Abstract
The Drosophila testis is a well-established system for studying stem cell self-renewal and competition. In this tissue, the niche supports two stem cell populations, germ line stem cells (GSCs), which give rise to sperm, and somatic stem cells called cyst stem cells (CySCs), which support GSCs and their descendants. It has been established that CySCs compete with each other and with GSCs for niche access, and mutations have been identified that confer increased competitiveness to CySCs, resulting in the mutant stem cell and its descendants outcompeting wild type resident stem cells. Socs36E, which encodes a negative feedback inhibitor of the JAK/STAT pathway, was the first identified regulator of niche competition. The competitive behavior of Socs36E mutant CySCs was attributed to increased JAK/STAT signaling. Here we show that competitive behavior of Socs36E mutant CySCs is due in large part to unbridled Mitogen-Activated Protein Kinase (MAPK) signaling. In Socs36E mutant clones, MAPK activity is elevated. Furthermore, we find that clonal upregulation of MAPK in CySCs leads to their outcompetition of wild type CySCs and of GSCs, recapitulating the Socs36E mutant phenotype. Indeed, when MAPK activity is removed from Socs36E mutant clones, they lose their competitiveness but maintain self-renewal, presumably due to increased JAK/STAT signaling in these cells. Consistently, loss of JAK/STAT activity in Socs36E mutant clones severely impairs their self-renewal. Thus, our results enable the genetic separation of two essential processes that occur in stem cells. While some niche signals specify the intrinsic property of self-renewal, which is absolutely required in all stem cells for niche residence, additional signals control the ability of stem cells to compete with their neighbors. Socs36E is node through which these processes are linked, demonstrating that negative feedback inhibition integrates multiple aspects of stem cell behavior.
Author Summary
Niches are specialized local environments that support stem cell self-renewal through the local production of short-range signals. In many tissues, resident stem cells compete with each other for niche access. Stem cells that receive multiple self-renewal cues have to integrate these discrete signals to prevent excessive competition or premature differentiation. Negative feedback inhibition between signaling pathways can provide a cohesive node through which such signals are interpreted. Here we show that a negative feedback inhibitor of the JAK/STAT pathway (Socs36E) acts as a brake on a second signaling pathway (MAPK). Stem cells lacking Socs36E have sustained MAPK signaling, which endows these cells with superior competitive skills that allow them to displace their normal neighbors and take over the entire niche. Our results show that dampening niche-derived signals is critical in maintaining balance in stem cell niches.
Introduction
Stem cell niches are complex environments that provide support for stem cells through molecular signals. Several well-characterized niches provide not just one but multiple signals which stem cells must integrate and interpret in order to remain at the niche and self-renew [1]. How this integration is achieved is not well understood at present. Furthermore, in order to maintain the appropriate number of stem cells and the homeostatic balance between self-renewal and differentiation, it is necessary that self-renewal cues be present in limiting amounts or that their activity be dampened to prevent excessive accumulation of stem cells. One general feature of many signal transduction pathways is the presence of feedback inhibitors [2–4]. These are dampeners of signaling, transcriptionally induced by the signaling itself, that prevent signal levels from being aberrantly high. One such family of feedback inhibitors is the Suppressor of Cytokine Signaling (SOCS) proteins, which were identified as inhibitors of JAK/STAT (Janus Kinase/Signal Transduction and Activator of Transcription) signal transduction, and are SH2- and E3-ligase domain-containing proteins (Fig 1A and [2]). The SH2 domain binds phosphorylated (i.e., activated) signal transduction components and the E3-ligase targets them for degradation by Ubiquitin-dependent proteolysis. In mammals, SOCS proteins can thus inhibit several tyrosine kinase-dependent signaling pathways, including JAK/STAT and Mitogen-Activated Protein Kinase (MAPK) [2,5].
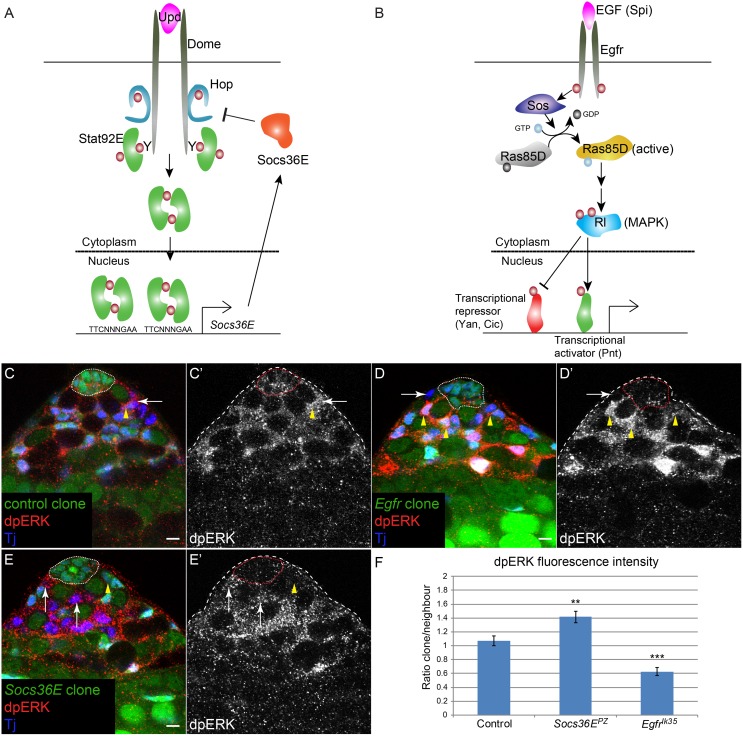
Fig 1. MAPK signaling is elevated in Socs36E mutant clones.
A) Model of the Drosophila JAK/STAT pathway. The ligand Unpaired (Upd, magenta) is produced by hub cells and binds to and activates the receptor Domeless (Dome, gray) on the surface of CySCs. This results in activation of the JAK Hopscotch (Hop) (blue), leading to tyrosine phosphorylation (maroon circles) of Dome. The phosphorylated cytoplasmic domain of the receptor serves as a docking site for a Stat92E dimer (green). Stat92E is phosphorylated leading to the generation of an active Stat92E dimer that translocates to the nucleus, binds to a consensus TTCNNNGAA site, and alters gene expression. Socs36E is one of the best-characterized Stat92E target genes and encodes a negative regulator of JAK/Receptor activity (orange). B) Model of the MAPK pathway. The EGF ligand Spitz (Spi, magenta) is produced by germ line cells. Spi activates the EGF receptor (Egfr, dark gray) on the surface of CySCs, which triggers the canonical MAPK pathway. Activation of Egfr leads to the recruitment of Son of Sevenless (Sos, dark blue), a guanine exchange factor for Ras85D (Ras) that converts Ras from an inactive GDP-loaded form (light gray) to an active GTP-loaded form (yellow). Ras activates Rolled (Rl, blue), a Drosophila MAPK, which inhibits transcriptional repressors (orange) Yan and Capicua (Cic) and activates Pointed (Pnt), a transcriptional activator (green). C-E) MAPK activity (dpERK, red) in control clones, Egfr mutant clones and Socs36E mutant clones. Clones are negatively-marked and are identified by the lack of GFP. C) dpERK can be detected in a control CySC clone (C,C’, arrow), which lacks GFP (green), at similar levels to a neighboring wild type CySC (C,C’, arrowhead). These data indicate that MAPK activity is normally present in CySCs. MAPK activity (dpERK) is decreased in an Egfr mutant CySC (D,D’, arrow), compared to neighboring wild type CySCs (D,D’, arrowheads). E) dpERK is elevated in Socs36E mutant CySCs (E,E’ arrows) compared to a neighboring wild type CySC (E,E’, arrowhead). F) Quantification of fluorescence intensity in clones, expressed as a ratio of intensity within a CySC compared to that of its immediate unmarked wild type neighbor CySC. Asterisks indicate statistical significance ** P<0.01 and *** P<0.001. Tj is blue in C-E. The hub is outlined by a dotted line. Scale bar = 5 μM.
The Drosophila testis is an ideal model system to study questions of signal regulation and integration in stem cells [6]. The testis niche, called the hub, supports two stem cell populations. The first, germ line stem cells (GSCs), gives rise to sperm after several transit-amplifying divisions leading up to meiosis. The second, somatic cyst stem cells (CySCs), gives rise to cyst cells, the essential support cells for germ line development. Many ligands for signaling pathways are produced by the hub, including the JAK/STAT pathway agonist, Unpaired (Upd), the Hedgehog (Hh) pathway ligand Hh and the Bone Morphogenetic Protein (BMP) homologs Decapentaplegic (Dpp) and Glass Bottom Boat (Gbb) [7–11]. The latter two signals are also produced by CySCs and are required in GSCs for self-renewal, indicating that CySCs constitute part of the niche for GSCs along with the hub [10,12]. CySCs require JAK/STAT and Hh activity for self-renewal [8,13–15].
