and metabolites can result in organ dysfunction, and has been well described in liver (leading to hepatic steatosis), pancreatic β-cells (leading to diabetes), and heart (leading to congestive failure) [96]. The association between renal tubular epithelial cell lipid deposition and diabetic nephropathy was described many years ago [97, 98], and lipotoxicity has been implicated in CKD progression [99-102]. At a molecular level, lipotoxicity has been associated primarily with long chain (C12-C22) NEFA accumulation, and cytotoxicity is proportional to acyl chain length and carbon bond saturation [103, 104]. Some, but not all studies indicate that unsaturated NEFA exposure could be cytoprotective [105], perhaps due to preferential partitioning to lipid droplets [106] (see discussion below). This is clinically relevant because plasma concentrations of saturated NEFAs are increased in CKD patients, and are associated with CKD progression, particularly in cohorts enriched for diabetes [107-112].
The mechanisms of NEFA accumulation in tubule cells is an area of active investigation. In cases of glomerular damage that result in albuminuria, a large fraction of the filtered albumin is reabsorbed by the megalin/cubilin/amnionless protein complex expressed on the proximal tubule brush border, and degraded by lysosomes [113]. It has been postulated that in the nephrotic syndrome massive quantities of intracellular albumin exceed lysosomal degradative capacity, which leads to proximal tubule ER stress and secretion of noxious cytokines and chemokines [114]. This protein overload hypothesis is attractive because it directly links glomerular and tubular pathology to inflammatory pathways that perpetuate further renal injury, as discussed in the previous paragraph. However, albumin is heavily bound by NEFA, which permits NEFA to circulate in a soluble complex. Several groups have shown that tubulointerstitial disease can be induced in rats by infusion of NEFA-loaded, but not delipidated albumin [115-117], and in vitro incubation with albumin-bound NEFAs stimulate proximal tubule apoptosis, whereas exposure to delipidated albumin does not [116-121]. The importance of NEFA-bound albumin filtration and internalization is further illustrated in minimal change disease, which does not progress, at least in part, due to absence of tubular atrophy. Despite massive albuminuria, the ultrafiltrate is relatively NEFA-depleted in minimal change disease [122]. In subjects with heavy albuminuria due to diabetic nephropathy or minimal change disease, urine NEFA: albumin ratio was significantly greater in the diabetic nephropathy group, and correlated with tubulointerstitial pathology [123]. These data suggest that proximal tubule uptake of filtered NEFAs, rather than albumin, is the source of tubular toxicity (Figure 2). NEFAs are internalized by vectorial acylation, which involves simultaneous transport (mostly likely by a member of the fatty acid transporter (FATP) family) and esterification to long chain acyl CoA (LC-CoA) by plasma membrane-localized long chain acyl CoA synthetases. Under normal conditions LC-CoAs are primarily converted to acylcarnitine by carnitine palmitoyl transferase (CPT), which facilitates LC-CoA transport into mitochondria for β-oxidation and ATP generation, as described below.
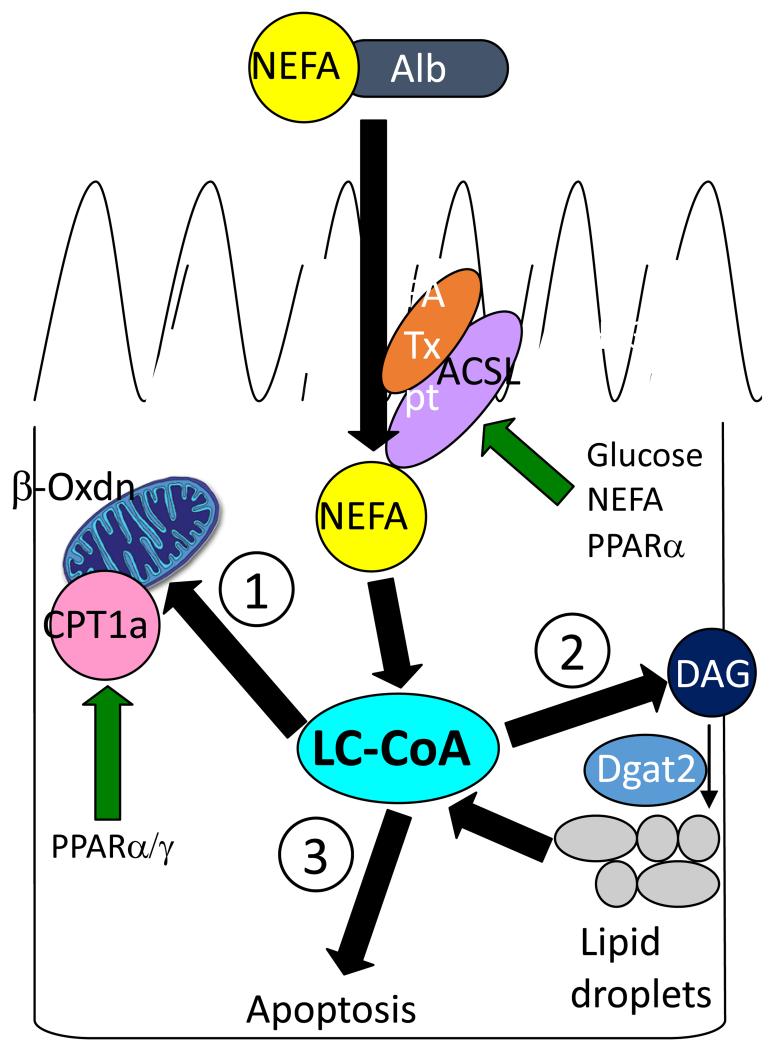
Figure 2.