CySCs and GSCs compete for space at the niche, a phenomenon that was revealed by the analysis of testes lacking the JAK/STAT feedback inhibitor Socs36E [16,17]. In these animals, excessive JAK/STAT activity was detected in CySCs, and Socs36E mutant CySCs displaced the resident wild type GSCs. Additionally, we recently showed that CySCs with sustained Hh signaling or sustained Yorkie (Yki) activity also outcompeted neighboring wild type GSCs, indicating that several signaling pathways can control niche competition [18]. Moreover, we showed that prior to out-competing GSCs, mutant CySCs displaced neighboring wild type CySCs, indicating that both intra- (CySC-CySC) and inter-lineage (CySC-GSC) competition take place in the testis. While the two types of competition appear related, in that one precedes the other, there are instances in which only intra-lineage competition takes place [19]. While the competitive phenotype of Socs36E mutant CySCs was ascribed to increased JAK/STAT signaling [16,17], we were surprised to find that clonal gain-of-function in JAK/STAT signaling in CySCs did not induce competitive behavior, and we concluded that loss of Socs36E did not mimic increased JAK/STAT signaling in CySCs [18].
Here, we address whether other mechanisms could account for the competitive behavior of Socs36E mutant CySCs. Because SOCS proteins can inhibit MAPK signaling in cultured cells and in Drosophila epithelial tissues [5,20,21], we examined if Socs36E repression of MAPK signaling underlied the Socs36E competitive phenotype. Indeed, we find that Socs36E inhibits MAPK signaling in CySCs during self-renewal, and that gain of MAPK activity induces CySCs to outcompete wild type CySCs and GSCs at the niche. We dissect the genetic relationship between Socs36E and the MAPK and JAK/STAT pathways and show that loss of Socs36E can compensate for decreased self-renewal signaling within CySCs. Thus, we show that CySCs integrate multiple self-renewal signals through the use of a feedback inhibitor that controls at least two signaling pathways regulating stem cell maintenance at the niche.
Results
Gain-of-function in MAPK resembles loss of Socs36E
Loss of Socs36E in the somatic lineage leads to the displacement of GSCs at the niche by mutant CySCs [16]. Although Socs36E is a well-described inhibitor of JAK/STAT signaling [22–25], we previously determined that elevating JAK/STAT pathway activity in CySCs did not cause the displacement of GSCs [18]. Therefore, we asked whether another signaling pathway known to be inhibited by Socs36E—the MAPK pathway—could be responsible for the niche colonization phenotype by Socs36E mutant CySCs in the testis. Previous work has found that over-expression of Socs36E inhibited MAPK activity and conversely Socs36E knock down enhanced MAPK-dependent tumorigenesis [20–22,24]. Increased MAPK activity in Socs36E-depleted cultured cells suggested that Socs36E directly regulated the MAPK pathway [20], but whether this occurs in vivo is yet to be established. MAPK signaling is activated by several extracellular ligands, the best characterized of which are epidermal growth factors (EGFs), acting through the EGF receptor (Egfr) (reviewed in [3]). Upon ligand binding to Egfr, intracellular adaptors recruit the Ras guanine exchange factor (GEF) Son of Sevenless (Sos). Sos activates Ras and initiates a phosphorylation cascade resulting in MAPK (called Rolled (Rl) in Drosophila) activation and subsequent gene transcription alterations through several ETS domain-containing transcription factors (Fig 1B). EGF ligands are present in the testis; germ cells produce Spitz (Spi) while somatic cells express vein [26,27].
First, we tested whether MAPK signaling levels were regulated by Socs36E in the testis. We induced mutant clones for Socs36E and stained for phosphorylated MAPK (dpERK), an established readout for pathway activity [28]. In testes with control clones, we observed dpERK staining in CySCs as well as in differentiating cyst cells (Fig 1C, arrow for stem cell clone and arrowhead for unmarked CySC). The dpERK staining in CySCs is dependent on Egfr activity because the dpERK signal was autonomously lost in Egfr mutant clones (Fig 1D, compare arrow to arrowheads). By contrast, we found that dpERK staining was autonomously increased in Socs36E mutant CySCs (Fig 1E, compare arrows to arrowhead). Because dpERK staining was variable, we compared the fluorescence intensity of the marked clone with that of its immediate wild type CySC neighbor (Fig 1F). This analysis revealed a significant decrease in dpERK intensity in Egfr mutant clones (P = 0.0008) and a significant increase in Socs36E mutant clones (P = 0.0071). While a previous study reported that Socs36E did not regulate dpERK using Socs36E mutant testes [17], our clonal analysis provides better resolution and allows for direct comparison of mutant and wild type CySCs in the same tissue. Taken together, these data suggest that Socs36E negatively regulates the MAPK pathway in normal CySC function, in addition to its known role in repressing JAK/STAT activity.
Next, we sought to establish whether increased MAPK signaling in somatic cells in the testis could cause CySCs to outcompete GSCs for space at the niche and recapitulate the loss of GSCs observed in Socs36E mutant testes (S1 Table and [16]). We used the somatic cell driver Traffic jam (Tj)-Gal4 to hyper-activate MAPK in all CySCs and their lineage. In controls, we found 13.6 GSCs contacting the hub, and the nuclei of CySCs, marked with Zfh1, were visible behind the GSCs (Fig 2A and 2F). When we over-expressed either a dominant-active form of the EGF receptor (λTop) or of MAPK, Rolled (RlSEM), we observed CySCs contacting the hub directly in the place of GSCs (Fig 2B and 2F). We counted the number of GSCs in these genotypes and found that hyper-activation of MAPK in CySCs resulted in a significant loss of GSCs non-autonomously (Fig 2F, S1 Table, 13.6 in control vs 9.4 in UAS-λTop and vs 9.8 in UAS-RlSEM, P<0.0013 and P<0.0025, respectively). We note that we did not see an increase in βPS-integrin when MAPK signaling was hyper-activated in CySCs or when Socs36E was lost from these cells (S1 Fig). Additionally, when we over-expressed a very strong activator of the pathway, RasV12, using Tj-Gal4 we observed a dramatic loss of GSCs (1.5 GSCs in UAS-RasV12 testes) (Fig 2C and 2F, S1 Table), indicating that the strength of competition between CySCs and GSCs depends on the level of MAPK activation in CySCs.
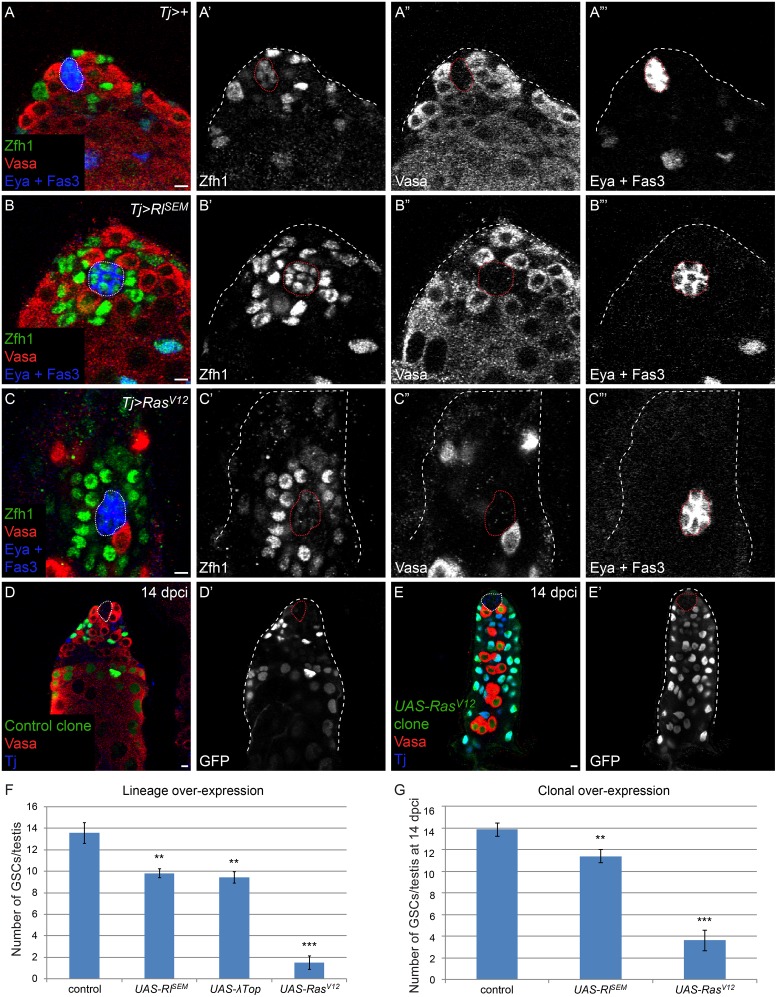
Fig 2. MAPK gain-of-function in CySCs causes them to out-compete GSCs at the niche.