Model of lipotoxicity-induced apoptosis and tubular atrophy. In progressive albuminuric renal diseases non-esterified fatty acid (NEFA) concentration is increased in proximal tubule cells through luminal reabsorption and/or lipogenesis. NEFA are rapidly converted to long chain fatty acyl CoA (LC-CoA) by long chain acyl CoA synthetases (ACSL). Preferential metabolic pathways include (1) carnitine palmitoyl transferase-1a (CPT1a)-regulated LC-CoA transport into mitochondria for β-oxidation and ATP generation or (2) conversion of diacylglycerol (DAG) to triacylglerol, which is stored in lipid droplets. If these pathways are blocked or saturated, accumulated LC-CoAs become detrimental and lead to apoptosis (3), through a variety of mechanisms, such as peroxisomal oxidation and reactive oxygen species (ROS) generation, disruption of NHE1-PI(4,5)P2 interaction-mediated cell survival, and ceramide formation.
In addition to NEFA uptake, tubular lipotoxicity may be amplified by increased proximal tubule NEFA synthesis, as shown in animal models of CKD due to type 1 and type 2 diabetes [124, 125]. Sterol regulatory element-binding protein (SREBP)-1c and carbohydrate response element-binding protein (ChREBP) transcription factors, which regulate fatty acid synthesis, have been investigated as likely mechanisms. On the other hand, SREBP-1c, ChREBP and fatty acid synthase mRNA expression have recently been shown to be significantly decreased with declining GFR in a diabetic nephropathy biopsy series [126], thereby casting some doubt regarding lipogenesis as a major mechanism of lipotoxicity in CKD progression.
NEFAs are the preferred substrate for proximal tubule ATP generation under normal circumstances [127-129]. However, in diabetic nephropathy proximal tubule β-oxidation of NEFA is decreased [124, 125], a finding that has been corroborated by human microarray studies, which demonstrate that GFR decline and interstitial fibrosis correlate with decreased NEFA β-oxidation, as well as decreased expression of CPT1a, PPAR-γ and PPAR-α transcripts [92, 126, 129], which encode for proteins that are critical for β-oxidation. One consequence of a block in β-oxidation is the accumulation of lipid precursor molecules, which are potentially pathogenic. For example, LC-CoAs, which are normally shuttled into the mitochondria for β-oxidation by CPT1a in the proximal tubule, are significantly increased in the renal cortex of mouse models of diabetic nephropathy and FSGS [120]. In vitro inhibition of CPT1a, which mimics the lipid metabolism abnormality in CKD, caused proximal tubule epithelial cell apoptosis [120, 129].
Numerous pathways buffer against NEFA accumulation and subsequent lipotoxicity. The first is metabolism of NEFA by β-oxidation to generate ATP (Figure 2). Once energy needs are met, excess NEFA are stored as cytoplasmic organelles comprised of triacylglycerol aggregates, termed lipid droplets [130] (Figure 2). While primarily functioning as an energy reserve by facilitating bidirectional NEFA trafficking throughout the cell, lipid droplets are also an adaptive depot for excess NEFA, and thereby shield against lipotoxicity and apoptosis [131-133]. In adipocytes, the cytoplasm is almost entirely filled with lipid droplets, whereas in epithelial cells lipid droplet capacity is limited, and saturation can therefore lead to lipotoxicity. The most alarming situation may be the combination of ROS generation and intracellular accumulation of NEFA, resulting in lipid droplet storage of peroxidated NEFA, which are cytotoxic [134].
Intracellular accumulation of NEFA and LC-CoA to levels that exceed tubule cell metabolism and storage thresholds can then lead to lipid-induced apoptosis (lipoapoptosis) (Figure 2). Multiple mechanisms of lipoapoptosis have been described in a variety of systems, including enhanced ceramide generation, ER stress, inhibition of several pro-survival kinases (Akt, PI-3 kinase, and AMP-activated protein kinase) [135-137] and ion transporters (see next paragraph). In diabetic nephropathy, β-oxidation may also be aberrant, resulting in electron transport chain defects and ROS generation [60]. Pharmacologic and genetic studies suggest that stimulation of PPAR-α or PPAR-γ, which enhance NEFA utilization, may improve histopathology and slow CKD progression in humans, as well as animal models of CKD [129, 138-143].
NHE1 inactivation
As mentioned previously, a final common pathway for tubular atrophy is tubular epithelial cell apoptosis, and an invariant feature of apoptosis is cell volume decrease. In response to shrinkage by non-apoptotic stimuli, cells undergo activation of regulatory volume increase (RVI) pathways in an effort to re-expand. It has been suggested that a similar pathway is activated to limit damage from apoptotic stress [144]. Of the well-characterized RVI-associated ion channels and transporters, only the NHE1 Na+/H+ exchanger is expressed in proximal tubules [144]. Apoptotic cells also undergo cytosol acidification, which catalyzes pro-apoptotic enzymes [145, 146], indicating that NHE1-regulated Na+/H+ exchange might defend against renal tubular epithelial cell apoptosis by intracellular alkalinization, as well as expanding cell volume.
Proximal tubule NHE1 is indeed activated by multiple different apoptotic stressors [147, 148]. NHE1 activation is dependent upon the anchoring of two Arg/Lys-rich cytoplasmic domains to the plasma membrane inner leaflet through binding to negatively charged phosphate groups on PI(4,5)P2 [149-152]. PI-3 kinase phosphorylates PI(4,5)P2, and the PI(3,4,5)P3 product docks the pro-survival kinase Akt, leading to its activation and phosphorylation of downstream targets that inhibit apoptosis. This docking and signal transduction function of NHE1, which is independent of Na+-H+ exchange activity, represents one mechanism of proximal tubule defense against apoptotic stress and tubular atrophy [152].
Multiple studies have demonstrated that tubular epithelial cell NHE1 becomes inactivated during apoptosis [147, 148, 153, 154], but the mechanism has been difficult to clarify. Decreased NHE1 mRNA and protein expression were noted in ureteral obstruction models [153, 154], and there is some evidence to indicate that the NHE1 cytosolic tail undergoes caspase-3-dependent degradation [147, 155]. However, the cleavage site has not been mapped, and there are no consensus caspase-3 cleavage sequences within the cytosolic tail. NHE1 inactivation has recently been associated with the previously discussed lipotoxicity model. The link is predicated upon the accumulation of LC-CoAs, which bear structural homology to phosphoinositides, and demonstration that LC-CoAs bind the NHE1 cytosolic tail with greater affinity compared to PI(4,5)P2 [120]. In cultured proximal tubule cells and microinjected oocytes, LC-CoAs compete with PI(4,5)P2 for binding to NHE1, and uncouple the NHE1-PI(4,5)P2 interaction, causing loss of NHE1 activity, which predisposes to apoptosis [120]. In this model, proximal tubule NHE1 therefore acts as a metabolic sensor for lipotoxicity.
Animal models of tubular atrophy
There are a number of rodent models of non-diabetic glomerular diseases that include a progressive phenotype with tubular atrophy (recently reviewed in [156]). In general, these models fall into the categories of transgenic animals, spontaneous/acquired mutations, or induction of a renal phenotype. In some cases, a combination of phenotype induction in a mutant animal has been fruitful for accentuating tubular atrophy [147, 157]. An important consideration is the genetic background of the animal, and in mice, C57BL/6 background tends to be resistant to disease, whereas FVB, 129/sv and Balb/c mice are relatively susceptible [158, 159].
Conclusions
The glomerulus has been the focus of CKD pathology and cell biology investigation for many years. More recently there has been much deserved attention to the podocyte, which appears to be the proximate source of dysfunction in a variety of glomerular diseases. A better understanding of podocyte pathophysiology will undoubtedly lead to treatments for prominent glomerular diseases, such as FSGS, which are badly needed, since state of the art therapeutic regimens have not substantially changed since the advent of ACE inhibitors in the 1980s, and current immunosuppressive agents have substantial toxicities, and may not be effective in a significant number of patients [160]. This review emphasizes the importance of the remainder of the nephron, which has been relatively neglected as a topic for CKD research, despite repeated demonstration over 50 years that tubular atrophy and interstitial fibrosis are better predictors of CKD progression than glomerular pathology.
A reductionist approach to identify CKD therapies, with separate focus on glomerular and tubular compartments, may be sensible for several reasons. First, a panacea (monotherapy) to simultaneously address CKD pathology for the entire nephron, which is comprised from over 20 cell types, is unlikely to be identified. Second, as highlighted in this review, several plausible pathophysiologic mechanisms of tubular atrophy are proposed, and although some may ultimately be proven incorrect, the emergence of a single dominant pathway is unlikely. Third, even if amelioration of tubular atrophy could be achieved, this would expose the glomerulus as the limiting factor, with necessary identification of separate glomerular therapies. As a result, a sanguine approach might be to develop glomerular and tubular therapies in tandem, in a manner similar to cancer chemotherapy regimens, which employ multiple drugs that target different mechanistic pathways. There are no current therapies specifically designed to treat tubulointerstitial pathology, but based upon the importance of tubular atrophy and interstitial fibrosis as drivers of CKD progression, it seems rational to begin the search, which should involve testing in adults and children.
Acknowledgments
I am grateful to Drs. Katherine Dell and John O’Toole for careful review of the manuscript. Dr. Schelling is supported by NIH grants NIH 2R01 DK067528 and 2U01 DK061021.