A) A normal complement of Vasa-positive GSCs and Zfh1-positive CySCs at the niche and Eya-positive differentiating cyst cells is observed in a control a Tj-Gal4 (Tj>+) testis. B) GSCs are displaced by CySCs over-expressing dominant-active form of MAPK (RlSEM). C) GSCs are almost entirely out-competed by CySCs over-expressing a dominant-active Ras (RasV12). In this image, only one GSC remains in contact with the niche and there are numerous CySCs immediately contacting hub cells. We note that the position of the hub frequently shifts slightly basally in Tj>RasV12 over-expressing testes. D) A testis with control MARCM clones at 14 dpci, identified by the expression of GFP within the clone. Note that some CySCs and their differentiating progeny are clonally marked with GFP and there is a normal complement of GSCs at the niche. E) A testis with UAS-RasV12 MARCM clones at 14 dpci. The entire somatic lineage is clonally marked, indicating that RasV12-expressing CySCs out-competed wild type CySCs. There are two GSCs in contact with the niche, indicating that RasV12-expressing CySCs also out-competed most GSCs in this testis. Germ line differentiation is also perturbed, presumably because somatic cells with high Ras activity cannot adequately support gonial cells [44]. F) Graph displaying the number of GSCs in control (Tj-Gal4), Tj>RlSEM, Tj>λTop, and Tj>RasV12 testes. GSC number is moderately but significantly reduced in Tj>RlSEM and Tj>λTop and substantially reduced in Tj>RasV12 testes. G) Graph displaying the number of GSCs in testes containing control, RlSEM-expressing and RasV12 -expressing CySC clones. Note the significant drop in GSCs when CySC clones expressing UAS-RlSEM and UAS-RasV12 are present. **P< 0.01, ***P<0.001. Vasa is red in all panels. Zfh1 is green and Fas3 and Eya are blue in A-C. MARCM clones, which express GFP, are green and Tj is blue in D and E. The hub is outlined by a dotted line. Scale bar = 5 μM.
Finally, as these experiments tested CySC-GSC competition using lineage-wide over-expression, we wanted to determine whether a single CySC clone with increased MAPK activation could outcompete wild type CySCs (CySC-CySC competition) and GSCs (CySC-GSC competition) for space at the niche. We used the MARCM technique [29] to generate control clones, or clones that over-expressed either RlSEM or RasV12. At 14 days post clone induction (dpci), we observed that wild type clones labelled a variable fraction of CySCs, consistent with CySCs undergoing stochastic loss and replacement (Fig 2D and [18]). However, clones in which MAPK was hyper-activated had replaced most wild type CySCs by 14 dpci (Fig 2E) and also had outcompeted resident GSCs, leading to a significant reduction in GSC numbers (Fig 2G). These phenotypes closely resemble the effects that we and others observed in Socs36E mutant clones (see below and [16]). Specifically, we observed 13.9 GSCs in testes with control clones and 8.4 GSCs in testes with Socs36E clones (P<5.2x10-8). In contrast, gain-of-function of JAK/STAT signaling in CySC clones did not lead to GSC loss [18]. Together, our results suggest that both CySC-CySC and CySC-GSC competition induced by Socs36E loss is due primarily to the increase in MAPK activity in these cells, rather than that of JAK/STAT.
MAPK pathway activity regulates CySC numbers
The results presented above suggest that CySCs undergo MAPK signaling and are responsive to changes in levels of its activity. Indeed, labelling with dpERK antibody reveals that the MAPK signaling pathway is active in wild type CySCs (Fig 1C and [26,27]). Although MAPK has been shown to be required during cyst cell differentiation in the testis [26,27,30,31], its role in the CySCs themselves is unclear. Previous work has reported that persistent Egfr or raf mutant clones are not recovered, but there is a debate as to whether this reflects a requirement for MAPK signaling in CySCs during self-renewal [17,27,30].
In order to clarify the role of MAPK in CySCs, we addressed whether MAPK signaling affected CySC numbers and self-renewal. First, we examined testes from flies carrying a temperature-sensitive mutation in Egfr in trans to a loss-of-function allele (referred to as Egfrts). When shifted to the restrictive temperature, these testes displayed the previously-described phenotype of a block in germ cell development, resulting in many small germ cells throughout the testes and a complete lack of differentiated spermatid fibers (Fig 3A and 3B and [27,30]). Notably, these testes also have fewer somatic cells near the hub (Fig 3A and 3B). We labelled these somatic cells with Zfh1 to mark the CySCs and their offspring and with Eya to mark differentiated somatic cells. In Egfrts testes, Eya expression was observed in somatic cells adjacent to the hub, suggesting that CySCs differentiate early in the absence of MAPK signaling (Fig 3B, arrows), corroborating prior observations [30,32]. We counted the number of CySCs (defined as Zfh1-positive, Eya-negative cells) in these samples and found that there were significantly fewer CySCs in Egfrts testes compared to control (Fig 3G, S1 Table, P<2.7x10-12). It was important to exclude Eya-expressing cells from the Zfh1 pool in this analysis because a prior study using only one somatic marker did not note a difference in somatic cell number when MAPK was decreased [32].
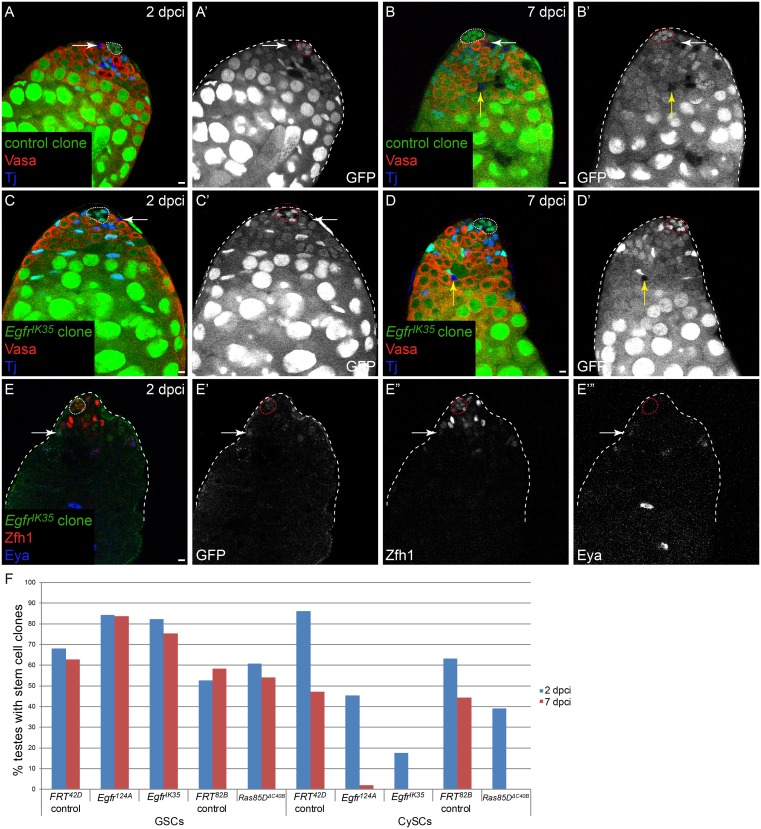
Fig 3. MAPK signaling regulates CySC numbers.
A) A representative testis from an Egfr/+ animal contains a rosette of GSCs in contact with the niche surrounded by a row of Zfh1-positive CySCs. The arrow highlights a differentiating cyst cell that has started to express Eya. B) A testis from an Egfrts animal that was upshifted to the restrictive temperature of 29°C for 10 days. Note the accumulation of many small Vasa-positive early germ cells and a reduction in the number of Zfh1-positive cells. Arrows mark somatic cells close to the niche that have turned on expression of the differentiation marker Eya. C) Markers of the somatic lineage in a control Tj-Gal4 (Tj>+) testis. Tj (blue) is expressed at low levels in the hub and at higher levels in CySCs and early cyst cells. Zfh1-positive CySCs (green) are observed near the niche and Eya (red) begins to be expressed in differentiating cyst cells. Arrow marks a differentiating cyst cell that is several rows away from the niche and that has upregulated expression of Eya. D) Tj>MAPK RNAi testis. Note the reduction in Zfh1-positive cells and upregulation of Eya in somatic cells close to the niche, which is not observed in controls (compare to arrow in C). E) A normal complement of Vasa-positive GSCs and large differentiating spermatogonia in a control Tj-Gal4 (Tj>+) testis. F) In a Tj>MAPK RNAi testis, there is a block in germ cell differentiation, an accumulation of many small early germ cells and a total lack of differentiating spermatogonia, similar to Egfrts testes at the restrictive temperature (see B). G,H) Graphs showing the number of CySCs, defined as Zfh1-positive, Eya-negative cells, in Egfr/+, Egfrts, control (Tj-Gal4), Tj>MAPK RNAi, Tj>λTop, and Tj>RlSEM testes. (G) Note a significant drop in CySCs in Egfrts testes compared to Egfr/+. H) Note significant decrease in CySCs in Tj>MAPK RNAi testes compared to control and a significant increase in the number of CySCs in Tj>λTop and Tj>RlSEM testes (see S1 Table for “n” values). Vasa is red in A,B,E,F. Zfh1 is green in all panels. Fas3 and Eya are blue in A,B,E,F and red in C,D. The hub is outlined by a dotted line. Scale bar = 5 μM.
To determine whether the requirement for MAPK in maintenance of CySCs is autonomous to the somatic lineage, we used Tj-Gal4 to over-express an RNAi against MAPK (Fig 3D). These testes displayed a phenotype similar to that which we observed in testes in which the entire animal was mutant for Egfr (Fig 3B). In Tj>MAPK RNAi testes, Eya-expressing cells were present close to the hub (Fig 3D”, arrow). However, in control Tj>+ testes, Eya-expressing cells were located several cell diameters from the hub (Fig 3C”, arrow). We counted significantly fewer Zfh1-positive, Eya-negative CySCs when MAPK signaling was inhibited autonomously within the somatic lineage (Fig 3H, S1 Table, P<2.3x10-12). Moreover, as expected, proper germ cell development was inhibited in Tj>MAPK RNAi testes, resulting in an accumulation of small early germ cells (Fig 3F). Conversely, when we hyper-activated MAPK in somatic cells by over-expressing λTop or RlSEM, there were significantly more Zfh1-expressing, Eya-negative cells (Fig 3H, S1 Table, P<1.4x10-11 for UAS-λTop and P<1.0x10-7 for UAS-RlSEM). Taken together, these experiments indicate that MAPK acts autonomously within the somatic lineage to regulate CySC numbers.
MAPK signaling is required autonomously for CySC self-renewal
As not all CySCs were lost when we inhibited MAPK within the whole somatic lineage, we generated mutant clones for components of the MAPK pathway to determine whether CySC clones with compromised MAPK signaling were able to self-renew. We generated both positively-marked and negatively-marked clones of several alleles of Egfr and the Drosophila Ras, Ras85D, and scored the presence of marked stem cells at 2 dpci, to verify that mutant clones could be induced, and at 7 dpci, to assess the ability of the mutant clones to self-renew at the niche. Egfr or Ras85D mutant GSCs were recovered and maintained at similar rates to control GSCs (Fig 4F, Table 1 for negatively-marked clones, S2 Table for positively-marked clones). However, CySCs mutant for Egfr or Ras85D were recovered less well at 2 dpci and were not maintained by 7 dpci (Fig 4F, Tables 1 and S2). We note that another group reported that Ras85DΔC40B null mutant clones were recovered at higher rates than control clones [17]. However, our results that CySCs lacking Ras85D function do not self-renew are supported by our use of multiple alleles of several pathway components (see below). Moreover, our results are consistent with prior reports that persistent Egfr or raf mutant somatic clones were not recovered [27,30]. We determined the fate of the clones that were induced but not recovered at later time points. We were able to detect Egfr mutant clones that expressed the differentiation marker Eya by 2 dpci (Fig 4E, arrow marks a positively-labeled clone), suggesting that Egfr mutant clones differentiate rapidly. Thus, our results indicate that MAPK activity is required autonomously in CySCs for self-renewal and that MAPK-deficient stem cells are rapidly lost from the niche and differentiate. The fact that somatic knock down of MAPK reduced CySC numbers by ~45% (Fig 3H), whereas all MAPK pathway mutant clones were lost, strongly suggests that CySCs lacking MAPK activity are primarily lost as a result of competition by their wild type CySC neighbors. Therefore, we conclude that MAPK signaling regulates the ability of CySCs to compete for space at the niche.
Fig 4. MAPK is required autonomously for CySC self-renewal.
In A-D, clones are marked by the absence of GFP and in E, MARCM clones are marked by the presence of GFP. A) Testis with a control CySC clone (arrow) at 2 dpci. B) Testis with a control CySC clone at 7 dpci (B,B’, white arrow). Yellow arrow denotes a labeled differentiating cyst cell. C) Testis with an Egfr mutant clone (arrow) at 2 dpci, indicating that clones of this genotype can be induced. D) At 7 dpci, Egfr mutant CySC clones cannot be recovered. However, the differentiating offspring of these mutant clones are observed (yellow arrow). E) At 2 dpci, an Egfr mutant CySC (arrow) has already begun to differentiate as it has low levels of Zfh1 and has started to express Eya. F) Graph showing clone recovery rates for negatively-marked clones (mutant GSCs and mutant CySCs) at 2 and 7 dpci. See Table 1 for “n” values. Loss of Egfr or Ras does not affect the maintenance of GSCs. By contrast, CySC clones lacking either gene have reduced clone recovery rates at 2 dpci and are not maintained at 7 dpci. Vasa is red and Tj is blue in A-D. Zfh1 is red and Eya is blue in E. The hub is outlined by a dotted line. Scale bar = 5 μM.
Table 1. Negatively marked Egfr or Ras85D mutant CySC clones are not recovered.
| 2 dpci | 7 dpci | |||
|---|---|---|---|---|
| % testes with marked clone | GSCs (n) | CySCs (n) | GSCs (n) | CySCs (n) |
| FRT42D control | 68 (72) | 86 (72) | 63 (70) | 47 (70) |
| Egfr124A | 84 (64) | 45 (64) | 84 (49) | 2 (49) |
| EgfrIK35 | 82 (51) | 18 (51) | 75 (53) | 0 (53) |
| FRT82B control | 53 (19) | 63 (19) | 58 (36) | 44 (36) |
| Ras85DΔC40B | 61 (23) | 39 (23) | 54 (24) | 0 (24) |
n: number of testes scored
Clones were negatively-marked and were identified by the absence of GFP.
Socs36E mutant clones lose their competitive advantage when MAPK signaling is impaired
We have shown that Socs36E represses MAPK activity in addition to its known role in repressing JAK/STAT signaling. Therefore, we sought to clarify the relationship between Socs36E and these two signaling pathways in CySC self-renewal, in particular to establish which pathway was the functionally relevant target of Socs36E regulation. We used the MARCM technique to generate GFP-expressing clones lacking both Socs36E and MAPK activity and monitored the ability of these clones to self-renew and compete with wild type CySCs for niche occupancy. CySCs mutant for either of two alleles of Socs36E (Socs36EEY and Socs36EPZ) self-renewed better than control clones at all time points, indicating that they are less likely to be lost through neutral competition (Fig 5J and 5K, Table 2 and [18]). We generated control clones expressing a dominant-negative form of Ras (RasN17) and determined that, while they were recovered at 2 dpci, few marked CySCs were found at 7 dpci (Fig 5A and 5J, Table 2, Fisher’s exact test P<0.0001, FRT40A, UAS-RasN17 compared to control FRT40A), consistent with our earlier observations that MAPK-deficient clones cannot be maintained in the niche. Surprisingly, Socs36E mutant clones expressing RasN17 were able to self-renew and were recovered robustly at 7 dpci (Fig 5B, arrows, Fig 5J, Table 2, Fisher’s exact test P<0.0001, Socs36EEY FRT40A, UAS-RasN17 compared to FRT40A, UAS-RasN17). One caveat of this experiment could be that MAPK activity was incompletely blocked by expression of RasN17. Therefore, we generated clones that were doubly mutant for Socs36E and a component of the MAPK pathway. Sos encodes a Ras GEF and is located on the same chromosome arm as Socs36E (2L), enabling the generation of clones doubly mutant for Socs36E and Sos (i.e., Socs36E mutant cells that are deficient for MAPK signal transduction).
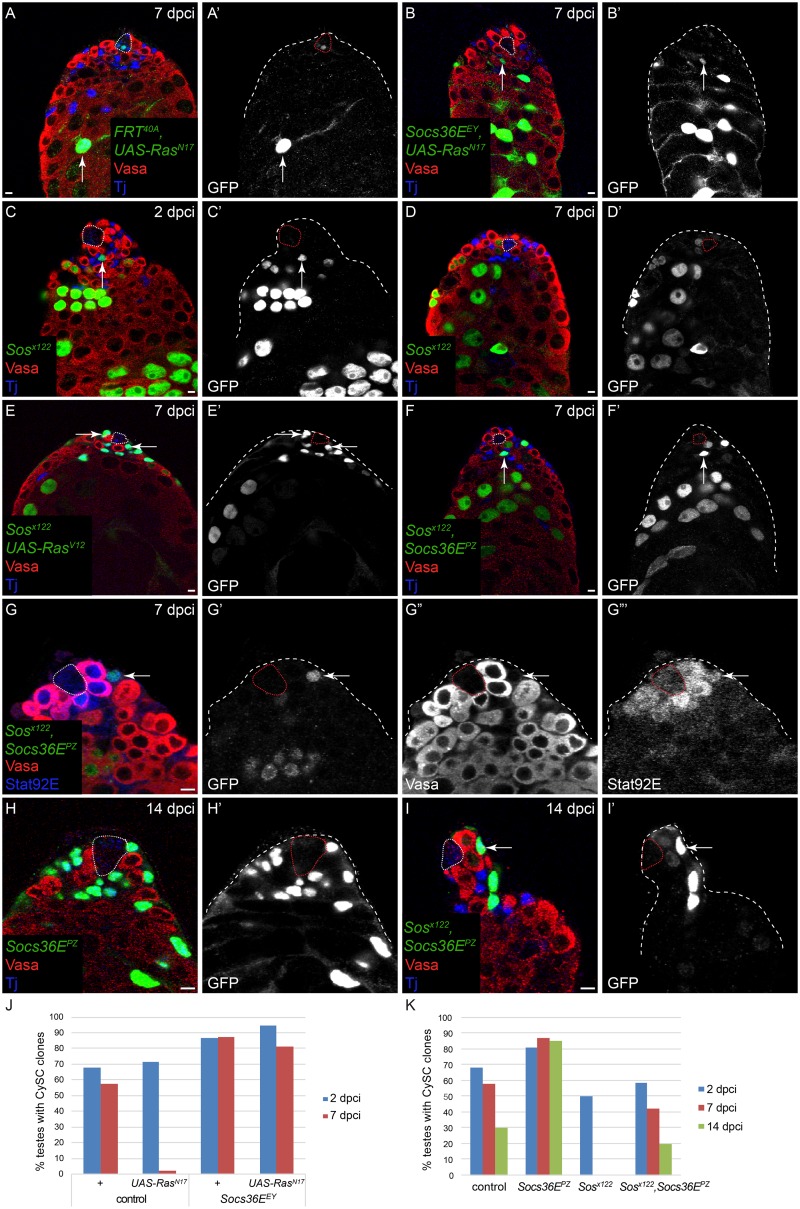
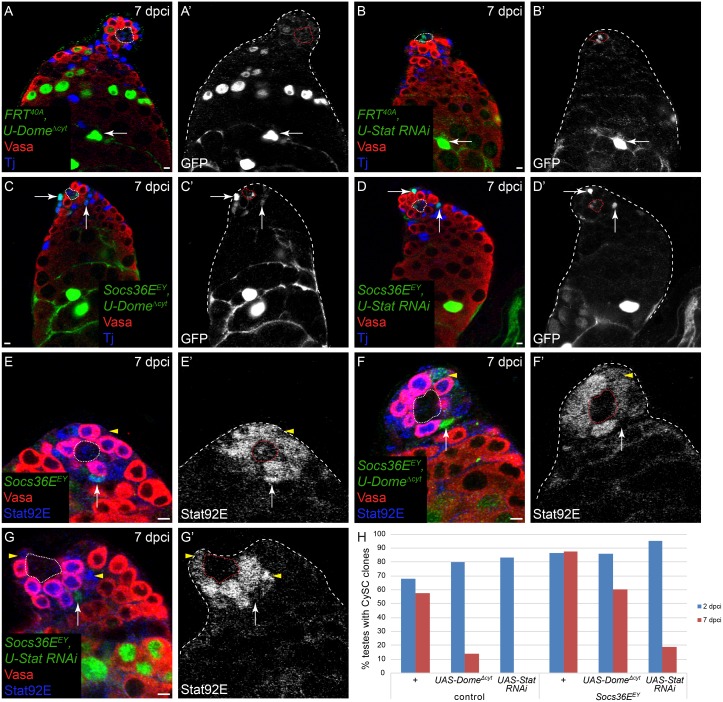
Fig 5. Socs36E regulates CySC competition via MAPK.