Footnotes
Conflict of interest - none
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van LF, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, Quinn RR, Wiebe N, Hemmelgarn BR. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21. doi: 10.7326/0003-4819-154-1-201101040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 4.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R, AASK Pilot Study Investigators Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. Kidney Int. 1997;51:244–252. doi: 10.1038/ki.1997.29. [DOI] [PubMed] [Google Scholar]
- 5.Zarif L, Covic A, Iyengar S, Sehgal AR, Sedor JR, Schelling JR. Inaccuracy of clinical phenotyping parameters for hypertensive nephrosclerosis. Nephrol Dial Transplant. 2000;15:1801–1807. doi: 10.1093/ndt/15.11.1801. [DOI] [PubMed] [Google Scholar]
- 6.Muehrcke RC, Kark RM, Pirani CL, Pollak VE. Lupus nephritis: a clinical and pathologic study based on renal biopsies. Medicine (Baltimore) 1957;36:1–145. [PubMed] [Google Scholar]
- 7.Rosenbaum JL, Mikail M, Wiedmann F. Further correlation of renal function with kidney biopsy in chronic renal disease. Am J Med Sci. 1967;254:156–160. doi: 10.1097/00000441-196708000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 9.Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. Hum Pathol. 1970;1:631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- 10.Bohle A, Mackensen-Haen S, von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: A morphometric contribution. Am J Nephrol. 1987;7:421–433. doi: 10.1159/000167514. [DOI] [PubMed] [Google Scholar]
- 11.Bunnag S, Einecke G, Reeve J, Jhangri GS, Mueller TF, Sis B, Hidalgo LG, Mengel M, Kayser D, Kaplan B, Halloran PF. Molecular correlates of renal function in kidney transplant biopsies. J Am Soc Nephrol. 2009;20:1149–1160. doi: 10.1681/ASN.2008080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases - Insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 13.Striker GE, Schainuck LI, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Hum Pathol. 1970;1:615–630. doi: 10.1016/s0046-8177(70)80060-0. [DOI] [PubMed] [Google Scholar]
- 14.Bohle A, Muller GA, Wehrmann M, Mackensen-Haen S, Xiao J-C. Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies, and chronic interstitial nephritides. Kidney Int. 1996;54:S2–S9. [PubMed] [Google Scholar]
- 15.Cravedi P, Heeger PS. Complement as a multifaceted modulator of kidney transplant injury. J Clin Invest. 2014;124:2348–2354. doi: 10.1172/JCI72273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seron D, Moreso F, Ramon JM, Hueso M, Condom E, Fulladosa X, Bover J, Gil-Vernet S, Castelao AM, Alsina J, Grinyo JM. Protocol renal allograft biopsies and the design of clinical trials aimed to prevent or treat chronic allograft nephropathy. Transplantation. 2000;69:1849–1855. doi: 10.1097/00007890-200005150-00019. [DOI] [PubMed] [Google Scholar]
- 17.Schlondorff DO. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 2008;74:860–866. doi: 10.1038/ki.2008.351. [DOI] [PubMed] [Google Scholar]
- 18.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis. 2003;35:39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 19.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz A, Ziyadeh FN, Neilson EG. Expression of apoptosis-regulatory genes in renal proximal tubular epithelial cells exposed to high ambient glucose and in diabetic kidneys. J Invest Med. 1997;45:50–56. [PubMed] [Google Scholar]
- 21.Schelling JR, Nkemere N, Kopp JB, Cleveland RP. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab Invest. 1998;78:813–824. [PubMed] [Google Scholar]
- 22.Khan S, Cleveland RP, Koch CJ, Schelling JR. Hypoxia induces renal tubular epithelial cell apoptosis in chronic renal disease. Lab Invest. 1999;79:1089–1099. [PubMed] [Google Scholar]
- 23.Schelling JR, Cleveland RP. Involvement of Fas-dependent apoptosis in renal tubular epithelial cell deletion in chronic renal failure. Kidney Int. 1999;56:1313–1316. doi: 10.1046/j.1523-1755.1999.00684.x. [DOI] [PubMed] [Google Scholar]
- 24.Kelly DJ, Cox AJ, Tolcos M, Cooper ME, Wilkinson-Berka JL, Gilbert RE. Attenuation of tubular apoptosis by blockade of the renin-angiotensin system in diabetic Ren-2 rats. Kidney Int. 2002;61:31–39. doi: 10.1046/j.1523-1755.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem. 2004;259:67–70. doi: 10.1023/b:mcbi.0000021346.03260.7e. [DOI] [PubMed] [Google Scholar]
- 26.Susztak K, Ciccone E, McCue P, Sharma K, Bottinger EP. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2:e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brezniceanu ML, Liu F, Wei CC, Tran S, Sachetelli S, Zhang SL, Guo DF, Filep JG, Ingelfinger JR, Chan JS. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int. 2007;71:912–923. doi: 10.1038/sj.ki.5002188. [DOI] [PubMed] [Google Scholar]
- 28.Brezniceanu ML, Liu F, Wei CC, Chenier I, Godin N, Zhang SL, Filepa JG, Ingelfingerb JR, Chan JS. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes. 2008;57:451–459. doi: 10.2337/db07-0013. [DOI] [PubMed] [Google Scholar]
- 29.Brezniceanu ML, Lau CJ, Godin N, Chenier I, Duclos A, Ethier J, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:943–954. doi: 10.1681/ASN.2009030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe M, Nakatsuka A, Murakami K, Inoue K, Terami T, Higuchi C, Katayama A, Teshigawara S, Eguchi J, Ogawa D, Watanabe E, Wada J, Makino H. Pemt deficiency ameliorates endoplasmic reticulum stress in diabetic nephropathy. PLoS One. 2014;9:e92647. doi: 10.1371/journal.pone.0092647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T, Kimura M, Asano M, Fujigaki Y, Hishida A. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am J Pathol. 2001;158:75–85. doi: 10.1016/S0002-9440(10)63946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura M, Asano M, Abe K, Miyazaki M, Suzuki T, Hishida A. Role of atrophic changes in proximal tubular cells in the peritubular deposition of type IV collagen in a rat renal ablation model. Nephrol Dial Transplant. 2005;20:1559–1565. doi: 10.1093/ndt/gfh872. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Fogo AB. Cell senescence in the aging kidney. J Am Soc Nephrol. 2010;21:1436–1439. doi: 10.1681/ASN.2010020205. [DOI] [PubMed] [Google Scholar]
- 35.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross ML, Serrano M, Schmitt R, Melk A. Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol. 2012;23:1467–1473. doi: 10.1681/ASN.2011100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbosa Junior Ade A, Zhou H, Hultenschmidt D, Totovic V, Jurilj N, Pfeifer U. Inhibition of cellular autophagy in proximal tubular cells of the kidney in streptozotocin-diabetic and uninephrectomized rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;61:359–366. doi: 10.1007/BF02890439. [DOI] [PubMed] [Google Scholar]
- 38.Takabatake Y, Kimura T, Takahashi A, Isaka Y. Autophagy and the kidney: health and disease. Nephrol Dial Transplant. 2014;29:1639–1647. doi: 10.1093/ndt/gft535. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–837. doi: 10.4161/auto.19419. [DOI] [PubMed] [Google Scholar]
- 40.Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Haneda M, Matsusaka T, Kashiwagi A, Maegawa H, Uzu T. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol. 2013;24:1769–1781. doi: 10.1681/ASN.2012111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120:1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19:1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohashi R, Kitamura H, Yamanaka N. Peritubular capillary injury during the progression of experimental glomerulonephritis in rats. J Am Soc Nephrol. 2000;11:47–56. doi: 10.1681/ASN.V11147. [DOI] [PubMed] [Google Scholar]
- 47.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 48.Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N. Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am SocNephrol. 2002;13:1795–1805. doi: 10.1097/01.asn.0000018408.51388.57. [DOI] [PubMed] [Google Scholar]
- 49.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlöndorff D, Cohen CD. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18:1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- 50.Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26:1132–1137. doi: 10.1093/ndt/gfq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2013;29:333–342. doi: 10.1007/s00467-013-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norman JT, Stidwill R, Singer M, Fine LG. Angiotensin II blockade augments renal cortical microvascular pO2 indicating a novel, potentially renoprotective action. Nephron Physiol. 2003;94:39–46. doi: 10.1159/000071289. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto M, Tanaka T, Yamamoto T, Noiri E, Miyata T, Inagi R, Fujita T, Nangaku M. Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J Am Soc Nephrol. 2004;15:1574–1581. doi: 10.1097/01.asn.0000128047.13396.48. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberger C, Khamaisi M, Abassi Z, Shilo V, Weksler-Zangen S, Goldfarb M, Shina A, Zibertrest F, Eckardt KU, Rosen S, Heyman SN. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 2008;73:34–42. doi: 10.1038/sj.ki.5002567. [DOI] [PubMed] [Google Scholar]
- 55.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 56.Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. doi: 10.1097/01.asn.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- 57.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haase VH. A breath of fresh air for diabetic nephropathy. J Am Soc Nephrol. 2015;26:239–241. doi: 10.1681/ASN.2014080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, Hansell P, Palm F. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol. 2015;26:328–338. doi: 10.1681/ASN.2013090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, Hoppel CL. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes. 2012;61:2074–2083. doi: 10.2337/db11-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxidants Redox Signaling. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 63.Nakashima I, Takeda K, Kawamoto Y, Okuno Y, Kato M, Suzuki H. Redox control of catalytic activities of membrane-associated protein tyrosine kinases. Arch Biochem Biophys. 2005;434:3–10. doi: 10.1016/j.abb.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nlandu Khodo S, Dizin E, Sossauer G, Szanto I, Martin PY, Feraille E, Krause KH, de Seigneux S. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J Am Soc Nephrol. 2012;23:1967–1976. doi: 10.1681/ASN.2012040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sedeek M, Nasrallah R, Touyz RM, Hebert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol. 2013;24:1512–1518. doi: 10.1681/ASN.2012111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S, Fanti P, Szyndralewiez C, Barnes JL, Block K, Abboud HE. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol. 2015;308:F1276–F1287. doi: 10.1152/ajprenal.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Godin N, Liu F, Lau GJ, Brezniceanu ML, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int. 2010;77:1086–1097. doi: 10.1038/ki.2010.63. [DOI] [PubMed] [Google Scholar]
- 69.Jun M, Venkataraman V, Razavian M, Cooper B, Zoungas S, Ninomiya T, Webster AC, Perkovic V. Antioxidants for chronic kidney disease. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD008176.pub2. Cd008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM, BEACON Trial Investigators Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oliver J. When is the kidney not a kidney? J Urol. 1950;63:373–402. [Google Scholar]