All clones were generated by the MARCM technique and are positively-marked by the expression of GFP. A) UAS-RasN17 CySC clones are not recovered at 7 dpci but their differentiating offspring are observed (arrow). B) By contrast, Socs36E, UAS-RasN17 CySCs are readily recovered at 7 dpci (arrow). C, D) A Sos mutant CySC is recovered at 2 dpci (C, arrow) but not 7 dpci (D). E) Sos mutant CySCs are rescued when an activated form of Ras (RasV12), which functions downstream of Sos, is also expressed in the clones (E,E’, arrows). F) Sos, Socs36E double mutant CySCs are also recovered at 7 dpci (arrow). G) Sos, Socs36E double mutant clones have robust staining for Stat92E (arrow). H) Socs36E mutant clones have colonized the niche at 14 dpci. I) Sos, Socs36E double mutant CySC clones are recovered at 14 dpci (arrow), indicating clone persistence. However, unlike Socs36E single mutant clones, Sos, Socs36E double mutant clones do not colonize the niche, indicating that MAPK signaling regulates the competitiveness of Socs36E mutant clones. J,K) Graphs showing CySC clone recovery rates at 2 (blue bars), 7 (red bars) and 14 (green bars in K) dpci. Whereas CySCs expressing a dominant-negative Ras (UAS-RasN17) are not recovered at 7 dpci, Socs36E, UAS-RasN17 CySCs are robustly recovered (J). In K, control CySC clones have reduced recovery rates over time, consistent with stochastic loss and replacement [18]. By contrast, Socs36E mutant CySCs are not lost over time, indicating their competitive advantage. While Sos single mutant clones are not recovered at 7 and 14 dpci, Sos, Socs36E double mutant clones are readily recovered and in a similar fashion to control clones. Vasa is red in all panels. Tj is blue in A-F, H,I. Stat92E is blue in G. The hub is outlined by a dotted line. Scale bar = 5 μM.
Table 2. Clone recovery rates for epistasis experiments between Socs36E and the JAK/STAT and MAPK pathways.
| 2 dpci | 7 dpci | 14 dpci | ||||
|---|---|---|---|---|---|---|
| % testes with marked clone | GSCs (n) | CySCs (n) | GSCs (n) | CySCs (n) | GSCs (n) | CySCs (n) |
| FRT40A control | 56 (50) | 68 (50) | 58 (26) | 58 (26) | 55 (53) | 30 (53) |
| Sose26D | 25 (36) | 39 (36) | 38 (53) | 0 (53) | ND | ND |
| Sosx122 | 86 (50) | 50 (50) | 89 (55) | 0 (55) | ND | ND |
| Sosx122, UAS-RasV12 | 80 (10) | 50 (10) | 86 (37) | 35 (37) | ND | ND |
| Sosx122, UAS-P35 | ND | ND | 94 (47) | 0 (47) | ND | ND |
| Socs36EPZ | 48 (42) | 81 (42) | 10 (30) | 87 (30) | 42 (59) | 84 (59) |
| Sosx122, Socs36EPZ | 86 (43) | 58 (43) | 84 (55) | 42 (55) | 89 (46) | 20 (46) |
| Socs36EEY | 29 (52) | 86 (52) | 31 (16) | 87 (16) | 38 (58) | 93 (58) |
| FRT40A, UAS-DomeΔcyt | 40 (15) | 80 (15) | 59 (51) | 14 (51) | ND | ND |
| Socs36EEY, UAS-DomeΔcyt | 14 (43) | 86 (43) | 29 (58) | 60 (58) | ND | ND |
| FRT40A, UAS-Stat92E RNAi | 15 (48) | 83 (48) | 5 (82) | 0 (82) | ND | ND |
| Socs36EEY, UAS-Stat92E RNAi | 10 (21) | 95 (21) | 5 (63) | 19 (63) | ND | ND |
| FRT40A, UAS-RasN17 | 57 (35) | 71 (35) | 75 (52) | 2 (52) | ND | ND |
| Socs36EEY, UAS-RasN17 | 19 (36) | 94 (36) | 25 (64) | 81 (64) | ND | ND |
ND: not determined. n: number of testes examined.
Clones were positively-marked and were identified by the expression of GFP.
First, we confirmed that Sos mutant clones were unable to self-renew using two independent alleles (Fig 5C, 5D and 5K, Table 2). In both cases mutant clones were induced and observed at 2 dpci (Fig 5C, arrow), but no mutant CySCs were recovered at 7 dpci (Fig 5D), suggesting that like other components of the MAPK pathway, Sos is required for CySC self-renewal. To verify that the lack of self-renewal observed in Sos mutant clones was due to loss of MAPK activity in these cells, we generated Sos mutant MARCM clones in which we over-expressed RasV12, a dominant-active form that acts downstream of Sos. CySCs of this genotype were recovered at 60% of control rates, compared to 0% for Sos alone (Fig 5E, arrows, Table 2, Fisher’s exact test P<0.0001, sosx122 FRT40A, UAS-RasV12 compared to sosx122 FRT40A), indicating that increasing MAPK pathway activity downstream of Sos is sufficient to rescue self-renewal in CySCs. We took advantage of the possibility of rescuing Sos mutant CySCs to determine whether cell death played a role in eliminating clones lacking MAPK activity. We expressed the baculovirus caspase inhibitor P35 to prevent apoptosis in Sos mutant MARCM clones and scored for CySC clones at 7 dpci. Blocking apoptosis did not increase recovery of Sos mutant CySCs (Table 2). Thus, caspase-dependent cell death cannot account for the loss of MAPK signaling-deficient CySCs.
Next we analyzed CySC clones that were doubly mutant for Socs36E and Sos (Fig 5F, 5I and 5K, Table 2). These mutant CySC clones were readily recovered at 7 dpci (Fig 5F, arrow, Fisher’s exact test P<0.0001, Sosx122, Socs36EPZ FRT40A compared to Sosx122 FRT40A), like CySCs lacking Socs36E and over-expressing dominant-negative RasN17. These clones persisted for at least 2 weeks (Fig 5I and 5K), indicating long-term stem cell function. However, we noted an important difference between Socs36E single mutant and Sos, Socs36E double mutant CySC clones. Socs36E single mutant clones maintained constant clone recovery rates, indicating that they have a robust ability to bias neutral replacement and colonize the niche (Fig 5K, Table 2). Indeed, by 14 dpci, most Socs36E clones had entirely replaced all wild type CySCs at the niche (33/42 clones were fixed, meaning that they had colonized the entire niche, Fig 5H and S3 Table). However, recovery rates of Sos, Socs36E double mutant clones decreased over time, similar to the normal turnover observed in control clones (Fig 5K, Table 2, Fisher’s exact test at 7 dpci P = 0.0026, Sosx122, Socs36EPZ FRT40A compared to Socs36EPZ FRT40A). In contrast to Socs36E mutant clones, Sos, Socs36E double mutant CySCs were not able to outcompete their neighbors and few mutant CySCs were present at the niche at 14 dpci. In these testes, wild type CySCs outnumbered mutant CySCs, and no Sos, Socs36E clones were fixed, indicating that they had not colonized the niche (Fig 5I, S3 Table, Fisher’s exact test for fixed clones P<0.0001, Sosx122, Socs36EPZ FRT40A compared to Socs36EPZ FRT40A). Finally, we note that as in the case of Hh- and Yki-induced competition, the CySC-CySC competition caused by Socs36E mutation could be suppressed by removing one copy of string (stg), which encodes the Drosophila Cdc25 protein and is a limiting factor for entry into mitosis [33]. Whereas 79% of Socs36E clones were fixed at 14 dpci, only 45% of Socs36E clones were fixed when stg was reduced (S3 Table, Fisher’s exact test for fixed clones P = 0.0183, Socs36EPZ FRT40A compared to Socs36EPZ FRT40A; stg/+).