- 72.Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol. 2008;19:197–206. doi: 10.1681/ASN.2007080862. [DOI] [PubMed] [Google Scholar]
- 73.Zuk A, Matlin KS, Hay ED. Type I collagen gel induces Madin-Darby canine kidney cells to become fusiform in shape and lose apical-basal polarity. J Cell Biol. 1989;108:903–919. doi: 10.1083/jcb.108.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hay ED, Zuk A. Transformations between epithelium and mesenchyme: Normal, pathological, and experimentally induced. Am J Kidney Dis. 1995;26:678–690. doi: 10.1016/0272-6386(95)90610-x. [DOI] [PubMed] [Google Scholar]
- 75.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zadran S, Arumugam R, Herschman H, Phelps ME, Levine RD. Surprisal analysis characterizes the free energy time course of cancer cells undergoing epithelial-to-mesenchymal transition. Proc Natl Acad Sci U S A. 2014;111:13235–13240. doi: 10.1073/pnas.1414714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-b regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 79.Rastaldi MP, Ferrario F, Giardino L, Dell’Antonio G, Grillo C, Grillo P, Strutz F, Müller GA, Colasanti G, D’Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62:137–146. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 80.Faulkner JL, Szcykalski LM, Springer F, Barnes JL. Origin of Interstitial Fibroblasts in an Accelerated Model of Angiotensin II-Induced Renal Fibrosis. Am J Pathol. 2005;167:1193–1205. doi: 10.1016/S0002-9440(10)61208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 84.Morel-Maroger Striker L, Killen PD, Chi E, Striker GE. The composition of glomerulosclerosis. I. Studies in focal sclerosis, crescentic glomerulonephritis, and membranoproliferative glomerulonephritis. Lab Invest. 1984;51:181–192. [PubMed] [Google Scholar]
- 85.Eddy AA. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl. 2014;4:S2–S8. doi: 10.1038/kisup.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 87.Castano AP, Lin SL, Surowy T, Nowlin BT, Turlapati SA, Patel T, Singh A, Li S, Lupher ML, Jr., Duffield JS. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Gröne HJ, Onuffer J, Horuk R, Nelson PJ, Schlöndorff D. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest. 2002;109:251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu ZH, Striker GE, Stetler-Stevenson M, Fukushima P, Patel A, Striker LJ. TNF-a and IL-1a induce mannose receptors and apoptosis in glomerular mesangial but not endothelial cells. Am J Physiol Cell Physiol. 1996;270:C1595–C1601. doi: 10.1152/ajpcell.1996.270.6.C1595. [DOI] [PubMed] [Google Scholar]
- 90.Lange-Sperandio B, Fulda S, Vandewalle A, Chevalier RL. Macrophages induce apoptosis in proximal tubule cells. Pediatr Nephrol. 2003;18:335–341. doi: 10.1007/s00467-003-1116-2. [DOI] [PubMed] [Google Scholar]
- 91.Zoja C, Abbate M, Remuzzi G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant. 2014;30:706–712. doi: 10.1093/ndt/gfu261. [DOI] [PubMed] [Google Scholar]
- 92.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354–2369. doi: 10.2337/db10-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 94.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 96.Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta. 2010;1801:246–251. doi: 10.1016/j.bbalip.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kimmelstiel P, Wilson C. Intercapillary Lesions in the Glomeruli of the Kidney. Am J Pathol. 1936;12:83–98. [PMC free article] [PubMed] [Google Scholar]
- 98.Oliver J, MacDowell M, Lee YC. Cellular mechanisms of protein metabolism in the nephron. I. The structural aspects of proteinuria; tubular absorption, droplet formation, and the disposal of proteins. J Exp Med. 1954;99:589–604. doi: 10.1084/jem.99.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 100.Thomas ME, Schreiner GF. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. Am J Nephrol. 1993;13:385–398. doi: 10.1159/000168653. [DOI] [PubMed] [Google Scholar]
- 101.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70:1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 102.Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713–721. doi: 10.1038/nrneph.2009.184. [DOI] [PubMed] [Google Scholar]
- 103.Riedel MJ, Light PE. Saturated and cis/trans unsaturated acyl CoA esters differentially regulate wild-type and polymorphic beta-cell ATP-sensitive K+ channels. Diabetes. 2005;54:2070–2079. doi: 10.2337/diabetes.54.7.2070. [DOI] [PubMed] [Google Scholar]
- 104.Riedel MJ, Baczko I, Searle GJ, Webster N, Fercho M, Jones L, Lang J, Lytton J, Dyck JR, Light PE. Metabolic regulation of sodium-calcium exchange by intracellular acyl CoAs. EMBO J. 2006;25:4605–4614. doi: 10.1038/sj.emboj.7601321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Timmeren MM, Gross ML, Hanke W, Klok PA, van GH, Stegeman CA, Bakker SJ. Oleic acid loading does not add to the nephrotoxic effect of albumin in an amphibian and chronic rat model of kidney injury. Nephrol Dial Transplant. 2008;23:3814–3823. doi: 10.1093/ndt/gfn417. [DOI] [PubMed] [Google Scholar]
- 106.Thomas ME, Morrison AR, Schreiner GF. Metabolic effects of fatty acid-bearing albumin on a proximal tubule cell line. Am J Physiol. 1995;268:F1177–F1184. doi: 10.1152/ajprenal.1995.268.6.F1177. [DOI] [PubMed] [Google Scholar]
- 107.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 108.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58:293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 109.Samuelsson O, Mulec H, Knight-Gibson C, Attman PO, Kron B, Larsson R, Weiss L, Wedel H, Alaupovic P. Lipoprotein abnormalities are associated with increased rate of progression of human chronic renal insufficiency. Nephrol Dial Transplant. 1997;12:1908–1915. doi: 10.1093/ndt/12.9.1908. [DOI] [PubMed] [Google Scholar]