Consistent with their increased competitiveness towards CySCs, Socs36E single mutant clones also out-competed resident GSCs for niche space, significantly reducing GSC numbers (S3 Table, 8.4 GSCs/testis with Socs36E mutant clones versus 13.9 GSCs/testis with control clones at 14 dpci, P<5.2x10-8). As in the case of Hh- and Yki-induced CySC-GSC competition, the GSC reduction caused by Socs36E mutant CySCs could be suppressed by removing one copy of string (stg) (S3 Table, 12 GSCs/testis for Socs36E clones in a stg/+ background vs. 8.4 GSCs/testis for Socs36E clones in a background that was wild type for stg, P<0.00032). In contrast to Socs36E single mutant clones, the Sos, Socs36E double mutant clones did not out-compete GSCs (S3 Table, P<0.17). Notably Sos, Socs36E double mutant CySCs displayed elevated levels of stabilized Stat92E protein (Fig 5G, arrow), indicating that the JAK/STAT pathway was activated in these cells. This latter observation suggests that elevating JAK/STAT signaling is not sufficient to confer competitive ability on CySCs, consistent with our prior clonal results [18].
Socs36E mutant CySCs are able to self-renew when JAK/STAT signaling is impaired
Next, we examined whether JAK/STAT pathway activity was required for self-renewal and/or competitiveness downstream of Socs36E. Unfortunately, there is no known JAK/STAT pathway component encoded by a gene on chromosome 2L, precluding double mutant analysis. However, we used the JAK/STAT target and effector chinmo, located on 2L, as a proxy for JAK/STAT activity in CySCs [34]. As previously described, chinmo mutant CySC clones were unable to self-renew and were likely out-competed by wild type neighbors (S2B and S2D Fig and [34,35]). Importantly, chinmo Socs36E double mutant clones were recovered frequently (S2C and S2D Fig), indicating that removing Socs36E from chinmo mutant CySCs restored their ability to compete with neighbors. Additionally, these double mutant clones over-proliferated and formed ectopic masses of somatic cells (S2C Fig, arrow), suggesting they were mis-specified, consistent with work showing that chinmo is required to maintain the male identity of CySCs [35].
In order to assess directly the role of JAK/STAT signaling downstream of Socs36E, we used the MARCM technique to inhibit pathway activity in Socs36E mutant clones. We used two approaches: first we expressed a dominant-negative form of the receptor Domeless (Dome [36]), called DomeΔcyt, and second we expressed an RNAi transgene against the transcription factor Stat92E. In control clones, knocking down JAK/STAT activity with either approach led to a marked loss of self-renewal: by 7 dpci very few clones expressing DomeΔcyt were maintained and clonal depletion of Stat92E was sufficient to abolish self-renewal (Fig 6A, 6B and 6H, Table 2). Socs36E mutant CySCs that expressed DomeΔcyt were recovered with high frequency, similar to controls (Fig 6C, arrows, Fig 6H, Table 2, Fisher’s exact test P<0.0001, FRT40A, UAS-DomeΔcyt compared to Socs36EEY FRT40A, UAS-DomeΔcyt). Socs36E mutant CySCs depleted for Stat92E were recovered at 7 dpci (Fig 6D, arrows, Fig 6H, Table 2, Fisher’s exact test P<0.0001, FRT40A, UAS-Stat92E RNAi compared to Socs36EEY FRT40A, UAS-Stat92E RNAi), albeit at rates that were lower than control clones. Since control clones lacking Stat92E were never covered at 7 dpci, it is notable that removing Socs36E from these cells resulted in a moderate but significant rescue of self-renewal. The more robust rescue of self-renewal of Socs36E, UAS-DomeΔcyt CySCs could be due to incomplete pathway inhibition. To address this possibility, we tested whether Stat92E activity was indeed lacking in these clones by staining testes carrying Socs36E, UAS-DomeΔcyt or Socs36E, UAS-Stat92E RNAi clones with an antibody against stabilized, activated Stat92E [34]. In Socs36E mutant clones alone, as expected, we observed increased Stat92E protein (Fig 6E’, arrow, compare with wild type CySC, arrowhead). In most Socs36E mutant CySCs expressing DomeΔcyt, we observed a lack of Stat92E staining (Fig 6F’, arrow). However, a few of these CySCs displayed reduced but detectable Stat92E immunoreactivity (Fig 6F’, arrowhead), suggesting that there may be residual JAK/STAT signaling in these clones. However, in Socs36E mutant CySCs expressing Stat92E RNAi, we never observed any Stat92E protein (Fig 6G’ arrow, compare with wild type CySCs, arrowheads), indicating robust inhibition of JAK/STAT signaling in these clones. Although we cannot exclude the possibility that there may be some remaining Stat92E protein below the threshold of detection, these results suggest that Socs36E mutant CySCs are capable of renewing in the absence of Stat92E, although at reduced rates.
Fig 6. Socs36E mutant CySCs can self-renew without JAK/STAT signaling.
All clones were generated by the MARCM technique and are positively-marked by the expression of GFP. A,B) CySC clones that express a dominant-negative form of Dome (DomeΔcyt) (A), which inhibits JAK/STAT signaling, or that are depleted for Stat92E (UAS-Stat RNAi) (B), are not recovered at 7 dpci. However, their differentiating cyst descendants are observed at this time point (A’,B’, arrow). C,D) By contrast, when Socs36E is also removed from DomeΔcyt (C, arrows) or from UAS-StatRNAi (D, arrows) CySC clones, these clones are now maintained as stem cells. E) Socs36E clones have elevated levels of Stat92E (E,E’, arrow) compared with wild type CySCs (E,E’, arrowhead). Note that GSCs have higher levels of stabilized Stat92E protein than wild type CySCs. F) Some Socs36E, DomeΔcyt CySCs have residual levels of stabilized Stat92E (F,F’, arrowhead) and some have undetectable levels of stabilized Stat92E (F,F’, arrow). G) Socs36E, UAS-Stat RNAi CySCs have undetectable Stat92E levels (G’, arrow) whereas wild type CySCs in the same testis have moderate to high levels of Stat92E (G’, arrowheads). H) Graph of CySC clone recovery rates at 2 (blue bars) and 7 (red bars) dpci. CySCs expressing DomeΔcyt or UAS-Stat RNAi are not recovered at 7 dpci. By contrast, Socs36E, UAS-DomeΔcyt CySCs are recovered at robust levels at 7 dpci. Socs36E, UAS-StatRNAi CySCs are maintained at moderate levels at 7 dpci. Vasa is red in A-G. Tj is blue in A-D and Stat92E is blue in E-G. The hub is outlined by a dotted line. Scale bar = 5 μM.
Discussion
The data presented here implicate MAPK signaling as a major regulator of CySC competition for niche access and establish that the competitiveness of CySCs lacking Socs36E is derived primarily from their increased MAPK activity. The ability of a stem cell to self-renew reflects not only intrinsic properties but also extrinsic relationships with its neighbors [37]. For instance, if a cell is unable to compete for space at the niche then it will be no longer able to receive short-range niche signals and will be more likely to differentiate. Conversely, if a cell is more competitive for niche space, this cell and its offspring will replace wild type neighbors and colonize the entire niche [18,38,39].
Our data show that CySCs with increased MAPK signaling out-compete neighboring stem cells in CySC-CySC as well as CySC-GSC competition and that CySCs with reduced MAPK activity are themselves out-competed. We favor the interpretation that MAPK regulates primarily competitiveness rather than self-renewal because while MAPK mutant clones are lost from the niche, lineage-wide inhibition of the pathway does not result in a complete loss of stem cells. This contrasts with the role of JAK/STAT signaling in CySCs. Stat92E mutant CySCs are lost and lineage-wide pathway inhibition results in pronounced and rapid stem cell loss [8,12,13,40]. Based on these results, we argue that JAK/STAT signaling in CySCs primarily controls their intrinsic self-renewal capability while MAPK signaling regulates their competitiveness. Interestingly, there are important similarities between Hh and MAPK function in CySCs in that CySCs lacking Hh signal transduction are out-competed and those with sustained Hh activity out-compete wild type neighbors [14,15,18]. Lastly, we note that CySCs mutant for the tumor suppressor Hippo (Hpo) (which leads to sustained Yki activation) or Abelson kinase (Abl) also have increased competitiveness [18,19], suggesting the existence of multiple inputs controlling the ability of stem cells to stay in the niche at the expense of their neighbors. In the future, it would be interesting to determine if genetic hierarchies exist between competitive pathways or if they independently converge on similar targets. One outstanding question is how altering the competitiveness of CySCs affects the maintenance of the germ line. In the case of Socs36E, MAPK, Hh and Hpo, the competitive CySC displaces not only wild type CySCs but also wild type GSCs (this study and [16,18]). While these observations suggest that out-competition of CySCs and GSCs is linked, the result that Abl mutant CySCs only compete with CySCs and not with GSCs indicates that these two competitive processes are separable genetically [19].