- 110.Cases A, Coll E. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl. 2005;99:S87–S93. doi: 10.1111/j.1523-1755.2005.09916.x. [DOI] [PubMed] [Google Scholar]
- 111.de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Brunzell JD, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. White NH, Danis RP, Davis MD, Hainsworth D, Hubbard LD, Nathan DM. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171:412–420. doi: 10.1001/archinternmed.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Makinen VP, Tynkkynen T, Soininen P, Peltola T, Kangas AJ, Forsblom C, Thorn LM, Kaski K, Laatikainen R, Ala-Korpela M, Groop PH. Metabolic diversity of progressive kidney disease in 325 patients with type 1 diabetes (the FinnDiane study) J Proteome Res. 2012;11:1782–1790. doi: 10.1021/pr201036j. [DOI] [PubMed] [Google Scholar]
- 113.Christensen EI, Birn H, Storm T, Weyer K, Nielsen R. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 2012;27:223–236. doi: 10.1152/physiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- 114.Abbate M, Zoja C, Corna D, Capitanio M, Bertani T, Remuzzi G. In progressive nephropathies, overload of tubular cells with filtered proteins translates glomerular permeability dysfunction into cellular signals of interstitial inflammation. J Am Soc Nephrol. 1998;9:1213–1224. doi: 10.1681/ASN.V971213. [DOI] [PubMed] [Google Scholar]
- 115.Thomas ME, Harris KPG, Walls J, Furness PN, Brunskill NJ. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol. 2002;283:F640–F647. doi: 10.1152/ajprenal.00001.2002. [DOI] [PubMed] [Google Scholar]
- 116.Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, Kaneko T, Hirata Y, Goto A, Fujita T, Omata M. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 2002;62:1628–1637. doi: 10.1046/j.1523-1755.2002.00618.x. [DOI] [PubMed] [Google Scholar]
- 117.van Timmeren MM, Bakker SJ, Stegeman CA, Gans RO, van GH. Addition of oleic acid to delipidated bovine serum albumin aggravates renal damage in experimental protein-overload nephrosis. Nephrol Dial Transplant. 2005;20:2349–2357. doi: 10.1093/ndt/gfh964. [DOI] [PubMed] [Google Scholar]
- 118.Arici M, Brown J, Williams M, Harris KP, Walls J, Brunskill NJ. Fatty acids carried on albumin modulate proximal tubular cell fibronectin production: a role for protein kinase C. Nephrol Dial Transplant. 2002;17:1751–1757. doi: 10.1093/ndt/17.10.1751. [DOI] [PubMed] [Google Scholar]
- 119.Arici M, Chana R, Lewington A, Brown J, Brunskill NJ. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-g. J Am Soc Nephrol. 2003;14:17–27. doi: 10.1097/01.asn.0000042167.66685.ea. [DOI] [PubMed] [Google Scholar]
- 120.Khan S, Abu Jawdeh BG, Goel M, Schilling WP, Parker MD, Puchowicz MA, Yadav SP, Harris RC, El-Meanawy A, Hoshi M, Shinlapawittayatorn K, Deschênes I, Ficker E, Schelling JR. Lipotoxic disruption of NHE1 interaction with PI(4,5)P2 expedites proximal tubule apoptosis. J Clin Invest. 2014;124:1057–1068. doi: 10.1172/JCI71863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruggiero C, Elks CM, Kruger C, Cleland E, Addison K, Noland RC, Stadler K. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am J Physiol Renal Physiol. 2014;306:F296–F306. doi: 10.1152/ajprenal.00484.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghiggeri GM, Ginevri F, Candiano G, Oleggini R, Perfumo F, Queirolo C, Gusmano R. Characterization of cationic albumin in minimal change nephropathy. Kidney Int. 1987;32:547–553. doi: 10.1038/ki.1987.243. [DOI] [PubMed] [Google Scholar]
- 123.Sasaki H, Kamijo-Ikemori A, Sugaya T, Yamashita K, Yokoyama T, Koike J, Sato T, Yasuda T, Kimura K. Urinary fatty acids and liver-type fatty acid binding protein in diabetic nephropathy. Nephron Clin Pract. 2009;112:c148–156. doi: 10.1159/000214210. [DOI] [PubMed] [Google Scholar]
- 124.Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277:18919–18927. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 125.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502–2509. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 126.Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2013;55:561–572. doi: 10.1194/jlr.P040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guder WG, Wagner S, Wirthensohn G. Metabolic fuels along the nephron: pathways and intracellular mechanisms of interaction. Kidney Int. 1986;29:41–45. doi: 10.1038/ki.1986.6. [DOI] [PubMed] [Google Scholar]
- 128.Meyer C, Nadkarni V, Stumvoll M, Gerich J. Human kidney free fatty acid and glucose uptake: evidence for a renal glucose-fatty acid cycle. Am J Physiol. 1997;273:E650–654. doi: 10.1152/ajpendo.1997.273.3.E650. [DOI] [PubMed] [Google Scholar]
- 129.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2014;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Farese RV, Jr., Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garbarino J, Padamsee M, Wilcox L, Oelkers PM, D’ AD, Ruggles K, Ramsey N, Jabado O, Turkish A, Sturley SL. Sterol and diacylglycerol acyltransferase deficiency triggers fatty acid mediated cell death. J Biol Chem. 2009;284:30994–31005. doi: 10.1074/jbc.M109.050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Urahama Y, Ohsaki Y, Fujita Y, Maruyama S, Yuzawa Y, Matsuo S, Fujimoto T. Lipid droplet-associated proteins protect renal tubular cells from fatty acid-induced apoptosis. Am J Pathol. 2008;173:1286–1294. doi: 10.2353/ajpath.2008.080137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, Bellen HJ. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 136.Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem. 2011;286:1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Simon-Szabo L, Kokas M, Mandl J, Keri G, Csala M. Metformin attenuates palmitate-induced endoplasmic reticulum stress, serine phosphorylation of IRS-1 and apoptosis in rat insulinoma cells. PLoS One. 2014;9:e97868. doi: 10.1371/journal.pone.0097868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M, FIELD study investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 139.Park CW, Kim HW, Ko SH, Chung HW, Lim SW, Yang CW, Chang YS, Sugawara A, Guan Y, Breyer MD. Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:885–893. doi: 10.2337/diabetes.55.04.06.db05-1329. [DOI] [PubMed] [Google Scholar]