It is well established that Egfr/MAPK signaling is required in somatic cells for their proper differentiation and for their encystment of the developing germ line [26,27,30,31]. In this study, we identity an additional function for Egfr/MAPK in the somatic stem cells, specifically that this pathway regulates competitiveness of CySCs, with each other and with GSCs. Regarding the latter, it is possible that the loss of GSCs when somatic cells have high MAPK signaling is linked to their possibly increased encystment by these cells. Indeed, recent work has shown that Egfr activity in CySCs regulates cytokinesis and maintenance stem cell fate in GSCs [41]. It is tempting to speculate that increased somatic Egfr activity leads to increased encystment of GSCs and loss of stem cell fate in GSCs.
MAPK may play a conserved role in niche competitiveness as mouse intestinal stem cells that acquire activating mutations in Ras bias normal stem cell replacement dynamics and colonize the niche [38,39]. Interestingly, the activating ligand Spi is produced by germ cells [26,27], suggesting that the germ line coordinates multiple behaviors in the somatic cell lineage. In addition to transducing signals from the germ line, CySCs also receive ligands from hub cells (including Hh and the JAK/STAT ligand Upd) and they have to integrate these various stimuli. If unmitigated, the combined effect of all of these signals could produce highly competitive CySCs, with overall negative effects on niche homeostasis. Our data are consistent with a model in which the induction of Socs36E by the primary self-renewal pathway (JAK/STAT) results in the restraint of a competitive trigger (MAPK) in CySCs. In this way, Socs36E acts to integrate signals from different sources and maintain homeostatic balance between resident cell populations that share a common niche (Fig 7).
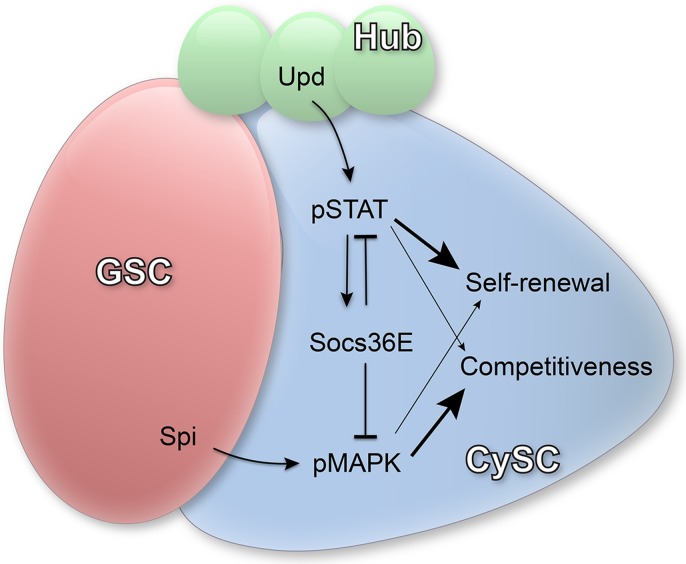
Fig 7. Model for the role of Socs36E in CySCs.
Upd is produced by hub cells (green) and activates Stat92E (pSTAT) in CySCs (blue). JAK/STAT signaling upregulates the expression of Socs36E, which negatively regulates both JAK/STAT and MAPK signaling. pSTAT in CySCs primarily controls self-renewal (bigger arrow) but may also impact competition (smaller arrow). Spi is produced by GSCs/germ cells (red) and results in the activation of MAPK (pMAPK), which primarily regulates competitive behavior (bigger arrow) of CySCs but may also impact self-renewal (smaller arrow). In the absence of Socs36E, both JAK/STAT and MAPK pathways have elevated activity, resulting in increased self-renewal and increased competition.
Materials and Methods
Fly stocks and husbandry
For a full list of genotypes, see S1 Text. The following stocks are described in fFybase (flybase.org): Egfrtsla; FRT42D EgfrIK35 (Egfrf2, Flybase); FRT82B Ras85Dx7b; FRT82B Ras85D ΔC40B; Sosx122 FRT40A (gift of N. Baker); chinmo1 FRT40A; Socs36EPZ1647 FRT40A (referred to as Socs36EPZ, gift of E. Matunis); Socs36EEY06665 FRT40A (referred to as Socs36EEY); Traffic jam (Tj)-Gal4; UAS-RlSEM;UAS-λTop 4.4 [42]; UAS-Rl RNAi (MAPK RNAi, VDRC#43123); UAS-Ras85DN17 (gift of D. Montell); UAS-DomeΔcyt (gift of J. Hombria); UAS-Stat92E RNAi (BL# 33637). FRT42D Egfr124A and Sose26D FRT40A were gifts of J. Treisman. Egfrts is Egfrtsla in trans to Egfr124A. The Sosx122 Socs36EPZ1647 FRT40A double mutant was generated by recombination and the presence of both mutations confirmed by the lack of complementation with Sose26D and the presence of β-gal. Crosses were maintained at 25°C except Tj-Gal4 crosses, which were raised at room temperature and males were shifted to 29°C after eclosion for 10 days to achieve maximum Gal4 activity. For Figs 1C–1F, 4A–4D and 4F, negatively marked clones were generated by FLP/FRT [43] and clones were scored by the absence of GFP. For Figs 2D, 2E, 2G, 4E, 5 and 6, positively marked clones were generated by the MARCM technique [29] and clones were scored by the expression of GFP. All clones were induced randomly using hs-FLP by heat shocking males at 37°C for one hour. Since these techniques rely on mitotic recombination, within the somatic lineage, clones can only be induced in CySCs, which are the only mitotic somatic cells. Within the germ-line, clones can be induced in GSCs and their transit-amplifying offspring, but only GSC clones will persist. Statistical analyses were carried out using Graph Pad Prism and MS Excel.
Immunohistochemistry
Immunohistochemistry was performed as previously described [34], except in the case of dpERK antibody, for which testes were dissected and fixed in 10 mM Tris-HCl, pH 6.8, 180 mM KCl, 50 mM NaF, 10 mM NaVO4 and 10 mM β-glycerophosphate as described in [26] and then treated as in the case of other antibodies. We used the following primary antibodies: guinea pig anti-Tj (1:3000, gift of D. Godt), rabbit anti-Zfh1 (1:5000, gift of R. Lehmann), rabbit anti-Stat92E (1:1000), rabbit anti-Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (1:200, Cell Signaling #9101), mouse anti-βPS Integrin, mouse anti-Eya, mouse anti-Fas3 (all 1:20, Developmental Studies Hybridoma Bank (DSHB), created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242), goat anti-Vasa (1:100, Santa Cruz Biotechnology), rabbit anti-GFP (1:500, Life Technologies), chicken anti-GFP (1:500, Aves Labs).
Supporting Information
A) In control testes (Tj>+), βPS-integrin (green) is observed in the muscle sheath as well as on somatic cell membranes. B) βPS-integrin is not increased in Tj>λTop testes. C,D) βPS-integrin staining is not increased in testes from Socs36EPZ/+ heterozygotes (C) or in those from Socs36EPZ homozygotes (D). (E) βPS-integrin staining is not increased in positively-marked Socs36EPZ mutant CySC clones. In E, at 14 dpci, most of the CySCs are descendants of Socs36EPZ mutant clones. βPS-integrin is green in A-D and red in E. Vasa is red and Zfh1 is blue in A-D. In E, Socs36E clones are green and Tj is blue. The hub is indicted by an asterisk. Scale bar = 5 μM.
(TIF)
A) Control CySC clones (arrow) are recovered at 7 dpci. B) By contrast, only differentiated chinmo mutant clones (arrow) are recovered at 7 dpci. C) chinmo, Socs36E double mutant CySC clones can be recovered at 7 dpci (C,C’, arrow), indicating that the loss of Socs36E rescues CySCs lacking chinmo. In fact, these double mutant clones aggregate and proliferate, consistent with the model that CySCs lacking chinmo are feminized and the lack of Socs36E rescues them for outcompetition. D) Graph showing CySC clone recovery rates at 2 (blue bars), 7 (red bars) and 14 (green bars) dpci for control clones, chinmo clones and chinmo, Socs36E double mutant clones. Removing Socs36E robustly rescues chinmo mutant CySCs at 7 dpci and these clones can still be recovered although at reduced rates at 14 dpci, indicating clone persistence. The hub is indicted by an asterisk. Scale bar = 5 μM.