- 140.Park CW, Zhang Y, Zhang X, Wu J, Chen L, Cha DR, Su D, Hwang MT, Fan X, Davis L, Striker G, Zheng F, Breyer M, Guan Y. PPARa agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 2006;69:1511–1517. doi: 10.1038/sj.ki.5000209. [DOI] [PubMed] [Google Scholar]
- 141.Fogo AB. PPARg and chronic kidney disease. Pediatr Nephrol. 2011;26:347–351. doi: 10.1007/s00467-010-1602-2. [DOI] [PubMed] [Google Scholar]
- 142.Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, Jenkins AJ, O’Connell RL, Whiting MJ, Glasziou PP, Simes RJ, Kesäniemi YA, Gebski VJ, Scott RS, Keech AC, Fenofibrate Intervention and Event Lowering in Diabetes Study investigators Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011;54:280–290. doi: 10.1007/s00125-010-1951-1. [DOI] [PubMed] [Google Scholar]
- 143.Hong YA, Lim JH, Kim MY, Kim TW, Kim Y, Yang KS, Park HS, Choi SR, Chung S, Kim HW, Kim HW, Choi BS, Chang YS, Park CW. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1a in db/db mice. PLoS ONE. 2014;9:e96147. doi: 10.1371/journal.pone.0096147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2:318–325. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 146.Segal MS, Beem E. Effect of pH, ionic charge, and osmolality on cytochrome c-mediated caspase-3 activity. Am J Physiol. 2001;281:C1196–C1204. doi: 10.1152/ajpcell.2001.281.4.C1196. [DOI] [PubMed] [Google Scholar]
- 147.Wu KL, Khan S, Lakhe-Reddy S, Wang LM, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR. Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol Renal Physiol. 2003;284:F829–F839. doi: 10.1152/ajprenal.00314.2002. [DOI] [PubMed] [Google Scholar]
- 148.Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR, Schelling JR. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem. 2004;279:26280–26286. doi: 10.1074/jbc.M400814200. [DOI] [PubMed] [Google Scholar]
- 149.Aharonovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S. Intracellular pH regulation by Na+/H+ exchange requires phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2000;150:213–224. doi: 10.1083/jcb.150.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fuster D, Moe OW, Hilgemann DW. Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods. Proc Natl Acad Sci U S A. 2004;101:10482–10487. doi: 10.1073/pnas.0403930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wakabayashi S, Nakamura TY, Kobayashi S, Hisamitsu T. Novel phorbol ester-binding motif mediates hormonal activation of Na+/H+ exchanger. J Biol Chem. 2010;285:26652–26661. doi: 10.1074/jbc.M110.130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abu Jawdeh BG, Khan S, Deschênes I, Hoshi M, Goel M, Lock JT, Shinlapawittayatorn K, Babcock G, Lakhe-Reddy S, DeCaro G, Yadav SP, Mohan ML, Naga Prasad SV, Schilling WP, Ficker E, Schelling JR. Phosphoinositide binding differentially regulates NHE1 Na+/H+ exchanger-dependent proximal tubule cell survival. J Biol Chem. 2011;286:42435–42445. doi: 10.1074/jbc.M110.212845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bocanegra V, Gil Lorenzo AF, Cacciamani V, Benardon ME, Costantino VV, Valles PG. RhoA and MAPKinase signal transduction pathways regulate NHE1 Na+/H+ exchanger-dependent proximal tubule cell apoptosis following mechanical stretch. Am J Physiol Renal Physiol. 2014;307:F881–F889. doi: 10.1152/ajprenal.00232.2014. [DOI] [PubMed] [Google Scholar]
- 154.Manucha W, Carrizo L, Ruete C, Valles PG. Apoptosis induction is associated with decreased NHE1 expression in neonatal unilateral ureteric obstruction. BJU Int. 2007;100:191–198. doi: 10.1111/j.1464-410X.2007.06840.x. [DOI] [PubMed] [Google Scholar]
- 155.Lupescu A, Geiger C, Zahir N, Aberle S, Lang PA, Kramer S, Wesselborg S, Kandolf R, Foller M, Lang F, Bock CT. Inhibition of Na+/H+ exchanger activity by parvovirus B19 protein NS1. Cell Physiol Biochem. 2009;23:211–220. doi: 10.1159/000204110. [DOI] [PubMed] [Google Scholar]
- 156.Yang HC, Zuo Y, Fogo AB. Models of chronic kidney disease. Drug Discov Today Dis Models. 2010;7:13–19. doi: 10.1016/j.ddmod.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Esposito C, He CJ, Striker GE, Zalups RK, Striker LJ. Nature and severity of the glomerular response to nephron reduction is strain-dependent in mice. Am J Pathol. 1999;154:891–897. doi: 10.1016/S0002-9440(10)65336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gharavi AG, Ahmad T, Wong RD, Hooshyar R, Vaughn J, Oller S, Frankel RZ, Bruggeman LA, D’Agati VD, Klotman PE, Lifton RP. Mapping a locus for susceptibility to HIV-1-associated nephropathy to mouse chromosome 3. Proc Natl Acad Sci U S A. 2004;101:2488–2493. doi: 10.1073/pnas.0308649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–F222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- 160.Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Hogan SL, Middleton JP, Vehaskari VM, Flynn PA, Powell LM, Vento SM, McMahan JL, Siegel N, D’Agati VD, Friedman AL. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]