(TIF)
(DOCX)
n: number of testes scored. Clones were generated by MARCM and scored by the presence of GFP. We note that the FRT82B MARCM stock does not enable accurate scoring of GSC recovery rates as the UAS-GFP in this stock is not expressed well in the germ line.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Jessica Treisman, Yannis Mavromatakis, Andrew Tomlinson, Nick Baker, Denise Montell, James Castelli-Gair Hombria, Erika Matunis, Guy Tanentzapf and Michael Fairchild, and the Bloomington Stock Center for stocks; Dorothea Godt, Ruth Lehmann and DSHB for antibodies; Cordula Schulz for the dpERK protocol; and the members of the Bach, Treisman and Ryoo labs for meaningful discussions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by NIGMS http://www.nigms.nih.gov/Pages/default.aspx Grant# R01GM085075; NYS Dept of Health/NYSTEM http://nystem.com/ Grant# N11G-292 Contract #C028132; and by The Helen L. and Martin S. Kimmel Stem Cell Postdoctoral Fellowship Program http://kimmelstem.med.nyu.edu/training-program/kimmel-senior-postdocs, Marc Amoyel - 2014 awardee. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21(1):159–71. 10.1016/j.devcel.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annual review of immunology. 2004;22:503–29. [DOI] [PubMed] [Google Scholar]
- 3.Shilo BZ. The regulation and functions of MAPK pathways in Drosophila. Methods. 2014;68(1):151–9. 10.1016/j.ymeth.2014.01.020 . [DOI] [PubMed] [Google Scholar]
- 4.Peterson AJ, O'Connor MB. Strategies for exploring TGF-beta signaling in Drosophila. Methods. 2014;68(1):183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazi JU, Kabir NN, Flores-Morales A, Ronnstrand L. SOCS proteins in regulation of receptor tyrosine kinase signaling. Cellular and molecular life sciences: CMLS. 2014;71(17):3297–310. 10.1007/s00018-014-1619-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development (Cambridge, England). 2011;138(14):2861–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science (New York, NY). 2001;294(5551):2546–9. . [DOI] [PubMed] [Google Scholar]
- 8.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science (New York, NY). 2001;294(5551):2542–5. . [DOI] [PubMed] [Google Scholar]
- 9.Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development (Cambridge, England). 1996;122(4):1125–35. Epub 1996/04/01. . [DOI] [PubMed] [Google Scholar]
- 10.Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development (Cambridge, England). 2004;131(6):1365–75. . [DOI] [PubMed] [Google Scholar]
- 11.Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr Biol. 2003;13(23):2065–72. . [DOI] [PubMed] [Google Scholar]
- 12.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nature cell biology. 2010;12(8):806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell stem cell. 2008;3(1):44–54. 10.1016/j.stem.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michel M, Kupinski AP, Raabe I, Bokel C. Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development (Cambridge, England). 2012;139(15):2663–9. Epub 2012/06/30. 10.1242/dev.075242 . [DOI] [PubMed] [Google Scholar]
- 15.Amoyel M, Sanny J, Burel M, Bach EA. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development (Cambridge, England). 2013;140(1):56–65. 10.1242/dev.086413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science (New York, NY). 2009;326(5949):153–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh SR, Zheng Z, Wang H, Oh SW, Chen X, Hou SX. Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. Journal of cellular physiology. 2010;223(2):500–10. 10.1002/jcp.22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amoyel M, Simons BD, Bach EA. Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. The EMBO journal. 2014;33(20):2295–313. 10.15252/embj.201387500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stine RR, Greenspan LJ, Ramachandran KV, Matunis EL. Coordinate regulation of stem cell competition by Slit-Robo and JAK-STAT signaling in the Drosophila testis. PLoS Genet. 2014;10(11):e1004713 10.1371/journal.pgen.1004713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herranz H, Hong X, Hung NT, Voorhoeve PM, Cohen SM. Oncogenic cooperation between SOCS family proteins and EGFR identified using a Drosophila epithelial transformation model. Genes & development. 2012;26(14):1602–11. Epub 2012/07/18. 10.1101/gad.192021.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almudi I, Stocker H, Hafen E, Corominas M, Serras F. SOCS36E specifically interferes with Sevenless signaling during Drosophila eye development. Developmental biology. 2009;326(1):212–23. 10.1016/j.ydbio.2008.11.014 . [DOI] [PubMed] [Google Scholar]
- 22.Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene. 2002;21(31):4812–21. 10.1038/sj.onc.1205618 . [DOI] [PubMed] [Google Scholar]
- 23.Stec W, Vidal O, Zeidler MP. Drosophila SOCS36E negatively regulates JAK/STAT pathway signaling via two separable mechanisms. Molecular biology of the cell. 2013;24(18):3000–9. 10.1091/mbc.E13-05-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawlings JS, Rennebeck G, Harrison SM, Xi R, Harrison DA. Two Drosophila suppressors of cytokine signaling (SOCS) differentially regulate JAK and EGFR pathway activities. BMC Cell Biol. 2004;5(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeg GH, Zhou R, Perrimon N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes & development. 2005;19(16):1861–70. 10.1101/gad.1320705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz C, Wood CG, Jones DL, Tazuke SI, Fuller MT. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development (Cambridge, England). 2002;129(19):4523–34. . [DOI] [PubMed] [Google Scholar]
- 27.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407(6805):750–4. . [DOI] [PubMed] [Google Scholar]
- 28.Gabay L, Seger R, Shilo BZ. In situ activation pattern of Drosophila EGF receptor pathway during development. Science (New York, NY). 1997;277(5329):1103–6. . [DOI] [PubMed] [Google Scholar]
- 29.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22(3):451–61. . [DOI] [PubMed] [Google Scholar]
- 30.Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407(6805):754–7. . [DOI] [PubMed] [Google Scholar]
- 31.Sarkar A, Parikh N, Hearn SA, Fuller MT, Tazuke SI, Schulz C. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr Biol. 2007;17(14):1253–8. . [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Chen X, Zheng Y. The nuclear lamina regulates germline stem cell niche organization via modulation of EGFR signaling. Cell stem cell. 2013;13(1):73–86. 10.1016/j.stem.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93(7):1183–93. . [DOI] [PubMed] [Google Scholar]
- 34.Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18(4):556–68. 10.1016/j.devcel.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Q, Wawersik M, Matunis EL. The Jak-STAT Target Chinmo Prevents Sex Transformation of Adult Stem Cells in the Drosophila Testis Niche. Dev Cell. 2014;31(4):474–86. 10.1016/j.devcel.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11(21):1700–5. . [DOI] [PubMed] [Google Scholar]
- 37.Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145(6):851–62. 10.1016/j.cell.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 38.Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science (New York, NY). 2013;342(6161):995–8. 10.1126/science.1243148 . [DOI] [PubMed] [Google Scholar]
- 39.Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO reports. 2014;15(1):62–9. 10.1002/embr.201337799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science (New York, NY). 2004;304(5675):1331–4. 10.1126/science.1097676 . [DOI] [PubMed] [Google Scholar]
- 41.Lenhart KF, DiNardo S. Somatic cell encystment promotes abscission in germline stem cells following a regulated block in cytokinesis. Dev Cell. 2015;34(2):192–205. 10.1016/j.devcel.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Queenan AM, Ghabrial A, Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development (Cambridge, England). 1997;124(19):3871–80. . [DOI] [PubMed] [Google Scholar]
- 43.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development (Cambridge, England). 1993;117(4):1223–37. . [DOI] [PubMed] [Google Scholar]
- 44.Hudson AG, Parrott BB, Qian Y, Schulz C. A temporal signature of epidermal growth factor signaling regulates the differentiation of germline cells in testes of Drosophila melanogaster. PloS one. 2013;8(8):e70678 10.1371/journal.pone.0070678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) In control testes (Tj>+), βPS-integrin (green) is observed in the muscle sheath as well as on somatic cell membranes. B) βPS-integrin is not increased in Tj>λTop testes. C,D) βPS-integrin staining is not increased in testes from Socs36EPZ/+ heterozygotes (C) or in those from Socs36EPZ homozygotes (D). (E) βPS-integrin staining is not increased in positively-marked Socs36EPZ mutant CySC clones. In E, at 14 dpci, most of the CySCs are descendants of Socs36EPZ mutant clones. βPS-integrin is green in A-D and red in E. Vasa is red and Zfh1 is blue in A-D. In E, Socs36E clones are green and Tj is blue. The hub is indicted by an asterisk. Scale bar = 5 μM.
(TIF)
A) Control CySC clones (arrow) are recovered at 7 dpci. B) By contrast, only differentiated chinmo mutant clones (arrow) are recovered at 7 dpci. C) chinmo, Socs36E double mutant CySC clones can be recovered at 7 dpci (C,C’, arrow), indicating that the loss of Socs36E rescues CySCs lacking chinmo. In fact, these double mutant clones aggregate and proliferate, consistent with the model that CySCs lacking chinmo are feminized and the lack of Socs36E rescues them for outcompetition. D) Graph showing CySC clone recovery rates at 2 (blue bars), 7 (red bars) and 14 (green bars) dpci for control clones, chinmo clones and chinmo, Socs36E double mutant clones. Removing Socs36E robustly rescues chinmo mutant CySCs at 7 dpci and these clones can still be recovered although at reduced rates at 14 dpci, indicating clone persistence. The hub is indicted by an asterisk. Scale bar = 5 μM.
(TIF)
(DOCX)
n: number of testes scored. Clones were generated by MARCM and scored by the presence of GFP. We note that the FRT82B MARCM stock does not enable accurate scoring of GSC recovery rates as the UAS-GFP in this stock is not expressed well in the germ line.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.